Abstract
Delivery system design and adjuvant development are crucially important areas of research for improving vaccines. Peptide amphiphile micelles are a class of biomaterials that have the unique potential to function as both vaccine delivery vehicles and self-adjuvants. In this study, peptide amphiphiles comprised of a group A streptococcus B cell antigen (J8) and a dialkyl hydrophobic moiety (diC16) were synthesized and organized into self-assembled micelles, driven by hydrophobic interactions among the alkyl tails. J8-diC16 formed cylindrical micelles with highly α-helical peptide presented on their surfaces. Both the micelle length and secondary structure were shown to be enhanced by annealing. When injected into mice, J8-diC16 micelles induced a strong IgG1 antibody response that was comparable to soluble J8 peptide supplemented with two classical adjuvants. It was discovered that micelle adjuvanticity requires the antigen be a part of the micelle since separation of J8 and the micelle was insufficient to induce an immune response. Additionally, the diC16 tail appears to be non-immunogenic since it does not stimulate a pathogen recognition receptor whose agonist (Pam3Cys) possesses a very similar chemical structure. The research presented in this paper demonstrates the promise peptide amphiphile micelles have in improving the field of vaccine engineering.
KEY WORDS: group A streptococcus, J8 peptide, peptide amphiphile micelles, self-adjuvant, vaccine
INTRODUCTION
Streptococcus pyogenes (group A streptococcus, GAS) is a Gram-positive bacterium restricted to natural growth in humans where it frequently elicits diseases that range in severity from mild infections of the pharyngeal mucosa and dermis to life-threatening invasive infections of connective and muscle tissues leading to necrotizing fasciitis, myonecrosis, and toxic shock (1). Additionally, postinfection sequelae diseases like acute rheumatic fever and glomerulonephritis can arise following localized infections of the nasopharynx and skin. Epidemiological studies estimate that each year, greater than 500,000 worldwide deaths are attributable to GAS infections, placing it among the top ten leading causes of death from infectious pathogens (2,3). In the USA alone, more than $600 million is spent annually treating diseases caused by this organism (4) with no effective preventative method established short of prophylactic antibiotic usage.
Despite decades worth of research, vaccines against GAS remain commercially unavailable (5). The primary barriers preventing the successful development of a vaccine include variability of surface proteins (6,7) and the autoreactivity of antibodies raised against GAS proteins like the highly immunogenic M protein (8). Safety concerns over protein-based GAS vaccine candidates have been addressed by utilizing peptide antigens from conserved protein domains that do not generate cross-reactive antibodies to host tissues. Specifically, the J8 peptide is a 29 amino acid sequence (QAEDKVKQSREAKKQVEKALKQLEDKVQK) from the C-terminal domain of the GAS M1 protein (M-5336-364) which possesses a conformationally dependent B cell epitope (SREAKKQVEKAL) and has been found to induce an opsonophagocytic, high-titer antibody response in mice (9,10) that does not react with human cardiac tissues (11,12).
Peptides are attractive vaccine candidates since they are typically safer than whole pathogen vaccines, but they are often weak immunogens (13). To enhance the corresponding host immune response, peptide vaccines are often delivered by aid of a delivery vehicle or with immune boosting substances termed adjuvants. While promising experiments have been published that employ J8 peptide delivery vehicles (14,15) or adjuvanted J8 peptide (10,16), this research has yet to lead to a commercially viable GAS vaccine, so novel systems need to be explored. An effective construct should concentrate the peptide antigen, protect it from degradation, enhance its cellular uptake, and adjuvant its immunogenicity in order to induce a robust immune response (17,18).
Peptide amphiphiles are a class of biomaterials comprised of peptide-lipid conjugates that undergo self-assembly into micelles in water. They have been shown capable of delivering biologically active peptides for a variety of applications including angiogenesis (19,20), osteogenesis (21,22), neurogenesis (23,24), atherosclerosis treatment (25), cancer therapy (26,27), and islet transplantation (28,29). Also, peptide amphiphile micelles are comprised of a high concentration of peptide (30), inhibit peptide degradation (31), and can greatly increase peptide intracellular delivery (32). Recent research by the Tirrell Group has shown that peptide amphiphile micelles that display a tumor-specific cytotoxic T cell epitope can function as a self-adjuvanting vaccine delivery system capable of inducing a tumor-suppressing immune response when given prophylactically (33). In this report, the potential for peptide amphiphile micelles to act as a self-adjuvanting platform for the delivery of a GAS peptide vaccine was investigated.
MATERIALS AND METHODS
Peptide and Peptide Amphiphile Synthesis
J8 peptide (QAEDKVKQSREAKKQVEKALKQLEDKVQK) was synthesized on Rink amide MBHA resin (Novabiochem) utilizing standard Fmoc solid-phase synthesis with the aid of a PS3 Peptide Synthesizer (Protein Technologies, Inc.). The resulting J8 peptide was treated using a concentrated trifluoroacetic acid solution to deprotect side groups and cleave the peptide from resin. High-pressure liquid chromatography with mass spectrometry controlled fraction collection (LCMS; Shimadzu Corp.) utilizing a reversed-phase C8 column (Waters) with a gradient of acetonitrile in Milli-Q water containing 0.1% formic acid was employed to purify J8 peptide. For J8 peptide amphiphiles, the hydrophobic moiety dipalmitoylglutamic acid (diC16) was synthesized by a previously established method (34). J8 peptide was synthesized similarly to above except the C-terminal lysine was protected with DDE instead of Boc which was used for the other lysines. The peptide was treated with 2% hydrazine in DMF to orthogonally deprotect the C-terminal lysine amine group which was covalently coupled to diC16 by an amidation reaction yielding J8-diC16 peptide amphiphiles. J8-diC16 was further processed and purified by the same methods as the J8 peptide. All samples were lyophilized and stored at −20°C until used. It should be noted that all peptide and peptide amphiphiles were created in a chemical synthesis laboratory using appropriate personal protective equipment to eliminate exposure to biological contaminants.
Micelle Formation and Characterization
To fabricate micelles, J8-diC16 peptide amphiphiles were film cast by dissolving them in methanol and evaporating the solvent using nitrogen as a drying gas. Hydration of the films with water or phosphate-buffered saline (PBS) followed by thorough vortexing induced spontaneous micelle formation. The micelles were then allowed to equilibrate overnight. J8-diC16 micelles were characterized by previously defined methodologies (33,35,36) including critical micelle concentration (CMC) analysis, transmission electron microscopy (TEM), and circular dichroism (CD). CMC was measured by fluorescent sequestration where varying concentrations of J8-diC16 were exposed to 1 mM 1,6-diphenyl-1,3,5-hexatriene (DPH) which greatly increases in fluorescence intensity when trapped within the micelle core. Solutions were prepared and allowed to equilibrate for 1 h prior to fluorescent measurement utilizing a TECAN Infinite M200 plate reader (ex. 350 nm, em. 428 nm). The data were fit with two trend lines which were set equal to one another to determine the fluorescence inflection point (i.e. CMC). Micelle morphology was investigated using negative stain TEM. J8-diC16 solution (1 uL of 200 μM) was allowed to incubate on Formvar-coated copper grids (Ted Pella, Inc.) for 1 min, after which excess liquid was wicked away with filter paper. Grids were then washed with Milli-Q water and then incubated with aqueous phosphotungstic acid (1 wt %) for 1 min before the solution was wicked away. Samples were allowed to air dry and then imaged on a FEI Tecnai 12 TEM using an accelerating voltage of 120 kV. The secondary structure of J8 peptide in solution and confined within the corona of J8-diC16 micelles was assessed using CD. CD spectra of 30 μM solutions of J8 and J8-diC16 were measured in water at 25°C a total of five times and the data was averaged. The data presented represents CD analysis performed at least three times per sample. Water was used alternatively to PBS since chloride ions from the PBS can interfere with CD measurements. The data were averaged and a curve from 190 to 250 nm was fit using a linear combination of polylysine basis spectra (37) to determine approximate α-helix, β-sheet, and random coil peptide secondary structure.
Micelle Annealing and Characterization
To investigate the effect of annealing on micelles, J8 peptide and J8-diC16 micelle solutions were prepared as described above. Preliminary experiments employing differential scanning calorimetry showed that J8-diC16 micelles anneal at 40°C and undergo an irreversible transition at 70°C as evidenced by the fact that further heat-cool annealing cycles showed no such transition behavior. To ensure annealing has gone to completion, J8 peptide and J8-diC16 micelle solutions were heating to 70°C for 1 h and allowed to cool back to room temperature and equilibrate overnight. Micelles were characterized for morphometric changes by TEM using the methodology described previously. Secondary structure of the peptide in solution and on the micelle surface was assessed by CD.
Murine Vaccination
Female BALB/c mice 6–8 weeks old were purchased from Charles River and immunized to investigate the capacity for micelles to induce an antibody-mediated immune response. Previous research has shown that the J8 peptide is a weak immunogen which requires adjuvants to be effective (38). The strong physical adjuvant Incomplete Freund’s Adjuvant (IFA) was purchased from Sigma-Aldrich. For control groups, a universal T helper peptide antigen termed KLIP (KLIPNASLIENCTKAEL) (39) and a mock peptide amphiphile (diC16-SK4) were synthesized and purified in the lab similarly to methods detailed above. To confirm that diC16-SK4 formed micelles, micelle characterization was conducted and a CMC of 4.53 μM was determined (data not shown). J8-diC16 and diC16-SK4 micelles used for vaccination were fabricated by the film deposition, rehydration, and annealing method outlined above. Mice were vaccinated in the nape of the neck at days 0 (prime), 21 (boost 1), 28 (boost 2), and 35 (boost 3) with one of three vaccine formulations:
Whole blood was collected from mice saphenous veins pre-vaccination on days 21, 28, and 35 as well as on day 42 to analyze for circulating J8-specific antibody response induced by the previous round of immunization. The blood was centrifuged at 10,000 RPM for 10 min to separate out red blood cells and the supernatant serum was harvested and stored at −20°C until analysis.
Antibody Response Characterization
An enzyme-linked immunosorbent assay (ELISA) was utilized to determine J8-specific antibody titers. Flat-bottom 96-well EIA microtiter plates (Costar) were coated overnight with 100 μL of 10 μg/mL J8 peptide in sodium bicarbonate coating buffer in each well at 4°C. The wells were washed with 200 μL of 0.05% Tween 20 in PBS (PBS-T) three times and then blocked with 200 μL of assay diluent (10% FBS in PBS) for 1 h. The blocking solution was removed and 100 μL of 1:1000 diluted sera samples was added to the top row and then serially diluted twofold with assay diluent down the plate. After 2 h incubation, wells were washed with PBS-T three times and incubated with 100 μL of 1:3000 diluted detection antibody (IgM, IgG, IgA, IgG1, IgG2a, IgG3, or IgG4; Invitrogen) for 1 h. PBS-T was used to wash wells three times, after which 100 uL of Ultra TMB-ELISA substrate solution (Pierce) was added to the wells. Plates were allowed to incubate for 15 min in darkness and then optical density (OD) was measured for each well at 650 nm using a TECAN Infinite M200 plate reader. End point antibody titers were defined as the greatest serum dilution where OD was at least twice that of normal mouse serum at the same dilution.
Toll-like Receptor 2 Stimulation
Toll-like receptor 2 (TLR-2) stimulation activity was characterized similarly to a previously established technique (33). Human embryonic kidney cells (HEK-293) transfected to express the TLR-2 receptor on their surface which when stimulated causes a luciferase reporter gene to fluoresce were generously provided as a gift by Professor Greg Barton. The cells were seeded at 104 cells per well in a 96-well plate in complete culture media (Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum, 4.5 g/L glucose, 4 mM L-glutamine, 1 mM sodium pyruvate, 100 μg/mL streptomycin, 100 units/mL penicillin, and 10 μg/mL Geneticin) and allowed to incubate stimulus-free for 16 h. Cells were exposed to PBS, 10 μM J8 peptide, 10 μM J8-diC16, or 1 μM synthetic triacetylated lipoprotein (Pam3Cys-SK4, Invitrogen), a known TLR-2 agonist, and incubated for 6 h. The Promega Luciferase Assay System was used according to manufacturer instructions and luminescence was measured at 560 nm using a TECAN Infinite M200 plate reader.
Statistical Analysis
JMP software (SAS Institute) was used to make comparisons between groups using an ANOVA followed by Tukey’s HSD test to determine pairwise statistically significant differences (p < 0.05). Within the figure graphs, groups that possess different letters have statistically significant differences in mean whereas those that possess the same letter are similar.
RESULTS
Synthesis and Characterization of GAS Vaccine Peptide Amphiphile Micelles
With the J8 peptide sequence possessing a C-terminal lysine, the hydrophobic dialkyl tail moiety (diC16) was able to be covalently tethered to the peptide via an amide bond yielding J8-diC16 peptide amphiphile (Fig. 1a). J8-diC16 amphiphiles were film cast by methanol evaporation and rehydrated to determine if they could self-assemble into micelles. DPH sequestration evident by an exponential increase in fluorescence as a function of increasing peptide amphiphile concentration indicated that J8-diC16 forms micelles and does so at a CMC of 1.71 μM (Fig. 1b). Negative stain TEM revealed that J8-diC16 formed short cylindrical micelles approximately 5–15 nm in diameter and 25–125 nm in length (Fig. 1c). In order to determine if micellization affected the J8 peptide, CD was employed to determine secondary structure for J8 peptide in solution and in micelles (Fig. 1d). Interestingly, tethering of J8 peptide within the micelle corona significantly decreased peptide random coil structure while greatly increasing its α-helicity from 0 to 42.5%.
Fig. 1.
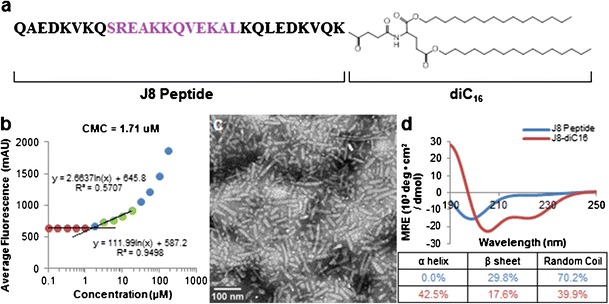
Physical characterization of self-assembled GAS vaccine peptide amphiphile micelles. a J8-diC16 peptide amphiphile structure with the B cell antigen shown in purple. b The capacity for J8-diC16 to form micelles was evaluated by a critical micelle concentration (CMC) assay and a CMC of 1.71 μM was calculated. c TEM of J8-diC16 revealed that PAs form short, rigid, cylindrical micelles. d CD showed that J8 peptide confined within the corona of micelles possessed altered secondary structure. Curve fitting revealed J8 peptide in solution had no detectable α-helicity whereas micelle-based J8 peptide exhibited significant α-helicity
Annealing Modulates GAS Vaccine Peptide Amphiphile Micelle Structure
Previous research has shown that micelle morphology and structure can change over time (40), which can be expedited by annealing (41,42). After heating J8-diC16 to 70°C and allowing it to cool to room temperature, TEM revealed that long, flexible, cylindrical micelles had been formed approximately 5–15 nm in diameter and 200 nm–2 um in length (Fig. 2a). CD revealed that annealing J8 peptide in solution and confined within micelles yielded 0 and 50.5% α-helicity, respectively (Fig. 2b).
Fig. 2.
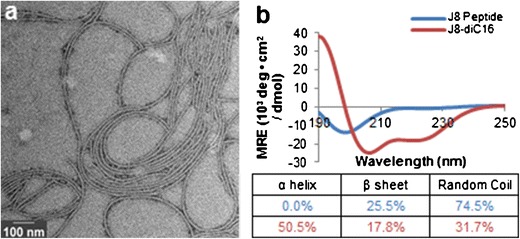
Annealing GAS vaccine peptide amphiphile micelles altered their physical structure. a TEM of J8-diC16 heated and then cooled to room temperature showed micelles transitioned from short, rigid, cylindrical micelles to long, flexible, cylindrical micelles. b CD revealed that annealing did not alter the α-helicity of J8 peptide in solution but did appreciably increase the α-helicity of micelle-based J8 peptide
GAS Vaccine Peptide Amphiphile Micelles Induce Strong Immune Responses
Mice were subcutaneously vaccinated with either GAS vaccine peptide supplemented with conventional adjuvants (J8 + KLIP in IFA), GAS vaccine peptide amphiphile micelles (J8-diC16), or GAS vaccine supplemented with mock micelles (J8 + diC16-SK4). Harvested serum samples were analyzed by ELISA to determine J8-specific antibody isotype titers (Fig. 3). Only the J8-diC16 vaccine was able to induce appreciable IgM titers which were found to be significantly higher than both J8 + KLIP in IFA and J8 + diC16-SK4 vaccines after the second boost vaccination. Both J8 + KLIP in IFA and J8-diC16 vaccines were able to cause antibody isotype switching as shown by their induction of appreciable IgG titers by the third boost and second boost vaccinations, respectively. The J8 + diC16-SK4 vaccine did not induce any IgM or IgG antibodies. No vaccine treatment induced an IgA response (data not shown). To further investigate the IgG response induced, ELISAs were conducted on the serum samples to determine IgG subtype titers (Fig. 4). The data show that IgG1 is the dominant antibody with either J8 + KLIP in IFA or J8-diC16 vaccine. A few mice vaccinated with J8 + KLIP in IFA showed appreciable IgG2a titers and a couple of mice given J8-diC16 had above background IgG3 titers, but neither of these results was significant. IgG4 was also assayed for but no titers were observed (data not shown).
Fig. 3.
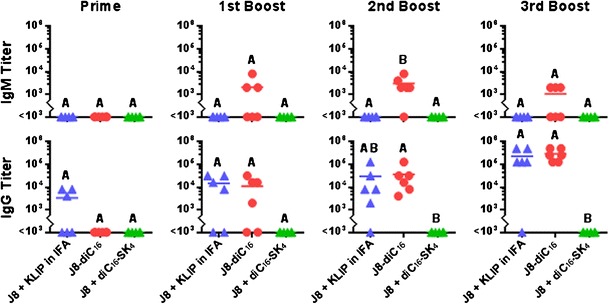
GAS vaccine peptide amphiphile micelles induced strong antibody isotype responses in vivo. Only mice given the J8-diC16 vaccine produced appreciable IgM titers. Mice given J8 + KLIP in IFA or J8-diC16 vaccines produced similar quantities of IgG antibody that directly correlated to the number of vaccinations. In contrast, no antibody titers were observed for J8 + diC16-SK4-vaccinated mice. While IgA titers were assessed, no mouse produced above background levels. Each point represents one mouse (N = 6); bars represent the mean. Within a graph, groups that possess different letters have statistically significant differences in mean (p ≤ 0.05) whereas those that possess the same letter are similar (p > 0.05)
Fig. 4.
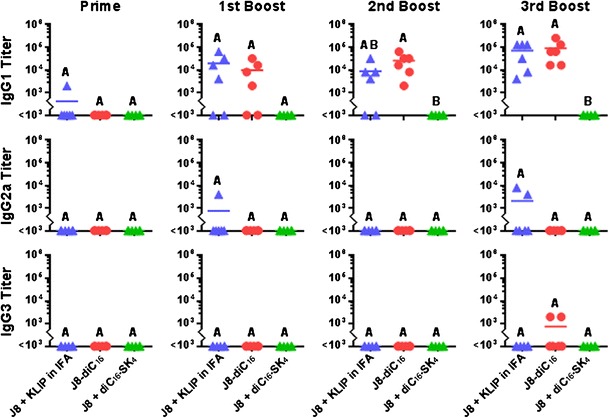
GAS vaccine peptide amphiphile micelles induced strong IgG1-dominant antibody subtype responses in vivo. The strong IgG response induced by the J8 + KLIP in IFA or J8-diC16 vaccines was found to be predominantly comprised of the IgG1 subtype. While a few mice possessed above background titers for IgG2a and IgG3 dependent on the vaccine formulation they were given, these responses were not statistically significant. Also, no mouse produced above background levels of IgG4 titers. Each point represents one mouse (N = 6); bars represent the mean. Within a graph, groups that possess different letters have statistically significant differences in mean (p ≤ 0.05) whereas those that possess the same letter are similar (p > 0.05)
GAS Vaccine Peptide Amphiphile Micelle Adjuvanticity Is Not TLR-2 Mediated
The hydrophobic moiety diC16 possesses a similar chemical structure to the known adjuvant Pam3Cys (Fig. 5a) which is a TLR-2 agonist. To determine if TLR-2 stimulation was responsible for J8-diC16 micelle self-adjuvanticity, TLR-2 expressing, luciferase reporter HEK-293 cells were treated in vitro with PBS, J8 peptide, J8-diC16 micelles, or Pam3Cys-SK4 (Fig. 5b). Incubation of cells with PBS or J8 peptide induced no fluorescence, indicating an absence of TLR-2 stimulation compared to the positive Pam3Cys-SK4 control. Cells incubated with J8-diC16 micelles had similar fluorescence levels to cells exposed to PBS and J8 peptide, implying the micelles did not stimulate TLR-2.
Fig. 5.
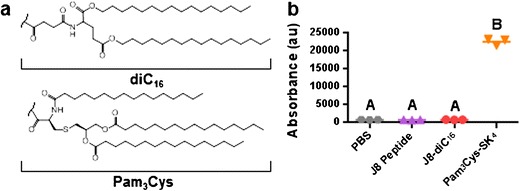
J8-diC16 micelles do not stimulate TLR-2 in vitro. a Chemical structure similarity between diC16 and Pam3Cys, a known TLR-2 stimulant. b HEK-293 cells transfected to express TLR-2 on their surface and luminesce when the TLR-2 is stimulated were incubated with PBS, 10 μM J8 peptide, 10 μM J8-diC16 micelles, or 1 μM Pam3Cys. Only cells incubated with Pam3Cys strongly fluoresced whereas cells exposed to J8-diC16 had similar fluorescence to the PBS and J8 peptide controls. Within a graph, groups that possess different letters have statistically significant differences in mean (p ≤ 0.05) whereas those that possess the same letter are similar (p > 0.05)
DISCUSSION
Vaccines are the most effective method for the prevention of pathogenic infections, yet a viable vaccine that protects against GAS has yet to make it to the market. While previous research employing the J8 peptide vaccine to induce a protective antibody response has been promising (9–12), common issues associated with peptide vaccines (i.e., immunogenicity, conformational dependence, localized high concentration delivery, and adjuvant supplementation) have kept it from moving into human clinical trials. The design of a self-adjuvanting delivery device that overcomes these problems could yield the first commercial vaccine against GAS. While a variety of systems could be designed, micelles possess several advantages over other nanoparticle-based systems. Micelles are water soluble, which makes them easy to deliver via injection in comparison to non-soluble particles like polymeric or metallic nanoparticles that must be suspended (43,44), increasing solution viscosity and which have a tendency to agglomerate complicating their injectability (45,46). Also, since the micelle delivery device is comprised of the peptide itself, it is more than 80% peptide vaccine by weight which is considerably higher than the ∼25% maximum peptide loading possible with other nanoparticle delivery devices (47). Previous research has shown peptide amphiphile micelles presenting a cytotoxic T cell epitope act as self-adjuvants that induce a strong immune response (33), so the same platform technology was tested for its ability to facilitate an antibody-mediated response.
J8-diC16 peptide amphiphiles were synthesized and shown to readily form micelles that possessed several desirable physical characteristics. Each J8-diC16 micelle shown in Fig. 1c traps hundreds to thousands of peptides together, which greatly increases local antigen concentration upon interaction with immune cells compared to soluble peptide which can rapidly disseminate from the injection site. Also, many B cell epitopes including J8 are conformationally dependent, meaning their secondary structure is crucial for their immunogenicity (48). A major issue with conformationally dependent peptide antigens is that since they lack the tertiary structure restraints of their native protein, they often lose their secondary structure. This is particularly evident with the J8 peptide which is highly α-helical in the context of the M1 protein (49), but possesses no α-helicity when isolated and in solution (Fig. 1d). Interestingly, confining and crowding the peptide within the micelle corona gives an artificial tertiary structure that facilitates J8 peptide becoming highly α-helical (42.5%, Fig. 1d), which agrees with previous research that has shown the capacity of peptide amphiphile micelles to reestablish native peptide secondary structure (50–52). Annealing the micelles induced them to form much longer structures (Fig. 2a) with even greater α-helicity (50.5%, Fig. 2b). This modulation allows for each micelle to deliver an order of magnitude greater number of peptides (thousands to tens of thousands) with nearly 20% more of them in the correct conformational state. It should also be noted that micelle peptide structure and elongation are interrelated phenomena. The increase in temperature allows for greater peptide folding into the native α-helical structure (51,53), which compacts the peptide head group of the amphiphile decreasing the overall molecular packing parameter (54) and allowing for longer micelle lengths.
Soluble J8 peptide supplemented with the strong physical adjuvant IFA and the universal T helper epitope KLIP induced a strong IgG response in mice after prime immunization followed by three booster immunizations (Fig. 3) While IFA has been used clinically in the past, there now exists serious concerns that it may cause autoimmunity (55,56) and minimize the effectiveness of induced immune cells (57). Vaccine adjuvanting systems capable of mediating similar responses to IFA have the potential to move peptide-based vaccines to the market. Annealed J8-diC16 micelles were found to induce a strong J8-specific antibody response comparative to the J8 + KLIP in IFA control vaccine formulation (Fig. 3). In specific, J8-diC16 micelles induced the production of IgM that peaked after the second booster vaccination and isotype switching that led to IgG titers that increased with each round of vaccination which is characteristic of a classical antibody-mediated vaccination response (58). Further investigation into the specific nature of the J8-diC16-induced IgG response revealed that it was almost entirely comprised of IgG1 subtype (Fig. 4) which has been found to facilitate complement activation and macrophage engulfment as well as protect against GAS infections (59). These results provide considerable evidence that peptide amphiphile micelles can act as self-adjuvants capable of inducing strong antibody responses against peptide antigens. While promising, the capacity for micelle-induced J8-specific antibodies to identify their cognate sequence within the M1 protein has not yet been studied. Future experiments will investigate whether J8-specific antibodies can preferentially bind native M1 protein presented extracellularly on wild-type GAS bacteria.
While the mechanism responsible for micelle adjuvanticity is currently unknown, a few theories do exist. First, micellization can induce native peptide secondary structure (e.g., α-helicity) and increase local peptide concentration which enhances conformationally correct antigen delivery at the injection site. Second, micelles are a nanoparticulate which may strongly interact with antigen presenting cells (APCs). In specific, hydrophobic moieties like diC16 can act as danger signals known as pathogen-associated molecular patterns (PAMP) which can interact with pathogen recognition receptors (PRRs) called Toll-like receptors (TLRs), C-type lectin receptors (CLRs), NOD-like receptors (NLRs), and RIG-I-like receptors (RLRs) found on the surface of APCs (13). The importance of micellization is evident by the lack of an immune response induced when J8 peptide was delivered in solution separately from micelles (J8 + diC16-SK4, Figs. 3 and 4). While some adjuvants like IFA can induce antibody responses for co-delivered antigens, peptide amphiphile micelles must directly deliver the peptide antigen in order to function as an adjuvant which is similar to other self-assembled peptide vaccines (60,61). With regard to PRR stimulation, while diC16 has a chemical structure quite similar to Pam3Cys, it was found unable to stimulate TLR-2, the cognate receptor for Pam3Cys (Fig. 5). This result combined with previous research that has shown a lack of TLR-2 stimulation and APC activation by peptide amphiphile micelles (33) provides convincing evidence that peptide amphiphile micelle adjuvanticity is conveyed by its ability to function as a delivery device instead of as a PRR stimulating system. However, other TLRs exist and will need to be tested in the future to confirm diC16-based micelles do not act as PAMPs.
CONCLUSIONS
While other work has shown lipopeptides and self-assembled peptides can be effective vaccines, this research is the first to demonstrate that peptide amphiphile micelles can be utilized as a self-adjuvanting vaccine delivery vehicle to induce a significant peptide-specific antibody response. Micellization allows for the fabrication of cylindrical nanomaterials mostly comprised of conformationally correct (i.e., α-helical) J8 peptide vaccine which can be enhanced by annealing. When delivered subcutaneously to mice, J8-diC16 peptide amphiphile micelles induced a strong IgG1 antibody response similar to immunization with a conventional gold-standard vaccine formulation. Additional experiments revealed that micelle adjuvanticity appears to be conveyed by its peptide delivery capacity instead of through stimulation of danger signal receptors. These results provide evidence that peptide amphiphile micelles are a novel biomaterials platform that can be utilized to achieve desirable immune responses.
Acknowledgments
The authors gratefully acknowledge support from funds provided from the University of California, Berkeley and the University of Chicago, as well as research funding from the University of Chicago Institute for Translational Medicine (CTSA UL1 TR000430). We thank Dr. Eva Ulery for her comments and valuable discussion regarding the manuscript.
Footnotes
Amanda Trent and Bret D. Ulery contributed equally to this work.
References
- 1.Tart AH, Walker MJ, Musser JM. New understanding of the group A streptococcus pathogenesis cycle. Trends Microbiol. 2007;15(7):318–25. doi: 10.1016/j.tim.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5(11):685–94. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 3.Eison TM, Ault BH, Jones DP, Chesney RW, Wyatt RJ. Post-streptococcal acute glomerulonephritis in children: clinical features and pathogenesis. Pediatr Nephrol. 2011;26(2):165–80. doi: 10.1007/s00467-010-1554-6. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence RS. Vaccines for the 21st century: a tool for decision making. Washington, D.C.: National Academies Press; 2000. [PubMed] [Google Scholar]
- 5.Steer AC, Batzloff MR, Mulholland K, Carapetis JR. Group A streptococcal vaccines: facts versus fantasy. Curr Opin Infect Dis. 2009;22(6):544–52. doi: 10.1097/QCO.0b013e328332bbfe. [DOI] [PubMed] [Google Scholar]
- 6.Sagar V, Bergmann R, Nerlich A, McMillan DJ, Nitsche-Schmitz DP, Chhatwal GS. Variability in the distribution of genes encoding virulence factors and putative extracellular proteins of Streptococcus pyogenes in India, a region with high streptococcal disease burden, and implication for development of a regional multisubunit vaccine. Clin Vaccine Immunol. 2012;19(11):1818–25. doi: 10.1128/CVI.00112-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Persson J, Beall B, Linse S, Lindahl G. Extreme sequence divergence but conserved ligand-binding specificity in Streptococcus pyogenes M protein. PLoS Pathog. 2006;2(5):e47. doi: 10.1371/journal.ppat.0020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lymbury RS, Olive C, Powell KA, Good MF, Hirst RG, LaBrooy JT, et al. Induction of autoimmune valvulitis in Lew rats following immunization with peptides from the conserved region of group A streptococcal M protein. J Autoimmun. 2003;20(3):211–7. doi: 10.1016/S0896-8411(03)00026-X. [DOI] [PubMed] [Google Scholar]
- 9.Olive C, Sun HK, Ho MF, Dyer J, Horvath A, Toth I, et al. Intranasal administration is an effective mucosal vaccine delivery route for self-adjuvanting lipid core peptides targeting the group A streptococcal M protein. J Infect Dis. 2006;194(3):316–24. doi: 10.1086/505580. [DOI] [PubMed] [Google Scholar]
- 10.Pandey M, Wykes MN, Hartas J, Good MF, Batzloff MR. Long-term antibody memory induced by synthetic peptide vaccination is protective against Streptococcus pyogenes infection and is independent of memory T cell help. J Immunol. 2013;190(6):2692–701. doi: 10.4049/jimmunol.1202333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Relf WA, Cooper J, Brandt ER, Hayman WA, Anders RF, Pruksakorn S, et al. Mapping a conserved conformational epitope from the M protein of group A streptococci. Pept Res. 1996;9(1):12–20. [PubMed] [Google Scholar]
- 12.Hayman WA, Brandt ER, Relf WA, Cooper J, Saul A, Good MF. Mapping the minimal murine T cell and B cell epitopes within a peptide vaccine candidate from the conserved region of the M protein of group A streptococcus. Int Immunol. 1997;9(11):1723–33. doi: 10.1093/intimm/9.11.1723. [DOI] [PubMed] [Google Scholar]
- 13.Black M, Trent A, Tirrell M, Olive C. Advances in the design and delivery of peptide subunit vaccines with a focus on toll-like receptor agonists. Expert Rev Vaccines. 2010;9(2):157–73. doi: 10.1586/erv.09.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simerska P, Abdel-Aal AB, Fujita Y, Moyle PM, McGeary RP, Olive C, et al. Development of a liposaccharide-based delivery system and its application to the design of group A streptococcal vaccines. J Med Chem. 2008;51(5):1447–52. doi: 10.1021/jm701410p. [DOI] [PubMed] [Google Scholar]
- 15.Middelberg AP, Rivera-Hernandez T, Wibowo N, Lua LH, Fan Y, Magor G, et al. A microbial platform for rapid and low-cost virus-like particle and capsomere vaccines. Vaccine. 2011;29(41):7154–62. doi: 10.1016/j.vaccine.2011.05.075. [DOI] [PubMed] [Google Scholar]
- 16.Pandey M, Batzloff MR, Good MF. Mechanism of protection induced by group A streptococcus vaccine candidate J8-DT: contribution of B and T-cells toward protection. PLoS One. 2009;4(4):e5147. doi: 10.1371/journal.pone.0005147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azmi F, Fuaad AAA, Skwarczynski M, Toth I. Recent progress in adjuvant discovery for peptide-based subunit vaccines. Hum Vaccines Immunother. 2013;10(3):27332. doi: 10.4161/hv.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao L, Seth A, Wibowo N, Zhao CX, Mitter N, Yu C, et al. Nanoparticle vaccines. Vaccine. 2014;32(3):327–37. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 19.Webber MJ, Tongers J, Newcomb CJ, Marquardt KT, Bauersachs J, Losordo DW, et al. Supramolecular nanostructures that mimic VEGF as a strategy for ischemic tissue repair. Proc Natl Acad Sci U S A. 2011;108(33):13438–43. doi: 10.1073/pnas.1016546108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mammadov R, Mammadov B, Toksoz S, Aydin B, Ragci Y, Tekinay AB, et al. Heparin mimetic peptide nanofibers promote angiogenesis. Biomacromolecules. 2011;12(10):3508–19. doi: 10.1021/bm200957s. [DOI] [PubMed] [Google Scholar]
- 21.Anderson JM, Kushwaha M, Tambralli A, Bellis SL, Camata RP, Jun HW. Osteogenic differentiation of human mesenchymal stem cells directed by extracellular matrix-mimicking ligands in a biomimetic self-assembled peptide amphiphile nanomatrix. Biomacromolecules. 2009;10(10):2935–44. doi: 10.1021/bm9007452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mata A, Geng Y, Henrikson KJ, Aparicio C, Stock SR, Satcher RL, et al. Bone regeneration mediated by biomimetic mineralization of a nanofiber matrix. Biomaterials. 2010;31(23):6004–12. doi: 10.1016/j.biomaterials.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song Y, Li Y, Zheng Q, Wu K, Guo X, Wu Y, et al. Neural progenitor cells survival and neuronal differentiation in peptide-based hydrogels. J Biomater Sci Polym Ed. 2011;22(4–6):475–87. doi: 10.1163/092050610X487756. [DOI] [PubMed] [Google Scholar]
- 24.Angeloni NL, Bond CW, Tang Y, Harrington DA, Zhang S, Stupp SI, et al. Regeneration of the cavernous nerve by Sonic hedgehog using aligned peptide amphiphile nanofibers. Biomaterials. 2011;32(4):1091–101. doi: 10.1016/j.biomaterials.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters D, Kastantin M, Kotamraju VR, Karmali PP, Gujraty K, Tirrell M, et al. Targeting atherosclerosis by using modular, multifunctional micelles. Proc Natl Acad Sci U S A. 2009;106(24):9815–9. doi: 10.1073/pnas.0903369106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Missirlis D, Krogstad DV, Tirrell M. Internalization of p53(14–29) peptide amphiphiles and subsequent endosomal disruption results in SJSA-1 cell death. Mol Pharm. 2010;7(6):2173–84. doi: 10.1021/mp100193h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Standley SM, Toft DJ, Cheng H, Soukasene S, Chen J, Raja SM, et al. Induction of cancer death by self-assembling nanostructures incorporating a cytotoxic peptide. Cancer Res. 2010;70(8):3020–6. doi: 10.1158/0008-5472.CAN-09-3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim DJ, Antipenko SV, Anderson JM, Jaimes KF, Viera L, Stephen BR, et al. Enhanced rat islet function and survival in vitro using a biomimetic self-assembled nanomatrix gel. Tissue Eng A. 2011;17(3–4):399–406. doi: 10.1089/ten.tea.2010.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan S, Sur S, Newcomb CJ, Appelt EA, Stupp SI. Self-assembling glucagon-like peptide 1-mimetic peptide amphiphiles for enhanced activity and proliferation of insulin-secreting cells. Acta Biomater. 2012;8(5):1685–92. doi: 10.1016/j.actbio.2012.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boato F, Thomas RM, Ghasparian A, Freund-REnard A, Moehle K, Robinson JA. Synthetic virus-like particles from self-assembling coiled-coil lipopeptides and their use in antigen display to the immune system. Angew Chem Int Ed. 2007;46(47):9015–8. doi: 10.1002/anie.200702805. [DOI] [PubMed] [Google Scholar]
- 31.Lee KC, Carlson PA, Goldstein AS, Yager P, Gelb MH. Protection of a decapeptide from proteolytic cleavage by lipidation and self-assembly into high-axial-ratio microstructures: a kinetic and structural study. Langmuir. 1999;15(17):5500–8. doi: 10.1021/la9900775. [DOI] [Google Scholar]
- 32.Missirlis D, Khant H, Tirrell M. Mechanisms of peptide amphiphile internalization by SJSA-1 cells in vitro. Biochemistry. 2009;48(15):3304–14. doi: 10.1021/bi802356k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Black M, Trent A, Kostenko Y, Lee JS, Olive C, Tirrell M. Self-assembled peptide amphiphile micelles containing a cytotoxic T-cell epitope promote a protective immune response in vivo. Adv Mater. 2012;24(28):3845–9. doi: 10.1002/adma.201200209. [DOI] [PubMed] [Google Scholar]
- 34.Berndt P, Fields GB, Tirrell M. Synthetic lipidation of peptides and amino acids: monolayer structure and properties. J Am Chem Soc. 1995;117(37):9515–22. doi: 10.1021/ja00142a019. [DOI] [Google Scholar]
- 35.Kastantin M, Ananthanarayanan B, Karmali P, Ruoslahti E, Tirrell M. Effect of the lipid chain melting transition on the stability of DSPE-PEG(2000) micelles. Langmuir. 2009;25(13):7279–86. doi: 10.1021/la900310k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mlinar LB, Chung EJ, Wonder EA, Tirrell M. Active targeting of early and mid-stage atherosclerosis plaques using self-assembled peptide amphiphile micelles. BIomaterials. 2014;35(30):8678–86. doi: 10.1016/j.biomaterials.2014.06.054. [DOI] [PubMed] [Google Scholar]
- 37.Greenfield NJ, Fasman GD. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969;8(10):4108–16. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- 38.Skwarcynski M, Kamaruzaman KA, Srinivasan S, Zaman M, Lin IC, Batzloff MR, et al. M-protein-derived conformational peptide epitope vaccine candidate against group A streptococcus. Curr Drug Deliv. 2013;10(1):39–45. doi: 10.2174/1567201811310010007. [DOI] [PubMed] [Google Scholar]
- 39.Jackson DC, Lau YF, Le T, Suhrbier A, Deliyannis G, Cheers C, et al. A totally synthetic vaccine of generic structure that targets Toll-like receptor 2 on dendritic cells and promotes antibody or cytotoxic T cell responses. Proc Natl Acad Sci U S A. 2004;101(43):15440–5. doi: 10.1073/pnas.0406740101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pashuck ET, Stupp SI. Direct observation of morphological transformation from twisted ribbons into helical ribbons. J Am Chem Soc. 2010;132(26):8819–21. doi: 10.1021/ja100613w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gore T, Dori Y, Talmon Y, Tirrell M, Biance-Peled H. Self-assembly of model collagen peptide amphiphiles. Langmuir. 2001;17(17):5352–60. doi: 10.1021/la010223i. [DOI] [Google Scholar]
- 42.Shimada T, Lee S, Bates FS, Hotta A, Tirrell M. Wormlike micelle formation in peptide-lipid conjugates driven by secondary structure transformation of the headgroups. J Phys Chem B. 2009;113(42):13711–4. doi: 10.1021/jp901727q. [DOI] [PubMed] [Google Scholar]
- 43.Ghendon Y, Markushin S, Akopova I, Koptiaeva I, Krivtsov G. Chitosan as an adjuvant for poliovaccine. J Med Virol. 2011;83(5):847–52. doi: 10.1002/jmv.22030. [DOI] [PubMed] [Google Scholar]
- 44.Allard-Vannier E, Cohen-Jonathan S, Gautlier J, Herve-Aubert K, Munnier E, Souce M, et al. Pegylated magnetic nanocarriers for doxorubicin delivery: a quantitative determination of stealthiness in vitro and in vivo. Eur J Pharm Biopharm. 2012;81(3):498–505. doi: 10.1016/j.ejpb.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Narayanan D, Anitha A, Jayakumar R, Nair SV, Chennazhi KP. Synthesis, characterization and preliminary in vitro evaluation of PTH 1–34 loaded chitosan nanoparticles for osteoporosis. J Biomed Nanotechnol. 2012;8(1):98–106. doi: 10.1166/jbn.2012.1367. [DOI] [PubMed] [Google Scholar]
- 46.Gosens I, Post JA, Fonteyne LJDL, Jansen EH, Geus JW, Cassee FR, et al. Impact of agglomeration state of nano- and submicron sized gold particles on pulmonary inflammation. Part Fibre Toxicol. 2010;7(1):37. doi: 10.1186/1743-8977-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu J, Kamaly N, Shi J, Zhao L, Xiao Z, Hollett G, et al. Development of multinuclear polymeric nanoparticles as robust protein nanocarriers. Angew Chem Int Ed. 2014;53(34):8975–9. doi: 10.1002/anie.201404766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao B, Zheng D, Liang S, Zhang C. Conformational B-cell epitope prediction on antigen protein structures: a review of current algorithms and comparison with common binding site prediction methods. PLoS One. 2013;8(4):e62249. doi: 10.1371/journal.pone.0062249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McNamara C, Zinkernagel AS, Macheboeuf P, Cunningham MW, Nizet V, Ghosh P. Coiled-coil irregularities and instabilities in group A streptococcus M1 are required for virulence. Science. 2008;319(5868):1405–8. doi: 10.1126/science.1154470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tu RS, Marullo R, Pynn R, Bitton R, Bianco-Peled H, Tirrell MV. Cooperative DNA binding and assembly by a bZip peptide-amphiphile. Soft Matter. 2010;6:1035–44. doi: 10.1039/b922295b. [DOI] [Google Scholar]
- 51.Trent A, Marullo R, Lin B, Black M, Tirrell M. Structural properties of soluble peptide amphiphile micelles. Soft Matter. 2011;7:9572–82. doi: 10.1039/c1sm05862b. [DOI] [Google Scholar]
- 52.Marullo R, Kastantin M, Drews LB, Tirrell M. Peptide contour length determines equilibrium secondary structure in protein-analogous micelles. Biopolymers. 2013;99(9):573–81. doi: 10.1002/bip.22217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hansmann UHE, Masuya M, Okamoto Y. Characteristic temperatures of folding of a small peptide. Proc Natl Acad Sci U S A. 1997;94(20):10652–6. doi: 10.1073/pnas.94.20.10652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagarajan R. Molecular packing parameter and surfactant self-assembly: the neglected role of the surfactant tail. Langmuir. 2002;18(1):31–8. doi: 10.1021/la010831y. [DOI] [Google Scholar]
- 55.Satoh M, Kuroda Y, Yoshida H, Behney KM, Mizutani A, Akaogi J, et al. Induction of lupus autoantibodies by adjuvants. J Autoimmun. 2003;21(1):1–9. doi: 10.1016/S0896-8411(03)00083-0. [DOI] [PubMed] [Google Scholar]
- 56.Kuroda Y, Nacionales DC, Akaogi J, Reeves WH, Satoh M. Autoimmunity induced by adjuvant hydrocarbon oil components of vaccine. Biomed Pharmacother. 2004;58(5):325–37. doi: 10.1016/j.biopha.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 57.Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang X-F, et al. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat Med. 2013;19(4):465–72. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schunk MK, Macallum GE. Applications and optimization of immunization procedures. ILAR J. 2005;46(3):241–57. doi: 10.1093/ilar.46.3.241. [DOI] [PubMed] [Google Scholar]
- 59.Zaman M, Abdel-Aal AB, Fujita Y, Phillipps KS, Batzloff MR, Good MF, et al. Immunological evaluation of lipopeptide group A streptococcus (GAS) vaccine: structure-activity relationship. PLoS One. 2012;7(1):e30146. doi: 10.1371/journal.pone.0030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rudra JS, Tian YF, Jung JP, Collier JH. A self-assembling peptide acting as an immune adjuvant. Proc Natl Acad Sci U S A. 2010;107(2):622–7. doi: 10.1073/pnas.0912124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen J, Pompano RR, Santiago FW, Maillat L, Sciammas R, Sun T, et al. The use of self-adjuvanting nanofiber vaccines to elicit high-affinity B cell responses to peptide antigens without inflammation. Biomaterials. 2013;34(34):8776–85. doi: 10.1016/j.biomaterials.2013.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]


