Abstract
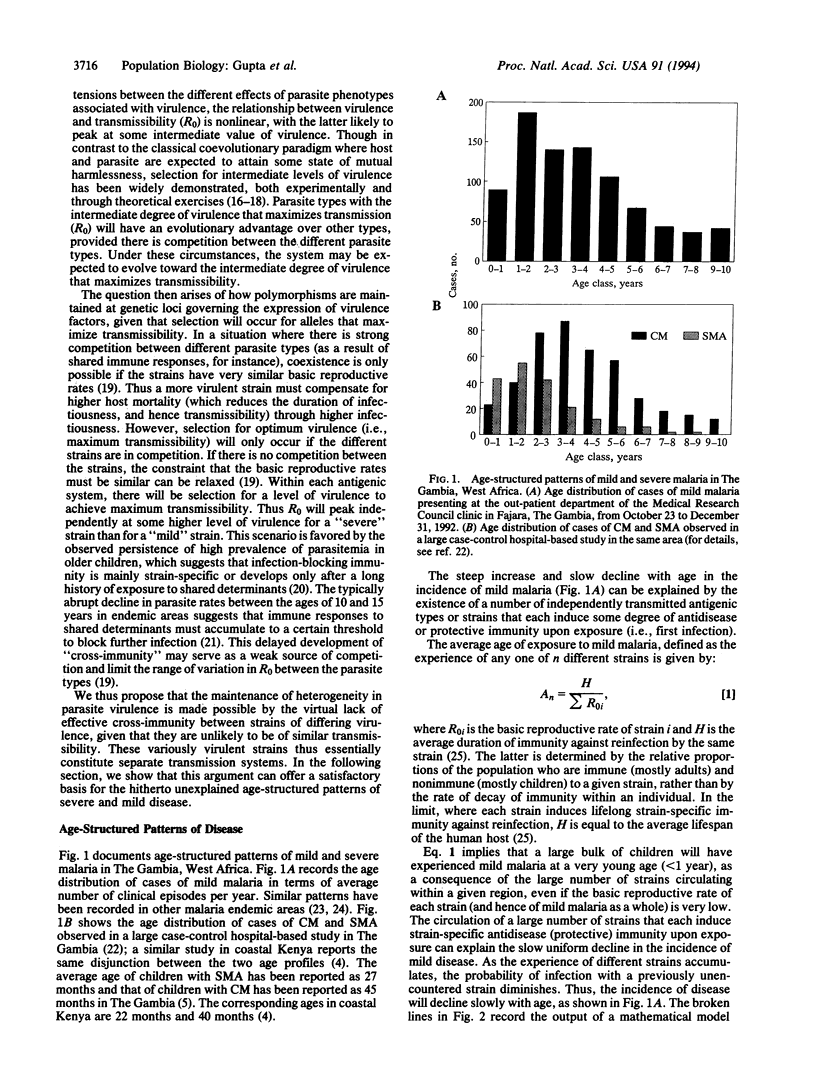
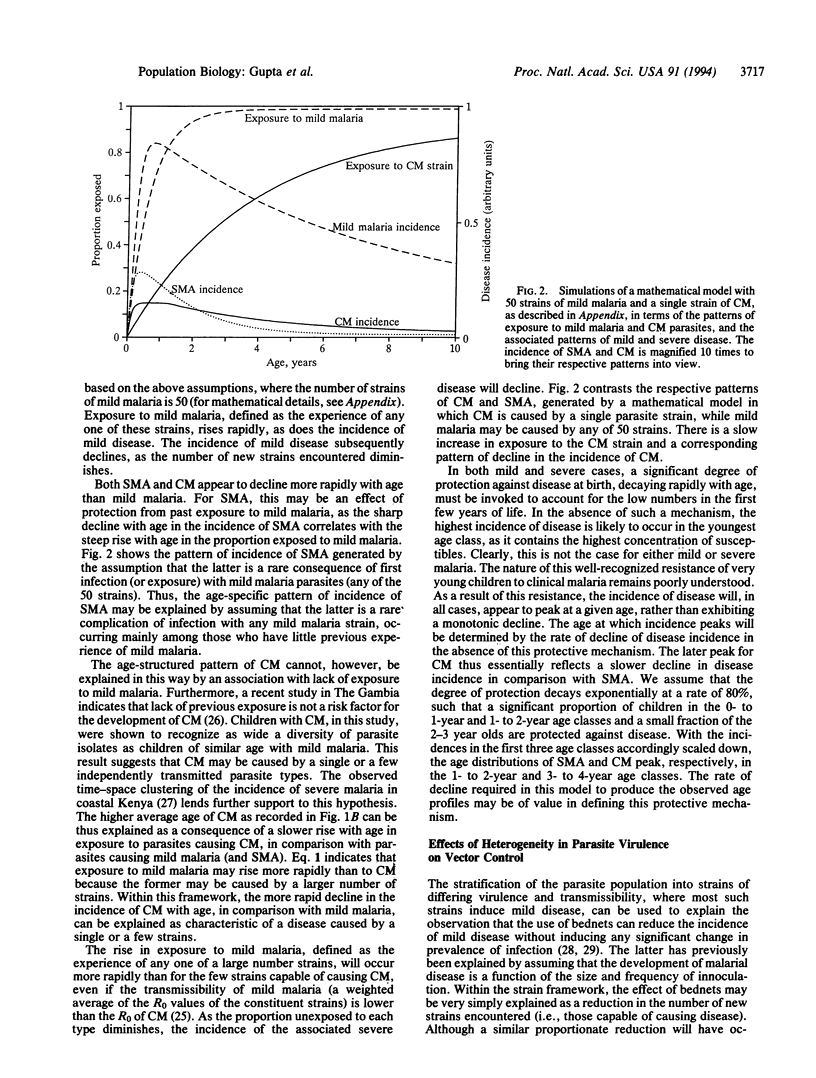
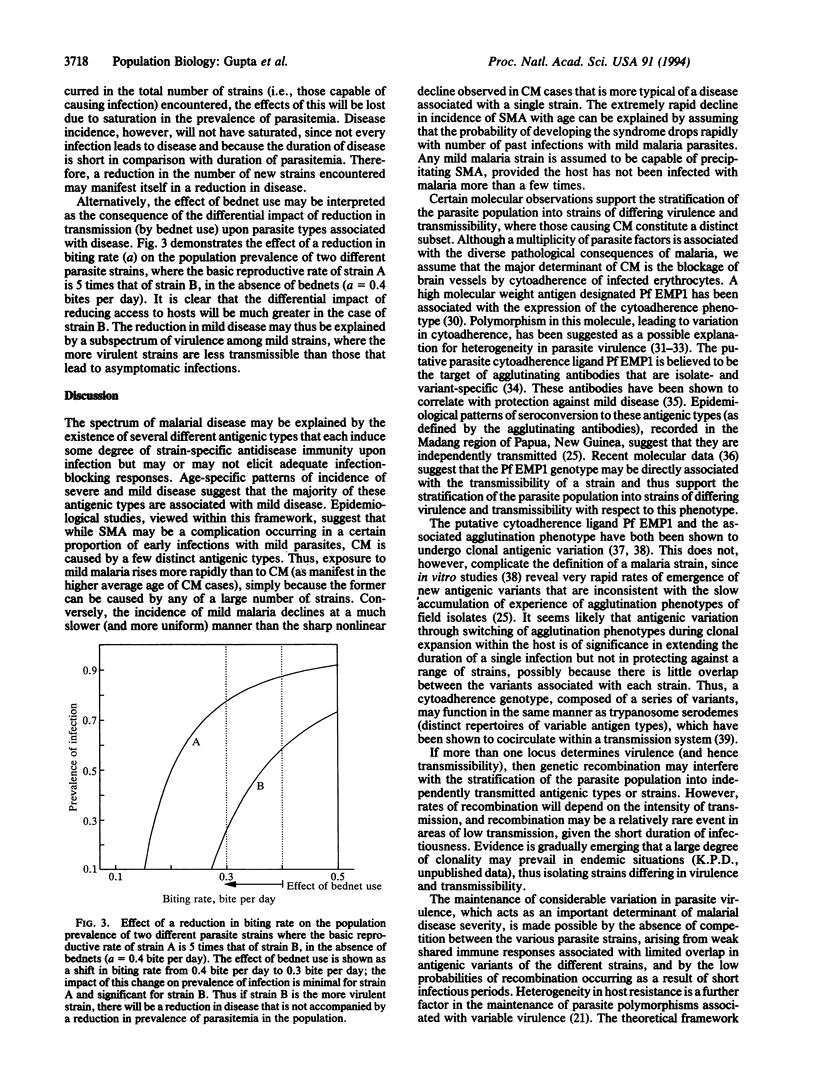
Heterogeneity in parasite virulence is one of several factors that have been proposed to contribute to the wide spectrum of disease severity in Plasmodium falciparum malaria. We used observed age-structured patterns of disease to define a population structure of P. falciparum, where the latter contains several independently transmitted antigenic types or "strains" that each induce some degree of strain-specific antidisease immunity upon infection. Patterns of incidence of severe and mild disease may be explained by assuming that a majority of these strains are associated with mild disease and that although severe malarial anemia is a complication occurring in a certain proportion of early infections with "mild" parasites, cerebral malaria is caused by a few distinct highly virulent strains. Considerable variation in parasite virulence, as a major factor of disease severity in malaria, is made possible by the absence of competition between the various parasite strains, arising from weak shared immune responses. The theoretical framework presented in this paper can explain other epidemiological observations, such as the results of interventions with insecticide-impregnated bednets.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso P. L., Lindsay S. W., Armstrong J. R., Conteh M., Hill A. G., David P. H., Fegan G., de Francisco A., Hall A. J., Shenton F. C. The effect of insecticide-treated bed nets on mortality of Gambian children. Lancet. 1991 Jun 22;337(8756):1499–1502. doi: 10.1016/0140-6736(91)93194-e. [DOI] [PubMed] [Google Scholar]
- Anderson R. M., May R. M. Coevolution of hosts and parasites. Parasitology. 1982 Oct;85(Pt 2):411–426. doi: 10.1017/s0031182000055360. [DOI] [PubMed] [Google Scholar]
- Berendt A. R., Ferguson D. J., Newbold C. I. Sequestration in Plasmodium falciparum malaria: sticky cells and sticky problems. Parasitol Today. 1990 Aug;6(8):247–254. doi: 10.1016/0169-4758(90)90184-6. [DOI] [PubMed] [Google Scholar]
- Biggs B. A., Goozé L., Wycherley K., Wollish W., Southwell B., Leech J. H., Brown G. V. Antigenic variation in Plasmodium falciparum. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9171–9174. doi: 10.1073/pnas.88.20.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster D. R., Kwiatkowski D., White N. J. Neurological sequelae of cerebral malaria in children. Lancet. 1990 Oct 27;336(8722):1039–1043. doi: 10.1016/0140-6736(90)92498-7. [DOI] [PubMed] [Google Scholar]
- COVELL G., NICOL W. D. Clinical, chemotherapeutic and immunological studies on induced malaria. Br Med Bull. 1951;8(1):51–55. doi: 10.1093/oxfordjournals.bmb.a074054. [DOI] [PubMed] [Google Scholar]
- Carter R., Miller L. H. Evidence for environmental modulation of gametocytogenesis in Plasmodium falciparum in continuous culture. Bull World Health Organ. 1979;57 (Suppl 1):37–52. [PMC free article] [PubMed] [Google Scholar]
- Cattani J. A., Tulloch J. L., Vrbova H., Jolley D., Gibson F. D., Moir J. S., Heywood P. F., Alpers M. P., Stevenson A., Clancy R. The epidemiology of malaria in a population surrounding Madang, Papua New Guinea. Am J Trop Med Hyg. 1986 Jan;35(1):3–15. doi: 10.4269/ajtmh.1986.35.3. [DOI] [PubMed] [Google Scholar]
- Day K. P., Karamalis F., Thompson J., Barnes D. A., Peterson C., Brown H., Brown G. V., Kemp D. J. Genes necessary for expression of a virulence determinant and for transmission of Plasmodium falciparum are located on a 0.3-megabase region of chromosome 9. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8292–8296. doi: 10.1073/pnas.90.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erunkulu O. A., Hill A. V., Kwiatkowski D. P., Todd J. E., Iqbal J., Berzins K., Riley E. M., Greenwood B. M. Severe malaria in Gambian children is not due to lack of previous exposure to malaria. Clin Exp Immunol. 1992 Aug;89(2):296–300. doi: 10.1111/j.1365-2249.1992.tb06948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamage-Mendis A. C., Rajakaruna J., Carter R., Mendis K. N. Transmission blocking immunity to human Plasmodium vivax malaria in an endemic population in Kataragama, Sri Lanka. Parasite Immunol. 1992 Jul;14(4):385–396. doi: 10.1111/j.1365-3024.1992.tb00013.x. [DOI] [PubMed] [Google Scholar]
- Gupta S., Trenholme K., Anderson R. M., Day K. P. Antigenic diversity and the transmission dynamics of Plasmodium falciparum. Science. 1994 Feb 18;263(5149):961–963. doi: 10.1126/science.8310293. [DOI] [PubMed] [Google Scholar]
- Hill A. V., Allsopp C. E., Kwiatkowski D., Anstey N. M., Twumasi P., Rowe P. A., Bennett S., Brewster D., McMichael A. J., Greenwood B. M. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991 Aug 15;352(6336):595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- Howard R. J. Malarial proteins at the membrane of Plasmodium falciparum-infected erythrocytes and their involvement in cytoadherence to endothelial cells. Prog Allergy. 1988;41:98–147. doi: 10.1159/000415221. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D. Febrile temperatures can synchronize the growth of Plasmodium falciparum in vitro. J Exp Med. 1989 Jan 1;169(1):357–361. doi: 10.1084/jem.169.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGREGOR I. A. CONSIDERATION OF SOME ASPECTS OF HUMAN MALARIA. Trans R Soc Trop Med Hyg. 1965 Mar;59:145–152. doi: 10.1016/0035-9203(65)90075-1. [DOI] [PubMed] [Google Scholar]
- Marsh K. Malaria--a neglected disease? Parasitology. 1992;104 (Suppl):S53–S69. doi: 10.1017/s0031182000075247. [DOI] [PubMed] [Google Scholar]
- Marsh K., Marsh V. M., Brown J., Whittle H. C., Greenwood B. M. Plasmodium falciparum: the behavior of clinical isolates in an in vitro model of infected red blood cell sequestration. Exp Parasitol. 1988 Apr;65(2):202–208. doi: 10.1016/0014-4894(88)90123-3. [DOI] [PubMed] [Google Scholar]
- Marsh K., Otoo L., Hayes R. J., Carson D. C., Greenwood B. M. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989 May-Jun;83(3):293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- Masake R. A., Nantulya V. M., Musoke A. J., Moloo S. K., Nguli K. Characterization of Trypanosoma congolense serodemes in stocks isolated from cattle introduced onto a ranch in Kilifi, Kenya. Parasitology. 1987 Apr;94(Pt 2):349–357. doi: 10.1017/s0031182000054007. [DOI] [PubMed] [Google Scholar]
- May R. M., Anderson R. M. Parasite-host coevolution. Parasitology. 1990;100 (Suppl):S89–101. doi: 10.1017/s0031182000073042. [DOI] [PubMed] [Google Scholar]
- Naotunne T. S., Karunaweera N. D., Mendis K. N., Carter R. Cytokine-mediated inactivation of malarial gametocytes is dependent on the presence of white blood cells and involves reactive nitrogen intermediates. Immunology. 1993 Apr;78(4):555–562. [PMC free article] [PubMed] [Google Scholar]
- Newbold C. I., Pinches R., Roberts D. J., Marsh K. Plasmodium falciparum: the human agglutinating antibody response to the infected red cell surface is predominantly variant specific. Exp Parasitol. 1992 Nov;75(3):281–292. doi: 10.1016/0014-4894(92)90213-t. [DOI] [PubMed] [Google Scholar]
- Ockenhouse C. F., Ho M., Tandon N. N., Van Seventer G. A., Shaw S., White N. J., Jamieson G. A., Chulay J. D., Webster H. K. Molecular basis of sequestration in severe and uncomplicated Plasmodium falciparum malaria: differential adhesion of infected erythrocytes to CD36 and ICAM-1. J Infect Dis. 1991 Jul;164(1):163–169. doi: 10.1093/infdis/164.1.163. [DOI] [PubMed] [Google Scholar]
- Roberts D. J., Craig A. G., Berendt A. R., Pinches R., Nash G., Marsh K., Newbold C. I. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature. 1992 Jun 25;357(6380):689–692. doi: 10.1038/357689a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooth I., Björkman A. Fever episodes in a holoendemic malaria area of Tanzania: parasitological and clinical findings and diagnostic aspects related to malaria. Trans R Soc Trop Med Hyg. 1992 Sep-Oct;86(5):479–482. doi: 10.1016/0035-9203(92)90076-o. [DOI] [PubMed] [Google Scholar]
- Snow R. W., Lindsay S. W., Hayes R. J., Greenwood B. M. Permethrin-treated bed nets (mosquito nets) prevent malaria in Gambian children. Trans R Soc Trop Med Hyg. 1988;82(6):838–842. doi: 10.1016/0035-9203(88)90011-9. [DOI] [PubMed] [Google Scholar]
- Snow R. W., Schellenberg J. R., Peshu N., Forster D., Newton C. R., Winstanley P. A., Mwangi I., Waruiru C., Warn P. A., Newbold C. Periodicity and space-time clustering of severe childhood malaria on the coast of Kenya. Trans R Soc Trop Med Hyg. 1993 Jul-Aug;87(4):386–390. doi: 10.1016/0035-9203(93)90007-d. [DOI] [PubMed] [Google Scholar]



