Abstract
Background
Predicting refractory coagulopathy early in resuscitation of injured patients may decrease a leading cause of preventable death. We hypothesized that clot strength (G) measured by point-of-care rapid thrombelastography (r-TEG) on arrival in the emergency department can predict massive transfusion (MT) and coagulation-related mortality (MT-death).
Methods
Trauma alerts/activations from May 2008 to September 2010 were reviewed. The variables included the following: age, sex, injury severity score (ISS), systolic blood pressure (SBP), base deficit (BD), traditional coagulation tests (international normalized ratio ([INR], partial thromboplastin time [PTT]), TEG-derived G, and blood products transfused within the first 6 hours. Independent predictors of 2 outcomes (MT [≥10 packed red blood cells units/6 h] and MT-related death) were identified using logistic regression. The individual predictive values of BD, INR, PTT, and G were assessed comparing the areas under the receiver operating characteristic curves (AUC ROC), while adjusting for age, ISS, and SBP.
Results
Among the 80 study patients, 48% required MT, and 21% died of MT-related complications. INR, ISS, and G were independent predictors of MT, whereas age, ISS, SBP, and G were independently associated with MT-death. The predictive power for outcome MT did not differ among INR (adjusted AUC ROC = 0.92), PTT (AUC ROC = 0.90, P = .41), or G (AUC ROC = 0.89, P = .39). For outcome MT-death, G had the greatest adjusted AUC ROC (0.93) compared with the AUC ROC for BD (0.87, P = .05), INR (0.88, P = .11), and PTT (0.89; P = .19).
Conclusion
These data suggest that the point-of-care TEG parameter clot strength (G) provides consistent, independent prediction of MT and MT-death early in the resuscitation of injured patients.
Uncontrolled hemorrhage remains one of the leading causes of preventable death after trauma worldwide.1,2 Predicting the development of refractory coagulopathy early in the injured patient’s hospital course has the potential to decrease morbidity and mortality related to this disorder. Several measures have been tested as predictors of this lethal condition, such as base deficit (BD).3 Although acidosis is a known risk factor for developing refractory coagulopathy,4 BD does not directly measure coagulation status. Prothrombin time (PT)/international normalized ratio (INR) and activated partial thromboplastin time (PTT) assess clotting function; however, these tests only measure the fluid phase of coagulation, neglecting the contribution of platelets and fibrin to clot formation. In addition, PT/INR and PTT testing are not typically available for the initial assessment of a severely injured patient.
Recently, we have implemented a point-of-care rapid thrombelastography (TEG) protocol for injured patients requiring immediate transfusion of red blood cells (RBC).5 TEG offers a comprehensive assessment of the coagulation system from the onset of clot formation through fibrinolysis. Our previous work6 identified the TEG parameter clot strength (G) as an accurate predictor of death within the first 3 hours of arrival to the emergency department.
Yet, we recognized the need for a faster identification of high-risk patients. Thus, we hypothesized that point-of-care measurement of clot strength (TEG parameter G) could predict the impending need for massive transfusion (MT) and coagulation-related mortality within 15 minutes of arrival to the hospital.
METHODS
Trauma activations at the Rocky Mountain Regional Trauma Center, housed at the Denver Health Medical Center, from May 2008 to September 2010 were reviewed. The Rocky Mountain Regional Trauma Center is a state-designated level 1 trauma center verified by the American College of Surgeons Committee on Trauma and the academic trauma center for the University of Colorado Denver. The data collection and storage processes were in compliance with Health Insurance Portability and Accountability Act and approved by our institutional review board (COMIRB 10-0477).
Study inclusion criteria were as follows: age greater than 15 years, injury severity score (ISS) greater than 15, and both BD and rapid TEG (r-TEG) obtained on arrival at the emergency department per our standard resuscitation protocols. Of the 732 consecutive patients reviewed, 83 had their resuscitation guided by TEG and their arterial BD values obtained at the direction of the attending physician. Of the 83 patients who met the criteria for study inclusion, 3 died from traumatic brain injury (TBI) and were excluded, leaving a total cohort size of 80 patients.
Patient outcomes of interest were MT and coagulation-related death. Based on our previously published work,7 the majority of blood products were given to this patient cohort within the first 6 hours; therefore, MT was defined as ≥10 units of PRBC in the first 6 hours after admission. Because a retrospective determination of the precise cause of death (ie, coagulopathy, exsanguination, or combination of lethal factors) was not possible, we proposed an objectively defined outcome, namely coagulation-related mortality to classify these deaths. Coagulation-related mortality (MT-death) was therefore defined as death after receiving a MT (≥10 PRBC units/first 6 hours postinjury).
Clinical data analyzed included the following: age, sex, mechanism of injury, ISS, and systolic blood pressure (SBP) on admission, as well as units of blood products transfused (ie, PRBC, fresh frozen plasma [FFP], platelets, cryoprecipitate) and crystalloid infusion within the first 6 hours. The ISS was derived from the Trauma Registry calculated at the time of patient discharge by a trauma coordinator. The INR, activated PTT, and BD were performed in our central laboratory.
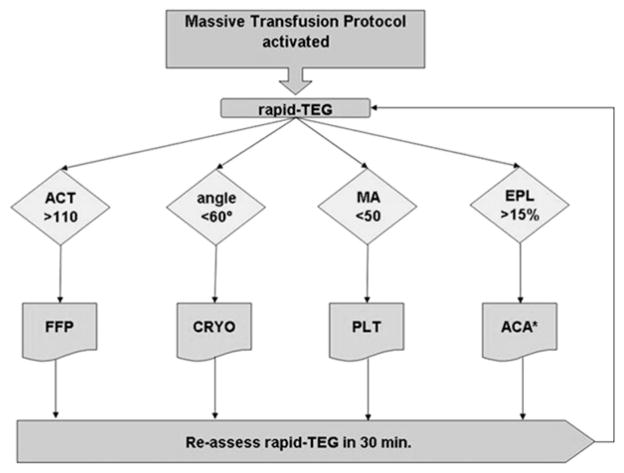
r-TEG was performed on patients deemed at risk for acute coagulopathy by the attending surgeon. Successive r-TEG analyses were performed per our MT protocol, which guides blood component transfusion using TEG variables (Fig 1). Noncitrated, whole blood samples were analyzed by r-TEG in our central laboratory (TEG-5000 series; Haemonetics, Boston, MA). A total of 10 μl of r-TEG solution (8% kaolin, human recombinant tissue factor, phospholipids, buffers, and stabilizers), used as an activator, were added to 0.36 mL of whole blood within 4 minutes of blood collection, placed in cuvettes, and warmed to 37.3°C.
Fig 1.
Algorithm for goal-directed massive transfusion. The transfusion algorithm relies on thrombelastography (TEG) tracing variables to direct blood component therapy. r-TEG, Rapid TEG; ACT, activated clotting time; MA, maximum amplitude; EPL, estimated per cent lysis; FFP, fresh frozen plasma; CRYO, cryoprecipitate; PLT, platelet; ACA, aminocaproiate.
A torsion wire suspended within the rotating cup measured the impedance of developing platelet-fibrin bonds. This impedance was displayed graphically on r-TEG tracings. The following parameters were recorded from the tracings of the r-TEG: TEG–activated clotting time (ACT, seconds), angle (α, degrees), coagulation time (K, seconds), maximum amplitude (MA, mm), clot strength (G, dynes/cm2) and lysis 30 minutes after MA (LY30, %). Results for all components of clot formation were available within 10 minutes.
The significance of each parameter listed above has been well described in the literature.8 The TEG parameter G, a global reflection of clot strength, is calculated from the amplitude (A) based on a curvilinear relationship: G = (5000 × A)/(100 − A). Our previous work5 has identified G within the first 3 hours of patient arrival as a significant predictor of death; thus, G was the r-TEG variable compared to conventional clinical tests.
The predictive power of the various clinical and laboratory variables were evaluated using multiple logistic regression. Age, ISS, SBP, BD, INR, PTT, and G were assessed as the independent variables. Categorical variables were reported as percentages and continuous variables as mean ± standard error of the mean (SEM) when normally distributed, or as median with interquartile range (IQR) if otherwise. Normally distributed continuous variables were evaluated using analysis of variance (ANOVA) or the Student t test. If the variables were not normally distributed, they were analyzed with the Wilcoxon nonparametric test. The predictive power of individual tests of each adverse outcome were examined by comparing the area under the receiver operating characteristic curves (AUC ROC) using the c statistic, while controlling for age, ISS, and SBP. All statistical analyses were performed using commercially available statistical software (SAS version 9.2 for Windows; SAS Institute, Cary, NC).
RESULTS
Demographics and the hospital course for the 80 study patients are depicted in Table I. Mean patient age was 34.2 ± 1.5 years, and 82% were men. The mean ISS was 29 ± 2, and 62% sustained penetrating wounds. On arrival at the emergency department, the mean SBP, diastolic blood pressure, and heart rate were 77 ± 6 mm Hg, 46 ± 4 mm Hg, and 88 ± 6 bpm, respectively. Patients received a median transfusion of 9 PRBC units (IQR, 3–16), 4 FFP units (IQR, 0–8), 1 apheresis pack of platelets (IQR, 0–2) and 2 cryoprecipitate units (IQR, 0–2) within the first 6 hours of arrival. A median 7.8 L of crystalloid (IQR, 4.9–10.0) were given during this interval.
Table I.
Demographics and hospital course
| All patients (N = 80) | |
|---|---|
| Age, y* | 34 ± 2 |
| Male, n (%) | 65 (81) |
| Activations, n (%) | 70 (88) |
| Mechanism, n (%) | |
| Blunt | 38 (30) |
| Penetrating | 62 (50) |
| ISS* | 29 ± 1 |
| SBP, mm Hg* | 77 ± 6 |
| DBP, mm Hg* | 46 ± 4 |
| HR, bpm* | 88 ± 5 |
| PRBC, first 6 h† | 9 (3–16) |
| FFP, first 6 h† | 4 (0–8) |
| Platelets, first 6 h† | 1 (0–2) |
| Cryo, first 6 h† | 2 (0–2) |
| Crystalloid, first 6 h† | 7.8 (4.9–10.0) |
| ICU-free, d* | 9.3 ± 1.9 |
| Ventilator-free, d* | 13.8 ± 1.8 |
| Adverse outcomes, n (%) | |
| Massive transfusion | 37 (46) |
| Death | 19 (24) |
| MT-related death | 17 (21) |
Data are presented as mean ± standard error of the mean (SEM).
Data are presented as median (interquartile range).
ISS, Injury severity score; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; PRBC, packed red blood cells; FFP, fresh frozen plasma; Cryo, cryoprecipitate; ICU, intensive care unit; MT, massive transfusion.
The median number of days that the patients were not in the intensive care unit was 11 (IQR, 0–22) and the median number of days they were not on a ventilator was 22 (IQR, 0–26). Of the 80 patients, 37 (46%) underwent MT (PRBC ≥ 10 units/6 hours). The overall mortality rate was 24%, and MT-related deaths occurred in 21% of patients.
To compare the clinical and laboratory variables of the patients on their arrival at the emergency department (Table II), the 80 patients were placed in 4 groups: (1) all patients (N = 80); (2) blunt without TBI (N = 30); (3) blunt with TBI (N = 15); and (4) penetrating injuries (N = 50). The mean BD was 15 ± 1 mEq/L and the mean INR and PTT on arrival were 2.2 ± 0.1 and 67 ± 7 seconds. From the initial r-TEG tracing obtained within 15 minutes after hospital admission, the mean G value was 4.9 ± 0.3 dynes/cm2. Table III uses the same variable to compare patients with no MT (N = 42) with patients with MT (N = 38) and patients who survived (N = 61) with patients who died (N = 19).
Table II.
On-arrival variables
| Variables* | All patients (N = 80) | Blunt (N = 30) | Blunt + TBI (N = 15) | Penetrating (N = 50) | Normal range |
|---|---|---|---|---|---|
| Age, y | 34 ± 2 | 39 ± 13 | 37 ± 12 | 31 ± 13 | NA |
| ISS | 29 ± 1 | 38 ± 15 | 41 ± 11 | 24 ± 8 | NA |
| SBP, mm Hg | 77 ± 6 | 81 ± 58 | 94 ± 59 | 69 ± 52 | 90–120 |
| BD, mmol/L | 15 ± 1 | 14 ± 7 | 13 ± 7 | 15 ± 7 | 0–6 |
| Lactate, mmol/L | 8.4 ± 0.7 | 8.2 ± 4.9 | 6.8 ± 3.9 | 8.5 ± 6.0 | 0–3 |
| INR, s | 2.2 ± 0.1 | 2.14 ± 1.36 | 2.06 ± 1.18 | 2.16 ± 1.14 | 0.82–1.49 |
| PTT, s | 67 ± 7 | 72 ± 69 | 69 ± 69 | 64 ± 61 | 20–40 |
| G, dynes/cm2 | 4.9 ± 0.3 | 4.9 ± 2.7 | 5.0 ± 2.7 | 4.9 ± 2.7 | 5.1–11.0 |
Data are presented as mean ± standard error of the mean (SEM).
TBI, Traumatic brain injury; NA, not applicable; ISS, injury severity score; SBP, systolic blood pressure; BD, base deficit; INR, international normalized ratio; PTT, activated partial thromboplastin time; G, clot strength.
Table III.
On-arrival variables, cont’d
| Variables* | No MT† (N = 42) | MT† (N = 38) | Survivors (N = 61) | Nonsurvivors (N = 19) | Normal range |
|---|---|---|---|---|---|
| Age, y | 36 ± 13 | 32 ± 14 | 33 ± 13 | 37 ± 16 | NA |
| ISS | 25 ± 9 | 34 ± 15 | 27 ± 11 | 37 ± 15 | NA |
| SBP, mm Hg | 101 ± 46 | 48 ± 49 | 89 ± 51 | 38 ± 46 | 90–120 |
| BD, mmol/L | 11 ± 6 | 18 ± 6 | 13 ± 7 | 19 ± 5 | 0–6 |
| Lactate, mmol/L | 5.2 ± 3.3 | 11.8 ± 5.6 | 6.6 ± 4.1 | 14.0 ± 5.9 | 0–3 |
| INR, s | 1.5 ± 0.4 | 2.86 ± 1.35 | 1.84 ± 0.79 | 3.14 ± 1.60 | 0.82–1.49 |
| PTT, s | 35 ± 15 | 101 ± 78 | 49 ± 32 | 122 ± 98 | 20–40 |
| G, dynes/cm2 | 6.5 ± 2.3 | 3.1 ± 1.7 | 5.6 ± 2.5 | 2.5 ± 1.3 | 5.1–11.0 |
Data are presented as mean ± standard error of the mean (SEM).
Massive transfusion (MR) >10 red blood cell units/2 hours).
MT, Massive transfusion; NA, not applicable; ISS, injury severity score; BD, base deficit; INR, international normalized ratio; PTT, activated partial throm-boplastin time; G, clot strength.
Independent predictors of each multiple logistic regression model are depicted in Table IV. INR, ISS, and G were significant independent predictors of MT with an AUC ROC of 0.92 (95% confidence interval [CI], 0.86–0.97), whereas age, ISS, SBP, and G were independently associated with overall mortality. When the 2 outcomes were combined to examine MT-specific mortality, age, ISS, SBP, and G emerged as significant independent predictors. Of note, ISS and G consistently predicted both adverse outcomes assessed in this study.
Table IV.
Independent predictors of massive transfusion and coagulation-related death*
| Model AUC ROC | 95% CI | P value | |
|---|---|---|---|
| Massive transfusion (MT) | 0.92 | 0.86–0.97 | |
| ISS | .026 | ||
| INR, s | .013 | ||
| G, dynes/cm2 | .019 | ||
| Coagulation-related death (MT + death) | 0.93 | 0.87–0.98 | |
| Age, y | .057 | ||
| ISS | .118 | ||
| SBP, mm Hg | .027 | ||
| G, dynes/cm2 | .004 |
Variables entered: Age, ISS, SBP, BD, INR, PTT, and G.
AUC ROC, Area under the receiver operating characteristic curve; CI, confidence interval; ISS, injury severity score; INR, international normalized ratio; G, clot strength; SBP, systolic blood pressure.
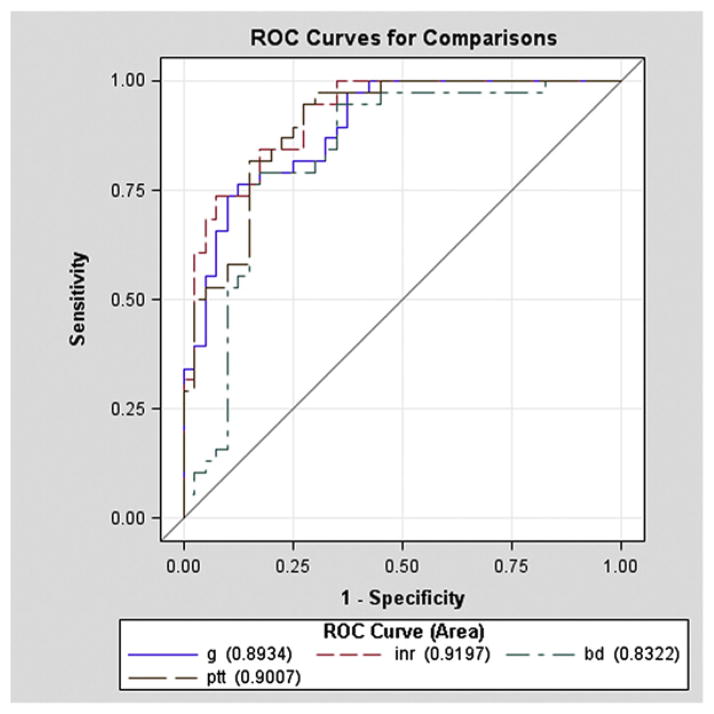
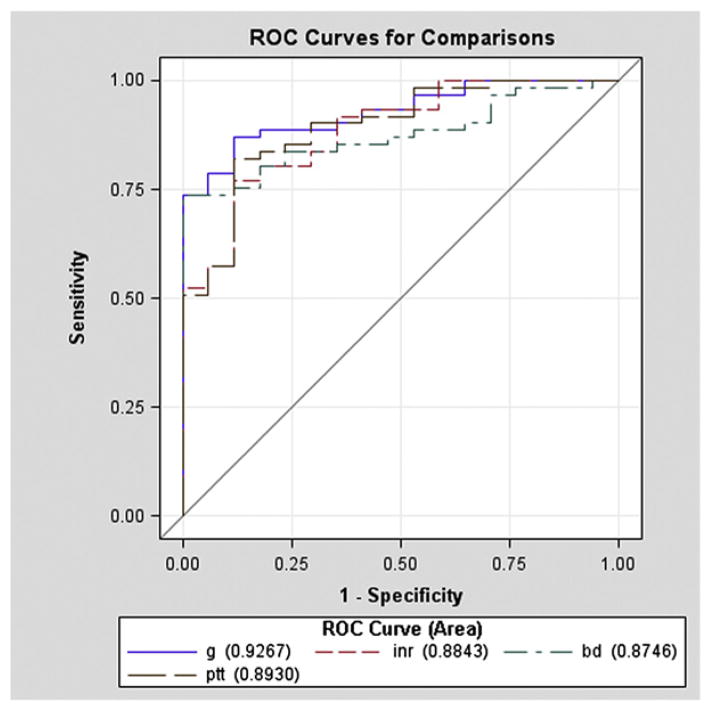
The individual discriminatory value of BD, INR, PTT, and G was determined by comparing the AUC ROC for each adverse outcome while adjusting for age, ISS, and SBP (Table V). For MT as the outcome, prognostic significance was no different when comparing INR (AUC ROC = 0.92), PTT (AUC ROC = 0.90; P = .41), and G (AUC ROC = 0.89; P = .39). BD had an AUC ROC of 0.83 for MT, which was significantly less compared to INR (P = .03) and only marginally less than the AUC ROC for G (P = .10) (Fig 2). For MT-related mortality as the outcome, G had the greatest AUC ROC (AUC ROC = 0.93), which was marginally greater than BD (AUC ROC = 0.87; P = .05) and INR (AUC ROC = 0.88; P = .11) and no different than PTT (AUC ROC = 0.89; P = .19 (Fig 3).
Table V.
Predictive power of base deficit, INR, PTT, and G*
| Model AUC ROC | 95% CI | |
|---|---|---|
| Massive transfusion (MT) | ||
| BD, mmol/L | 0.83 | 0.74–0.93 |
| INR, s | 0.92 | 0.86–0.98 |
| PTT, s | 0.90 | 0.83–0.97 |
| G, dynes/cm2 | 0.89 | 0.83–0.96 |
| Coagulation-related death (MT + death) | ||
| BD, mmol/L | 0.87 | 0.80–0.95 |
| INR, s | 0.88 | 0.80–0.97 |
| PTT, s | 0.89 | 0.81–0.97 |
| G, dynes/cm2 | 0.93 | 0.87–0.98 |
Adjusted for age, ISS, and SBP.
AUC ROC, Area under the receiver operating characteristic curve; CI, confidence interval; BD, base deficit; INR, international normalized ratio; PTT, activated partial thromboplastin time; G, clot strength.
Fig 2.
The predictive power of clot strength (G), international normalized ratio (INR) (INR), partial thromboplastin time (PTT), and base deficit (BD) with massive transfusion (MT) as the outcome (adjusting for age, injury severity score [ISS], and systolic blood pressure [SBP]). With MT as the outcome, INR was not different using PTT and G. BD had an area under the receiver operating curve (AUC ROC) of 0.83, which was significantly less than the ROC for INR and was marginally less compared to G.
Fig 3.
The predictive power of clot strength (G), international normalized ratio (INR) (INR), partial thromboplastin time (PTT), and base deficit (BD) with coagulation-related death as the outcome (adjusting for age, injury severity score [ISS], and systolic blood pressure [SBP]). After investigating coagulation-related mortality as the outcome, G had the best predictive power, which was marginally greater than BD and INR and no different than PTT.
DISCUSSION
Recent studies document that approximately 25% of severely injured patients have an established coagulopathy on arrival to the emergency department, underscoring the necessity for prompt recognition and treatment.9,10 While the precise cause of the acute coagulopathy of trauma continues to be debated,11,12 blood component therapy remains its foremost treatment. Yet, nationwide blood product shortages and the risks associated with blood transfusions highlight the need for a judicious transfusion strategy in the trauma population.13–15 Thus, the development of quick, precise, and dynamic assessments of the coagulation status to support point-of-care protocols remains of great importance.
Several recent civilian and military studies have sought to find early clinical and laboratory predictors of MT and resulting death. A multicenter, retrospective review of 5,700 injured patients in a civilian trauma center evaluated the predictive power of the following variables on arrival: injury pattern, SBP, BD, and PT, achieving an AUC ROC of 0.81.16 A combat study found that anemia, abnormal INR, and penetrating torso injuries independently predicted the need for MT with an AUC ROC curve of 0.80.17 A study carried out by Larson et al18 reached a similar conclusion when they examined similar variables (ie, SBP, heart rate, BD, and hemoglobin), although the sensitivity was only 69%.
Leemann et al19 evaluated TEG and demonstrated that abnormal maximal clot firmness, which reflects G and a low hemoglobin value (≤10 g/dL) on admission were independently associated with the need for MT (AUC ROC = 0.82). Encouraged by our previous experience identifying abnormal clot strength (TEG parameter G) in the first 3 hours of arrival as predictive of mortality,5 we designed the present study to address the role of r-TEG to identify the patient at risk for MT and coagulation-related mortality within a lesser time interval (ie, 15 minutes after admission).
Traditionally, the initial assessment of injured patients has included SBP, injury pattern, and BD, because these variables are widely available within 15 minutes of patient arrival. Because of its availability, BD has been used as an independent predictor of mortality.1,20–22 Recent clinical evidence, however, suggests that endothelial ischemia initiates an endogenous coagulopathy through activation of protein C via increased thrombomodulin expression.8 Although BD greater than 6 mEq/L correlates with prolonged clotting times,23 BD does not predict MT. Indeed, BD is an indirect measure of tissue hypoperfusion and does not reflect the patient’s coagulation status, and thus BD lacks specificity in identifying coagulopathy and predicting related outcomes. In contrast, r-TEG measures global clot formation and, according to our findings, serves as an early, accurate marker of coagulopathy-related outcomes.
While G, INR, and PTT were comparable in predicting both outcomes, r-TEG results were available within 15 minutes, whereas INR and PTT required 30 minutes or more for results. r-TEG results were available early enough during resuscitation to initiate and guide component therapy, which is a clear limitation of both INR and PTT. Although the increasing availability of point-of-care coagulation assays (INR and PTT) could eliminate this time constraint, INR and PTTare still limited in their analysis of clot formation. Current concepts of coagulation recognize a comprehensive model involving plasma (clotting/fibrinolysis factors), platelets, and potentially vascular endothelium (eg, activated protein C pathway). Both INR and PTT overlook the contribution of fibrinolysis and platelets to hemostasis and thus are unable to accurately guide component transfusion.
Our study had several limitations. First, we carried out a retrospective assessment. Although every patient had a detailed medical record, determination of the exact cause of death (ie, coagulopathy, bleeding, or acidosis) was inconclusive in some patients. Although INR and PTT have previously defined coagulopathy, this definition was unsuitable for our purposes given the current model of coagulation. In light of this change in terminology, we used MT-death to provide an appropriate term to classify these phenomena while remaining objective to the coagulation assessment.
Second, the evaluation of the coagulation status was performed only in severely injured patients and was based on the assessment of the attending trauma surgeon; however, our trauma center relies on protocol-based care, which minimizes the potential for selection bias. In addition, our study population included a great proportion of penetrating torso injuries, which are a known, independent risk factor for life-threatening coag-ulopathy,16 whereas other studies, for example, included predominately blunt head injuries.18
Third, the cohort size remains small despite the duration of 2.5 years. The incorporation of TEG as a cornerstone of the MT protocol required cooperation of multiple hospital departments and came with a considerable learning curve. As the new MT protocol developed, analysis with r-TEG on patient arrival became more consistent and extensive.
In spite of these limitations, this study suggests an effective, early approach to identifying and treating patients who are at risk for coagulation-related bleeding at the time of hospital arrival. While previous studies have relied predominantly on physiologic parameters and mechanism of injury to predict MT,24,25 ordering blood products and high-ratio plasma based solely on these parameters would have led to an unnecessarily high transfusion rate in this study population.
Acknowledgments
Supported in part by research grants from the National Institutes of Health (P50GM49222 and T32GM08315).
Footnotes
Presented at the Society of University Surgeons annual meeting, Huntington Beach, CA, February 3, 2001.
References
- 1.Cosgriff N, Moore EE, Sauaia A, Kenny-Moynihan M, Burch JM, Galloway B. Predicting life-threatening coagulopathy in the massively transfused trauma patient: hypothermia and acidoses revisited. J Trauma. 1997;42:857–61. doi: 10.1097/00005373-199705000-00016. discussion 861–2. [DOI] [PubMed] [Google Scholar]
- 2.Teixeira PG, Inaba K, Hadjizacharia P, Brown C, Salim A, Rhee P, et al. Preventable or potentially preventable mortality at a mature trauma center. J Trauma. 2007;63:1338–46. doi: 10.1097/TA.0b013e31815078ae. discussion 1346–7. [DOI] [PubMed] [Google Scholar]
- 3.Parr MJ, Bouillon B, Brohi K, Dutton RP, Hauser CJ, Hess JR, et al. Traumatic coagulopathy: where are the good experimental models? J Trauma. 2008;65:766–71. doi: 10.1097/TA.0b013e31818606d2. [DOI] [PubMed] [Google Scholar]
- 4.Kashuk JL, Moore EE, Millikan JS, Moore JB. Major abdominal vascular trauma–a unified approach. J Trauma. 1982;22:672–9. doi: 10.1097/00005373-198208000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Kashuk JL, Moore EE, Sawyer M, Le T, Johnson J, Biffl WL, Cothren CC, et al. Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Ann Surg. 2010;251:604–14. doi: 10.1097/SLA.0b013e3181d3599c. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez E, Pieracci FM, Moore EE, Kashuk JL. Coagulation abnormalities in the trauma patient: the role of point-of-care thromboelastography. Semin Thromb Hemost. 2010;36:723–37. doi: 10.1055/s-0030-1265289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, et al. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65:261–70. doi: 10.1097/TA.0b013e31817de3e1. [DOI] [PubMed] [Google Scholar]
- 8.Salooja N, Perry DJ. Thrombelastography. Blood Coagul Fibrinolysis. 2001;12:327–37. doi: 10.1097/00001721-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 9.MacLeod JB, Jynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 10.Moore EE, Moore FA, Fabian TC, Bernard AC, Fulda GJ, Hoyt DB, et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA multicenter trial. J Am Coll Surg. 2009;208:1–13. doi: 10.1016/j.jamcollsurg.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 11.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. 2007;245:812–8. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gando S. Acute coagulopathy of trauma shock and coagulopathy of trauma: a rebuttal. You are now going down the wrong path. J Trauma. 2009;67:381–3. doi: 10.1097/TA.0b013e3181a84f63. [DOI] [PubMed] [Google Scholar]
- 13.Johnson JL, Moore EE, Kashuk JL, Banerjee A, Cothren CC, Biffl WL, et al. Effect of blood products transfusion on the development of postinjury multiple organ failure. Arch Surg. 2010;145:973–7. doi: 10.1001/archsurg.2010.216. [DOI] [PubMed] [Google Scholar]
- 14.Watson GA, Sperry JL, Rosengart MR, Minei JP, Harbrecht BG, Moore EE, et al. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma. 2009;67:221–7. doi: 10.1097/TA.0b013e3181ad5957. discussion 228–30. [DOI] [PubMed] [Google Scholar]
- 15.Moore FA, Moore EE, Sauaia A. Blood transfusion. An independent risk factor for postinjury multiple organ failure. Arch Surg. 1997;132:620–4. discussion 624–5. [PubMed] [Google Scholar]
- 16.Stanworth SJ, Morris TP, Gaarder C, Goslings JC, Maegele M, Cohen MJ, et al. Reappraising the concept of massive transfusion in trauma. Crit Care. 2010;14:R239. doi: 10.1186/cc9394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schreiber MA, Perkins J, Kiraly L, Underwood S, Wade C, Holcomb JB. Early predictors of massive transfusion in combat casualties. J Am Coll Surg. 2007;205:541–5. doi: 10.1016/j.jamcollsurg.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Larson CR, White CE, Spinella PC, Jones JA, Holcomb JB, Blackbourne LH, et al. Association of shock, coagulopathy, and initial vital signs with massive transfusion in combat casualties. J Trauma. 2010;69(Suppl 1):26–32. doi: 10.1097/TA.0b013e3181e423f4. [DOI] [PubMed] [Google Scholar]
- 19.Leemann H, Lustenberger T, Talving P, Kobayashi L, Bukur M, Brenni M, et al. The role of rotation thromboelastometry in early prediction of massive transfusion. J Trauma. 2010;69:1403–8. doi: 10.1097/TA.0b013e3181faaa25. discussion 1408–9. [DOI] [PubMed] [Google Scholar]
- 20.Paladino L, Sinert R, Wallace D, Anderson T, Yadav K, Zehtabchi S. The utility of base deficit and arterial lactate in differentiating major from minor injury in trauma patients with normal vital signs. Resuscitation. 2008;77:363–8. doi: 10.1016/j.resuscitation.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Rixen D, Siegel JH. Bench-to-bedside review: oxygen debt and its metabolic correlates as quantifiers of the severity of hemorrhagic and post-traumatic shock. Crit Care. 2005;9:441–53. doi: 10.1186/cc3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutherford EJ, Morris JA, Jr, Reed GW, Hall KS. Base deficit stratifies mortality and determines therapy. J Trauma. 1992;33:417–23. doi: 10.1097/00005373-199209000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Martini WZ, Pusateri AE, Uscilowicz JM, Delgado AV, Holcomb JB. Independent contributors of hypothermia and acidosis to coagulopathy in swine. J Trauma. 2005;58:1002–9. doi: 10.1097/01.ta.0000156246.53383.9f. discussion 1009–10. [DOI] [PubMed] [Google Scholar]
- 24.Cotton BA, Dossett LA, Haut ER, Shafi S, Nunez TC, Au BK, et al. Multicenter validation of a simplified score to predict massive transfusion in trauma. J Trauma. 2010;69(Suppl 1):33–9. doi: 10.1097/TA.0b013e3181e42411. [DOI] [PubMed] [Google Scholar]
- 25.McLaughlin DF, Niles SE, Salinas J, Perkins JG, Cox ED, Wade CE, et al. A predictive model for massive transfusion in combat casualty patients. J Trauma. 2008;64(2 Suppl):57–63. doi: 10.1097/TA.0b013e318160a566. discussion 63. [DOI] [PubMed] [Google Scholar]