SUMMARY
An organism’s behavioral decisions often depend upon the relative strength of appetitive and aversive sensory stimuli, the relative sensitivity to which can be modified by internal states like hunger. However, whether sensitivity to such opposing influences is modulated in a unidirectional or bidirectional manner is not clear. Starved flies exhibit increased sugar and decreased bitter sensitivity. It is widely believed that only sugar sensitivity changes, and that this masks bitter sensitivity. Here we use gene- and circuit-level manipulations to show that sweet- and bitter-sensitivity are independently and reciprocally regulated by starvation in Drosophila. We identify orthogonal neuromodulatory cascades that oppositely control peripheral taste sensitivity for each modality. Moreover, these pathways are recruited at increasing hunger levels, such that low-risk changes (higher sugar sensitivity) precede high-risk changes (lower sensitivity to potentially toxic resources). In this way, state intensity-dependent, reciprocal regulation of appetitive and aversive peripheral gustatory sensitivity permits flexible, adaptive feeding-decisions.
Keywords: Neuromodulation, Drosophila, gustatory system, decision making, dopamine, neuropeptide
INTRODUCTION
Changes in internal states, such as emotion, arousal, starvation and sleep, affect behavioral choices in animals (Blanchard and Blanchard, 1989; Sternson et al., 2013; Taghert and Nitabach, 2012). Typically, these state-dependent influences are multidimensional, scalable and time-variant: one state can modulate multiple physiological and behavioral parameters; and the quantitative or qualitative changes caused by such modulation can vary with the intensity or duration of the state (Anderson and Adolphs, 2014). These prominent features of state-control enable animals to adjust their behavioral responses properly according to context or internal demands. However, understanding how these features are instantiated in the nervous system is challenging because it requires a comprehensive analysis of state-control pathways, including the identification of interoceptive mechanisms, neuromodulatory influences, targets of neuromodulation, and consequent behavioral changes (Bargmann, 2012).
The control of feeding in starved Drosophila melanogaster provides an attractive model for state-dependent control of behavior, because of the organism’s relatively simple nervous system, easily quantified behavioral responses, and our growing understanding of the gustatory, interoceptive, and neuromodulatory systems in this species (reviewed in (Itskov and Ribeiro, 2013; Pool and Scott, 2014)). Drosophila detects gustatory cues in foods with their taste bristles on the labellum and other parts of the body (Montell, 2009; Thorne et al., 2004). Sugar, low concentrations of salt, fatty acids and other attractive tastants are detected by gustatory receptor 5a and 64f (Gr5a and Gr64f)-expressing gustatory receptor neurons (GRNs), while toxic compounds, such as bitter substances and high concentrations of salt, are detected by Gr66-expressing GRNs (Dahanukar et al., 2007; Marella et al., 2006; Masek and Keene, 2013; Scott et al., 2001; Wang et al., 2004; Weiss et al., 2011; Zhang et al., 2013). Multiple candidate interoceptive receptors and cells have been also identified in Drosophila (Dus et al., 2013; Kim and Rulifson, 2004; Kreneisz et al., 2010; Miyamoto et al., 2012). As in mammals (Andrews et al., 2008; Luquet et al., 2005; Sternson et al., 2013), some of these interoceptive neurons express neuropeptides/neurohormones, such as adipokinetic hormone (AKH) and Drosophila insulin-like peptides (DILPs) (Kim and Rulifson, 2004; Kreneisz et al., 2010). In addition, various other neuromodulators have been shown to regulate feeding responses in starved adult Drosophila (Itskov and Ribeiro, 2013; Nassel and Wegener, 2011; Pool and Scott, 2014; Taghert and Nitabach, 2012). In particular, dNPF and sNPF, neuropeptides related to mammalian NPY, modulate multiple feeding related behaviors, including the formation and expression of food-associated memory, enhancement of food-related olfactory sensitivity, and control of food intake during starvation (Beshel and Zhong, 2013; Hergarden et al., 2012; Krashes et al., 2009; Lee et al., 2004; Root et al., 2011).
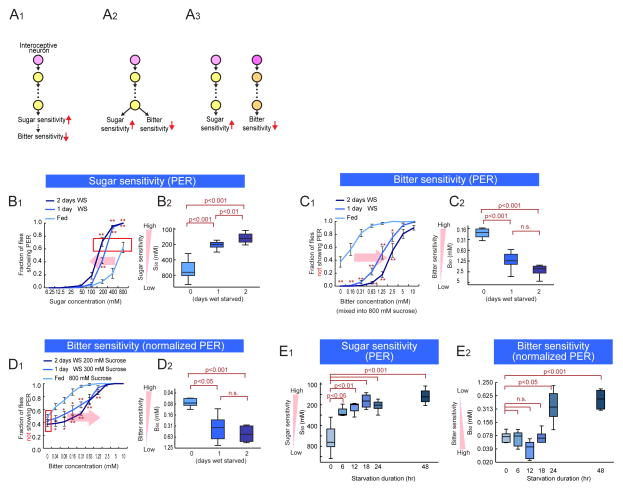
Many animal species become less selective in their food choices during periods of energy deficit. They do so by enhancing their sensitivity to nutritious resources, such as sugar (Dethier, 1976; Gillette et al., 2000; Inagaki et al., 2012; Kawai et al., 2000; Page et al., 1998; Sengupta, 2013). In Drosophila, starvation enhances behavioral sensitivity to sugar, at least in part, via increased dopamine (DA) release onto Gr5a-expressing sugar-sensing GRNs, which increases calcium responses to GR activation (Inagaki et al., 2012; Marella et al., 2012). Starvation also decreases sensitivity to unpalatable and potentially toxic compounds, such as bitter tastants. The prevailing view is that this decrease in bitter sensitivity is not independently controlled, but rather is an indirect consequence of the “masking effect” of enhanced sugar sensitivity (Figure 1A1) (Moss and Dethier, 1983).
Figure 1. Modulation of Sugar and Bitter Sensitivity During Starvation.
(A) Schematics illustrating different models to explain the reciprocal control of sugar and bitter sensitivity during starvation. (B) Fraction of flies showing PER to different concentration of sucrose at different starvation levels. (B1) Average responses. Error bars represent SEM. Two-way ANOVA followed by post hoc t-test with Bonferroni correction at each sugar concentration. *p<0.05; **p<0.005. n>5 for each experimental group. (B2) S50 (the sugar concentration at which 50% of flies show PER) plotted as a function of starvation duration. One-way ANOVA followed by post hoc t-test with Bonferroni correction. The same plotting and statistical analysis of PER assay are used throughout this paper. Red box indicates the sucrose concentrations that yield the equivalent PER responses at different starvation levels. (C, D) Fraction of flies not showing PER to different concentration of lobeline mixed into 800mM sucrose (C) or different concentrations of sucrose (D). n>5 for each experimental group. (E) S50 and B50 measured and plotted as a function of starvation duration. One-way ANOVA followed by post hoc t-test with Bonferroni correction (n>5 for each experimental group). Panels B1 and B2 are independent replications of results previously reported in (Inagaki et al, 2012) and are presented here for purposes of comparison. See also Figure S1.
Here we identify a pathway in Drosophila controlling the reduction of bitter taste sensitivity during starvation, which is mechanistically independent of the increase in sweet tastant sensitivity. This pathway combines with the masking effect of enhanced sugar sensitivity, to increase acceptance of resources containing unpalatable, potentially toxic contaminants, during periods of energy deficit (Figure 1A3). Thus the multi-dimensional features of the “hunger” state reflect bidirectional, independent and reciprocal neuromodulatory mechanisms, rather than a unidirectional control process.
RESULTS
Bitter sensitivity decreases during starvation independently of increased sugar sensitivity
To quantify food acceptance behavior, we presented a drop of solution containing sugar and/or bitter tastants to the labellum, where GRNs are located. When sugar is presented, Drosophila extend their proboscis, a reaction known as the proboscis extension reflex (PER) (Dethier, 1976). We selected this method over others because it provides quantification of gustatory sensitivity independently of food intake. As previously reported (Inagaki et al., 2012; Meunier et al., 2007), when flies are wet starved (WS; deprived of food but not water), sugar sensitivity is increased, as indicated by a leftward shift in the PER dose-response curve (Figure 1B1). In addition, the mean acceptance threshold to sugar, S50 (the sucrose concentration at which 50% of the flies show a PER) (Inagaki et al., 2012) is decreased (Figure 1B2; note that the y-axis is inverted: as sensitivity increases, S50 decreases). Importantly, the magnitude of both effects increased significantly with longer starvation times (1 day vs. 2 days), suggesting a scalable, time-variant underlying state change (Fig. 1B1–2).
Next, we tested behavioral sensitivity to unpalatable tastants by presenting a sugar solution mixed with various concentrations of bitter substances. Consistent with a previous report (Meunier et al., 2003), the admixture of a bitter tastant (lobeline) suppressed the PER to sugar in fed flies, in a dose dependent manner (Figure 1C1,“Fed”). We quantified this effect by measuring the fraction of flies not showing a PER; thus a higher value of this metric reflects a stronger suppression of the PER by bitter compounds in the presence of a fixed amount of sucrose. Genetic silencing experiments indicated that Gr66a GRNs are required for the effect of bitter substances to suppress the PER (Figure S1A), consistent with earlier studies (Gordon and Scott, 2009; Wang et al., 2004). Interestingly, during starvation there was a progressive reduction in bitter sensitivity, as indicated by a rightward shift in the dose-response curve for PER inhibition as a function of lobelline concentration (Figure 1C1,“WS”). Consistent with this, themean threshold response to bitter, B50 (the bitter concentration required to inhibit the PER in 50% of the flies that responded to a given, fixed concentration of sugar; Figure S1B), significantly increased with starvation duration (Figure 1C2; note that the y axis is inverted).
Because bitter sensitivity during starvation is quantified as the suppression of a behavioral response to sucrose, it was possible that when flies are starved, their absolute bitter sensitivity does not change, but is relatively reduced as an indirect consequence of “masking” by increased sugar sensitivity. Studies in the blowfly, Phormia regina, support this idea (Moss and Dethier, 1983), and we confirmed the masking effect for Drosphila (Figure S1C). In order to determine whether there were any independent changes in bitter sensitivity during starvation, it was necessary to offset the effect of increased sugar sensitivity in our PER assays. To do this, we reduced the fixed concentrations of sucrose used in our bitter titration experiments, at different starvation times. Thus in fed, 1 day WS and 2 day WS flies, we used 800mM, 300mM and 200mM sucrose, respectively, concentrations that yielded equivalent sub-saturating PER responses (50–60 %; see red boxes in Figure 1B1 and 1D1). Using such a “sugar-normalized PER assay”, we still observed a statistically significant decrease in lobeline sensitivity following food deprivation (Figure 1D1,2). Absolute sensitivity to other bitter tastants (caffeine and coumamine) also decreased during starvation (data not shown). Therefore during starvation, sensitivity to bitter tastants is reduced, in part, independently of the increase in sugar sensitivity (c.f. Fig. 1B2 vs. 1D2).
We next compared the kinetics of these reciprocal changes in gustatory sensitivity. Sugar sensitivity increased most strongly during the first 6 hours of starvation and continued more gradually from 6 to 48 hours (Figure 1E1). In contrast bitter sensitivity, as measured using sugar-normalized PER assays, did not decrease until after 24 hours of starvation (Figure 1E2). Thus, bitter sensitivity decreased more slowly than the increase in sweet sensitivity during starvation, suggestive of independent and inverse regulation (Fig. 1A2,3). In order to confirm the independence of these bi-directional changes in gustatory sensitivity, we investigated their underlying cellular and molecular mechanisms.
dNPF acts upstream of DA to control sugar but not bitter sensitivity
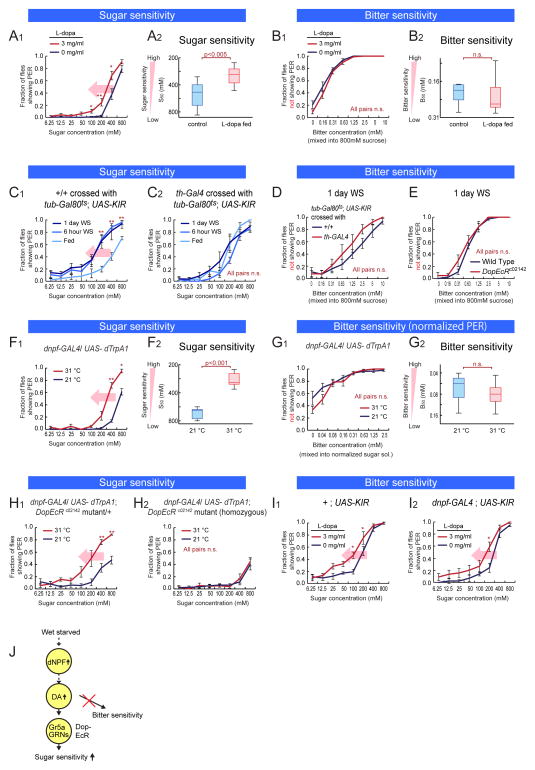
We first asked whether DA, a neuromodulator that increases sugar sensitivity during starvation (Inagaki et al., 2012; Marella et al., 2012), also decreased bitter sensitivity. To do this, we fed non-starved flies with L-dopa, a precursor of DA, which is known to increase DA levels in the fly brain (Bainton et al., 2000). As previously reported, L-dopa feeding increased sugar sensitivity in non-starved wild-type flies, mimicking the effect of starvation (Figure 2A1–2) (Inagaki et al., 2012). In contrast, L-dopa feeding did not cause a decrease in bitter sensitivity (Figure 2B1–2).
Figure 2. Neuronal Pathway Regulating Sugar Sensitivity During Starvation.
(A–B) Sugar and bitter sensitivity of non-starved wild type flies fed with L-dopa. (C–E) Sugar and bitter sensitivity of flies with genetic perturbation of dopaminergic signal. (F–G) Sugar and bitter sensitivity of flies with thermogenetic activation of dNPF neurons (w-; dnpf-GAL4 (II) crossed with w-; UAS-dTrpA1 (II); UAS-dTrpA1 (III)). For 31 °C experiments, flies were pre-incubated in 31 °C for 30 min. Bitter sensitivity was measured using normalized-sugar PER assay (sucrose concentration used: 800 mM for 21 °C and 400 mM for 31 °C). Data from non-normalized PER responses are shown in Figure S2B1. (H) Sugar sensitivity of flies with thermogenetic activation of dNPF neurons combined with DopEcR mutation (w-; dnpf-GAL4 (II); DopEcRc02142 crossed with w-; UAS-dTrpA1 (H1) or w-; UAS-dTrpA1 (II); DopEcRc02142 (H2)). (I) Sugar sensitivity of flies with L-dopa feeding combined with genetic silencing of NPF neurons. (J) Schematic illustrating neuromodulatory pathway regulating sugar sensitivity but not affecting bitter sensitivity. n>5 for all experimental groups. Panels A1–2 are independent replications of results previously reported in (Inagaki et al, 2012), and are presented here for purposes of comparison. See also Figure S2.
To further investigate this issue, we genetically silenced DA neurons by expressing the inwardly rectifying potassium channel KIR2.1 under the control of th-GAL4, a GAL4 line driven by the tyrosine hydroxylase (th) promoter. Expression of Kir2.1 was restricted to the adult phase using tub-Gal80ts, to avoid developmental lethality (Riemensperger et al., 2011). Consistent with a previous report (Marella et al., 2012), inactivation of DA neurons attenuated the increase in sugar sensitivity during starvation (Figure 2C1–2 and S2A1–2). In contrast, silencing of DA neurons did not affect bitter sensitivity (Figure 2D). The effect of DA to increase sugar sensitivity (Inagaki et al., 2012; Marella et al., 2012) is mediated by the receptor DopEcR (Srivastava et al., 2005), expressed on Gr5a GRNs (Inagaki et al., 2012). A hypomorphic mutation in DopEcR also did not affect bitter sensitivity (Figure 2E). Together, these data indicate that DA modulates sugar but not bitter sensitivity during starvation.
We next investigated neuropeptides that might control bitter sensitivity during starvation. dNPF, an orthologue of mammalian neuropeptide Y, has been shown to promote ingestion of unpalatable foods in both larval and adult Drosophila (Hergarden et al., 2012; Wu et al., 2003; Wu et al., 2005). To determine whether dNPF might directly suppress bitter sensitivity in adult flies, we artificially stimulated dNPF-expressing (dNPF+) neurons using dTrpA1 (Hamada et al., 2008) and performed PER assays at 31°C. Activation of dNPF+ neurons enhanced the sugar sensitivity of fed flies, as if they were starved, in comparison to flies of the same genotype tested at 21oC (Figure 2F1–2). In contrast, activation of dNPF+ neurons did not affect behavioral sensitivity to bitter tastants in sugar-normalized PER assays (Figure 2G1–2 and S2C1–4). None of the genetic control flies exhibited different sugar sensitivities at the permissive and non-permissive temperatures (Figure S2B1–4: note that genetic background has a significant effect on baseline gustatory sensitivity. For genetic manipulations using the GAL4-UAS system, +/UAS-effector, GAL4/+, and genetic background-matched +/+ controls were always tested in parallel to show that the behavioral effects were specific to the GAL4/UAS-effector genotype). Conversely, Kir2.1-mediated silencing of dNPF+ neurons inhibited the starvation-dependent increase in sugar sensitivity (Supplementary Figure S2D1–5), but did not interfere with the starvation-dependent decrease in bitter sensitivity (Supplementary Figure S2D6–8 and S2E). Thus, as in the case of DA neurons, the activity of dNPF+ neurons enhances sugar sensitivity, but does not independently influence bitter sensitivity.
Since both dNPF+ neurons and DA enhance sugar sensitivity during starvation, we sought to determine whether these neuromodulators function in the same or in parallel neuronal pathway(s). Immunostaining experiments indicated that dNPF and DA neurons are distinct (Figure S2F1–3). We therefore combined thermogenetic activation of dNPF+ neurons (dnpf-GAL4/UAS-dTrpA1) with a hypomorphic mutation in DopEcR, which is expressed in sugar-sensing GRNs and mediates the influence of DA on these cells (Inagaki et al., 2012). A homozygous DopEcR mutation completely blocked the increase in sugar sensitivity caused by activation of dNPF+ neurons in fed flies (Figure 2H1–2). Conversely, genetic inhibition of dNPF+ neurons did not block the effect of L-dopa feeding to increase sugar sensitivity (Figure 2I1–2). These data suggest that dNPF+ neurons act genetically upstream of DA neurons to increase sugar sensitivity (Figure 2J). Importantly, perturbations of this dNPF-DA pathway had no effect on sugar-independent changes in bitter sensitivity during starvation.
sNPF modulates bitter sensitivity during starvation without affecting sugar sensitivity
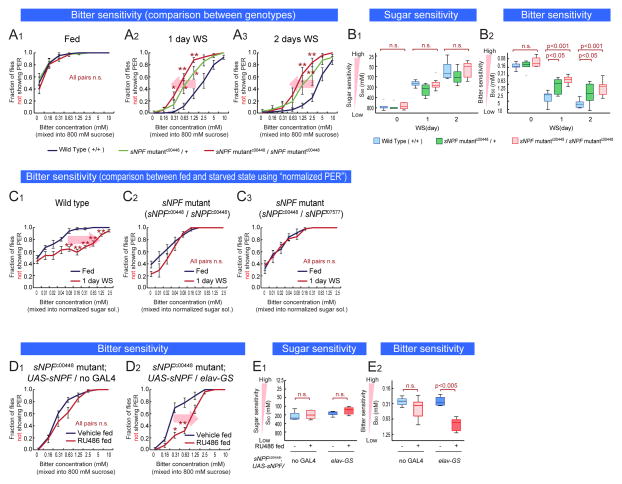
Next, we sought to identify neuromodulatory systems that mediate the decrease in bitter sensitivity during starvation. sNPF, an NPY-related protein in Drosophila, has been implicated in many hunger related behaviors (Nassel and Wegener, 2011), including the control of food intake in larvae (Lee et al., 2008; Lee et al., 2004) and food-related olfactory sensitivity in adults (Root et al., 2011). To ascertain whether sNPF is also involved in starvation-mediated control of gustatory sensitivity, we tested the behavioral sensitivity of sNPF mutant flies to bitter and sugar using the PER assay. We used two independent hypomorphic piggyBac transposon insertion (Thibault et al., 2004) alleles of sNPF, sNPFc00448 (Lee et al., 2008), and sNPFf07577 (Figure S3A: These sNPF mutant flies were introgressed into a wild type CS gentic background for at least six generations).
Food-deprived, homozygous sNPFc00448 and sNPFf07577 mutant flies were more bitter sensitive than starved genetic controls, but showed normal changes in sugar sensitivity (Figure 3A2–3 and 3B, red curves/bars; Figure S3A, S3B and S3C). Interestingly, sNPF/+ heterozygotes also showed a similar phenotype (Figure 3A2–3 and 3B2, green curves/bars), indicating haploinsufficiency of this neuropeptide gene. Importantly under fed conditions, sNPF mutant flies did not show any change in bitter sensitivity (Figure 3A1), indicating that the mutation affected starvation-dependent changes rather than baseline responsiveness. Consistent with this, sugar- normalized PER assays indicated that 1-day WS vs. unstarved homozygous sNPF mutant flies showed no difference in bitter sensitivity, in contrast to genetic background-matched wild-type controls (Figure 3C1–2). Flies trans-heterozygous for sNPFc00448 and sNPFf07577 also showed a similar phenotype (Figure 3C3).
Figure 3. sNPF is Necessary and Sufficient for Bitter Sensitivity Control During Starvation.
(A–B) Sugar and bitter sensitivity of wild type and sNPFc00448 mutant flies in the same genetic background. (C) Bitter sensitivity measured with normalized-sugar PER assays in wild type flies (C1), sNPF mutant flies (w-; sNPFc00448 (C2) and w-; sNPFc00448/sNPFf07577 (C3)). Lobeline was mixed into 800 mM sucrose solution for fed flies, or 200 mM sucrose solution for 1-day WS flies. (D–E) Sugar and bitter sensitivity of sNPF mutant flies with pan-neuronal, adult rescue of sNPF expression (w-; sNPFc00448; UAS-sNPF crossed with w-; sNPFc00448; + (D1) or w-; sNPFc00448; elav-GeneSwitch (D2)). Sucrose solution with or wihout 0.5 mM RU486 was fed to flies for 2days before experiments. n>5 for all experimental groups. See also Figure S3.
In larvae, sNPF regulates food intake and growth (Lee et al., 2008; Lee et al., 2004). To show that the bitter sensitivity phenotype in adult flies is not due to developmental effects, we rescued the expression of sNPF specifically in the adult nervous system. We expressed sNPF protein in neurons of sNPF hypomophic mutant flies using UAS-sNPF under the control of elav-GeneSwitch (elav-GS), a pan-neuronally expressed, hormone (RU486) inducible form of GAL4 (Osterwalder et al., 2001). Rescue of sNPF expression by RU486 feeding in adult flies resulted in a recovery of the starvation-induced decrease in bitter sensitivity (Figure 3D2 and 3E2), without affecting sugar sensitivity (Figure 3E1 and 3SD2). RU486 feeding did not affect bitter sensitivity in control flies lacking elav-GS, showing this is not an artifact caused by the inducer (Figure 3D1, 3E1–2, and S3D1). Altogether these results indicate that, 1) sNPF expression is necessary for the decrease in bitter sensitivity during starvation, 2) this effect is not due to a developmental function, and 3) neuronal sNPF regulates bitter sensitivity. Importantly, none of the genetic manipulations of sNPF described above affected sugar sensitivity (Figure 3B1, 3E1, S3B, S3C and S3D), suggesting that sNPF independently modulates bitter sensitivity during starvation.
Subsets of sNPF-expressing neurons regulate bitter sensitivity
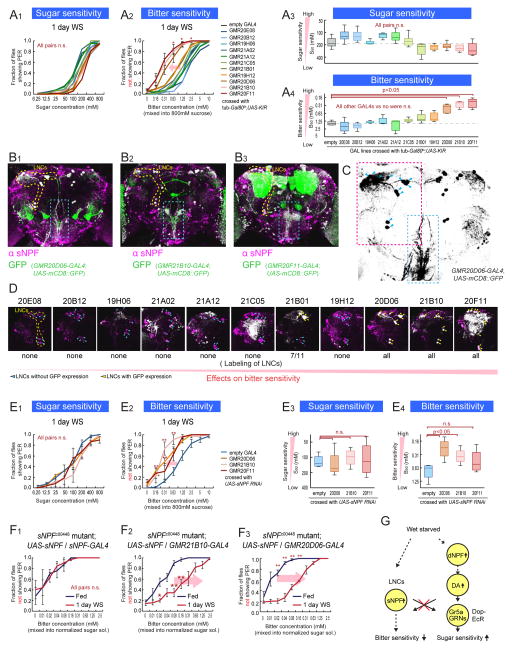
There is a large number of sNPF positive neurons, including ~4000 Kenyon cells and ~280 other neurons in the brain (Nassel et al., 2008; Nassel and Wegener, 2011) (Figure S4). To identify the subset of sNPF-expressing neurons (sNPF+ neurons) that controls bitter sensitivity, we genetically silenced different subsets of sNPF+ neurons by driving KIR2.1 expression using a panel of 11 GAL4 lines each containing different DNA fragments from the sNPF gene (Lee et al., 2009; Pfeiffer et al., 2008). Expression of KIR2.1 was restricted to adulthood using Gal80ts, and bitter sensitivity was analyzed after 1 day of wet starvation. Silencing neurons in 3 of these lines, GMR20D06-GAL4, GMR21B10-GAL4 and GMR20F11-GAL4, attenuated the starvation-induced decrease in bitter sensitivity (Figure 4A2 and 4A4), without affecting sugar sensitivity (Figure 4A1 and 4A3).
Figure 4. Subsets of sNPF Neurons Regulate Bitter Sensitivity During Starvation.
(A) Sugar and bitter sensitivity of flies with genetic silencing of different subsets of sNPF neurons. For this experiment, w-; UAS-KIR2.1; tub-Gal80ts flies were crossed with the indicated GAL4 lines or promoterless BDP-GAL4 flies (empty-GAL4). Flies were incubated at 31 °C for 2 days to inactivate Gal80ts before experiments. (B) Representative confocal projections of whole mount brains of sNPF promoter GAL4 lines crossed with UAS-mCD8::GFP flies and stained with anti-sNPF precursor antibody. Overlap of signals are shown in white color. LNCs are surrounded by yellow dotted lines. Axonal projection of LNCs are surrounded by blue dotted boxes. (C) Structure of LNCs. Blue arrowheads indicate cell bodies of LNCs. (D) Enlarge representative confocal projections of dorso-posterior side of the sNPF promoter GAL4 lines crossed with UAS-mCD8::GFP. LNCs are surrounded by yellow dotted line in the left panel. White color indicates the locations with overlap of GFP and anti-sNPF signals (Raw GFP signals in green are not shown to clarify the locations with the overlap. See Figure S4 for raw data). Blue arrowheads and yellow arrowheads indicate LNCs without and with GFP expression, respectively. (E) Sugar and bitter sensitivity of flies with UAS-sNPFR RNAi driven under the control of sNPF promoter GAL4 lines or BDP-GAL4 flies (No-GAL4). (F) Bitter sensitivity measured with normalized-sugar PER assays in sNPF mutant flies with genetic rescue of sNPF expression in different subsets of neurons (w-; sNPFc00448; UAS-sNPF crossed with w-; sNPFc00448; sNPF-GAL4 (F2) or w-; sNPFc00448; GMR21B10-GAL4 (F3)). See also Figure 3C2–3 for comparison. n>5 for all experimental groups. (G) Schematic summarizing results. See also Figure S4.
We next sought to identify the cells within these GAL4 lines responsible for the phenotype. None of the lines labeled GRNs projecting to the SEZ (Figure 4B1–3 and S4A). Instead, these lines labeled small numbers of neurons in the central brain, some of which were stained by an anti-sNPF antibody (Figure 4B1–3). Notably all 3 lines exhibited co-expression of anti-sNPF immunoreactivity and GAL4-driven GFP in a cluster of 11–12 so-called lateral neurosecretory cells (LNCs) (Nassel et al., 2008) (Cells surrounded by yellow dashed line in Figure 4B1–3 and red dashed rectangle in Figure 4C). Importantly, anti-sNPF immunocreactivity in these neurons was reduced in sNPF mutants (Figure S4B1–2). Consistent with previous observations using another GAL4 line (Kapan et al., 2012), axonal projections from LNCs to the SEZ were observed in all 3 lines (blue dashed rectangle in Figure 4B1–3 and 4C). In contrast, sNPF promoter lines whose silencing did not effect bitter sensitivity did not exhibit expression in LNCs (with the exception of GMR21B01-GAL4 (Figure 4D and Figure S4C), which is expressed in a subset of LNCs and only weakly decreased bitter sensitivity (Figure 4A)). Although it is formally possible that different subsets of neurons in each of the 3 GAL4 lines (GMR20D06-, GMR21B10- and GMR20F11- GAL4 lines) are responsible for the common phenotype, the simplest interpretation is that the effect is due in all 3 cases to silencing of the LNC sNPF+ neurons.
To investigate whether the sNPF gene itself regulates bitter sensitivity in these LNCs, we knocked down sNPF using an sNPF RNAi under the control of the GMR20D06-, GMR21B10-, and GMR20F11-GAL4 lines. This manipulation decreased bitter sensitivity during starvation, again without affecting sugar sensitivity (Figure 4E1–4). Line GMR20F11-GAL4 showed a non-significant trend overall, but showed a significant decrease in the B50 value at a concentration of 0.16mM lobeline (Figure 4E2, brown line,, asterisk). Furthermore, selective rescue of sNPF expression under the control of GMR20D06- or GMR21B10-GAL4 in the sNPF hypomorphic mutant background restored the starvation-dependent decrease in bitter sensitivity (Figure 4F2–3; compare to the phenotype of sNPF hypomorphic mutants in Figure 3C2–3). By contrast, driving UAS-sNPF expression in a different subset of sNPF+ neurons labeled by a line called sNPF-GAL4 (Lee et al., 2009) did not rescue the mutant phenotype (Figure 4F1 and S4D). Therefore, sNPF expression in a specific subset of sNPF+ neurons (LNCs) is necessary and sufficient for the effect of this neuropeptide to regulate bitter sensitivity.
Importantly, none of LNCs labeled by line 21B10-GAL4 co-expressed dNPF or TH (Figure S4E and S4F). These data, together with the lack of any effect of sNPF mutations or neuronal silencing on sugar sensitivity, suggest that the sNPF system and the dNPF-DA pathway independently regulate bitter- and sugar-sensitivity, respectively, at the neural circuit level (Figure 4G).
sNPFR is necessary and sufficient for bitter sensitivity control
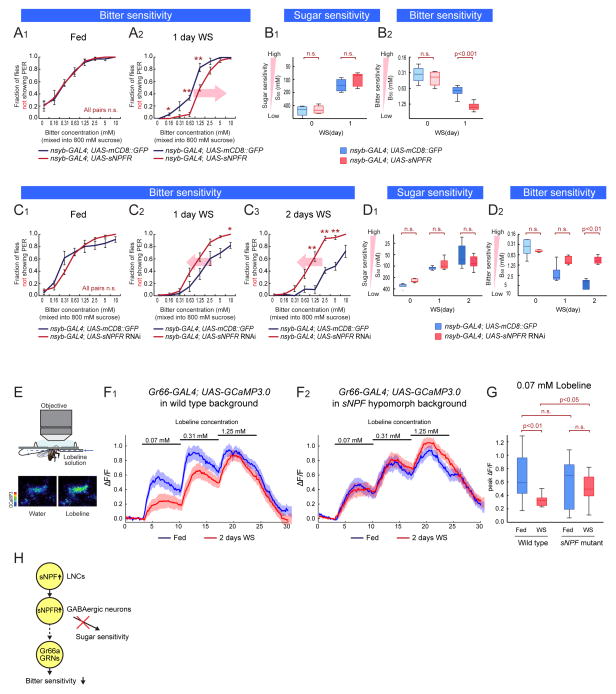
If sNPF controls the starvation-dependent decrease in bitter sensitivity, one might predict that its receptor should have a similar function. sNPF receptor (sNPFR) is the only identified G-protein coupled receptor for sNPF in Drosophila (Feng et al., 2003; Mertens et al., 2002; Reale et al., 2004). Over-expression of sNPFR using UAS-sNPFR under the control of the pan-neuronal nsyb-GAL4 driver (Fei et al., 2010) enhanced the starvation-dependent decrease in bitter sensitivity (Figure 5A1–2, B2, and S5B). Conversely, pan-neuronal knock-down of sNPFR using an sNPFR RNAi attenuated the starvation-dependent decrease in bitter sensitivity (Figure 5C1–3, and 5D2: these transgenic flies also contained UAS-Dicer2 to enhance the effects of RNAi). Importantly, these manipulations did not affect bitter sensitivity in fed flies (Figure 5A1 and 5C1), or sugar sensitivity (Figure 5B1, 5D1, S5A1–3 and S5C1–3). These data support the conclusion that sNPF plays an important role in modulating starvation-dependent changes in bitter sensitivity.
Figure 5. Modulation Target of sNPF Pathway.
(A–B) Sugar and bitter sensitivity of flies with genetic over-expression of sNPFR (w-;; nsyb-GAL4 crossed with UAS-mCD8::GFP or UAS-sNPFR. UAS-mCD8::GFP and UAS-sNPFR flies are in the same genetic background). (C–D) Sugar and bitter sensitivity of flies with genetic knock-down of sNPFR (UAS-Dicer2;; nsyb-GAL4 crossed with UAS-mCD8::GFP or UAS-sNPFR RNAi. UAS-mCD8::GFP and UAS-sNPFR RNAi flies are in the same genetic background). n>5 for each experimental group in A–D. (E) The experimental setup for calcium imaging of bitter-sensing GRNs. Blue arrow indicates direction of flow of bitter solution. The two images below the diagram are representative fields of view showing the GCaMP response of Gr66 GRNs. The fluorescent intensity of GCaMP3 is shown in pseudo-color (scale bar on left). (F) Responses (ΔF/F) to different concentrations of lobeline solution in the central projections of bitter sensing GRNs. The solid lines represent average traces, and envelopes indicate SEM (n>12 for each condition). w-; Gr66-GAL4; UAS-GCaMP3.0 (F1) and w-; Gr66-GAL4/sNPFc00448; UAS-GCaMP3.0 (F2) were used. (G) Quantification of peak fluorescent changes (ΔF/F) in response to 0.07 mM lobeline solution. One-way ANOVA followed by post hoc t-test with Bonferroni correction. (H) Schematic illustrating neuronal pathway regulating bitter sensitivity. See also Figure S5.
Downstream cellular targets of sNPF modulation
We next investigated potential cellular targets of modulation by sNFP/sNPFR in the control of bitter sensitivity. As LNCs, sNPF and sNPFR have been implicated in the regulation of insulin -producing cells (IPCs) (Kapan et al., 2012; Lee et al., 2008), we first tested whether IPCs may control bitter sensitivity in response to sNPF. However, neither IPC-specific knock down of sNPFR expression, using an Ins3P-GAL4 driver (Buch et al., 2008), nor ablation of IPCs using UAS-hid (Grether et al., 1995), affected bitter sensitivity in starved flies (Figure S5D1–2 S5E1–4; cell ablation was histologically confirmed; see Figure S5F1–2). Therefore IPCs are unlikely to mediate the modulatory influence of sNPF/sNPFR on bitter sensitivity during starvation.
Since LNCs project to the SEZ (Figure 4B1–3), we next asked whether the sNPF-sNPFR pathway might modulate the sensitivity of primary bitter-sensing GRNs. To test this hypothesis, we performed functional calcium imaging of bitter-sensing GRNs in wild type and sNPF hypomorphic mutant flies. To monitor calcium transients in bitter-sensing GRNs, we expressed a genetically encoded calcium indicator, GCaMP3.0 (Tian et al., 2009), under the control of Gr66-GAL4 (Scott et al., 2001). Consistent with previous reports (Marella et al., 2006), the axonal terminals of bitter-sensing GRNs in the subesophageal zone (SEZ) exhibited increased GCaMP3.0 fluorescence in response to increasing concentrations of lobeline applied to the labellum (Figure 5E and 5F1, blue line). Strikingly, 2 day wet starved wild-type flies showed a statistically significant reduction in GCaMP3.0 fluorescence evoked by application of 0.07mM lobeline, and a non-significant decrease at 0.31 mM lobeline (Figure 5F1, red line and 5G). Importantly, this starvation-dependent reduction in bitter responsiveness was virtually abolished in heterozygous sNPFc00448 mutant flies (Figure 5F2 and 5G), paralleling the effect of this mutation on behavioral sensitivity to bitter tastants (Fig. 3A and 3B).
To determine whether sNPF acts directly on Gr66 GRNs, we manipulated levels of sNPFR in these cells. Neither over-expression nor knock-down of sNPFR in bitter-sensing GRNs, using Gr66 and Gr33-GAL4 drivers (Moon et al., 2009) and UAS-Dicer2, affected bitter sensitivity in starved flies (Figure S5G2, S5). However, knock-down of sNPFR in GABAergic neurons using dvgat-GAL4 (Fei et al., 2010), a GAL4 line under the control of drosophila vesicular GABA transporter (dvgat) promoter, significantly attenuated the decrease in bitter sensitivity caused by starvation (Fig. S5J2, red line), without any effect on sugar sensitivity (Figure S5J1). Thus, sNPF likely modulates bitter sensing GRNs indirectly, perhaps by stimulating inhibitory neurons (Figure 5H).
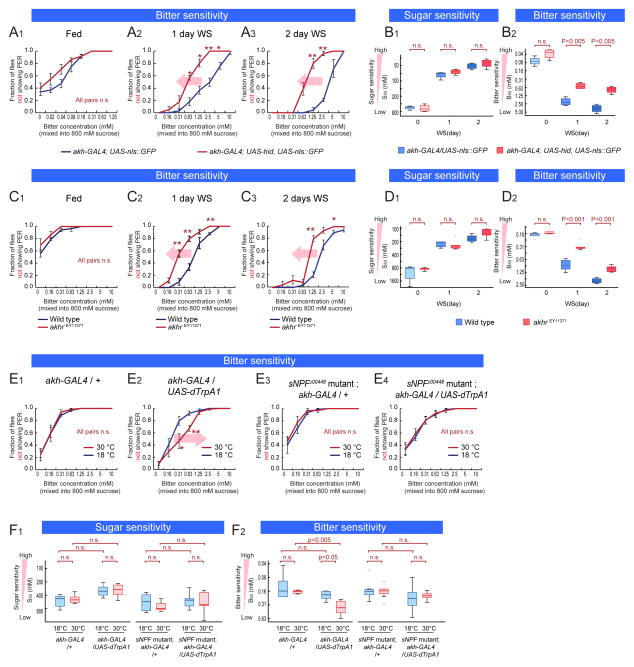
Interoceptive AKH+ neuroendocrine cells act upstream of sNPF to control bitter sensitivity
We next searched for interoceptive neurons that might act upstream of the sNPF pathway to regulate bitter sensitivity during starvation. The corpora cardiaca (CC) contains interoceptive neuroendocrine cells that release the peptide adipokinetic hormone (AKH), a fly analog of glucagon, during starvation (Kim and Rulifson, 2004). Genetic ablation of these cells using akh-GAL4 and UAS-hid attenuated the starvation-dependent decrease in bitter sensitivity, without affecting sugar sensitivity (Figure 6A1–3, B1–2, and S6A1–3; ablation was confirmed; see Figure S6B1–2). Consistent with this result, a hypomorphic mutation in the AKH receptor gene (akhr) (Lee et al., 2006; Shohat-Ophir et al., 2012), akhrEY11371 (Bharucha et al., 2008) (Figure S6C), also attenuated the starvation-dependent decrease in bitter sensitivity relative to genetic background-matched wild-type controls (Figure 6C1–3, 6D2). In contrast, bitter sensitivity under fed conditions (Figure 6C1 and 6D2), and sugar sensitivity regardless of starvation level (Figure 6D1 and S6D1–3) were not affected. Normalized-sugar PER assay comparing 1-day WS flies and unstarved flies revealed that akhrEY11371 flies showed no change in bitter sensitivity during starvation (Figure S6E1–2).
Figure 6. AKH Acts Genetically Upstream of the sNPF Pathway.
(A–B) Sugar and bitter sensitivity of flies with or without genetic ablation of AKH neuroendocrine cells (w-; akh-GAL4 (III) crossed with w-; UAS-nls::GFP or w-;UAS-nls::GFP, UAS-hid). (C–D) Sugar and bitter sensitivity of wild type and AKHREY11371 mutant flies in the same genetic background. (E–F) Sugar and bitter sensitivity of flies with genetic thermoactivation of AKH-producing cells (w-; +; akh-GAL4 (III) crossed with w-; +; + (E1) or w-; UAS-dTrpA1 (II); UAS-dTrpA1 (III) (E2). w-; sNPFc00448; akh-GAL4 (III) crossed with w-; +; + (E3) or w-; UAS-dTrpA1 (II); UAS-dTrpA1 (III) (E4)). Flies were preincubated in 30 °C or 18 °C for 30 min and PER was performed in 18 °C. n>5 for all experimental groups. See also Figure S5.
As an independent approach to investigating the role of AKH+ cells, we asked whether thermogenetic activation of these neuroendocrine cells, using dTrpA1, would suffice to decrease bitter sensitivity. We performed this experiment in 18 hr wet starved flies, which exhibit increased sugar sensitivity, but which do not yet exhibit decreased bitter sensitivity (Figure 1E1–2). Flies expressing dTrpA1 in AKH+ neurons were pre-incubated at 30 °C for 30 minutes, after which gustatory sensitivity was tested at 18 °C. Indeed, thermogenetic activation of AKH+ cells significantly decreased bitter sensitivity (Figure 6E2 and 6F2, red lines and boxes), relative to controls pre-incubated at 18°C (blue lines and boxes). This effect was not observed in genetic control flies subjected to 30°C pretreatment (Figure 6E1 and S6F1–2) and did not affect sugar sensitivity (Figure 6F1, and S6G1–4). Therefore activation of AKH+ neuroendocrine cells is sufficient to specifically decrease bitter sensitivity in partially starved flies.
Because both AKH and sNPF regulate bitter sensitivity in the same direction, we investigated whether these neuropeptides act in a common pathway. Antibody staining experiments have indicated that AKH+ neuroendocrine cells in the CC (Kim and Rulifson, 2004; Lee and Park, 2004) do not co-express sNPF (Kahsai et al., 2010). Consistent with this, expression of sNPF in AKH+ cells did not rescue the sNPF mutant phenotype (Figure S6H). To test whether sNPF functions genetically downstream of AKH+ cells, we asked whether the sNPF mutation would suppress the effect of activating AKH+ cells. Indeed, in a heterozygous sNPFc00448 background thermogenetic activation of AKH+ cells was unable to reduce bitter sensitivity (cf. Figure 6E2 vs. 6E3–4, 6F1–2 and S6F1–2). Thus, haploinsufficiency of sNPF is epistatic to artificial activation of AKH+ neuroendocrine cells. This suggests that sNPF-expressing neurons may mediate the effect of AKH+ cells to control bitter sensitivity (Fig. 7). However, an indirect, permissive role for sNPF in AKH action is not excluded by these data (Figure 7A, dashed curved arrow).
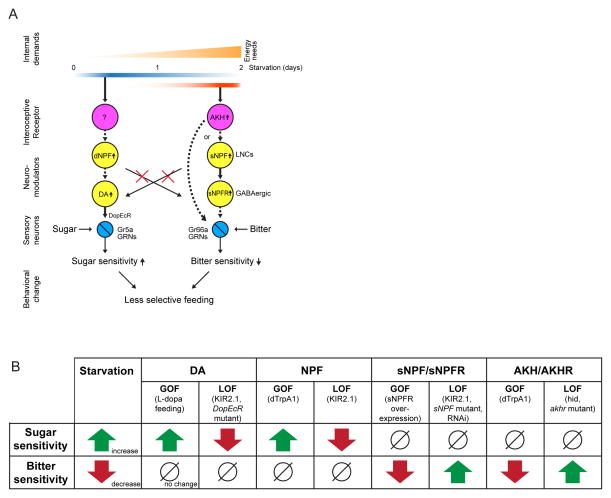
Figure 7. Distinct Neuronal Pathways Modulating Sugar and Bitter Sensitivity During Starvation.
(A) Schematic illustrating the two distinct neuronal pathways we identified to control sugar and bitter sensitivity in an independent manner. Dashed arrows indicate genetic interactions that we have not shown to be direct. (B) Table summarizing findings in this paper.
DISCUSSION
We have used starvation in Drosophila as a model system to understand how changes in internal states influence behavioral decisions. Starved animals exhibit enhanced sugar sensitivity and decreased bitter sensitivity, allowing them to accept food resources they would otherwise reject as insufficiently caloric or potentially toxic. Here we provide behavioral, cellular and genetic evidence that bitter sensitivity is independently modulated during food deprivation, in the opposite direction as sugar sensitivity. We identify parallel neuromodulatory pathways that control these changes, and show that they are recruited at different levels of energy deficit (Figure 7). This independent and reciprocal control of sweet vs. bitter taste sensitivity during starvation affords a greater dynamic range of control over feeding decisions. More generally, our data illustrate how different components of a time-varying, scalable multi-dimensional internal state can be independently regulated to achieve robust state-dependent changes in behavior.
Sugar and bitter sensitivities are independently modulated in starved flies
The idea that changes in gustatory sensitivity during starvation in flies exclusively reflect an increase in sugar sensitivity has been the dominant view in the field, based on behavioral studies in the blowfly Phormia (Moss and Dethier, 1983). Here we present several lines of evidence that bitter sensitivity decreases during starvation in Drosophila, independently of the increase in sugar sensitivity. First, we measured the sensitivity of PER behavior to inhibition by bitter compounds at different times of starvation, using progressively lower fixed concentrations of sucrose to offset the increase in sugar sensitivity. Such “normalized PER assays” revealed a decrease in behavioral sensitivity to bitter tastants during starvation, even when compensating for increased sugar sensitivity (Fig. 1D, E).
Second, we identified two independent neuromodulatory pathways (Fig. 7A), loss- and gain-of-function genetic manipulations of which repeatedly revealed a double-dissociation of the control of sugar- and bitter-taste sensitivity during starvation, at multiple levels of regulation (Fig. 7B). Sugar sensitivity is increased by a pathway in which dNPF+ neurons act genetically upstream of DA+ neurons, which increase their release of DA onto sweet-sensitive GRNs, thereby enhancing their physiological responsiveness to sugars (Inagaki et al., 2012; Marella et al., 2012). Bitter sensitivity is decreased, conversely, by AKH+ interoceptive neuroendocrine cells, which act genetically upstream of sNPF+ LNCs; in turn LNCs indirectly (perhaps via inhibitory interneurons) reduce the physiological responsiveness of Gr66a GRNs to bitter tastants. In each of close to two dozen genetic manipulations of these different pathway components, changes were observed in either bitter or sugar sensitivity, but not both.
Moreover, the phase of these genetic phenotypes was opposite: gain-of-function manipulations of the dNPF→DA pathway increased sugar sensitivity, while in the AKH→sNPF pathway they decreased bitter sensitivity, and vice-versa (Fig. 7B).
Orthogonal regulation of gustatory sensitivity by NPF family members
Previous studies have implicated dNPF and sNPF in the control of starvation dependent changes in physiology and behavior (Beshel and Zhong, 2013; Hergarden et al., 2012; Krashes et al., 2009; Lee et al., 2004; Root et al., 2011). Here we show that these neuropeptides control reciprocal changes in sweet and bitter sensitivity during starvation. dNPF+ neurons promote increased sugar sensitivity, while sNPF promotes decreased bitter sensitivity. While may be coincidental that these neuropeptides and their receptors are genetically related, it is tempting to speculate that this reciprocal regulation arose in evolution by duplication and modification of a single neuromodulatory pathway that originally regulated a single taste modality.
That said, the functions of dNPF and sNPF in controlling starvation-dependent changes in gustatory sensitivity may not necessarily be analogous. dNPF is expressed by very few neurons in the brain (Krashes et al., 2009), while sNPF is expressed in several hundred cells (Nassel et al., 2008). It is possible that this difference reflects a qualitative distinction in the type of function that these two neuropeptides perform (e.g., state control vs. signal amplification). Alternatively, it may simply reflect a difference in their frequency of utilization in different circuits. Whatever the case, both dNPF and sNPF have been implicated in additional functions besides hunger modulation (Kahsai et al., 2010; Kapan et al., 2012; Kim et al., 2013; Knapek et al., 2013; Lee et al., 2006; Shohat-Ophir et al., 2012), indicating that their effects on behavior are context-dependent.
Our studies identify a subset of neurons, called lateral neurosecretory cells (LNCs), in which sNPF acts to regulate bitter sensitivity. Although LNCs are known to express other neuropeptides (Kahsai et al., 2010; Kapan et al., 2012), our loss- and gain-of-function genetic experiments indicate that sNPF in LNCs is necessary and sufficient for the starvation-dependent decrease in bitter sensitivity. In addition to this function, however, sNPF expressed in LNCs has been reported to exert multiple influences during food-deprivation: LNCs regulate IPCs, sensitivity to starvation and metabolism (Kahsai et al., 2010; Kapan et al., 2012). Interestingly, LNCs project not only to the SEZ, but also to other brain areas and the CC (where AKH is produced (Kapan et al., 2012)). It is possible that sNPF and AKH form a feedback circuit to regulate starvation-driven behavioral changes.
Our studies leave unanswered a number of questions regarding the function and site of action of NPF family members in the starvation-dependent control of gustatory sensitivity. For example, it is not yet clear whether dNPF peptides themselves, or rather dNPF+ neurons acting through some other neurotransmitter or neuromodulator, control changes in sugar sensitivity. It is also not clear whether dNPF+ neurons act directly on DA neurons that control Gr5a GRN sugar sensitivity, or indirectly via intermediate connections. Interestingly previous research has shown that dNPF+ neurons have a direct inhibitory effect on dopaminergic MB-MP1 neurons to regulate starvation driven memory (Krashes et al., 2009). Experiments to knock down NPFR expression in DA neurons, however, did not affect sugar sensitivity (data not shown), implying an indirect effect. Similarly, the site at which sNPF reduces the sensitivity of Gr66 GRNs to bitter compounds remains unclear. Elucidation of these missing details may clarify the extent to which dNPF and sNPF exert analogous but orthogonal roles in controlling gustatory sensitivity during starvation.
Food deprivation recruits neuromodulatory cascades that modify the sensitivities of primary gustatory neurons
Our earlier (Inagaki et al., 2012) and present findings suggest that, in Drosophila, hunger modulates the sensitivity of two orthogonal classes of primary gustatory neurons to regulate feeding choices. Modulation of primary sensory neurons enables the state-dependent tuning of each sensory modality before the signal is integrated with other inputs at higher levels in the brain. Interestingly, in mice, it has been reported that multiple neuromodulators and hormones modulate the sensitivity of taste cells (Elson et al., 2010; Kawai et al., 2000), although whether this modulation causes starvation-dependent behavioral changes is not clear (Sternson et al., 2013).
Risk vs. benefit may determine the sequence of state-dependent changes recruited during food-deprivation
Animals continuously compare potential gains and risks to determine their behaviors (Dethier, 1976; Gillette et al., 2000; Itskov and Ribeiro, 2013). Because the need to feed increases with starvation, it is reasonable that the decision to accept or reject a potential food resource is modulated by energy deficit. A comparison of fed vs. 2 days starved flies revealed that sugar sensitivity alone changes 4.6 fold (Figure 1B2) and bitter sensitivity alone changes 4.8 fold (Figure 1D2); while the relative preference for food containing both sugar and bitter increases 10.2 fold (Figure 1C2). The increased acceptance of unpalatable food during starvation reflects both the “masking” effect of increased sugar sensitivity on the detection of bitter compounds (Moss and Dethier, 1983), and, as shown here, an independent decrease in bitter taste sensitivity. This reciprocal tuning of both sugar and bitter sensitivity contributes to a dramatic increase in the acceptance of food resources containing both reduced caloric value and potentially toxic compounds, by a starving fly.
Food-deprivation creates a type of “global organismal state change” (Kim et al., 2013; LeDoux, 2012) that is multi-dimensional: it involves multiple physiological and behavioral changes. Interestingly, these different changes occur with different kinetics during food deprivation. Some of them, such as the increase in sugar sensitivity (6 hours of starvation; present results), feeding amount (6–12 hours of starvation: (Farhadian et al., 2012; Hergarden et al., 2012), and food-related olfactory sensitivity (several hours of starvation: (Root et al., 2011)) are initiated during mild starvation, while others, such as the decrease in bitter sensitivity (1–2 days of starvation; present results) and increase in locomotion (2 days of starvation: (Isabel et al., 2005; Lee and Park, 2004))) are recruited during severe starvation just before death.
Interestingly, changes occurring during mild starvation appear to be low-risk, in that their premature implementation is unlikely to kill the animal, whereas changes accompanying severe starvation place the animal at higher-risk for damage or death: e.g., the decrease in bitter sensitivity allows intake of potentially toxic substances. A similar increase in risky behaviors has been observed in other settings involving state-dependent escalation (Blanchard and Blanchard, 1989; Potegal, 2012). These considerations may explain why the brain has evolved multiple mechanisms for the adaptive control of behavior in response to organismal state changes. One mechanism first activates lower risk responses, when energy demands are mild, while the other recruits higher risk responses when energy demands are severe and no other options are available.
The results presented here outline two parallel, orthogonal pathways that translate changing energy needs into the decision to accept a food resource. These data add to a growing body of evidence that neuromodulatory cascades serve as key mediators of state-dependent control (Bargmann, 2012; Flavell et al., 2013; Komuniecki et al., 2014; Taghert and Nitabach, 2012). The widespread projections of neuromodulatory neurons, and the specific expression of their receptors, allows them to coordinate the activity of multiple, behaviorally distinct sub-circuits in parallel. This property, and the ability of such modulators to alter the response properties of neurons and circuits (Marder and Bucher, 2007) are well suited to a mediating function in state control. Cascades of neuromodulators, moreover, afford multiple regulation points, allowing dynamic state control with potential feedback and/or feedforward regulation (Taghert and Nitabach, 2012). Our results may provide entry points to study in more detail the dynamics of neuromodulatory cascades and their impact on organismal physiology and behavior.
EXPERIMENTAL PROCEDURES
Fly Strains
Adult female Drosophila melanogaster were used for all experiments. Since genetic background affects the basal sugar and bitter sensitivities, all the comparisons were made within the same genetic background. Flies were backcrossed for at least 6 generations to ensure the same genetic background. Descriptions of detail genotypes are in the Supplemental Experimental Procedures.
PER Assays
For PER assays, 3–7 day-old female flies were wet-starved or fed in vials. Wet starvation was performed by keeping flies in a vial with a water-soaked filter paper. PER was tested as described previously (Inagaki et al., 2012). Detail procedures are in the Supplemental Experimental Procedures.
Supplementary Material
Figure S1. Modulation of Bitter Sensitivity During Starvation
(A) Gr66 GRNs is necessary for the bitter-dependent suppression of PER. Fraction of flies not showing PER to different concentrations of lobeline mixed into 800mM sucrose are plotted. (B) Multiple representative examples of sigmoidal fitting (red curves) of fraction of flies not showing PER (raw data in blue curves). See Supplemental Experimental Procedures for sigmoidal fitting. (C) Fraction of flies not showing PER in response to bitter mixed into different concentrations of sucrose solution. (A, C) n>4 for all experimental groups.
Figure S2. Neuronal Pathway Regulating Sugar Sensitivity Does Not Affect Bitter Sensitivity
(A) Genetic control of Figure 2C. Note that these genetic manipulations do no affect sugar sensitivity. (B–C) Sugar (B) and bitter (C) sensitivity of flies with thermogenetic activation of NPF neurons. Genotypes: w-; dnpf-GAL4 (II) flies were crossed with w-; UAS-dTrpA1 (II); UAS-dTrpA1 (III) (B1 and C1) or w- flies in the same genetic background (B2 and C2); w- flies were crossed with w-; UAS-dTrpA1 (II); UAS-dTrpA1 (III) (B3 and C3) or w- flies in the same genetic background (B4 and C4). B1 is copied from figure 2F1 for purposes of comparison. In (C1), note that there is statistically significant difference only when bitter is not mixed into sucrose solution (0 mM). Therefore, no difference in bitter sensitivity was observed. (D) Sugar and bitter sensitivity of flies with genetic silencing of dNPF neurons. UAS-mCD8::GFP was crossed with either w-; dnpf-GAL4 (II) flies (D1) or w- flies in the same genetic background (D2). UAS-KIR2.1 was crossed with either w-; dnpf-GAL4 (II) flies (D4 and D7) or w- flies in the same genetic background (D3 and D6). (E) Comparison of bitter sensitivity of 1-day WS dnpf-GAL4; UAS-KIR flies and +; UAS-KIR flies using the sugar-normalized PER assay (200 mM and 100 mM sucrose solution were used, respectively). No difference in bitter sensitivity was observed between two genotypes. (F) Representative confocal projections of whole mount brains from dnpf-GAL4; UAS-mCD8::GFP flies (gfp in green: F1,3) immunostained with anti-Tyrosine hydroxylase antibody (magenta: F2–3), which labels DA neurons. (A–E) n>4 for all experimental groups.
Figure S3. Genetic Manipulations of sNPF Do Not Affect Sugar Sensitivity
(A) Insertion of two piggyBac transposons in sNPF gene locus (top) and relative sNPF mRNA expression level of these strains compared to wild type flies in the same genetic background (bottom) measured by qPCR. One-way ANOVA followed by post hoc t-test with Bonferroni correction (n=3). (B) Sugar sensitivity of wild type and sNPF mutant flies. Data is acquired from the same flies that were used in Figure 3A1–3. S50 of these experiments are summarized in Figure 3B1. (C) Sugar and bitter sensitivity of sNPFf07577 flies compared with wild type flies in the same genetic background. (D) Sugar sensitivity of flies with pan-neuronal rescue of sNPF. Data is acquired from the same flies that were used in Figure 3D1–2. S50 of these experiments are summarized in Figure 3E1. n>5 for all experimental groups.
Figure S4. Expression patterns of sNPF-promoter GAL4s
(A) Representative confocal projections of the proboscis from GMR21B10-GAL4; UAS-mCD8::GFP flies (Green: GFP; gray: DIC image of proboscis). Note that there is no GFP positive cell in labellum where GRNs exist. There are two cells in other parts of labellum (white arrow head). (B) Representative confocal projections of whole mount brains from wild type (B1) or sNPFc00448 (B2) flies immunostained with anti-sNPF antibody. Scale bar to the left represents relative intensity of immunostaining in pseudocolor. (C) Representative confocal projections of whole mount brains from sNPF promoter GAL4 lines crossed with UAS-mCD8::GFP. Green: GFP; Magenta: anti-sNPF signal; white shows the overlap. (D) Representative confocal projections of whole mount brains from snpf-GAL4; UAS-mCD8::GFP flies (GFP: green) immunostained with anti-sNPF antibody (magenta). Note that huge populations of neurons in the brain are labeled by this GAL4. Some of them are sNPF positive. 3–4 LCNs are labeled by this GAL4 line. (E–F) Representative confocal projections of whole mount brains from GMR21B10-GAL4; UAS-mCD8::GFP flies immunostained with anti-Tyrosine hydroxylase (TH) (E) or anti-dNPF (F) antibodies. Note that LNCs are not TH or dNPF positive.
Figure S5. Genetic Manipulations of sNPFR Do Not Affect Sugar Sensitivity
(A–B) Sugar and bitter sensitivity of flies with genetic over-expression of sNPFR. Data is acquired from the same flies that were used in Figure 5A1–2. S50 of these experiments are summarized in Figure 5B1. (C) Sugar sensitivity of flies with genetic knock-down of sNPFR. Data is acquired from the same flies that were used in Figure 5C1–3. S50 of these experiments are summarized in Figure 5D1. (D) Sugar and bitter sensitivity of flies with genetic knock-down of sNPFR in IPCs by using InsP3-GAL4, GAL4 line specifically labeling IPCs, crossed with UAS-sNPFR RNAi or UAS-mCD8::GFP in the same genetic background. Note that no change was observed in gustatory sensitivity. (E–F) Sugar and bitter sensitivity of flies with genetic cellular ablation of IPCs by using InsP3-GAL4 (or control wild type flies in the same genetic background) crossed with UAS-hid or UAS-nls::GFP in the same genetic background. Ablation of IPCs were confirmed as a loss of nls::GFP signal (F). (G–I) Sugar and bitter sensitivity of flies with over-expression or genetic knock-down of sNPFR in bitter-sensing GRNs. Both Gr66-GAL4 and Gr33-GAL4 drivers were tested for RNAi also combined with UAS-Dicer2 (UAS-Dicer2; Gr66-GAL4 or UAS-Dicer2; Gr33-GAL4 crossed with UAS-sNPFR RNAi or UAS-mCD8::GFP in the same genetic background). Similar result (no effect on gustatory sensitivity) was observed for 1-day WS flies (data not shown). (J) Sugar and bitter sensitivity of flies with genetic knock-down of sNPFR in GABA positive neurons. dVGAT-GAL4 line is combined with UAS-sNPFR RNAi or UAS-mCD8::GFP in the same genetic background. UAS-Dicer2 was not used for this experiment. n>5 for all experimental groups.
Figure S6. Genetic Manipulations of AKH Do Not Affect Sugar Sensitivity
(A–B) Sugar sensitivity of flies with genetic cell-ablation of AKH neuroendocrine cells. Data is acquired from the same flies that were used in Figure 6A1–3. S50 of these experiments are summarized in Figure 6B1. Cell ablation was confirmed with loss of nls::GFP signal (B1–2). (C) Relative akhr mRNA expression level of AKHREY11371 compared to wild type flies in the same genetic background (bottom) measured by qPCR. P-value represents t-test (n=3). (D) Sugar sensitivity of wild type and AKHREY11371 mutant flies in the same genetic background. Data is acquired from the same flies that were used in Figure 6C1–3. S50 of these experiments are summarized in Figure 6D1. (E) Results of the sugar-normalized PER assay comparing bitter sensitivity between fed and 1-day WS. Wild type flies (E1) and akhrEY11371 mutant flies (E2) in the same genetic background were tested. Lobeline was mixed into 800 mM sucrose solution for fed flies, or 200 mM sucrose solution for 1-day WS flies. E1 is the same as Figure 3C1 (duplicated for purposes of comparison). (F–G) Sugar and bitter sensitivity of flies with genetic thermoactivation of AKH-producing cells (w-; +; akh-GAL4 (III) crossed with w-; +; + (G3) or w-; UAS-dTrpA1 (II); UAS-dTrpA1 (III) (G4). w-; sNPFc00448; akh-GAL4 (III) crossed with w-; +; + (G5) or w-; UAS-dTrpA1 (II); UAS-dTrpA1 (III) (G6)) and its genetic control flies (Wild flies crossed with w-; UAS-dTrpA1 (II); UAS-dTrpA1 (III) (F2, G2) or wild type flies in the same genetic background (F1, G1)). (H) Bitter sensitivity measured with the normalized-sugar PER assay in sNPF mutant flies with genetic rescue of sNPF expression in AKH neuroendocrine cells (w-; sNPFc00448; UAS-sNPF crossed with w-; sNPFc00448; akh-GAL4). Note that rescuing of sNPF expression in AKH neuroendocrine cells does not rescue the starvation-dependent decrease in bitter sensitivity. (A, D–H) n>4 for all experimental groups.
Acknowledgments
Fly stocks were generously provided by the Bloomington Stock Center, the VDRC stock center, Drosophila RNAi Screening Center, Drs. K. Yu, J. W. Wang, P. Shen, J. H. Park, M. J. Pankratz G. M. Rubin, B. Pfeiffer, J. Simpson, L. L Looger, H. Keshishian, K. Scott, C. Montell, H. Amrein, G. S. B. Suh, H. Keshishian, P. A. Garrity, T. Kitamoto, H. Ishimoto, and B. J. Dickson. We also thank Dr. D. R. Nässel for anti-sNPF precursor serum. H.K.I. is supported by the Nakajima Foundation. D.J.A. is an investigator of the Howard Hughes Medical Institute. This work was supported in part by NIH grant 1RO1 DA031389 to D.J. Anderson.
Footnotes
Supplemental Information includes Extended Experimental Procedures, figures can be found with this article online at xxx
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson DJ, Adolphs R. A framework for studying emotions across species. Cell. 2014;157:187–200. doi: 10.1016/j.cell.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, Tschop MH, Shanabrough M, Cline G, Shulman GI, et al. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LT, Singh CM, Moore MS, Neckameyer WS, Heberlein U. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Current biology: CB. 2000;10:187–194. doi: 10.1016/s0960-9822(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Bargmann CI. Beyond the connectome: how neuromodulators shape neural circuits. Bioessays. 2012;34:458–465. doi: 10.1002/bies.201100185. [DOI] [PubMed] [Google Scholar]
- Beshel J, Zhong Y. Graded Encoding of Food Odor Value in the Drosophila Brain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:15693–15704. doi: 10.1523/JNEUROSCI.2605-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharucha KN, Tarr P, Zipursky SL. A glucagon-like endocrine pathway in Drosophila modulates both lipid and carbohydrate homeostasis. The Journal of experimental biology. 2008;211:3103–3110. doi: 10.1242/jeb.016451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Attack and defense in rodents as ethoexperimental models for the study of emotion. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13(Suppl):S3–14. doi: 10.1016/0278-5846(89)90105-x. [DOI] [PubMed] [Google Scholar]
- Buch S, Melcher C, Bauer M, Katzenberger J, Pankratz MJ. Opposing effects of dietary protein and sugar regulate a transcriptional target of Drosophila insulin-like peptide signaling. Cell Metab. 2008;7:321–332. doi: 10.1016/j.cmet.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Lei YT, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethier VG. The hungry fly: a physiological study of the behavior associated with feeding. Cambridge, Mass: Harvard University Press; 1976. [Google Scholar]
- Dus M, Ai M, Suh GS. Taste-independent nutrient selection is mediated by a brain-specific Na+/solute co-transporter in Drosophila. Nat Neurosci. 2013;16:526–528. doi: 10.1038/nn.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson AE, Dotson CD, Egan JM, Munger SD. Glucagon signaling modulates sweet taste responsiveness. Faseb J. 2010;24:3960–3969. doi: 10.1096/fj.10-158105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadian SF, Suarez-Farinas M, Cho CE, Pellegrino M, Vosshall LB. Post-fasting olfactory, transcriptional, and feeding responses in Drosophila. Physiology & behavior. 2012;105:544–553. doi: 10.1016/j.physbeh.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei H, Chow DM, Chen A, Romero-Calderon R, Ong WS, Ackerson LC, Maidment NT, Simpson JH, Frye MA, Krantz DE. Mutation of the Drosophila vesicular GABA transporter disrupts visual figure detection. The Journal of experimental biology. 2010;213:1717–1730. doi: 10.1242/jeb.036053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Reale V, Chatwin H, Kennedy K, Venard R, Ericsson C, Yu K, Evans PD, Hall LM. Functional characterization of a neuropeptide F-like receptor from Drosophila melanogaster. Eur J Neurosci. 2003;18:227–238. doi: 10.1046/j.1460-9568.2003.02719.x. [DOI] [PubMed] [Google Scholar]
- Flavell SW, Pokala N, Macosko EZ, Albrecht DR, Larsch J, Bargmann CI. Serotonin and the neuropeptide PDF initiate and extend opposing behavioral states in C. elegans. Cell. 2013;154:1023–1035. doi: 10.1016/j.cell.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette R, Huang RC, Hatcher N, Moroz LL. Cost-benefit analysis potential in feeding behavior of a predatory snail by integration of hunger, taste, and pain. Proc Natl Acad Sci U S A. 2000;97:3585–3590. doi: 10.1073/pnas.97.7.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MD, Scott K. Motor control in a Drosophila taste circuit. Neuron. 2009;61:373–384. doi: 10.1016/j.neuron.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grether ME, Abrams JM, Agapite J, White K, Steller H. The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev. 1995;9:1694–1708. doi: 10.1101/gad.9.14.1694. [DOI] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergarden AC, Tayler TD, Anderson DJ. Allatostatin-A neurons inhibit feeding behavior in adult Drosophila. Proc Natl Acad Sci U S A. 2012;109:3967–3972. doi: 10.1073/pnas.1200778109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki HK, Ben-Tabou de-Leon S, Wong AM, Jagadish S, Ishimoto H, Barnea G, Kitamoto T, Axel R, Anderson DJ. Visualizing neuromodulation in vivo: TANGO-mapping of dopamine signaling reveals appetite control of sugar sensing. Cell. 2012;148:583–595. doi: 10.1016/j.cell.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isabel G, Martin JR, Chidami S, Veenstra JA, Rosay P. AKH-producing neuroendocrine cell ablation decreases trehalose and induces behavioral changes in Drosophila. Am J Physiol Regul Integr Comp Physiol. 2005;288:R531–538. doi: 10.1152/ajpregu.00158.2004. [DOI] [PubMed] [Google Scholar]
- Itskov PM, Ribeiro C. The dilemmas of the gourmet fly: the molecular and neuronal mechanisms of feeding and nutrient decision making in Drosophila. Front Neurosci. 2013;7:12. doi: 10.3389/fnins.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahsai L, Kapan N, Dircksen H, Winther AM, Nassel DR. Metabolic stress responses in Drosophila are modulated by brain neurosecretory cells that produce multiple neuropeptides. PLoS One. 2010;5:e11480. doi: 10.1371/journal.pone.0011480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapan N, Lushchak OV, Luo J, Nassel DR. Identified peptidergic neurons in the Drosophila brain regulate insulin-producing cells, stress responses and metabolism by coexpressed short neuropeptide F and corazonin. Cell Mol Life Sci. 2012 doi: 10.1007/s00018-012-1097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai K, Sugimoto K, Nakashima K, Miura H, Ninomiya Y. Leptin as a modulator of sweet taste sensitivities in mice. Proc Natl Acad Sci U S A. 2000;97:11044–11049. doi: 10.1073/pnas.190066697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Rulifson EJ. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature. 2004;431:316–320. doi: 10.1038/nature02897. [DOI] [PubMed] [Google Scholar]
- Kim WJ, Jan LY, Jan YN. A PDF/NPF neuropeptide signaling circuitry of male Drosophila melanogaster controls rival-induced prolonged mating. Neuron. 2013;80:1190–1205. doi: 10.1016/j.neuron.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapek S, Kahsai L, Winther AM, Tanimoto H, Nassel DR. Short neuropeptide F acts as a functional neuromodulator for olfactory memory in Kenyon cells of Drosophila mushroom bodies. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:5340–5345. doi: 10.1523/JNEUROSCI.2287-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuniecki R, Hapiak V, Harris G, Bamber B. Context-dependent modulation reconfigures interactive sensory-mediated microcircuits in Caenorhabditis elegans. Current opinion in neurobiology. 2014;29C:17–24. doi: 10.1016/j.conb.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–427. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreneisz O, Chen X, Fridell YW, Mulkey DK. Glucose increases activity and Ca2+ in insulin-producing cells of adult Drosophila. Neuroreport. 2010;21:1116–1120. doi: 10.1097/WNR.0b013e3283409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. Rethinking the emotional brain. Neuron. 2012;73:653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Bahn JH, Park JH. Sex- and clock-controlled expression of the neuropeptide F gene in Drosophila. Proc Natl Acad Sci U S A. 2006;103:12580–12585. doi: 10.1073/pnas.0601171103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167:311–323. doi: 10.1534/genetics.167.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Hong SH, Kim AK, Ju SK, Kwon OY, Yu K. Processed short neuropeptide F peptides regulate growth through the ERK-insulin pathway in Drosophila melanogaster. FEBS Lett. 2009;583:2573–2577. doi: 10.1016/j.febslet.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Lee KS, Kwon OY, Lee JH, Kwon K, Min KJ, Jung SA, Kim AK, You KH, Tatar M, Yu K. Drosophila short neuropeptide F signalling regulates growth by ERK-mediated insulin signalling. Nat Cell Biol. 2008;10:468–475. doi: 10.1038/ncb1710. [DOI] [PubMed] [Google Scholar]
- Lee KS, You KH, Choo JK, Han YM, Yu K. Drosophila short neuropeptide F regulates food intake and body size. J Biol Chem. 2004;279:50781–50789. doi: 10.1074/jbc.M407842200. [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Marella S, Mann K, Scott K. Dopaminergic modulation of sucrose acceptance behavior in Drosophila. Neuron. 2012;73:941–950. doi: 10.1016/j.neuron.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masek P, Keene AC. Drosophila Fatty Acid Taste Signals through the PLC Pathway in Sugar-Sensing Neurons. PLoS Genet. 2013;9:e1003710. doi: 10.1371/journal.pgen.1003710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens I, Meeusen T, Huybrechts R, De Loof A, Schoofs L. Characterization of the short neuropeptide F receptor from Drosophila melanogaster. Biochem Biophys Res Commun. 2002;297:1140–1148. doi: 10.1016/s0006-291x(02)02351-3. [DOI] [PubMed] [Google Scholar]
- Meunier N, Belgacem YH, Martin JR. Regulation of feeding behaviour and locomotor activity by takeout in Drosophila. J Exp Biol. 2007;210:1424–1434. doi: 10.1242/jeb.02755. [DOI] [PubMed] [Google Scholar]
- Meunier N, Marion-Poll F, Rospars JP, Tanimura T. Peripheral coding of bitter taste in Drosophila. Journal of neurobiology. 2003;56:139–152. doi: 10.1002/neu.10235. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Slone J, Song X, Amrein H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell. 2012;151:1113–1125. doi: 10.1016/j.cell.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. A taste of the Drosophila gustatory receptors. Curr Opin Neurobiol. 2009;19:345–353. doi: 10.1016/j.conb.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon SJ, Lee Y, Jiao Y, Montell C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr Biol. 2009;19:1623–1627. doi: 10.1016/j.cub.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss CF, Dethier VG. Central nervous system regulation of finicky feeding by the blowfly. Behav Neurosci. 1983;97:541–548. doi: 10.1037//0735-7044.97.4.541. [DOI] [PubMed] [Google Scholar]
- Nassel DR, Enell LE, Santos JG, Wegener C, Johard HA. A large population of diverse neurons in the Drosophila central nervous system expresses short neuropeptide F, suggesting multiple distributed peptide functions. BMC Neurosci. 2008;9:90. doi: 10.1186/1471-2202-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassel DR, Wegener C. A comparative review of short and long neuropeptide F signaling in invertebrates: Any similarities to vertebrate neuropeptide Y signaling? Peptides. 2011;32:1335–1355. doi: 10.1016/j.peptides.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci U S A. 2001;98:12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RE, Jr, Erber J, Fondrk MK. The effect of genotype on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.) J Comp Physiol A. 1998;182:489–500. doi: 10.1007/s003590050196. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BD, Jenett A, Hammonds AS, Ngo TT, Misra S, Murphy C, Scully A, Carlson JW, Wan KH, Laverty TR, et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc Natl Acad Sci U S A. 2008;105:9715–9720. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool AH, Scott K. Feeding regulation in Drosophila. Curr Opin Neurobiol. 2014;29C:57–63. doi: 10.1016/j.conb.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potegal M. Temporal and frontal lobe initiation and regulation of the top-down escalation of anger and aggression. Behavioural brain research. 2012;231:386–395. doi: 10.1016/j.bbr.2011.10.049. [DOI] [PubMed] [Google Scholar]
- Reale V, Chatwin HM, Evans PD. The activation of G-protein gated inwardly rectifying K+ channels by a cloned Drosophila melanogaster neuropeptide F-like receptor. Eur J Neurosci. 2004;19:570–576. doi: 10.1111/j.0953-816x.2003.03141.x. [DOI] [PubMed] [Google Scholar]
- Riemensperger T, Isabel G, Coulom H, Neuser K, Seugnet L, Kume K, Iche-Torres M, Cassar M, Strauss R, Preat T, et al. Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc Natl Acad Sci U S A. 2011;108:834–839. doi: 10.1073/pnas.1010930108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Ko KI, Jafari A, Wang JW. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell. 2011;145:133–144. doi: 10.1016/j.cell.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K, Brady R, Jr, Cravchik A, Morozov P, Rzhetsky A, Zuker C, Axel R. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell. 2001;104:661–673. doi: 10.1016/s0092-8674(01)00263-x. [DOI] [PubMed] [Google Scholar]
- Sengupta P. The belly rules the nose: feeding state-dependent modulation of peripheral chemosensory responses. Current opinion in neurobiology. 2013;23:68–75. doi: 10.1016/j.conb.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohat-Ophir G, Kaun KR, Azanchi R, Mohammed H, Heberlein U. Sexual deprivation increases ethanol intake in Drosophila. Science. 2012;335:1351–1355. doi: 10.1126/science.1215932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Yu EJ, Kennedy K, Chatwin H, Reale V, Hamon M, Smith T, Evans PD. Rapid, nongenomic responses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. J Neurosci. 2005;25:6145–6155. doi: 10.1523/JNEUROSCI.1005-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM, Nicholas Betley J, Cao ZF. Neural circuits and motivational processes for hunger. Current opinion in neurobiology. 2013;23:353–360. doi: 10.1016/j.conb.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghert PH, Nitabach MN. Peptide neuromodulation in invertebrate model systems. Neuron. 2012;76:82–97. doi: 10.1016/j.neuron.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault ST, Singer MA, Miyazaki WY, Milash B, Dompe NA, Singh CM, Buchholz R, Demsky M, Fawcett R, Francis-Lang HL, et al. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat Genet. 2004;36:283–287. doi: 10.1038/ng1314. [DOI] [PubMed] [Google Scholar]
- Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Dahanukar A, Kwon JY, Banerjee D, Carlson JR. The molecular and cellular basis of bitter taste in Drosophila. Neuron. 2011;69:258–272. doi: 10.1016/j.neuron.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Wen T, Lee G, Park JH, Cai HN, Shen P. Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron. 2003;39:147–161. doi: 10.1016/s0896-6273(03)00396-9. [DOI] [PubMed] [Google Scholar]
- Wu Q, Zhao Z, Shen P. Regulation of aversion to noxious food by Drosophila neuropeptide Y- and insulin-like systems. Nat Neurosci. 2005;8:1350–1355. doi: 10.1038/nn1540. [DOI] [PubMed] [Google Scholar]
- Zhang YV, Ni J, Montell C. The molecular basis for attractive salt-taste coding in Drosophila. Science. 2013;340:1334–1338. doi: 10.1126/science.1234133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Modulation of Bitter Sensitivity During Starvation
(A) Gr66 GRNs is necessary for the bitter-dependent suppression of PER. Fraction of flies not showing PER to different concentrations of lobeline mixed into 800mM sucrose are plotted. (B) Multiple representative examples of sigmoidal fitting (red curves) of fraction of flies not showing PER (raw data in blue curves). See Supplemental Experimental Procedures for sigmoidal fitting. (C) Fraction of flies not showing PER in response to bitter mixed into different concentrations of sucrose solution. (A, C) n>4 for all experimental groups.
Figure S2. Neuronal Pathway Regulating Sugar Sensitivity Does Not Affect Bitter Sensitivity
(A) Genetic control of Figure 2C. Note that these genetic manipulations do no affect sugar sensitivity. (B–C) Sugar (B) and bitter (C) sensitivity of flies with thermogenetic activation of NPF neurons. Genotypes: w-; dnpf-GAL4 (II) flies were crossed with w-; UAS-dTrpA1 (II); UAS-dTrpA1 (III) (B1 and C1) or w- flies in the same genetic background (B2 and C2); w- flies were crossed with w-; UAS-dTrpA1 (II); UAS-dTrpA1 (III) (B3 and C3) or w- flies in the same genetic background (B4 and C4). B1 is copied from figure 2F1 for purposes of comparison. In (C1), note that there is statistically significant difference only when bitter is not mixed into sucrose solution (0 mM). Therefore, no difference in bitter sensitivity was observed. (D) Sugar and bitter sensitivity of flies with genetic silencing of dNPF neurons. UAS-mCD8::GFP was crossed with either w-; dnpf-GAL4 (II) flies (D1) or w- flies in the same genetic background (D2). UAS-KIR2.1 was crossed with either w-; dnpf-GAL4 (II) flies (D4 and D7) or w- flies in the same genetic background (D3 and D6). (E) Comparison of bitter sensitivity of 1-day WS dnpf-GAL4; UAS-KIR flies and +; UAS-KIR flies using the sugar-normalized PER assay (200 mM and 100 mM sucrose solution were used, respectively). No difference in bitter sensitivity was observed between two genotypes. (F) Representative confocal projections of whole mount brains from dnpf-GAL4; UAS-mCD8::GFP flies (gfp in green: F1,3) immunostained with anti-Tyrosine hydroxylase antibody (magenta: F2–3), which labels DA neurons. (A–E) n>4 for all experimental groups.
Figure S3. Genetic Manipulations of sNPF Do Not Affect Sugar Sensitivity
(A) Insertion of two piggyBac transposons in sNPF gene locus (top) and relative sNPF mRNA expression level of these strains compared to wild type flies in the same genetic background (bottom) measured by qPCR. One-way ANOVA followed by post hoc t-test with Bonferroni correction (n=3). (B) Sugar sensitivity of wild type and sNPF mutant flies. Data is acquired from the same flies that were used in Figure 3A1–3. S50 of these experiments are summarized in Figure 3B1. (C) Sugar and bitter sensitivity of sNPFf07577 flies compared with wild type flies in the same genetic background. (D) Sugar sensitivity of flies with pan-neuronal rescue of sNPF. Data is acquired from the same flies that were used in Figure 3D1–2. S50 of these experiments are summarized in Figure 3E1. n>5 for all experimental groups.
Figure S4. Expression patterns of sNPF-promoter GAL4s
(A) Representative confocal projections of the proboscis from GMR21B10-GAL4; UAS-mCD8::GFP flies (Green: GFP; gray: DIC image of proboscis). Note that there is no GFP positive cell in labellum where GRNs exist. There are two cells in other parts of labellum (white arrow head). (B) Representative confocal projections of whole mount brains from wild type (B1) or sNPFc00448 (B2) flies immunostained with anti-sNPF antibody. Scale bar to the left represents relative intensity of immunostaining in pseudocolor. (C) Representative confocal projections of whole mount brains from sNPF promoter GAL4 lines crossed with UAS-mCD8::GFP. Green: GFP; Magenta: anti-sNPF signal; white shows the overlap. (D) Representative confocal projections of whole mount brains from snpf-GAL4; UAS-mCD8::GFP flies (GFP: green) immunostained with anti-sNPF antibody (magenta). Note that huge populations of neurons in the brain are labeled by this GAL4. Some of them are sNPF positive. 3–4 LCNs are labeled by this GAL4 line. (E–F) Representative confocal projections of whole mount brains from GMR21B10-GAL4; UAS-mCD8::GFP flies immunostained with anti-Tyrosine hydroxylase (TH) (E) or anti-dNPF (F) antibodies. Note that LNCs are not TH or dNPF positive.
Figure S5. Genetic Manipulations of sNPFR Do Not Affect Sugar Sensitivity
(A–B) Sugar and bitter sensitivity of flies with genetic over-expression of sNPFR. Data is acquired from the same flies that were used in Figure 5A1–2. S50 of these experiments are summarized in Figure 5B1. (C) Sugar sensitivity of flies with genetic knock-down of sNPFR. Data is acquired from the same flies that were used in Figure 5C1–3. S50 of these experiments are summarized in Figure 5D1. (D) Sugar and bitter sensitivity of flies with genetic knock-down of sNPFR in IPCs by using InsP3-GAL4, GAL4 line specifically labeling IPCs, crossed with UAS-sNPFR RNAi or UAS-mCD8::GFP in the same genetic background. Note that no change was observed in gustatory sensitivity. (E–F) Sugar and bitter sensitivity of flies with genetic cellular ablation of IPCs by using InsP3-GAL4 (or control wild type flies in the same genetic background) crossed with UAS-hid or UAS-nls::GFP in the same genetic background. Ablation of IPCs were confirmed as a loss of nls::GFP signal (F). (G–I) Sugar and bitter sensitivity of flies with over-expression or genetic knock-down of sNPFR in bitter-sensing GRNs. Both Gr66-GAL4 and Gr33-GAL4 drivers were tested for RNAi also combined with UAS-Dicer2 (UAS-Dicer2; Gr66-GAL4 or UAS-Dicer2; Gr33-GAL4 crossed with UAS-sNPFR RNAi or UAS-mCD8::GFP in the same genetic background). Similar result (no effect on gustatory sensitivity) was observed for 1-day WS flies (data not shown). (J) Sugar and bitter sensitivity of flies with genetic knock-down of sNPFR in GABA positive neurons. dVGAT-GAL4 line is combined with UAS-sNPFR RNAi or UAS-mCD8::GFP in the same genetic background. UAS-Dicer2 was not used for this experiment. n>5 for all experimental groups.
Figure S6. Genetic Manipulations of AKH Do Not Affect Sugar Sensitivity
(A–B) Sugar sensitivity of flies with genetic cell-ablation of AKH neuroendocrine cells. Data is acquired from the same flies that were used in Figure 6A1–3. S50 of these experiments are summarized in Figure 6B1. Cell ablation was confirmed with loss of nls::GFP signal (B1–2). (C) Relative akhr mRNA expression level of AKHREY11371 compared to wild type flies in the same genetic background (bottom) measured by qPCR. P-value represents t-test (n=3). (D) Sugar sensitivity of wild type and AKHREY11371 mutant flies in the same genetic background. Data is acquired from the same flies that were used in Figure 6C1–3. S50 of these experiments are summarized in Figure 6D1. (E) Results of the sugar-normalized PER assay comparing bitter sensitivity between fed and 1-day WS. Wild type flies (E1) and akhrEY11371 mutant flies (E2) in the same genetic background were tested. Lobeline was mixed into 800 mM sucrose solution for fed flies, or 200 mM sucrose solution for 1-day WS flies. E1 is the same as Figure 3C1 (duplicated for purposes of comparison). (F–G) Sugar and bitter sensitivity of flies with genetic thermoactivation of AKH-producing cells (w-; +; akh-GAL4 (III) crossed with w-; +; + (G3) or w-; UAS-dTrpA1 (II); UAS-dTrpA1 (III) (G4). w-; sNPFc00448; akh-GAL4 (III) crossed with w-; +; + (G5) or w-; UAS-dTrpA1 (II); UAS-dTrpA1 (III) (G6)) and its genetic control flies (Wild flies crossed with w-; UAS-dTrpA1 (II); UAS-dTrpA1 (III) (F2, G2) or wild type flies in the same genetic background (F1, G1)). (H) Bitter sensitivity measured with the normalized-sugar PER assay in sNPF mutant flies with genetic rescue of sNPF expression in AKH neuroendocrine cells (w-; sNPFc00448; UAS-sNPF crossed with w-; sNPFc00448; akh-GAL4). Note that rescuing of sNPF expression in AKH neuroendocrine cells does not rescue the starvation-dependent decrease in bitter sensitivity. (A, D–H) n>4 for all experimental groups.