Abstract
The acute respiratory distress syndrome (ARDS) is a major cause of acute respiratory failure. Its development leads to high rates of mortality, as well as short- and long-term complications, such as physical and cognitive impairment. Therefore, early recognition of this syndrome and application of demonstrated therapeutic interventions are essential to change the natural course of this devastating entity. In this review article, we describe updated concepts in ARDS. Specifically, we discuss the new definition of ARDS, its risk factors and pathophysiology, and current evidence regarding ventilation management, adjunctive therapies, and intervention required in refractory hypoxemia.
During the Vietnam War in 1960s, military physicians encountered a distinctive form of hypoxemic respiratory failure involving both lungs simultaneously. During the same period, civilian physicians who came across this form of lung injury called it adult respiratory distress syndrome (1). This term was later modified to acute respiratory distress syndrome (ARDS), when similar cases were reported across all age groups. In the United States, the most recent population-based data estimated an incidence of 190,000 cases per year (2). Mortality from ARDS has been estimated at 26% to 58% (3–6). Advances in supportive care have led to improvements in patient outcomes (7, 8). Nevertheless, the mortality associated with this syndrome remains unacceptably high.
DEFINITION AND DIAGNOSIS
ARDS and what was previously called acute lung injury (ALI) are both characterized by rapid onset of respiratory failure following a variety of direct and indirect lung insults. Since these entities were originally described, multiple definitions or diagnostic criteria have been proposed. In 1988, Murray et al introduced the lung injury score, which included chest radiograph, the ratio of the partial pressure of arterial oxygen and the fraction of inspired oxygen (PaO2/FiO2), total respiratory system compliance, and positive end-expiratory pressure (PEEP). Despite its clinical utility, the score was unable to differentiate between cardiogenic and noncardiogenic edema (9). In 1994, the American and European Consensus Conference established specific clinical criteria for ARDS and ALI (10). There were three diagnostic criteria: 1) PaO2/FiO2 ≤ 200, 2) bilateral infiltrates on chest radiograph, and 3) pulmonary artery occlusion pressure < 18 mm Hg when measured by pulmonary artery catheterization, or no clinical evidence of left atrial hypertension. The term ALI was adopted from the lung injury score to include patients with less severe forms of the same pathological entity. Therefore, patients with a PaO2/FiO2 of 200 to 300 were included within this group.
Since its description, the American and European Consensus Conference definition has been widely used for enrollment of ARDS patients in therapeutic clinical trials (11–15). Nevertheless, the aforementioned definition also presented several shortcomings. First, the reliability in reading chest radiographs was questionable. Second, the definition did not explicitly define the time interval for “acute.” Third, the level of PEEP utilized during ventilation was not incorporated in the definition. Last, the use of pulmonary artery catheters has been decreasing over the last few years, precluding measurements of pulmonary artery occlusion pressures.
Based on the aforementioned limitations, and after reviewing current epidemiologic evidence and results of clinical trials, in 2011 the European Society of Intensive Care Medicine proposed the Berlin ARDS definition (16), which considered the factors of timing, chest imaging, origin of edema, and oxygenation:
Timing: Within 1 week of a known clinical insult or new or worsening respiratory symptoms
Imaging: A chest radiograph or computed tomography scan showing bilateral opacities not fully explained by effusions, lobar/lung collapse, or nodules
Origin of edema: Respiratory failure not fully explained by cardiac failure or fluid overload; objective assessment (e.g., echocardiography) needed to exclude hydrostatic edema if no risk factor present
Oxygenation: Divided into mild (PaO2/FIO2 >200 to ≤ 300 mm Hg with PEEP or continuous positive airway pressure ≥ 5 cm H2O), moderate (PaO2/FIO2 >100 to ≤ 200 mm Hg with PEEP ≥ 5 cm H2O) or severe (PaO2/FIO2 ≤ 100 mm Hg with PEEP ≥ 5 cm H2O)
Of note, the term ALI has been eliminated. The categories of mild, moderate, and severe correlate with mortalities of 27%, 32%, and 45%, respectively (16).
RISK FACTORS
Multiple conditions may cause ARDS (Table 1). Sepsis remains the most common cause of ARDS, with 46% of the cases triggered by pulmonary entities (2). Mortality also varies according to the cause. Particularly, mortality in patients with ARDS due to severe trauma (injury severity score > 15) is 24.1%, whereas mortality in patients with severe sepsis with a pulmonary source is 40.6% (2). Notably, certain patient-related variables have been associated with the risk of developing ARDS and with mortality. Among these risk factors, age (2, 17–19), male gender, African American race (20), and history of alcoholism are associated with a higher incidence and mortality (21–23). Active and passive smoking exposure increases the incidence of ARDS as well (24, 25). Patients with a higher body-mass index have an increased incidence of ARDS, but its association with mortality is not clearly defined (26–28). Both diabetes mellitus and prehospital antiplatelet therapy seem to have a protective effect on development of ARDS (29–31).
Table 1.
Common risk factors for acute respiratory distress syndrome/acute lung injury
| Direct | Indirect |
|---|---|
|
|
Interestingly, the Acute Lung Injury Verification of Epidemiology (ALIVE) study (32) reported that ALI occurred in 16.1% of patients who were mechanically ventilated for other reasons. Hence, several groups have investigated a variety of methods to predict ARDS. Particularly, Gajic et al described the Lung Injury Prediction Score (LIPS, Table 2) using a prospective cohort study of 5584 patients (33). A LIPS score higher than 4 was associated with risk of developing ARDS within a median time of 2 days. The score has a sensitivity of 69% and a specificity of 78%, with a positive predictive value of 18% and a negative predictive value of 97%.
Table 2.
Lung Injury Prediction Score calculation worksheet
| LIPS points | |
|---|---|
| Predisposing conditions | |
| Shock | 2 |
| Aspiration | 2 |
| Sepsis | 1 |
| Pneumonia | 1.5 |
| High-risk surgerya | |
| Orthopedic spine | 1 |
| Acute abdomen | 2 |
| Cardiac | 2.5 |
| Aortic vascular | 3.5 |
| High-risk trauma | |
| Traumatic brain injury | 2 |
| Smoke inhalation | 2 |
| Near drowning | 2 |
| Lung contusion | 1.5 |
| Multiple fractures | 1.5 |
| Risk modifiers | |
| Alcohol abuse | 1 |
| Obesity (body mass index >30) | 1 |
| Hypoalbuminemia | 1 |
| Chemotherapy | 1 |
| Fraction of inspired oxygen > 0.35 (>4 L/min) | 2 |
| Tachypnea (respiratory rate >30/min) | 1.5 |
| Oxygen saturation < 95% | 1 |
| Acidosis (pH <7.35) | 1.5 |
| Diabetes mellitusb | –1 |
Add 1.5 points if emergency surgery.
Only if sepsis.
Reprinted from Gajic et al, 2011 (33) with permission of the American Thoracic Society. Copyright © American Thoracic Society.
The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society.
PATHOPHYSIOLOGY OF VENTILATOR-INDUCED LUNG INJURY
Gattinoni et al (34) described three general regions of the lung: normal lung tissue, a region densely consolidated, and a region that collapses during expiration and is recruitable during inspiration. When these heterogeneous lungs are ventilated at low tidal volumes, in the absence of PEEP they present a repetitive opening and closing of airways and lung units (35). This type of injury is called “atelectrauma” (35). Conversely, when heterogeneous lungs are ventilated with high tidal volumes, overdistension of alveoli is produced, leading to “barotrauma,” which involves complications such as pneumothorax (36). A third form of ventilator-induced lung injury is called “biotrauma,” which is a systemic inflammatory response syndrome as a consequence of a release of lung cytokines (tumor necrosis factor–alpha, interleukin-6, interleukin-8, matrix metallopeptidase 9, nuclear factor kappa-light-chain-enhancer of activated B cells) (37).
TREATMENT
Standard treatment
Low-tidal volume strategy. The aim of mechanical ventilation in ARDS is to provide oxygenation and ventilation, while reducing the risk of ventilator-induced lung injury. A multicenter National Heart, Lung, and Blood Institute ARDSnet trial randomly assigned 861 patients with ARDS to receive low-tidal volume ventilation (initial tidal volume of 6 mL/kg) or conventional mechanical ventilation (initial tidal volume of 12 mL/kg) (11). Tidal volumes were titrated to keep plateau pressures (alveolar pressure at the end of a paused inspiration) lower than 50 cm H2O in the conventional ventilation group, and lower than 30 cm H2O in the low-tidal volume group. Results showed that the intervention group (low-tidal volume) had a lower mortality rate (31% vs. 40%) and more ventilator-free days (12 days vs. 10 days). A recent meta-analysis of four randomized trials, which included 1149 patients, confirmed these findings with a reduction of hospital mortality from 41% to 34.2% (38). Since the publication of this landmark study, a low-tidal volume strategy, which involves a tidal volume of 6 mL/kg predicted body weight, is considered the standard of care. In certain circumstances, tidal volumes may be further decreased to 4 mL/kg in order to limit inspiratory plateau pressures to levels lower than 30 cm H2O (11).
Positive end-expiratory pressure. The utilization of PEEP improves gas exchange and lung function in a number of ways. PEEP recruits collapsed alveoli, improving oxygenation and lung compliance, and reduces cyclic atelectasis, decreasing atelectrauma and biotrauma. Despite these benefits, the appropriate dose of PEEP is still a matter of controversy. In the ARDSnet trial (11), patients using tidal volumes of 4 mL/kg required significantly higher levels of PEEP. Therefore, some have argued that this could have been the reason for the positive outcomes of the study. However, the subsequent Higher vs. Low PEEP in Patients with ARDS (ALVEOLI) study (4), which was a prospective, multicenter trial with 549 patients randomized to either lower or higher levels of PEEP, set according to predefined tables, showed no differences in outcomes among groups. Importantly, the study design of the ALVEOLI trial was highly criticized, as many providers believe that PEEP levels cannot universally be set for all patients, but rather must be individualized based on lung mechanics.
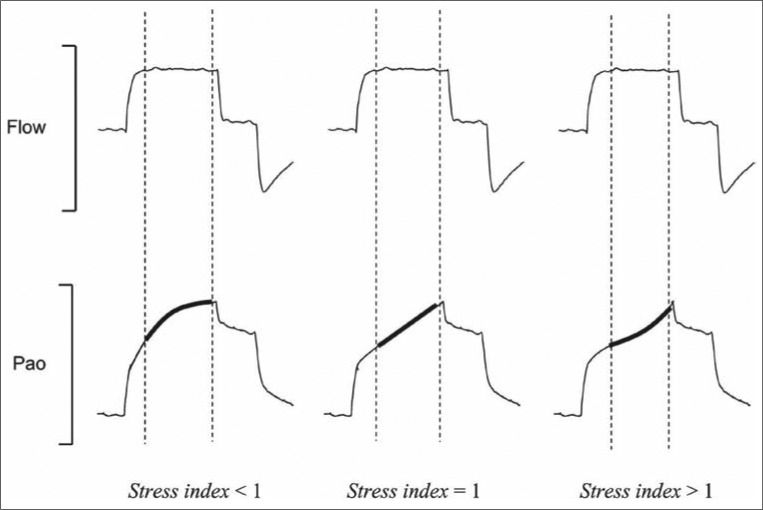
The analysis of the static lung compliance curve has been proposed to titrate PEEP. Both the lower inflection point on the aforementioned curve and the stress index calculated from the pressure-time curve have been employed with varying results (39, 40). However, in ARDS the lung does not function as a single compartment model but rather as a multiple one. Therefore, setting PEEP considering the lower and upper inflection points may not be the most reliable strategy. The stress index has been advocated as a favorable parameter to select PEEP level, avoiding potential hyperinflation (Figure 1) (41). To measure it, the ventilator should be set under conditions of constant flow and volume-limited ventilation. The stress index defines the slope of the airway opening pressure during a period of constant flow. Values lower than 1 suggest a continuous decrease in elastance during lung inflation. This is consistent with potential recruitability and, therefore, PEEP can be increased. Values higher than 1 suggest an increase in lung elastance, consistent with lung hyperinflation. In these situations, PEEP should be decreased to avoid overstretching. Even though the stress index represents an interesting physiologic concept, more investigations are needed to validate it as an optimal technique for PEEP titration.
Figure 1.
Graphic representation of the stress index concept. The stress index is the coefficient b of a power equation (airway pressure = a inspiratory time b + c), fitted on the airway opening pressure (Pao) segment (bold lines) corresponding to the period of constant-flow inflation (dotted lines), during constant-flow, volume-cycled mechanical ventilation. For stress index values <1, the Pao curve presents a downward concavity, suggesting a continuous decrease in elastance during constant-flow inflation. For stress index values >1, the curve presents an upward concavity suggesting a continuous increase in elastance. Finally, for a stress index value equal to 1, the curve is straight, suggesting the absence of tidal variations in elastance. Reprinted from Grasso et al, 2007 (41) with permission of the American Thoracic Society. Copyright © American Thoracic Society. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society.
Two other trials have evaluated the optimal level of PEEP in the treatment of ARDS. The Lung Open Ventilation Study (LOVS) was a multicenter randomized controlled trial that included 983 patients (42). The control group was ventilated with low tidal volume, plateau pressures not exceeding 30 cm H2O, and low levels of PEEP. The intervention group used low tidal volumes, plateau pressures not exceeding 40 cm H2O, and higher levels of PEEP. In addition, the intervention group performed recruiting maneuvers (40-sec breath holds at pressures of 40 cm H20). This last strategy resulted in reduced refractory hypoxemia and lower utilization of rescue techniques for hypoxemia, such as inhaled nitric oxide (iNO), prone ventilation, extracorporeal membrane oxygenation (ECMO), or high-frequency oscillatory ventilation (HFOV). The EXPRESS trial was also a multicenter randomized controlled trial, which included 767 patients from 37 French intensive care units (ICUs) (43). Patients were randomized to a minimal distension group (PEEP 5–9 cm H2O) or a maximal recruitment group (PEEP increased to reach plateau pressure of 28–30 cm H2O). The high PEEP recruitment strategy had no mortality benefit, but resulted in better oxygenation, higher compliance values, and more ventilator-free days (7 vs. 3 days; P = 0.04) and organ failure–free days (6 vs. 2 days; P = 0.04) in the subgroup of patients with refractory hypoxemia. A recent meta-analysis, which included data from ALVEOLI, LOVS, and EXPRESS trials, revealed that higher levels of PEEP were associated with improved survival among patients with moderate to severe ARDS (44).
Hemodynamic monitoring and fluid management. Avoidance of intrathoracic fluid accumulation is thought to be beneficial in patients with ARDS. Based on this premise, the Comparison of Two Fluid-Management Strategies in ARDS trial (FACTT) evaluated the hemodynamic management of patients with ARDS guided by a pulmonary artery catheter or a central line catheter, plus an explicit hemodynamic management protocol (45, 46). The FACTT study included 1000 patients, who were randomized to 1 of 4 hemodynamic protocols for a period of 7 days. The conservative hemodynamic strategy aimed for a central venous pressure <4 mm Hg or a pulmonary artery occlusion pressure <8 mm Hg. The liberal hemodynamic strategy aimed for a central venous pressure of 10 to 14 mm Hg or pulmonary artery occlusion pressure of 14 to 18 mm Hg. The mean (± standard error [SE]) cumulative fluid balance during the first 7 days was –136 ± 491 mL in the conservative strategy group and 6992 ± 502 mL in the liberal strategy group (P < 0.001). Also, the conservative strategy improved the oxygenation index and increased the number of ventilator-free days (14.6 ± 0.5 vs. 12.1 ± 0.5; P < 0.001) during the first 28 days. Interestingly, despite restrictions in the use of fluids in the conservative group, there was no increase in the incidence of shock or need for dialysis during the first 60 days (10% vs. 14%; P = 0.06) (46). These results support a conservative fluid strategy in the management of patients with ARDS.
Refractory hypoxemia
In certain situations, in which patients with ARDS do not improve their oxygenation with conventional therapies, other treatment options deemed as “salvage therapies” or “rescue therapies” have been advocated.
High-frequency oscillatory ventilation. HFOV delivers very low tidal volumes (equal to or less than anatomic dead space) at frequencies of 3 to 15 Hz. It also maintains a high airway pressure to permit recruitment. Ventilation is inversely related to the respiratory frequency and is directly related to the pressure amplitude of oscillation. Ideally, this strategy permits a more homogenous distribution of ventilation by maintaining mean airway pressure (47, 48), but avoiding hyperinflation (49, 50) and ventilator-induced lung injury by minimizing swings in tidal volumes (51).
Several randomized controlled trials have failed to show a mortality benefit with HFOV. Two large multicenter randomized controlled trials were recently published. The OSCILLATE trial was a multicenter randomized controlled trial conducted at 39 ICUs in five countries (52). The study included 548 patients with moderate to severe ARDS who were randomly assigned to HFOV targeting lung recruitment or a conventional low tidal volume–high PEEP ventilation strategy. The HFOV group had increased in-hospital mortality (47% vs. 35%; P = 0.005). Also, those in the HFOV group required more sedation, paralytics, and vasopressor agents. The OSCAR trial included nearly 800 patients in 17 United Kingdom ICUs. This study also failed to demonstrate a survival benefit at 30 days (41.7% mortality in the HFOV group and 41.1% mortality in the control group; P = 0.85) (53).
Airway pressure release ventilation. Airway pressure release ventilation (APRV) is a pressure-targeted, time-cycled mode of mechanical ventilation that permits spontaneous breathing across the full breathing cycle. It involves a long inspiratory time followed by a very short expiratory time, creating inverse ratio ventilation. By increasing the inflation period, the mean airway pressure is increased without an increase in the peak pressure. The superimposed spontaneous breathing has the advantage of providing more even ventilation distribution as well as augmentation of cardiac filling (54). In a randomized controlled trial, 30 mechanically ventilated trauma patients were randomly assigned to either APRV or pressure-limited ventilation (55). APRV was found to be associated with shorter duration of mechanical ventilation, a shorter ICU length of stay, and use of less sedatives and paralytics. Numerous studies have shown that APRV can decrease the peak airway pressure, improve alveolar recruitment, and improve oxygenation (56–60). Nevertheless, there is no evidence of an improved mortality outcome by using this mode, as compared to other modes of mechanical ventilation.
Extracorporeal membrane oxygenation. ECMO is used in ARDS patients with very severe hypoxemia, uncompensated hypercapnia (pH < 7.15), or excessively high end-inspiratory plateau pressures (>35–45 cm H2O) despite the use of standard-of-care treatments for the management of ARDS (61–64). Despite earlier negative trials (65), the Conventional Ventilator Support vs. ECMO for Severe Adult Respiratory Failure (CESAR) study suggests there may be some benefit with extracorporeal lung support in patients with severe ARDS (66). In this randomized controlled study, 180 patients were randomized to receive veno-venous ECMO (after being transferred to a specialized center) or conventional mechanical ventilation (in regional centers). The former group had a higher 6-month survival than the latter (63% vs. 47%; P = 0.03). Nevertheless, it is important to note that the intervention group underwent mechanical ventilation using a lung protective strategy, whereas it was used in only 70% of patients in the control group. Also, despite mortality benefits in the intervention group, only 75% of these patients actually received ECMO upon arrival to the specialized center. Therefore, the CESAR study demonstrated a mortality benefit in a specialized center vs. a regional center, but not necessarily a clear benefit of ECMO.
Vasodilator therapy. The rationale for using selective inhaled pulmonary vasodilators is to cause selective vasodilation in normal lung segments and recruit blood flow to these areas, where it can be oxygenated (67). Due to their local action and short half-lives, selective pulmonary vasodilators do not usually have systemic side effects, such as hypotension. Two metaanalyses compared iNO to either placebo or conventional management and found a modest and transient improvement in oxygenation, without improvement in survival, duration of mechanical ventilation, or ventilator-free days (68). It was also noted that patients without sepsis or septic shock responded more frequently to iNO than patients with septic shock (69). Inhaled epoprostenol has also been used in patients with ARDS, and it has similar physiologic effects as iNO. As with iNO, no study has demonstrated a clear survival benefit.
Recruitment maneuvers. Recruitment maneuvers can be defined as a strategy to increase transpulmonary pressure transiently with the goal of reexpansion of previously collapsed but recruitable lung alveolar units. This strategy can be performed by using conventional ventilators or oscillators. Gattitoni et al showed that the amount of lung mass that can be recruited averages 9% of the total lung mass, with pressures between 5 and 45 cm H2O (70). Recruitment maneuvers can increase the aerated lung mass and prevent atelectrauma caused by repeated opening and closing of terminal respiratory units (71). Two commonly used recruitment maneuvers are the sigh and sustained inflation. “Sigh” involves increasing tidal volume or PEEP for one or several breaths per minute to a prespecified plateau pressure. The other form of recruitment maneuver is the sustained inflation method, which consists of pressurizing the airways at a specific level and maintaining it for a given duration. A common combination is the application of 40 cm H2O of airway pressure for 40 seconds. Despite the physiological advantages associated with recruitment maneuvers, three randomized controlled trials and one metaanalysis were not able to demonstrate a beneficial effect of recruitment maneuvers on oxygenation. Current evidence does not recommend their routine use, but recruitment maneuvers remain an option as a rescue therapy in severe hypoxemic patients (72–74).
Prone positioning. Conceptually, prone position may lead to a more uniform distribution of lung stress and strain, leading to improved ventilation-perfusion matching and regional improvement in lung and chest wall mechanics. However, prior reports indicated that prone positioning was associated with a variety of complications, such as hardware displacement and pressure ulcers. Prior clinical trials showed that prone positioning improved oxygenation in patients with ARDS, without benefits in terms of survival (75–77). In those studies, investigators used either repeated sessions of prone ventilation lasting 6 to 8 hours per day (14, 78) or prolonged prone ventilation lasting 17 to 20 hours (79–81) with similar results. While previous randomized controlled trials had not shown a survival benefit in patients with ARDS (80, 82), some observation studies and metaanalysis revealed a positive signal in a subset of patients with severe ARDS (83, 84). A recent multicenter prospective controlled trial (the PROSEVA study) randomized 466 patients with severe ARDS (PaO2:FiO2 <150, FiO2 ≥ 0.6, PEEP ≥ 5 cm H2O) to undergo early (within 33 hours of intubation) prone-positioning sessions of at least 16 hours, or to be left in the supine position (79). Prone positioning decreased 28-day mortality (16% vs. 33%; P < 0.001), decreased 90-day mortality (24% vs. 41%; P < 0.001), increased ventilator–free days (14 vs. 10 days at day 28), and decreased time to extubation. The incidence of complications did not differ significantly between the groups, except for the incidence of cardiac arrests, which was higher in the supine group. Absolute and relative contraindications for prone positioning include spinal instability, elevated intracranial pressure, hemodynamic and cardiac abnormalities, massive hemoptysis, thoracic and abdominal surgeries, anterior chest tubes with leaks, and deep venous thrombosis treated for <2 days.
Adjunctive therapy
Neuromuscular blocking agents. Lung-protective mechanical ventilation has become the cornerstone management strategy for ARDS (85). However, patients with ARDS are still exposed to the risk of atelectrauma and barotrauma due to suboptimal ventilator strategies. Neuromuscular blocking agents have been proposed as adjuvant therapy in ARDS, as they may decrease patient-ventilator asynchrony and, potentially, avoid the risk of barotrauma and biotrauma (86).
A recent multicenter double-blinded randomized controlled trial (ACURASYS) was conducted with 340 patients with severe ARDS. The study compared cisatracurium with placebo (13). All patients were sedated, titrating the Ramsay sedation score to 6 (no response on glabellar tap). Muscle paralysis monitoring, using train-of-four testing, was not allowed in order to maintain study blinding. Cisatracurium was associated with decreased adjusted 90-day mortality (31.6% vs. 40.7%; P = 0.08). Furthermore, mortality at 28 days was 23.7% in the cisatracurium group and 33.3% in the placebo group (P = 0.05). In this study, there was no difference in the rate of myopathy between the two groups.
Steroids. Inflammation is a key component in ARDS. Multiple studies have investigated the role of steroids in the prevention of ARDS and in the treatment of its different phases. Four trials have assessed the use of methylprednisolone for prevention of ARDS in a high-risk group of patients (sepsis/septic shock) (87–90). Specifically, Weigelt et al looked at high-risk surgical ICU patients (90). In this study, methylprednisolone at a dose of 30 mg/kg every 6 hours for 2 days increased the incidence of ARDS (64% vs. 33%), as well as the rate of infections (77% vs. 43%). Similarly, Bone et al demonstrated an increased 14-day mortality in the steroid group compared with a control group (52% vs. 22%) (88).
Multiple controlled studies have evaluated the role of glucocorticoid therapy in early and late ARDS. Bernard et al performed the first multicenter double-blinded prospective randomized controlled trial to assess the role of a short course of steroids given for 24 hours to patients with early ARDS (91). The study showed that there was a small decrease in 45-day mortality (60% vs. 63%) and an increased chance of ARDS reversal (39% vs. 36%) among patients receiving methylprednisolone compared with placebo. A large multicenter randomized controlled trial was conducted by the National Heart, Lung, and Blood Institute ARDSnet group to determine the efficacy and safety of a moderate dose of steroids for a period of 21 days in patients with persistent ARDS (>7 days). The study showed no survival benefit at 60 or 180 days (92). Similar results were reported by Annane et al in the same year (93). Meduri et al performed a multicenter double-blinded randomized controlled trial with 91 patients with ARDS who received steroids within 72 hours of entry into the study (94). The authors used a prolonged course of methylprednisolone (a loading dose of 1 mg/kg, followed by an infusion of 1 mg/kg/day from day 1 to day 14, 0.5 mg/kg/day from day 15 to day 21, 0.25 mg/kg/day from day 22 to day 25, and 0.125 mg/kg/day from day 26 to day 28). This study showed a significant decrease in mortality (20.6% vs. 42.9%; P = 0.03), reduction in the duration of mechanical ventilation (P = 0.002), and reduction in ICU stay (P = 0.007).
The contradictory results of the ARDSnet and Meduri trials are likely due to the rapid taper of steroids in the ARDSnet study and the use of steroids during different phases of the disease. Clinical trials evaluating the effect of a prolonged course of steroids in ARDS have consistently shown a significant improvement in oxygenation (PaO2/FiO2 ratio) (92, 95–97) and a reduction in systemic inflammation (95–97), organ dysfunction score (92, 95–97), duration of mechanical ventilation (95–97), and ICU length of stay (95–97). Data from five recently conducted large trials were analyzed and showed that patients who received corticosteroids early (<14 days after onset of ARDS) had reduced mortality (38% vs. 52.5%; P = 0.02) (98). Both the ARDSnet group and Meduri showed an increase in the number of ventilator-free days and decreased length of ICU stay. Review of available data shows that the beneficial effect of corticosteroids is seen only when used in the early phase of ARDS and not in the late phase. Therefore, a recent consensus statement recommended early initiation of prolonged glucocorticoid therapy for patients with moderate to severe ARDS (PaO2/FiO2 < 200 mm Hg on PEEP 10 cm H20), and before day 14 (99).
CONCLUSION
ARDS continues to be associated with a high mortality. Despite multiple randomized controlled trials, only lung protective ventilation strategies, neuromuscular blocking agents, and prone ventilation have been shown to decrease mortality. Many trials are underway looking at nebulized heparin, aspirin, stem cell therapy, growth factors, interferon-β, and vascular endothelial growth factor. The new Berlin definition of ARDS may assist future trials of novel therapies by improving diagnostic reliability and allowing more precise stratification of patients according to severity.
References
- 1.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2(7511):319–323. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.MacCallum NS, Evans TW. Epidemiology of acute lung injury. Curr Opin Crit Care. 2005;11(1):43–49. doi: 10.1097/00075198-200502000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 5.Estenssoro E, Dubin A, Laffaire E, Canales H, Saenz G, Moseinco M, Pozo M, Gomez A, Baredes N, Jannello G, Osatnik J. Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Crit Care Med. 2002;30(11):2450–2456. doi: 10.1097/00003246-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Bersten AD, Edibam C, Hunt T, Moran J. Australian and New Zealand Intensive Care Society Clinical Trials Group. Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian states. Am J Respir Crit Care Med. 2002;165(4):443–448. doi: 10.1164/ajrccm.165.4.2101124. [DOI] [PubMed] [Google Scholar]
- 7.Milberg JA, Davis DR, Steinberg KP, Hudson LD. Improved survival of patients with acute respiratory distress syndrome (ARDS): 1983–1993. JAMA. 1995;273(4):306–309. [PubMed] [Google Scholar]
- 8.Suchyta MR, Orme JF, Jr, Morris AH. The changing face of organ failure in ARDS. Chest. 2003;124(5):1871–1879. doi: 10.1378/chest.124.5.1871. [DOI] [PubMed] [Google Scholar]
- 9.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138(3):720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 10.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 11.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 12.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369(9572):1553–1564. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 13.Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, Jaber S, Arnal JM, Perez D, Seghboyan JM, Constantin JM, Courant P, Lefrant JY, Guerin C, Prat G, Morange S, Roch A. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 14.Gattinoni L, Tognoni G, Pesenti A, Taccone P, Mascheroni D, Labarta V, Malacrida R, Di Giulio P, Fumagalli R, Pelosi P, Brazzi L, Latini R. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001;345(8):568–573. doi: 10.1056/NEJMoa010043. [DOI] [PubMed] [Google Scholar]
- 15.Derdak S, Mehta S, Stewart TE, Smith T, Rogers M, Buchman TG, Carlin B, Lowson S, Granton J. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166(6):801–808. doi: 10.1164/rccm.2108052. [DOI] [PubMed] [Google Scholar]
- 16.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 17.Suchyta MR, Clemmer TP, Elliott CG, Orme JF, Jr, Morris AH, Jacobson J, Menlove R. Increased mortality of older patients with acute respiratory distress syndrome. Chest. 1997;111(5):1334–1339. doi: 10.1378/chest.111.5.1334. [DOI] [PubMed] [Google Scholar]
- 18.Ely EW, Wheeler AP, Thompson BT, Ancukiewicz M, Steinberg KP, Bernard GR. Recovery rate and prognosis in older persons who develop acute lung injury and the acute respiratory distress syndrome. Ann Intern Med. 2002;136(1):25–36. [PubMed] [Google Scholar]
- 19.Eachempati SR, Hydo LJ, Shou J, Barie PS. Outcomes of acute respiratory distress syndrome (ARDS) in elderly patients. J Trauma. 2007;63(2):344–350. doi: 10.1097/TA.0b013e3180eea5a1. [DOI] [PubMed] [Google Scholar]
- 20.Moss M, Mannino DM. Race and gender differences in acute respiratory distress syndrome deaths in the United States: an analysis of multiple-cause mortality data (1979–1996) Crit Care Med. 2002;30(8):1679–1685. doi: 10.1097/00003246-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA. 1996;275(1):50–54. [PubMed] [Google Scholar]
- 22.Moss M, Parsons PE, Steinberg KP, Hudson LD, Guidot DM, Burnham EL, Eaton S, Cotsonis GA. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med. 2003;31(3):869–877. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- 23.Iscimen R, Cartin-Ceba R, Yilmaz M, Khan H, Hubmayr RD, Afessa B, Gajic O. Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med. 2008;36(5):1518–1522. doi: 10.1097/CCM.0b013e31816fc2c0. [DOI] [PubMed] [Google Scholar]
- 24.Iribarren C, Jacobs DR, Jr, Sidney S, Gross MD, Eisner MD. Cigarette smoking, alcohol consumption, and risk of ARDS: a 15-year cohort study in a managed care setting. Chest. 2000;117(1):163–168. doi: 10.1378/chest.117.1.163. [DOI] [PubMed] [Google Scholar]
- 25.Calfee CS, Matthay MA, Eisner MD, Benowitz N, Call M, Pittet JF, Cohen MJ. Active and passive cigarette smoking and acute lung injury after severe blunt trauma. Am J Respir Crit Care Med. 2011;183(12):1660–1665. doi: 10.1164/rccm.201011-1802OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong MN, Bajwa EK, Thompson BT, Christiani DC. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax. 2010;65(1):44–50. doi: 10.1136/thx.2009.117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anzueto A, Frutos-Vivar F, Esteban A, Bensalami N, Marks D, Raymondos K, Apezteguia C, Arabi Y, Hurtado J, Gonzalez M, Tomicic V, Abroug F, Elizalde J, Cakar N, Pelosi P, Ferguson ND. Influence of body mass index on outcome of the mechanically ventilated patients. Thorax. 2011;66(1):66–73. doi: 10.1136/thx.2010.145086. [DOI] [PubMed] [Google Scholar]
- 28.O'Brien JM, Jr, Phillips GS, Ali NA, Lucarelli M, Marsh CB, Lemeshow S. Body mass index is independently associated with hospital mortality in mechanically ventilated adults with acute lung injury. Crit Care Med. 2006;34(3):738–744. doi: 10.1097/01.CCM.0000202207.87891.FC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moss M, Guidot DM, Steinberg KP, Duhon GF, Treece P, Wolken R, Hudson LD, Parsons PE. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Crit Care Med. 2000;28(7):2187–2192. doi: 10.1097/00003246-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Honiden S, Gong MN. Diabetes, insulin, and development of acute lung injury. Crit Care Med. 2009;37(8):2455–2464. doi: 10.1097/CCM.0b013e3181a0fea5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erlich JM, Talmor DS, Cartin-Ceba R, Gajic O, Kor DJ. Prehospitalization antiplatelet therapy is associated with a reduced incidence of acute lung injury: a population-based cohort study. Chest. 2011;139(2):289–295. doi: 10.1378/chest.10-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brun-Buisson C, Minelli C, Bertolini G, Barzzi L, Pimentel J, Lewandowski K, Bion J, Romand JA, Villar J, Thorsteinsson A, Damas P, Armaganidis A, Lemaire F. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med. 2004;30(1):51–61. doi: 10.1007/s00134-003-2022-6. [DOI] [PubMed] [Google Scholar]
- 33.Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, Anderson H, 3rd, Hoth JJ, Mikkelsen ME, Gentile NT, Gong MN, Talmor D, Bajwa E, Watkins TR, Festic E, Yilmaz M, Iscimen R, Kaufman DA, Esper AM, Sadikot R, Douglas I, Sevransky J, Malinchoc M. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183(4):462–470. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gattinoni L, Caironi P, Pelosi P, Goodman LR. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med. 2001;164(9):1701–1711. doi: 10.1164/ajrccm.164.9.2103121. [DOI] [PubMed] [Google Scholar]
- 35.Slutsky AS. Lung injury caused by mechanical ventilation. Chest. 1999;116(1 Suppl):9S–15S. doi: 10.1378/chest.116.suppl_1.9s-a. [DOI] [PubMed] [Google Scholar]
- 36.Boussarsar M, Thierry G, Jaber S, Roudot-Thoraval F, Lemaire F, Brochard L. Relationship between ventilatory settings and barotrauma in the acute respiratory distress syndrome. Intensive Care Med. 2002;28(4):406–413. doi: 10.1007/s00134-001-1178-1. [DOI] [PubMed] [Google Scholar]
- 37.Halbertsma FJ, Vaneker M, Scheffer GJ, van der Hoeven JG. Cytokines and biotrauma in ventilator-induced lung injury: a critical review of the literature. Neth J Med. 2005;63(10):382–392. [PubMed] [Google Scholar]
- 38.Putensen C, Theuerkauf N, Zinserling J, Wrigge H, Pelosi P. Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann Intern Med. 2009;151(8):566–576. doi: 10.7326/0003-4819-151-8-200910200-00011. [DOI] [PubMed] [Google Scholar]
- 39.Maggiore SM, Jonson B, Richard JC, Jaber S, Lemaire F, Brochard L. Alveolar derecruitment at decremental positive end-expiratory pressure levels in acute lung injury: comparison with the lower inflection point, oxygenation, and compliance. Am J Respir Crit Care Med. 2001;164(5):795–801. doi: 10.1164/ajrccm.164.5.2006071. [DOI] [PubMed] [Google Scholar]
- 40.Harris RS, Hess DR, Venegas JG. An objective analysis of the pressure-volume curve in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;161(2 Pt 1):432–439. doi: 10.1164/ajrccm.161.2.9901061. [DOI] [PubMed] [Google Scholar]
- 41.Grasso S, Stripoli T, De Michele M, Bruno F, Moschetta M, Angelelli G, Munno I, Ruggiero V, Anaclerio R, Cafarelli A, Driessen B, Fiore T. ARDSnet ventilatory protocol and alveolar hyperinflation: role of positive end-expiratory pressure. Am J Respir Crit Care Med. 2007;176(8):761–767. doi: 10.1164/rccm.200702-193OC. [DOI] [PubMed] [Google Scholar]
- 42.Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, Davies AR, Hand LE, Zhou Q, Thabane L, Austin P, Lapinsky S, Baxter A, Russel J, Skrobik Y, Ronco JJ, Stewart TE. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):637–645. doi: 10.1001/jama.299.6.637. [DOI] [PubMed] [Google Scholar]
- 43.Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, Lefrant JY, Prat G, Richecoeur J, Nieszkowaska A, Gervais C, Baudot J, Bouadma L, Brochard L. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):646–655. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 44.Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, Slutsky AS, Pullenayegum E, Zhou Q, Cook D, Brochard L, Richard JC, Lamontagne F, Bhatnagar N, Stewart TE, Guyatt G. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303(9):865–873. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 45.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Jr, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 46.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Jr, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 47.Pillow JJ. Tidal volume, recruitment and compliance in HFOV: same principles, different frequency. Eur Respir J. 2012;40(2):291–293. doi: 10.1183/09031936.00020012. [DOI] [PubMed] [Google Scholar]
- 48.Tsuzaki K, Hales CA, Strieder DJ, Venegas JG. Regional lung mechanics and gas transport in lungs with inhomogeneous compliance. J Appl Physiol. 1993;75(1):206–216. doi: 10.1152/jappl.1993.75.1.206. [DOI] [PubMed] [Google Scholar]
- 49.Bauer K, Brucker C. The role of ventilation frequency in airway reopening. J Biomech. 2009;42(8):1108–1113. doi: 10.1016/j.jbiomech.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 50.Pillow JJ. High-frequency oscillatory ventilation: mechanisms of gas exchange and lung mechanics. Crit Care Med. 2005;33(3 Suppl):S135–S141. doi: 10.1097/01.ccm.0000155789.52984.b7. [DOI] [PubMed] [Google Scholar]
- 51.van Genderingen HR, van Vught AJ, Jansen JR. Regional lung volume during high-frequency oscillatory ventilation by electrical impedance tomography. Crit Care Med. 2004;32(3):787–794. doi: 10.1097/01.ccm.0000114823.16604.19. [DOI] [PubMed] [Google Scholar]
- 52.Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, Austin P, Zhou Q, Matte A, Walter SD, Lamontagne F, Granton JT, Arabi YM, Arroliga AC, Stewart TE, Slutsky AS, Meade MO. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368(9):795–805. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]
- 53.Young D, Lamb SE, Shah S, MacKenzie L, Tunnicliffe W, Lall R, Rowan K, Cuthbertson BH. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med. 2013;368(9):806–813. doi: 10.1056/NEJMoa1215716. [DOI] [PubMed] [Google Scholar]
- 54.Modrykamien A, Chatburn RL, Ashton RW. Airway pressure release ventilation: an alternative mode of mechanical ventilation in acute respiratory distress syndrome. Cleve Clin J Med. 2011;78(2):101–110. doi: 10.3949/ccjm.78a.10032. [DOI] [PubMed] [Google Scholar]
- 55.Putensen C, Zech S, Wrigge H, Zinserling J, Stuber F, Von Spiegel T, Mutz N. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med. 2001;164(1):43–49. doi: 10.1164/ajrccm.164.1.2001078. [DOI] [PubMed] [Google Scholar]
- 56.Putensen C, Mutz NJ, Putensen-Himmer G, Zinserling J. Spontaneous breathing during ventilatory support improves ventilation-perfusion distributions in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1241–1248. doi: 10.1164/ajrccm.159.4.9806077. [DOI] [PubMed] [Google Scholar]
- 57.Neumann P, Golisch W, Strohmeyer A, Buscher H, Burchardi H, Sydow M. Influence of different release times on spontaneous breathing pattern during airway pressure release ventilation. Intensive Care Med. 2002;28(12):1742–1749. doi: 10.1007/s00134-002-1522-0. [DOI] [PubMed] [Google Scholar]
- 58.Bugedo G, Bruhn A, Hernandez G, Rojas G, Varela C, Tapia JC, Castillo L. Lung computed tomography during a lung recruitment maneuver in patients with acute lung injury. Intensive Care Med. 2003;29(2):218–225. doi: 10.1007/s00134-002-1618-6. [DOI] [PubMed] [Google Scholar]
- 59.Rasanen J, Cane RD, Downs JB, Hurst JM, Jousela IT, Kirby RR, Rogove HJ, Stock MC. Airway pressure release ventilation during acute lung injury: a prospective multicenter trial. Crit Care Med. 1991;19(10):1234–1241. doi: 10.1097/00003246-199110000-00004. [DOI] [PubMed] [Google Scholar]
- 60.Valente Barbas CS. Lung recruitment maneuvers in acute respiratory distress syndrome and facilitating resolution. Crit Care Med. 2003;31(4 Suppl):S265–S271. doi: 10.1097/01.CCM.0000057902.29449.29. [DOI] [PubMed] [Google Scholar]
- 61.Beiderlinden M, Eikermann M, Boes T, Breitfeld C, Peters J. Treatment of severe acute respiratory distress syndrome: role of extracorporeal gas exchange. Intensive Care Med. 2006;32(10):1627–1631. doi: 10.1007/s00134-006-0262-y. [DOI] [PubMed] [Google Scholar]
- 62.Lewandowski K, Rossaint R, Pappert D, Gerlach H, Slama KJ, Weidemann H, Frey DJ, Hoffmann O, Keske U, Falke KJ. High survival rate in 122 ARDS patients managed according to a clinical algorithm including extracorporeal membrane oxygenation. Intensive Care Med. 1997;23(8):819–835. doi: 10.1007/s001340050418. [DOI] [PubMed] [Google Scholar]
- 63.Patroniti N, Zangrillo A, Pappalardo F, Peris A, Cianchi G, Braschi A, Iotti GA, Arcadipane A, Panarello G, Ranieri VM, Terragni P, Antonelli M, Gattinoni L, Oleari F, Pesenti A. The Italian ECMO network experience during the 2009 influenza A (H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med. 2011;37(9):1447–1457. doi: 10.1007/s00134-011-2301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pranikoff T, Hirschl RB, Steimle CN, Anderson HL, 3rd, Bartlett RH. Mortality is directly related to the duration of mechanical ventilation before the initiation of extracorporeal life support for severe respiratory failure. Crit Care Med. 1997;25(1):28–32. doi: 10.1097/00003246-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 65.Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, Morris AH, Peirce EC, 2nd, Thomas AN, Proctor HJ, Drinker PA, Pratt PC, Bagniewski A, Miller RG., Jr Extracorporeal membrane oxygenation in severe acute respiratory failure: a randomized prospective study. JAMA. 1979;242(20):2193–2196. doi: 10.1001/jama.242.20.2193. [DOI] [PubMed] [Google Scholar]
- 66.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 67.Rossaint R, Falke KJ, Lopez F, Slama K, Pison U, Zapol WM. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med. 1993;328(6):399–405. doi: 10.1056/NEJM199302113280605. [DOI] [PubMed] [Google Scholar]
- 68.Adhikari NK, Burns KE, Friedrich JO, Granton JT, Cook DJ, Meade MO. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ. 2007;334(7597):779. doi: 10.1136/bmj.39139.716794.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Manktelow C, Bigatello LM, Hess D, Hurford WE. Physiologic determinants of the response to inhaled nitric oxide in patients with acute respiratory distress syndrome. Anesthesiology. 1997;87(2):297–307. doi: 10.1097/00000542-199708000-00017. [DOI] [PubMed] [Google Scholar]
- 70.Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354(17):1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 71.Dreyfuss D, Saumon G. Ventilator-induced lung injury: Lessons from experimental studies. Am J Respir Crit Care Med. 1998;157(1):294–323. doi: 10.1164/ajrccm.157.1.9604014. [DOI] [PubMed] [Google Scholar]
- 72.Meade MO, Cook DJ, Griffith LE, Hand LE, Lpinsky SE, Stewart TE, Killian KJ, Slutsky AS, Guyatt GH. A study of the physiologic responses to a lung recruitment maneuver in acute lung injury and acute respiratory distress syndrome. Respir Care. 2008;53(11):1441–1449. [PubMed] [Google Scholar]
- 73.Brower RG, Morris A, MacIntyre N, Matthay MA, Hayden D, Thompson T, Clemmer T, Lanken PN, Schoenfeld D. Effects of recruitment maneuvers in patients with acute lung injury and acute respiratory distress syndrome ventilated with high positive end-expiratory pressure. Crit Care Med. 2003;31(11):2592–2597. doi: 10.1097/01.CCM.0000090001.91640.45. [DOI] [PubMed] [Google Scholar]
- 74.Fan E, Wilcox ME, Brower RG, Stewart TE, Mehta S, Lapinsky SE, Meade MO, Ferguson ND. Recruitment maneuvers for acute lung injury: a systematic review. Am J Respir Crit Care Med. 2008;178(11):1156–1163. doi: 10.1164/rccm.200802-335OC. [DOI] [PubMed] [Google Scholar]
- 75.Pelosi P, Tubiolo D, Mascheroni D, Vicardi P, Crotti S, Valenza F, Gattinoni L. Effects of the prone position on respiratory mechanics and gas exchange during acute lung injury. Am J Respir Crit Care Med. 1998;157(2):387–393. doi: 10.1164/ajrccm.157.2.97-04023. [DOI] [PubMed] [Google Scholar]
- 76.Mure M, Martling CR, Lindahl SG. Dramatic effect on oxygenation in patients with severe acute lung insufficiency treated in the prone position. Crit Care Med. 1997;25(9):1539–1544. doi: 10.1097/00003246-199709000-00022. [DOI] [PubMed] [Google Scholar]
- 77.Blanch L, Mancebo J, Perez M, Martinez M, Mas A, Betbese AJ, Joseph D, Ballus J, Lucangelo U, Bak E. Short-term effects of prone position in critically ill patients with acute respiratory distress syndrome. Intensive Care Med. 1997;23(10):1033–1039. doi: 10.1007/s001340050453. [DOI] [PubMed] [Google Scholar]
- 78.Guerin C, Gaillard S, Lemasson S, Ayzac L, Girard R, Beuret P, Palmier B, Le QV, Sirodot M, Rosselli S, Cadiergue V, Sainty JM, Barbe P, Combourieu E, Debatty D, Rouffineau J, Ezingeard E, Millet O, Guelon D, Rodriguez L, Martin O, Renault A, Sibille JP, Kaidomar M. Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial. JAMA. 2004;292(19):2379–2387. doi: 10.1001/jama.292.19.2379. [DOI] [PubMed] [Google Scholar]
- 79.Guerin C, Reignier J, Richard JC. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 80.Taccone P, Pesenti A, Latini R, Polli F, Vagginelli F, Mietto C, Caspani L, Raimondi F, Bordone G, Iapichino G, Mancebo J, Guerin C, Ayzac L, Blanch L, Fumagalli R, Tognoni G, Gattinoni L. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2009;302(18):1977–1984. doi: 10.1001/jama.2009.1614. [DOI] [PubMed] [Google Scholar]
- 81.Mancebo J, Fernandez R, Blanch L, Rialp G, Gordo F, Ferrer M, Rodriguez F, Garro P, Ricart P, Vallverdu I, Gich I, Castano J, Saura P, Dominguez G, Bonet A, Albert RK. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;173(11):1233–1239. doi: 10.1164/rccm.200503-353OC. [DOI] [PubMed] [Google Scholar]
- 82.Guerin C, Gaillard S, Lemasson S, Ayzac L, Girard R, Beuret P, Palmier B, Le QV, Sirodot M, Rosselli S, Cadiergue V, Sainty JM, Barbe P, Combourieu E, Debatty D, Rouffineau J, Ezingeard E, Millet O, Guelon D, Rodriguez L, Martin O, Renault A, Sibille JP, Kaidomar M. Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial. JAMA. 2004;292(19):2379–2387. doi: 10.1001/jama.292.19.2379. [DOI] [PubMed] [Google Scholar]
- 83.Alsaghir AH, Martin CM. Effect of prone positioning in patients with acute respiratory distress syndrome: a meta-analysis. Crit Care Med. 2008;36(2):603–609. doi: 10.1097/01.CCM.0000299739.98236.05. [DOI] [PubMed] [Google Scholar]
- 84.Sud S, Friedrich JO, Taccone P, Polli F, Adhikari NK, Latini R, Pesenti A, Guerin C, Mancebo J, Curley MA, Fernandez R, Chan MC, Beuret P, Voggenreiter G, Sud M, Tognoni G, Gattinoni L. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med. 2010;36(4):585–599. doi: 10.1007/s00134-009-1748-1. [DOI] [PubMed] [Google Scholar]
- 85.Artigas A, Bernard GR, Carlet J, Dreyfuss D, Gattinoni L, Hudson L, Lamy M, Marini JJ, Matthay MA, Pinsky MR, Spragg R, Suter PM. The American-European Consensus Conference on ARDS, part 2. Ventilatory, pharmacologic, supportive therapy, study design strategies and issues related to recovery and remodeling. Intensive Care Med. 1998;24(4):378–398. doi: 10.1007/s001340050585. [DOI] [PubMed] [Google Scholar]
- 86.Vender JS, Szokol JW, Murphy GS, Nitsun M. Sedation, analgesia, and neuromuscular blockade in sepsis: an evidence-based review. Crit Care Med. 2004;32(11 Suppl):S554–S561. doi: 10.1097/01.ccm.0000145907.86298.12. [DOI] [PubMed] [Google Scholar]
- 87.Schein RM, Bergman R, Marcial EH, Schultz D, Duncan RC, Arnold PI, Sprung CL. Complement activation and corticosteroid therapy in the development of the adult respiratory distress syndrome. Chest. 1987;91(6):850–854. doi: 10.1378/chest.91.6.850. [DOI] [PubMed] [Google Scholar]
- 88.Bone RC, Fisher CJ, Jr, Clemmer TP, Slotman GJ, Metz CA. Early methylprednisolone treatment for septic syndrome and the adult respiratory distress syndrome. Chest. 1987;92(6):1032–1036. doi: 10.1378/chest.92.6.1032. [DOI] [PubMed] [Google Scholar]
- 89.Luce JM, Montgomery AB, Marks JD, Turner J, Metz CA, Murray JF. Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis. 1988;138(1):62–68. doi: 10.1164/ajrccm/138.1.62. [DOI] [PubMed] [Google Scholar]
- 90.Weigelt JA, Norcross JF, Borman KR, Snyder WH., 3rd Early steroid therapy for respiratory failure. Arch Surg. 1985;120(5):536–540. doi: 10.1001/archsurg.1985.01390290018003. [DOI] [PubMed] [Google Scholar]
- 91.Bernard GR, Luce JM, Sprung CL, Rinaldo JE, Tate RM, Sibbald WJ, Kariman K, Higgins S, Bradley R, Metz CA. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med. 1987;317(25):1565–1570. doi: 10.1056/NEJM198712173172504. [DOI] [PubMed] [Google Scholar]
- 92.Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, Thompson BT, Ancukiewicz M. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354(16):1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 93.Annane D, Sebille V, Bellissant E, Ger-Inf-05 Study Group. Effect of low doses of corticosteroids in septic shock patients with or without early acute respiratory distress syndrome. Crit Care Med. 2006;34(1):22–30. doi: 10.1097/01.ccm.0000194723.78632.62. [DOI] [PubMed] [Google Scholar]
- 94.Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, Gibson M, Umberger R. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131(4):954–963. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 95.Meduri GU, Headley AS, Golden E, Carson SJ, Umberger RA, Kelso T, Tolley EA. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1998;280(2):159–165. doi: 10.1001/jama.280.2.159. [DOI] [PubMed] [Google Scholar]
- 96.Confalonieri M, Urbino R, Potena A, Piatttella M, Parigi P, Puccio G, Della Porta R, Giorgio C, Blasi F, Umberger R, Meduri GU. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005;171(3):242–248. doi: 10.1164/rccm.200406-808OC. [DOI] [PubMed] [Google Scholar]
- 97.Varpula T, Pettila V, Rintala E, Takkunen O, Valtonen V. Late steroid therapy in primary acute lung injury. Intensive Care Med. 2000;26(5):526–531. doi: 10.1007/s001340051199. [DOI] [PubMed] [Google Scholar]
- 98.Meduri GU, Marik PE, Chrousos GP, Pastores SM, Arlt W, Beishuizen A, Bokhari F, Zaloga G, Annane D. Steroid treatment in ARDS: A critical appraisal of the ARDS network trial and the recent literature. Intensive Care Med. 2008;34(1):61–69. doi: 10.1007/s00134-007-0933-3. [DOI] [PubMed] [Google Scholar]
- 99.Marik PE, Pastores SM, Annane D, Meduri GU, Sprung CL, Arlt W, Keh D, Briegel J, Beishuizen A, Dimopoulou I, Tsagarakis S, Singer M, Chrousos GP, Zaloga G, Bokhari F, Vogeser M. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36(6):1937–1949. doi: 10.1097/CCM.0b013e31817603ba. [DOI] [PubMed] [Google Scholar]