Abstract
Endothelial dysfunction has been recognized as a pathophysiologic mechanism in the progression of heart failure (HF). However, little attention has been given to the ability of dietary approaches to improve endothelial function. This study examined the effects of the Dietary Approaches to Stop Hypertension (DASH) diet on endothelial function, exercise capacity, and quality of life in patients with chronic symptomatic (stage C) HF. Forty-eight patients were randomized to follow the DASH diet (n = 24) or the general HF dietary recommendations (n = 24). Endothelial function was assessed by measuring large and small arterial elasticity (LAE and SAE) at rest. Exercise capacity (measured with the 6-minute walk test) and quality of life (measured with the Minnesota Living with Heart Failure Questionnaire) at baseline and 3 months were also evaluated. Patients were older adults with an average HF duration of 5 years. LAE at 1 month improved significantly in the DASH diet group (P < 0.01). Overall LAE and SAE scores at 3 months also improved; however, the net changes were not statistically significant. The DASH group had better exercise capacity (292 m vs 197 m; P = 0.018) and quality of life scores (21 vs 39; P = 0.006) over time, while sodium intake levels at 1, 2, and 3 months were comparable between the groups. Adhering to the DASH diet improved arterial compliance initially and improved exercise capacity and quality of life scores at 3 months. The DASH diet may be an important adjunctive therapy for patients with symptomatic HF.
Heart failure (HF) remains a leading illness responsible for significant morbidity and mortality in the United States. Current HF treatment guidelines provide no specific recommendations for dietary intake. Patients with HF typically follow dietary guidelines for prevention of coronary heart disease (1). The Dietary Approaches to Stop Hypertension (DASH), a carbohydrate-rich and low-fat diet that emphasizes fruits and vegetables, has been formally adopted into the 2013 dietary guidelines for cardiovascular risk prevention (2, 3). Endothelial dysfunction underlies pathophysiologic mechanisms in the progression of HF and may be an important therapeutic tool in the management and prevention of HF. Several components of the DASH diet may be partly responsible for its salutary effects and influence on endothelial function (4–8). This study examined the effects of the DASH diet on endothelial function, exercise capacity, and quality of life in patients with chronic symptomatic (stage C) HF.
METHODS
A single-center randomized controlled study was conducted. Patients were randomly assigned via a computerized assignment log into the DASH diet regiment or the comparison group, which required patients to follow the general HF dietary recommendations for 3 months. The study comprised 48 stable patients with chronic symptomatic (stage C) HF from the outpatient adult HF clinic at Advocate Christ Medical Center between February and July 2013. Institutional review board approval was obtained in January 2013. The study duration was 6 months. Inclusion criteria included adults over 18 years of age with stage C/New York Heart Association (NYHA) functional classes I–III and systolic or diastolic HF for at least 6 months. The patients were taking recommended medications for HF. Exclusion criteria included serum creatinine >3 mg/dL, allergy or intolerance to components of the DASH diet, chronic inflammatory bowel disease affecting gastrointestinal absorption, inability to perform the 6-minute walk test due to severe musculoskeletal disease, and dependency on using a walker or a cane for ambulation.
After written informed consent was obtained, patients were randomized, using a block-randomization algorithm, to be in the DASH group (n = 24) or the comparison group (n = 24) for 3 months in addition to receiving their standard HF medical therapy. Patients in the DASH group were provided with an educational packet consisting of a copy of the US Department of Health and Human Services DASH eating plan guidebook (9); a DASH shopping list (10), containing various DASH diet components from which patients could choose and a reference for the dietary components they were encouraged to consume for the duration of the study; and a daily and weekly food diary. Patients in the comparison group had no changes to their dietary habits other than the current general admonitions for diet in HF.
At the initial visit, all patients received further education regarding their diet from our outpatient dietitian. The same dietitian conducted monthly in-person visits and weekly or biweekly phone calls to counsel patients and reinforce their respective diets. In-person sessions were approximately 15 minutes in duration, in a one-on-one setting, and tailored to the individual patient's comorbidities. To more accurately assess the degree of concordance with the DASH diet, patients were assessed monthly using a DASH diet index developed by Folsom et al (11). The scoring system was based on the premise that each of the major DASH diet guidelines should contribute equally to the total index score. The index has 11 items, and the score ranges from 0 to 11, with higher scores indicating higher levels of concordance. During the interview with the dietitian, the reported intake of sodium was estimated from the patients' corresponding food diaries and included in a food frequency questionnaire. Baseline data included demographic characteristics, clinical history, physical exam, NYHA functional class, body mass index, quality of life as measured by the Minnesota Living with Heart Failure Questionnaire (MLHFQ) (12), ejection fraction available from recent transthoracic echocardiogram, and serum markers known to influence HF: electrolyte measurements (sodium, potassium, magnesium), creatinine, B-type natriuretic peptide (BNP), galectin-3, and peripheral (SpO2) and cerebral (SctO2) oxygen saturations.
Data collected throughout the study included exercise capacity as measured by the 6-minute walk test (13), weight, and DASH diet index score for compliance. To assess endothelial function, large and small arterial elasticity (LAE and SAE) at rest were measured using the pressure pulse contour analysis technique with the HDI/PulseWaveTM (Hypertension Diagnostics, Inc., Eagan, MN). The device obtains radial artery waveforms with a calibrated proprietary tonometer. The tonometer consists of a 1.27 cm-diameter stainless-steel canister with a 0.15 mm-thick stainless-steel diaphragm internally connected to a double-plated ceramic piezoelectric element used to amplify the waveform signal. The tonometer is housed inside a holding and positioning device that is wrapped around the patient's arm, supported by an angulated “wrist stabilizer,” achieving complete stability of position after the tonometer has been applied. The obtained waveforms are calibrated to the systolic and diastolic cuff pressure values of an integrated oscillometric device (cuff placed on the contralateral arm with respect to the tonometer). A computer-based Windkessel model of the circulation was used to match the diastolic pressure decay of the tonometrically obtained waveforms and to quantify changes in arterial waveform morphology in terms of LAE and SAE. The values were obtained during a recording period of approximately 2 minutes. The instrument provides separate measurements of LAE and SAE, which are sensitive markers for endothelial dysfunction (14–16).
Hemodynamic parameters were also obtained using the HDI/PulseWaveTM, including mean arterial blood pressure, heart rate, systemic vascular resistance, stroke volume, estimated cardiac output, and estimated cardiac index. The primary endpoint was the impact of the DASH diet on endothelial function, measured by LAE and SAE. The secondary endpoints were changes in hemodynamic parameters, exercise capacity, and quality of life during the 3-month follow-up period.
A power analysis was performed with the Student t test to account for differences in endothelial function between the groups. Based on the results of Fuentes et al (5), and assuming a mean change in endothelial function of 0.5 to 3.0 mL/mm Hg, estimated group standard deviations of 2.0 and 2.3, and α of 0.05, a minimum of 11 patients in each group was required to achieve 80% power to detect a difference of 3.5 between the groups. To account for potential differences in the secondary outcomes of interest and for the attrition rate throughout the 3-month period, the sample was increased to 24 patients in each group.
Descriptive analyses were calculated on all variables as appropriate. LAE and SAE were compared with baseline values using repeated-measures analysis of variance. The baseline characteristics and secondary outcomes of interest were compared using Student's t test, chi-square or Fisher's exact test, Mann-Whitney U test, or Wilcoxon signed rank test depending on variable type and distribution. All data were analyzed using the SPSS software package, version 20.0 (SPSS Inc., Chicago, IL), and a P value of 0.05 was defined as statistically significant.
RESULTS
Table 1 describes patients' demographic and clinical characteristics. Overall, they were older adults, with an average HF duration of 5 years. No significant differences in demographic and clinical characteristics were found between the groups (P values > 0.05). Table 2 compares the results of the groups' outcomes. Although patients in the DASH group reported better LAE and SAE scores over time, the net changes did not reach statistical significance (P values > 0.05). The dietary compliance (as reported by the DASH index score) improved over time, but this improvement was not statistically significant (P > 0.05). The LAE and SAE measurements over time for both groups are also summarized in Figure 1. No statistically significant differences were reported for 6-minute walk test scores at baseline. However, patients in the DASH group achieved better distances over time than those in the comparison group (Figure 2). MLHFQ scores at baseline were similar between the groups (P = 0.056); however, patients in the DASH group reported improved MLHFQ scores at 3-month follow-up (21 vs. 39; P = 0.006) (Figure 3). Although more patients in the comparison group had sodium intake levels >1500 mg/day at baseline, groups were comparable regarding the changes in sodium intake levels at 1, 2, and 3 months (P values > 0.05). No statistically significant differences between the DASH and comparison groups were found for weight, body mass index, BNP, or SctO2 at baseline or for hemodynamic parameters, SctO2, or BNP at any time during the study. The DASH dietary plan was well tolerated in our patients; no serious adverse effects were noted. None of the patients had a significant health event, including HF exacerbation requiring hospitalization. All participating patients were alive 6 months after study completion.
Table 1.
Baseline characteristics of patients in the DASH and comparison group*
| Variable | DASH (n = 24) | Comparison (n = 24) |
|---|---|---|
| Men | 13 (54%) | 16 (67%) |
| Women | 11 (46%) | 8 (33%) |
| Age (years), mean (SD) | 60 (11) | 64 (12) |
| White | 9 (38%) | 13 (54%) |
| Black | 15 (63%) | 11 (46%) |
| Weight (kg), mean (SD) | 112.3 (28.9) | 106.4 (27.9) |
| Body mass index (kg/m2), mean (SD) | 37.2 (8.6) | 35.1 (8.1) |
| Heart failure duration (years), mean (SD) | 6.5 (5.3) | 4.7 (4.0) |
| Etiology/type of heart failure | ||
| Nonischemic | 15 (63%) | 9 (38%) |
| Diastolic dysfunction | 10 (42%) | 11 (46%) |
| Ischemic | 8 (33%) | 9 (38%) |
| New York Heart Association functional class | ||
| I | 1 (4%) | 1 (4%) |
| II | 7 (29%) | 4 (17%) |
| III | 16 (67%) | 19 (79%) |
| Systemic hypertension | 21 (88%) | 23 (96%) |
| Dyslipidemiab | 12 (50%) | 17 (71%) |
| Coronary artery disease | 10 (42%) | 11 (46%) |
| Diabetes mellitus | 16 (67%) | 10 (42%) |
| Pulmonary hypertension | 3 (13%) | 5 (21%) |
| Previous stroke | 2 (8%) | 3 (13%) |
| Current smoker | 1 (4%) | 2 (8%) |
| Left ventricular ejection fraction (%), mean (SD) | 41 (13) | 40 (15) |
| Peripheral tissue oxygenation (%), mean (SD) | 97 (2) | 98 (2) |
| Cerebral tissue oxygenation (%), mean (SD) | 67 (5) | 67 (6) |
| Systolic blood pressure (mm Hg), mean (SD) | 136 (20) | 129 (18) |
| Diastolic blood pressure (mm Hg), mean (SD) | 71 (11) | 68 (8) |
| Mean arterial pressure (mm Hg), mean (SD) | 95 (14) | 90 (10) |
| Sodium (mEq/L), mean (SD)a | 137 (3) | 136 (4) |
| Creatinine (mg/dL), mean (SD)a | 1.3 (0.4) | 1.6 (0.6) |
| B-type natriuretic peptide (pg/mL), mean (SD)a | 102 (97) | 253 (472) |
| Hemoglobin (g/dL), mean (SD)a | 12.9 (1.4) | 12.3 (1.5) |
| Albumin (g/dL), mean (SD)a | 3.4 (1.0) | 3.5 (0.3) |
| Total protein (g/dL), mean (SD)a | 7.5 (0.8) | 7.6 (0.4) |
| Low-density lipoprotein (mg/dL), mean (SD)a | 88 (35) | 75 (17) |
| High-density lipoprotein (mg/dL), mean (SD)a | 52 (20) | 41 (10) |
| Cholesterol (mg/dL), mean (SD)a | 163 (46) | 142 (24) |
| Triglyceride (mg/dL), mean (SD)a | 115 (84) | 124 (96) |
| Galectin-3 (ng/mL), mean (SD)a | 19.1 (5.2) | 23.0 (13.8) |
| Medications | ||
| Beta-blockers | 23 (96%) | 23 (96%) |
| Diuretics | 24 (100%) | 23 (96%) |
| ACE/ARB | 17 (71%) | 19 (79%) |
| Aldosterone blocker | 14 (58%) | 13 (54%) |
| Statin | 9 (38%) | 13 (54%) |
| Nitrate | 6 (25%) | 5 (21%) |
All P values > 0.05. SD indicates standard deviation; ACE, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Data were missing for <8% of the patients.
Dyslipidemia was defined as fasting levels of low-density lipoprotein >100 mg/dL, or high-density lipoprotein <40 mg/dL, or triglyceride >150 mg/dL.
Table 2.
Comparative results of study outcomes over time*
| Variable | DASH | Comparison | P value |
|---|---|---|---|
| Absolute large arterial elasticity (mL/mm Hg × 10), mean (SD) | 15.1 (1.4) | 14.9 (1.2) | 0.905 |
| Absolute small arterial elasticity (mL/mm Hg × 100), mean (SD) | 6.3 (0.8) | 5.3 (0.7) | 0.366 |
| Six-minute walk test (meters), mean (SD) | |||
| Baseline | 254 (119) | 202 (77) | 0.158 |
| 3 months | 292 (124) | 197 (81) | 0.018 |
| Minnesota Living with Heart Failure Questionnaire, mean (SD) | |||
| Baseline | 29 (20) | 38 (4) | 0.056 |
| 3 months | 21 (15) | 39 (22) | 0.006 |
| Weight (kg), mean (SD) | 110.0 (6.8) | 101.1 (6.0) | 0.333 |
| Body mass index (kg/m2), mean (SD) | 35.8 (1.8) | 33.0 (1.6) | 0.256 |
| B-type natriuretic peptide (pg/mL), mean (SD) | |||
| Baseline | 102 (97) | 253 (472) | 0.138 |
| 3 months | 94 (97) | 314 (510) | 0.081 |
| Cerebral oxygen saturation (%), mean (SD) | 68 (1) | 67 (1) | 0.984 |
| DASH Index Score, mean (SD) | |||
| 1 month | 6.8 (2.1) | – | NA |
| 2 month | 7.3 (2.5) | – | NA |
| 3 month | 7.4 (2.3) | – | NA |
| Average | 6.8 (1.9) | – | NA |
| Sodium intake (mg/day) | |||
| Baseline | |||
| <1500 | 5 (21%) | 0 (0%) | |
| >1500 | 19 (79%) | 24 (100%) | 0.050 |
| 1 month | |||
| <1500 | 4 (14%) | 4 (14%) | |
| >1500 | 16 (57%) | 18 (64%) | 1.000 |
| 2 months | |||
| <1500 | 4 (14%) | 2 (7%) | |
| >1500 | 9 (32%) | 16 (57%) | 0.208 |
| 3 months | |||
| <1500 | 5 (21%) | 5 (21%) | |
| >1500 | 10 (36%) | 14 (50%) | 0.718 |
*Data are missing for 13% to 30% of the patients. NA indicates not applicable; SD, standard deviation.
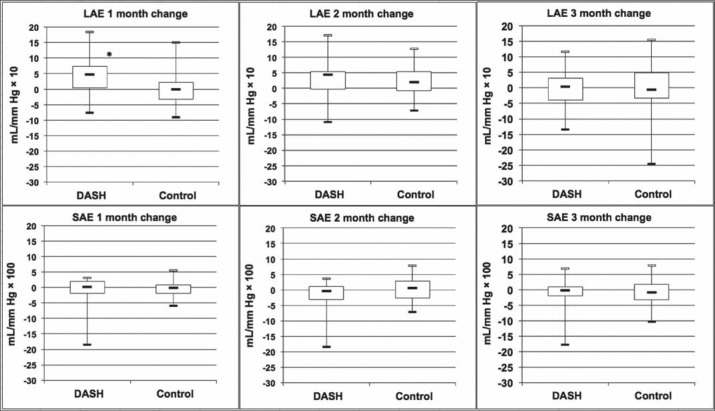
Figure 1.
Net change in large arterial elasticity (LAE) and small arterial elasticity (SAE) over time for the DASH group and comparison group. Central horizontal bars represent the median; boxes represent the 25th to 75th quartiles; vertical bars represent the range. *P < 0.01.
Figure 2.
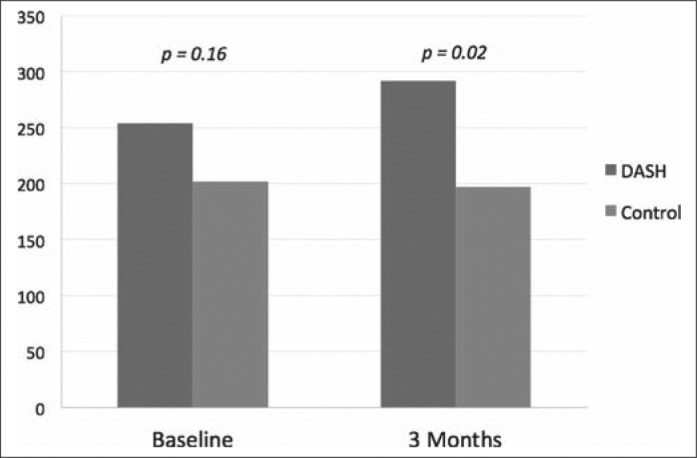
Six-minute walk distance at baseline and 3 months for DASH and comparison group.
Figure 3.
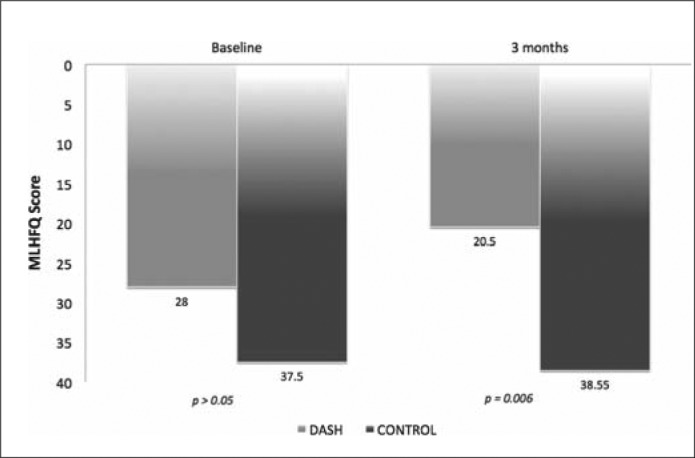
Minnesota Living with Heart Failure Questionnaire scores at baseline and 3 months for DASH and comparison group.
DISCUSSION
The clinical practice guidelines for HF endorse with reasonable evidence dietary recommendations for people at risk of HF (stages A and B), while few patients with a diagnosis of HF (stages C and D) meet the general nutritional recommendations (17, 18). Endothelial dysfunction has been recognized as a pathophysiologic mechanism in the progression of HF, and several pharmacologic and nonpharmacologic prevention and treatment strategies have been proposed (19–22). The effects of the DASH diet on endothelial function in patients with chronic symptomatic (stage C) HF using pulse contour analysis were assessed. Significant improvement in LAE was observed in patients who received the DASH diet for 1 month, but the change was less prominent at 2 and 3 months. No significant change in SAE over time was found in either group.
Since dietary patterns are complex mixtures of foods and nutrients, it is not clear whether the salutary effects of DASH on endothelial function are due to the vasodilatory effect of micronutrients (e.g., magnesium) in the DASH diet, the possible mediation of nitric oxide release, direct vasodilation, the interaction between foods and micronutrients, or a combination of these mechanisms. Another possible explanation is the antioxidant properties of components of the DASH diet, conceivably producing protective effects through preservation of endothelial function and antiinflammatory processes. Hummel et al supported the desirable influence of the DASH diet in HF on patients' blood pressure, arterial stiffness, oxidative stress (23), as well as ventricular diastolic function and arterial coupling (24).
Although the change in LAE and SAE in the DASH diet group was more prominent in the short term and was not sustained beyond 1 month, an improvement in clinical parameters of prognostic value in HF (6-minute walk test and MLHFQ) was evident at 3 months. These results are in line with recent studies demonstrating short-term effects of the DASH diet in mechanisms contributing to HF (23, 24), thus suggesting that the DASH diet may also improve HF through autonomic and cardiac mechanisms other than improving endothelial function.
Patients with HF continue to consume large quantities of sodium daily. Based on previous recommendations and in accordance with the DASH diet index score for assessment of adherence, the sodium dietary goal for the study was set at 1500 mg/day (11, 25). Although most patients consumed more than 1500 mg/day of sodium, the changes observed in LAE and SAE cannot be attributed to sodium, as no statistically significant changes in estimated sodium intake were found in the groups during the study time. However, more data are needed to support a specific sodium intake level, especially considering that emerging evidence appears to be conflicting (4, 26, 27). Newer and more reliable means of assessing longer-term sodium intake are needed to replace current measures that are episodic, are prone to recall bias (e.g., food frequency questionnaires), and may not be reflective of the overall sodium intake patterns (e.g., 24-hour urinary sodium measurements) (28).
The DASH index score trended toward greater compliance over time. This may reflect a more realistic expectation of transitioning into a new dietary lifestyle, in addition to implementing proper reinforcement to patient education. The DASH diet index was chosen over other available indexes for its simplicity and to supplement patients' self-reported food diaries. However, to improve adherence and behavioral modification rates, further exploration is needed to understand how to best implement the DASH dietary recommendations. Also, since the DASH diet is recommended to the public for the prevention of cardiovascular disease, a metric to assess consistency with this diet may be useful in clinical practice.
This study has several limitations. The results are hypothesis generating and cannot be generalized to all patients with HF. No blinding techniques were used, potentially affecting the interpretation of the results, especially the endothelial function levels and 6-minute walk test results. A dietary intervention other than the general dietary recommendations was used to evaluate the effect on endothelial function in HF over time. A limitation of dietary intervention studies is the lack of accurate measurement of individual components. It is unclear which specific DASH components contributed to the observed effects. Although multiple educational and compliance interventions were made available to the patients, they selected and consumed the food at their own discretion, allowing for extraneous variables to influence the study results. Adherence was self-reported rather than confirmed via objective tests, e.g., collection of 24-hour urine to assure equivalence in sodium intake. Food diaries and DASH diet scores, however, indicated good dietary adherence that improved over time from baseline dietary patterns for the DASH group. It is unknown whether our short-term results could be sustained in a cohort with complete freedom of food choice. Although no significant differences in demographics were found between the groups, more black patients enrolled in this study compared with previous dietary intervention studies. This is of interest since black patients are especially sensitive to the effects of micronutrients in the DASH diet (29). Finally, a repeat measurement of left ventricular ejection fraction was not part of this study.
In patients with HF, the DASH diet was associated with favorable changes in LAE, exercise capacity, and quality of life scores. Integrating the DASH diet into the dietary patterns of patients with HF could hold potential beneficial effects in decreasing the progression of endothelial dysfunction. To determine whether the particular positive effect on endothelial dysfunction and other possible salutary effects of DASH can be translated to clinical practice merits further investigation. These findings could inform the design of larger studies in the future that could incorporate well-characterized, more rigorous, and individualized nutritional management, while being mindful of the complex pathophysiology of HF. This study is one of the few randomized controlled trials to evaluate a dietary pattern in patients with HF, where little information is available to define optimal nutrient intakes and food patterns.
Acknowledgments
We would like to thank Christopher Blair, Director of Research Services, Advocate Health Care, Oak Brook, IL, for his help with data analysis. We thank Jennifer Swanson for her help with manuscript review and editing. We thank the patients and the nursing staff of the Heart Failure Institute, Oak Lawn, IL, for their cooperation and participation in this study.
References
- 1.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, Winston M, Wylie-Rosett J. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 2.Vogt TM, Appel LJ, Obarzanek E, Moore TJ, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Cutler JA, Windhauser MM, Lin PH, Karanja NM. Dietary Approaches to Stop Hypertension: rationale, design, and methods. DASH Collaborative Research Group. J Am Diet Assoc. 1999;99(8 suppl):S12–S18. doi: 10.1016/s0002-8223(99)00411-3. [DOI] [PubMed] [Google Scholar]
- 3.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC, Jr, Svetkey LP, Wadden TA, Yanovski SZ, Kendall KA, Morgan LC, Trisolini MG, Velasco G, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF. American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129(25 Suppl 2):S76–S99. doi: 10.1161/01.cir.0000437740.48606.d1. [DOI] [PubMed] [Google Scholar]
- 4.Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, Seals DR. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol. 2013;61(3):335–343. doi: 10.1016/j.jacc.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuentes JC, Salmon AA, Silver MA. Acute and chronic oral magnesium supplementation: effects on endothelial function, exercise capacity, and quality of life in patients with symptomatic heart failure. Congest Heart Fail. 2006;12(1):9–13. doi: 10.1111/j.1527-5299.2006.04692.x. [DOI] [PubMed] [Google Scholar]
- 6.Hornig B, Arakawa N, Kohler C, Drexler H. Vitamin C improves endothelial function of conduit arteries in patients with chronic heart failure. Circulation. 1998;97(4):363–368. doi: 10.1161/01.cir.97.4.363. [DOI] [PubMed] [Google Scholar]
- 7.Fuentes F, López-Miranda J, Sánchez E, Sánchez F, Paez J, Paz-Rojas E, Marín C, Gómez P, Jimenez Perepérez J, Ordovás JM, Pérez-Jiménez F. Mediterranean and low-fat diets improve endothelial function in hypercholesterolemic men. Ann Intern Med. 2001;134(12):1115–1119. doi: 10.7326/0003-4819-134-12-200106190-00011. [DOI] [PubMed] [Google Scholar]
- 8.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55(5):1075–1085. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Department of Health and Human Services Your Guide to Lowering Blood Pressure. Available at http://www.nhlbi.nih.gov/files/docs/public/heart/hbp_low.pdf; accessed November 3, 2014.
- 10.Nutrition Education Services Oregon Dairy Council. DASH shopping list. 2008. Available at www.dashdietoregon.org/files/dash/pdf/dash_shopping_list.pdf; accessed November 3, 2014.
- 11.Folsom AR, Parker ED, Harnack LJ. Degree of concordance with DASH diet guidelines and incidence of hypertension and fatal cardiovascular disease. Am J Hypertens. 2007;20(3):225–232. doi: 10.1016/j.amjhyper.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rector TS, Kubo SH, Cohn JN. Patients' self-assessment of their congestive heart failure: content, reliability and validity of a new measure, the Minnesota Living With Heart Failure questionnaire. Heart Fail. 1987;3:198–209. [Google Scholar]
- 13.Bittner V. Determining prognosis in congestive heart failure: role of the 6-minute walk test. Am Heart J. 1999;138(4 Pt 1):593–596. doi: 10.1016/s0002-8703(99)70166-3. [DOI] [PubMed] [Google Scholar]
- 14.Gilani M, Kaiser DR, Bratteli CW, Alinder C, Rajala S, Bank AJ, Cohn JN. Role of nitric oxide deficiency and its detection as a risk factor in prehypertension. J Am Soc Hypertens. 2007;1(1):45–55. doi: 10.1016/j.jash.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Cohn JN, Finkelstein S, McVeigh G, Morgan D, LeMay L, Robinson J, Mock J. Noninvasive pulse wave analysis for the early detection of vascular disease. Hypertension. 1995;26(3):503–508. doi: 10.1161/01.hyp.26.3.503. [DOI] [PubMed] [Google Scholar]
- 16.Manning TS, Shykoff BE, Izzo JL., Jr Validity and reliability of diastolic pulse contour analysis (Windkessel model) in humans. Hypertension. 2002;39(5):963–968. doi: 10.1161/01.hyp.0000016920.96457.7c. [DOI] [PubMed] [Google Scholar]
- 17.Arcand J, Floras V, Ahmed M, Al-Hesayen A, Ivanov J, Allard JP, Newton GE. Nutritional inadequacies in patients with stable heart failure. J Am Diet Assoc. 2009;109(1):1909–1913. doi: 10.1016/j.jada.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Grossniklaus DA, O'Brien MC, Clark PC, Dunbar SB. Nutrient intake in heart failure patients. J Cardiovasc Nurs. 2008;23(4):357–363. doi: 10.1097/01.JCN.0000317433.52210.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torre-Amione G, Young JB, Colucci WS, Lewis BS, Pratt C, Cotter G, Stangl K, Elkayam U, Teerlink JR, Frey A, Rainisio M, Kobrin I. Hemodynamic and clinical effects of tezosentan, an intravenous dual endothelin receptor antagonist, in patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2003;42(1):140–147. doi: 10.1016/s0735-1097(03)00556-4. [DOI] [PubMed] [Google Scholar]
- 20.Vogel RA, Corretti MC, Plotnick GD. The postprandial effect of components of the Mediterranean diet on endothelial function. J Am Coll Cardiol. 2000;36(5):1455–1460. doi: 10.1016/s0735-1097(00)00896-2. [DOI] [PubMed] [Google Scholar]
- 21.Brown AA, Hu FB. Dietary modulation of endothelial function: implications for cardiovascular disease. Am J Clin Nutr. 2001;73(4):673–686. doi: 10.1093/ajcn/73.4.673. [DOI] [PubMed] [Google Scholar]
- 22.Landberg R, Naidoo N, van Dam RM. Diet and endothelial function: from individual components to dietary patterns. Curr Opin Lipidol. 2012;23(2):147–155. doi: 10.1097/MOL.0b013e328351123a. [DOI] [PubMed] [Google Scholar]
- 23.Hummel SL, Seymour EM, Brook RD, Kolias TJ, Sheth SS, Rosenblum HR, Wells JM, Weder AB. Low-sodium dietary approaches to stop hypertension diet reduces blood pressure, arterial stiffness, and oxidative stress in hypertensive heart failure with preserved ejection fraction. Hypertension. 2012;60(5):1200–1206. doi: 10.1161/HYPERTENSIONAHA.112.202705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hummel SL, Seymour EM, Brook RD, Sheth SS, Ghosh E, Zhu S, Weder AB, Kovács SJ, Kolias TJ. Low-sodium DASH diet improves diastolic function and ventricular-arterial coupling in hypertensive heart failure with preserved ejection fraction. Circ Heart Fail. 2013;6(6):1165–1171. doi: 10.1161/CIRCHEARTFAILURE.113.000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev. 2013;4:CD004937. doi: 10.1002/14651858.CD004937.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colin Ramirez E, Castillo Martinez L, Orea Tejeda A, Rebollar Gonzalez V, Narvaez David R, Asensio Lafuente E. Effects of a nutritional intervention on body composition, clinical status, and quality of life in patients with heart failure. Nutrition. 2004;20(10):890–895. doi: 10.1016/j.nut.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the Trials of Hypertension Prevention (TOHP) BMJ. 2007;334(7599):885–888. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta D, Georgiopoulou VV, Kalogeropoulos AP, Dunbar SB, Reilly CM, Sands JM, Fonarow GC, Jessup M, Gheorghiade M, Yancy C, Butler J. Dietary sodium intake in heart failure. Circulation. 2012;126(4):479–485. doi: 10.1161/CIRCULATIONAHA.111.062430. [DOI] [PubMed] [Google Scholar]
- 29.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER, 3rd, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, Charleston J, McCarron P, Bishop LM. OmniHeart Collaborative Research Group The effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294(19):2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]