Abstract
A patient in Washington State harbored a fish tapeworm most likely acquired from eating raw salmon. Diphyllobothrium nihonkaiense was identified by cox1 sequence analysis. Although this is the first documented human D. nihonkaiense infection in the United States, the parasite may have been present earlier but misidentified as Diphyllobothrium latum.
TEXT
Diphyllobothrium spp. are tapeworms acquired by the ingestion of raw or undercooked fish. Approximately 20 million people worldwide are believed to harbor fish tapeworms (1). Since the description of an infection with Diphyllobothrium latum in Minnesota in 1906 (2), diphyllobothriasis in the United States has been attributed to this species. The majority of reported cases since that time have followed the ingestion of freshwater fish, such as perch or pike from the Great Lakes region.
However, more than 50 Diphyllobothrium species have been described, and at least 14 of these have been detected in humans (1). Species identification on the basis of morphology is unreliable, and molecular assays for distinguishing individual species have been developed but are not widely available (3, 4). Of particular note, Diphyllobothrium nihonkaiense is commonly found in Japan (5, 6) and has subsequently been reported in South Korea (7), in China (8), and in Europe in people who ingested raw fish originating from Canada (9, 10).
The life cycle of Diphyllobothrium spp. is complex and involves copepods as the first intermediate hosts, freshwater, anadromous, or marine fish as second intermediate hosts, and fish-eating birds or mammals as definitive hosts (1). D. nihonkaiense has been found in Pacific salmon (Oncorhynchus spp.), including in the pink, chum, and sockeye species. Brown bears are believed to be the natural definitive host (11).
We recently encountered a 20-year-old previously healthy resident of Washington State who passed a parasite that was identified as a section of strobila from a Diphyllobothrium sp. on morphological grounds. Due to the absence of risk factors for D. latum exposure, such as ingestion of raw freshwater fish or travel to areas in which D. latum is endemic, molecular analysis of DNA extracted from a proglottid was performed, and the parasite was identified as D. nihonkaiense. The patient was otherwise asymptomatic, was not taking any medications, and had not traveled outside the Pacific Northwest with the exception of a visit to Costa Rica at the age of 15 years. She admitted to eating sushi, including raw salmon in particular. After identification of the Diphyllobothrium sp., she received a single dose of praziquantel.
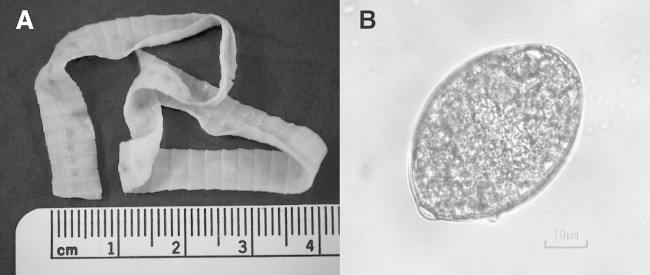
The proglottids were approximately 2.5 mm long by 7.5 mm wide after fixation, with a rosette-shaped central uterus characteristic of Diphyllobothrium spp. (Fig. 1). Ova were expressed from a gravid proglottid. Thirty ova were measured, yielding a mean (± standard deviation [SD]) size of 44.9 μm (±2.4 μm) by 67.2 μm (±3.3 μm).
FIG 1.
Diphyllobothrium nihonkaiense: proglottids (A) and ovum (B). Scale bar, 10 μm (magnification, ×400).
A section of the parasite was fixed in formalin-acetic acid-alcohol (FAA) prior to microscopic examination, and an equivalent portion was submitted in 70% ethanol without fixation for molecular analysis. DNA was extracted from the unfixed sample using the animal tissue protocol for the DNeasy blood and tissue kit (Qiagen, Hilden, Germany). A custom primer pair was designed to amplify a 349-bp region of the Diphyllobothrium cytochrome c oxidase subunit 1 (cox1) gene based on publicly available sequences (forward primer, 5′-TGCACTCTCTACACAGCGTTT-3′; reverse primer, 5′-ATGCCCCCACACAACACTAC-3′). This specific region was selected because it contained residues that discriminate between D. nihonkaiense and Diphyllobothrium klebanovskii (12). The PCR was performed using the AccuPrime Pfx DNA polymerase kit (Invitrogen, Carlsbad, CA) with the following cycling conditions: 95°C for 2 min followed by 35 cycles at 95°C for 15 s, 55°C for 30 s, and 68°C for 90 s, and a final extension at 68°C for 2 min.
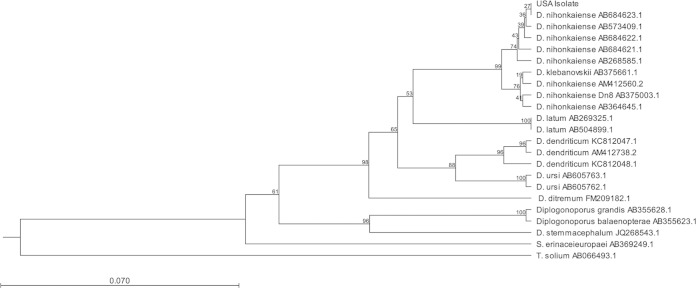
A DNA band of ∼350 bp was isolated from a 1.5% agarose gel from the clinical sample. A control PCR performed on a known Taenia solium sample did not produce a PCR product under the same conditions. Sequencing of the isolate revealed multiple differences from the D. latum cox1 gene and 100% identity to a known D. nihonkaiense sequence (Table 1) (Fig. 2A). The phylogeny of this U.S. D. nihonkaiense isolate was consistent with that of other reported D. nihonkaiense isolates (Fig. 2B).
TABLE 1.
Cox1 sequence alignment of a U.S. Diphyllobothrium isolate compared to sequences of other Diphyllobothrium spp. by ClustalW
| Isolate (GenBank accession no.)a | Sequence alignmentb by cox1 gene position: |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 663 | 669 | 678 | 684 | 717 | 720 | 723 | 756 | 765 | 792 | 795 | 801 | 807 | 825 | 846 | 855 | |
| U.S. isolate | C | A | A | C | C | C | C | A | C | A | T | T | G | T | C | T |
| D. nihonkaiense (AB684623.1) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| D. klebanovskii (AB375661.1) | X | X | X | T | X | T | X | X | X | X | X | X | X | C | X | X |
| D. nihonkaiense Dn8 (AB375003.1) | X | X | X | T | X | T | X | X | X | X | C | X | X | X | X | X |
| D. latum (AB504899.1) | T | G | G | T | T | T | T | G | G | T | X | C | A | X | T | A |
Sample D. nihonkaiense Dn8 (GenBank accession no. AB375003.1) was determined by the Hashimoto group to be of a distinct lineage closely related to D. klebanovskii. The D. latum sequence was included as a reference.
X, homology with the U.S. isolate sequence.
FIG 2.
Phylogenetic relationship of the D. nihonkaiense U.S. isolate and related isolates. Phylogenetic tree of the U.S. isolate in relation to other tapeworms. Unweighted-pair group method using average linkages (UPGMA) tree on amplified 349-bp fragment of cox1 gene, with Kimura 80 parameters. Bootstrap values for 10,000 replicates are shown. Sample AB375003.1 was determined by the Hashimoto group to be D. klebanovskii (12).
Although this represents the first documented D. nihonkaiense infection in the United States, it is possible that this species has been present in the country for a long time without being recognized as distinct from D. latum. A cluster of 52 cases of diphyllobothriasis were investigated in association with fresh salmon ingestion in California, Hawaii, Oregon, and Washington in 1980 to 1981 (13), but the parasites were not identified to the species level, and D. nihonkaiense had not yet been described as a species at that time (6). Morphological discrimination of Diphyllobothrium spp. can be difficult, and the diagnosis of diphyllobothriasis is usually made on morphological grounds. The ova of D. nihonkaiense have been reported to be somewhat smaller (mean size, 45 by 57 μm) than those of D. latum (mean size, 57 by 72 μm) (14), and it is noteworthy that the usual size criteria for the identification of D. latum ova essentially encompass both species (40 to 50 μm by 58 to 75 μm) (15). The ova from our specimen averaged 45 by 67 μm.
Human Diphyllobothrium infections may cause no symptoms, as in the present case, or may be associated with vague gastrointestinal symptoms. Vitamin B12 deficiency has been described as a complication of longstanding D. latum infection but has not been reported with D. nihonkaiense. Mild anemia or eosinophilia may occasionally occur (11), but a causal relationship has not been established. Patients harboring D. nihonkaiense may repeatedly shed large segments over extended periods of time, resulting in substantial psychological distress for the patient and the family members (1, 16).
Diphyllobothriasis has not been a reportable condition in the United States since 1982. The parasite appears to be reemerging in Russia, South Korea, Japan, South America, and alpine regions of Europe (1). Treatment with praziquantel appears effective (5). The U.S. Food and Drug Administration regulations (Code of Federal Regulations Part 123, Title 21) require ready-to-eat raw, raw-marinated, or partially cooked fish other than molluscan shellfish and tuna to be frozen at −20°C for 7 days or −35°C for 15 h prior to service or sale. Thus, salmon sushi served in commercial establishments should be safe to eat. However, it is notable that Diphyllobothrium spp. are not reliably killed by smoking (17), so cold-smoked salmon represents a potential source of infection. This report documents the presence of D. nihonkaiense in the United States and underscores the ability of molecular methods to reveal unappreciated diversity in human pathogens.
REFERENCES
- 1.Scholz T, Garcia HH, Kuchta R, Wicht B. 2009. Update on the human broad tapeworm (genus Diphyllobothrium), including clinical relevance. Clin Microbiol Rev 22:146–260. doi: 10.1128/CMR.00033-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nickerson WS. 1906. The broad tapeworm in Minnesota, with the report of a case of infection acquired in the state. JAMA 46:711–713. doi: 10.1001/jama.1906.62510370017001e.. [DOI] [Google Scholar]
- 3.Kim KH, Jeon HK, Kang S, Sultana T, Kim GJ, Eom K, Park JK. 2007. Characterization of the complete mitochondrial genome of Diphyllobothrium nihonkaiense (Diphyllobothriidae: Cestoda), and development of molecular markers for differentiating fish tapeworms. Mol Cells 23:379–390. [PubMed] [Google Scholar]
- 4.Wicht B, Yanagida T, Scholz T, Ito A, Jimenez JA, Brabec J. 2010. Multiplex PCR for differential identification of broad tapeworms (Cestoda: Diphyllobothrium) infecting humans. J Clin Microbiol 48:3111–3116. doi: 10.1128/JCM.00445-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohnishi K, Murata M. 1993. Single dose treatment with praziquantel for human Diphyllobothrium nihonkaiense infections. Trans R Soc Trop Med Hyg 87:482–483. doi: 10.1016/0035-9203(93)90049-V. [DOI] [PubMed] [Google Scholar]
- 6.Yamane Y, Kamo H, Bylund G, Wikgren BJ. 1986. Diphyllobothrium nihonkaiense sp. nov. (Cestoda: Diphyllobothriidae): revised identification of Japanese broad tapeworm. Shimane J Med Sci 10:29–48. [Google Scholar]
- 7.Jeon HK, Kim KH, Huh S, Chai JY, Min DY, Rim HJ, Eom KS. 2009. Morphologic and genetic identification of Diphyllobothrium nihonkaiense in Korea. Korean J Parasitol 47:369–375. doi: 10.3347/kjp.2009.47.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S, Ai L, Zhang Y, Chen J, Zhang W, Li Y, Muto M, Morishima Y, Sugiyama H, Xu X, Zhou X, Yamasaki H. 2014. Molecular detection of Diphyllobothrium nihonkaiense in humans, China. Emerg Infect Dis 20:315–318. doi: 10.3201/eid2002.121889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wicht B, Scholz T, Peduzzi R, Kuchta R. 2008. First record of human infection with the tapeworm Diphyllobothrium nihonkaiense in North America. Am J Trop Med Hyg 78:235–238. [PubMed] [Google Scholar]
- 10.Yera H, Estran C, Delaunay P, Gari-Toussaint M, Dupouy-Camet J, Marty P. 2006. Putative Diphyllobothrium nihonkaiense acquired from a Pacific salmon (Oncorhynchus keta) eaten in France; genomic identification and case report. Parasitol Int 55:45–49. doi: 10.1016/j.parint.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Arizono N, Yamada M, Nakamura-Uchiyama F, Ohnishi K. 2009. Diphyllobothriasis associated with eating raw Pacific salmon. Emerg Infect Dis 15:866–870. doi: 10.3201/eid1506.090132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arizono N, Shedko M, Yamada M, Uchikawa R, Tegoshi T, Takeda K, Hashimoto K. 2009. Mitochondrial DNA divergence in populations of the tapeworm Diphyllobothrium nihonkaiense and its phylogenetic relationships with Diphyllobothrium klebanovskii. Parasitol Int 58:22–28. doi: 10.1016/j.parint.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Ruttenber AJ, Weniger BG, Sorvillo F, Murray RA, Ford SL. 1984. Diphyllobothriasis associated with salmon consumption in Pacific Coast states. Am J Trop Med Hyg 33:455–459. [DOI] [PubMed] [Google Scholar]
- 14.Wicht B, de Marval F, Peduzzi R. 2007. Diphyllobothrium nihonkaiense (Yamane et al., 1986) in Switzerland: first molecular evidence and case reports. Parasitol Int 56:195–199. doi: 10.1016/j.parint.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Garcia LS, Bruckner DA. 1993. Diagnostic medical parasitology, 2nd ed, p 268 American Society for Microbiology, Washington, DC. [Google Scholar]
- 16.Desvois L, Gregory A, Ancelle T, Dupouy-Camet J, Yamada M, Nakamura-Uchiyama F, Ohnishi K. 2001. Enquête sur l'incidence de la bothriocéphalose en Haute-Savoie (1993–2000). Bull Epidemiol Hebd 45:211–213. [Google Scholar]
- 17.Beldsoe GE, Oria MP, Murata M. 2001. Potential hazards in cold-smoked fish: parasites. J Food Sci 66(suppl s7):S1100–S1103. doi: 10.1111/j.1365-2621.2001.tb15529.x. [DOI] [Google Scholar]