Abstract
We show here that matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) accurately and quickly identified the five high-risk clones of Pseudomonas aeruginosa sequence type 111 (ST111), ST175, ST235, ST253, and ST395. The use of this screening technique by clinical microbiology laboratories may tackle the spread of high-risk clones by the quick implementation of hygiene control procedures for relevant patients.
TEXT
Pseudomonas aeruginosa has a nonclonal population structure with a few multidrug-resistant clusters, called “high-risk clones,” that frequently produce acquired β-lactamases with an extended spectrum and are responsible for outbreaks in hospitals worldwide (1–13). The quick implementation of infection control measures for relevant patients may tackle the spread of these epidemic clones, but current identification is long and complex since it is based on the analysis of nucleotidic sequences. A quick and easy method for identifying high-risk clones of P. aeruginosa is then needed. Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) was recently integrated into the routine workflow of medical microbiology laboratories for microbial identification and has been further used for subspecies characterization (14). Here, we evaluated the ability of MALDI-TOF MS to identify high-risk clones of P. aeruginosa.
Identification of peak biomarkers for five major high-risk clones of P. aeruginosa.
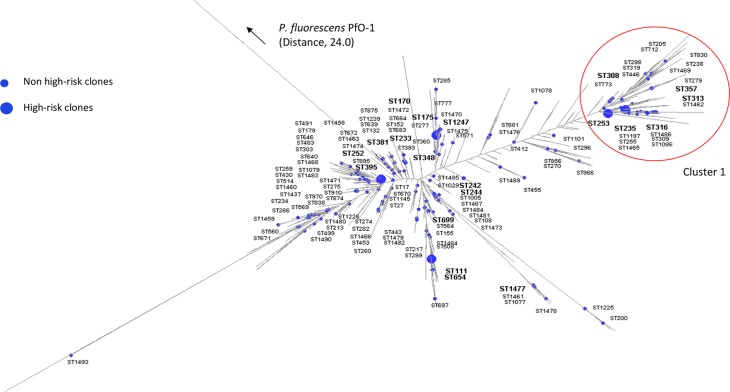
In order to identify five major high-risk clones of P. aeruginosa (sequence type 111 [ST111], ST175, ST235, ST253, and ST395), we first defined recognition models with a training set of 46 isolates with known STs distributed homogeneously in the phylogenetic tree (Table 1 and Fig. 1). Frozen bacteria were streaked onto Mueller-Hinton agar (Bio-Rad) and incubated for 18 h at 37°C. As previously reported (15), each isolate was extracted with the ethanol-formic acid method recommended by Bruker Daltonik and analyzed with a Microflex LT mass spectrometer (Bruker Daltonik), which generated 24 raw spectra. We analyzed the spectra with the software ClinProTools 3.0 (Bruker Daltonik), which defined six models based on peak biomarkers (Table 1). The reliability and accuracy of each model were assessed through the recognition capabilities and the cross-validation values. The models identified all the tested high-risk clones of P. aeruginosa with high recognition capabilities (>96%) and cross-validation values ranging from 72.5% to 100% (Table 1). Four models directly identified ST111, ST175, ST253, and ST395, while the identification of ST235 required a preliminary stage to identify cluster 1, which encompasses ST235 (Fig. 1).
TABLE 1.
Characteristics and performances of the models for the detection of P. aeruginosa high-risk clones
| Model | Training set |
Validation seta |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (no.) | Peak biomarkers (m/z) | Recognition capability (%) | Cross-validation (%) | Total (no.) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
| Cluster 1 | 6,734 | 100 | 99.86 | 92.5 | 95.9 | 87.3 | 97.6 | ||
| Cluster 1 | 11 | 9,586 | 67 | ||||||
| Other STs | 35 | 9,617 | 219 | ||||||
| 11,037 | |||||||||
| ST235b | 5,813 | 96.75 | 94.08 | 96.3 | 82.5 | 78.8 | 97.0 | ||
| ST235 | 5 | 11,611 | 27 | ||||||
| Other STs | 6 | 40 | |||||||
| ST111 | 3,657 | 100 | 99.94 | 81.8 | 98.9 | 81.8 | 99.2 | ||
| ST111 | 5 | 6,508 | 11 | ||||||
| Other STs | 41 | 8,502 | 275 | ||||||
| 10,041 | |||||||||
| 10,538 | |||||||||
| 12,579 | |||||||||
| ST175 | 5,211 | 96.86 | 84.26 | 100 | 92.6 | 41.2 | 100 | ||
| ST175 | 3 | 5,738 | 14 | ||||||
| Other STs | 43 | 7,203 | 272 | ||||||
| 7,329 | |||||||||
| 7,359 | |||||||||
| 7,580 | |||||||||
| 7,613 | |||||||||
| 12,154 | |||||||||
| ST253 | 5,813 | 99.09 | 72.5 | 100 | 97.5 | 58.8 | 100 | ||
| ST253 | 2 | 10 | |||||||
| Other STs | 44 | 276 | |||||||
| ST395 | 7,718 | 100 | 100 | 100 | 99.3 | 89.5 | 100 | ||
| ST395 | 8 | 8,550 | 17 | ||||||
| Other STs | 38 | 11,582 | 269 | ||||||
| 16,799 | |||||||||
PPV, positive predictive value; NPV, negative predictive value.
Within the isolates belonging to cluster 1 (see Fig. 1).
FIG 1.
Distribution of the 157 STs of the isolates of Pseudomonas aeruginosa used for the typing strategy on a dendrogram built with the data of all known STs (n = 1,595). STs belonging to the training set are indicated by bold type. Isolates were genotyped by multilocus sequence typing as previous described (4). The maximum-likelihood tree was constructed with RAxML 7.2.8 and visualized with Dendroscope 3.2.10 (13). In every case, 1,000 bootstrap repetitions gave values higher than 900 for most branches. Pseudomonas fluorescens PfO-1 was used as the outgroup.
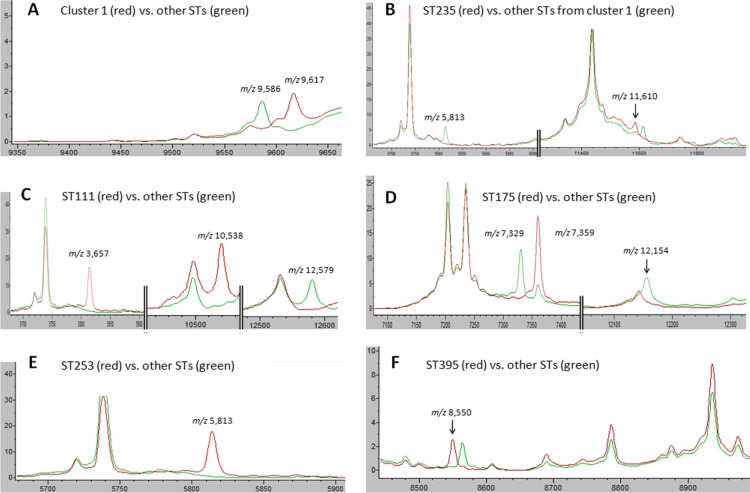
We then manually examined the spectra with ClinProTools 3.0 to assess the relevance of the peak biomarkers of each model (Table 1). Peaks were defined as biomarkers in a model because of their presence or absence or relative abundance between two tested classes. Figure 2 details only the peak biomarkers in which presence or absence was specific for a class.
FIG 2.
Peak biomarkers allowing MALDI-TOF MS to identify ST111, ST175, ST235, ST253, and ST395. The mass spectra were visualized with ClinProTools 3.0 (Bruker Daltonik). Units on the x axis represent the mass per charge (m/z), and those of the y axes represent the relative intensity in arbitrary units. (A) Peak at m/z 9,617 was specific to the STs of cluster 1, while that at m/z 9,586 was specific to the other STs. (B) Within cluster 1, isolates of ST235 specifically displayed a peak at m/z 11,610 and lacked a peak at m/z 5,813. (C) Isolates of ST111 were characterized by two specific peaks at m/z 3,657 and m/z 10,538 and the absence of a peak at m/z 12,579. (D) Absence of peaks at m/z 7,329 and m/z 12,154 combined with a peak at m/z 7,359 with a high intensity were specific to the ST175 isolates. (E) Isolates of ST253 lacked a specific peak at m/z 5,813. (F) Peak at m/z 8,550 was specific to isolates of ST395.
We further assessed the performances of the six models with an independent validation set of 295 isolates homogeneously distributed in the phylogenetic tree (Table 1 and Fig. 1). Isolates were prepared with the direct-transfer method, which is routinely used in clinical laboratories (15). One raw spectrum was collected per isolate and classified with the six models. We identified the high-risk clone ST111 with a sensitivity of 81.8% and ST175, ST235, ST253, and ST395 with higher sensitivities (≥96.3%). The specificities of our typing method were high (≥96.6%) for ST111, ST175, ST253, and ST395 and lower for ST235 (82.5%). The positive predictive values ranged from 41.2% to 89.5%, and the negative predictive values ranged from 97.0% to 100% (Table 1).
Strengths and limitations of the method.
MALDI-TOF MS distinguished the five tested intercontinental high-risk clones, ST111, ST175, ST235, ST253, and ST395. We deliberately included as many different STs (n = 157) of P. aeruginosa as possible from various hosts (humans [n = 128]; animals [n = 66]) to take into account the genetic diversity of the species (Fig. 1). Although other high-risk clones (e.g., ST277, ST357) are good candidates for identification by MALDI-TOF MS, the low number of isolates of these STs in our collection made it impossible to calculate and validate dedicated models. Nonetheless, given that MALDI-TOF MS successfully identified all the tested high-risk clones, this technique can presumably detect other clones. Interestingly, the few isolates that were misclassified by the models were scattered across the phylogenetic tree of P. aeruginosa (data not shown). The MALDI-TOF MS method had satisfactory positive predictive values for the routine detection of ST111, ST235, and ST395. Although MALDI-TOF MS detected ST175 and ST253 with low positive predictive values (<60%), the two corresponding models had a 100% negative predictive value, thereby excluding these high-risk clones with certitude.
Clinical laboratories use various culture media provided by various manufacturers. We tested the robustness of the method with a subset of representative isolates (n = 51) grown on various culture media. Briefly, we found that MALDI-TOF MS identified all tested high-risk clones grown on sheep blood agar (blood from Thermo Fisher Scientific, Columbia agar base from Mast) and that ST235, ST253, and ST395 were accurately identified from other manufacturers' Mueller-Hinton agar (bioMérieux) and MacConkey medium (Mast).
One limitation in the broad use of this typing strategy is that the ClinProTools 3.0 software has to be purchased separately from the MALDI-TOF MS apparatus. Equipped laboratories can freely download the method-containing files from our website (http://projet.chu-besancon.fr/rfclin/ClinProTools/) to skip the time-consuming steps of strategy design. However, each laboratory must validate the technique with its own instruments and culture media.
In conclusion, although multilocus sequence typing (MLST) remains the gold standard for the analysis of the genetic population of P. aeruginosa, the delay required for this sequence-based ST determination is not compatible with efficient management in an outbreak. Hence, the quick identification of high-risk clones that are more prone to disseminate would facilitate the management of a P. aeruginosa outbreak. We showed here that MALDI-TOF MS can detect high-risk clones of P. aeruginosa accurately, quickly (<1 min), and inexpensively. MALDI-TOF MS can type the isolates from the very spot used for species identification and prepared by a direct-transfer method. This may help limit the spread of high-risk clones by the quick implementation of hygiene control procedures for relevant patients.
ACKNOWLEDGMENTS
The Biological Resource Center Ferdinand Cabanne (BB-0033-00044) is partially supported by European Grant “Fonds européen de développement régional” (FEDER 34534). The funders had no role in study design, data collection, or analysis, in the decision to publish, or in the preparation of the manuscript.
We declare no competing interests.
REFERENCES
- 1.Maatallah M, Cheriaa J, Backhrouf A, Iversen A, Grundmann H, Do T, Lanotte P, Mastouri M, Elghmati MS, Rojo F, Mejdi S, Giske CG. 2011. Population structure of Pseudomonas aeruginosa from five Mediterranean countries: evidence for frequent recombination and epidemic occurrence of CC235. PLoS One 6:e25617. doi: 10.1371/journal.pone.0025617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edelstein MV, Skleenova EN, Shevchenko OV, D'Souza JW, Tapalski DV, Azizov IS, Sukhorukova MV, Pavlukov RA, Kozlov RS, Toleman MA, Walsh TR. 2013. Spread of extensively resistant VIM-2-positive ST235 Pseudomonas aeruginosa in Belarus, Kazakhstan, and Russia: a longitudinal epidemiological and clinical study. Lancet Infect Dis 13:867–876. doi: 10.1016/S1473-3099(13)70168-3. [DOI] [PubMed] [Google Scholar]
- 3.Castanheira M, Deshpande LM, Costello A, Davies TA, Jones RN. 2014. Epidemiology and carbapenem resistance mechanisms of carbapenem-non-susceptible Pseudomonas aeruginosa collected during 2009-11 in 14 European and Mediterranean countries. J Antimicrob Chemother 69:1804–1814. doi: 10.1093/jac/dku048. [DOI] [PubMed] [Google Scholar]
- 4.Cholley P, Thouverez M, Hocquet D, van der Mee-Marquet N, Talon D, Bertrand X. 2011. Most multidrug-resistant Pseudomonas aeruginosa isolates from hospitals in eastern France belong to a few clonal types. J Clin Microbiol 49:2578–2583. doi: 10.1128/JCM.00102-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Castillo M, Del Campo R, Morosini MI, Riera E, Cabot G, Willems R, van Mansfeld R, Oliver A, Canton R. 2011. Wide dispersion of ST175 clone despite high genetic diversity of carbapenem-nonsusceptible Pseudomonas aeruginosa clinical strains in 16 Spanish hospitals. J Clin Microbiol 49:2905–2910. doi: 10.1128/JCM.00753-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright LL, Turton JF, Livermore DM, Hopkins KL, Woodford N. 2015. Dominance of international “high-risk clones” among metallo-β-lactamase-producing Pseudomonas aeruginosa in the UK. J Antimicrob Chemother 70:103–110. doi: 10.1093/jac/dku339. [DOI] [PubMed] [Google Scholar]
- 7.Seok Y, Bae IK, Jeong SH, Kim SH, Lee H, Lee K. 2011. Dissemination of IMP-6 metallo-β-lactamase-producing Pseudomonas aeruginosa sequence type 235 in Korea. J Antimicrob Chemother 66:2791–2796. doi: 10.1093/jac/dkr381. [DOI] [PubMed] [Google Scholar]
- 8.Kim MJ, Bae IK, Jeong SH, Kim SH, Song JH, Choi JY, Yoon SS, Thamlikitkul V, Hsueh PR, Yasin RM, Lalitha MK, Lee K. 2013. Dissemination of metallo-β-lactamase-producing Pseudomonas aeruginosa of sequence type 235 in Asian countries. J Antimicrob Chemother 68:2820–2824. doi: 10.1093/jac/dkt269. [DOI] [PubMed] [Google Scholar]
- 9.Van der Bij AK, Van der Zwan D, Peirano G, Severin JA, Pitout JD, Van Westreenen M, Goessens WH, MBL-PA Surveillance Study Group . 2012. Metallo-β-lactamase-producing Pseudomonas aeruginosa in the Netherlands: the nationwide emergence of a single sequence type. Clin Microbiol Infect 18:E369–E372. doi: 10.1111/j.1469-0691.2012.03969.x. [DOI] [PubMed] [Google Scholar]
- 10.Elias J, Schoen C, Heinze G, Valenza G, Gerharz E, Gerharz H, Vogel U. 2010. Nosocomial outbreak of VIM-2 metallo-β-lactamase-producing Pseudomonas aeruginosa associated with retrograde urography. Clin Microbiol Infect 16:1494–1500. doi: 10.1111/j.1469-0691.2010.03146.x. [DOI] [PubMed] [Google Scholar]
- 11.Gomila M, Del Carmen Gallegos M, Fernandez-Baca V, Pareja A, Pascual M, Diaz-Antolin P, Garcia-Valdes E, Lalucat J. 2013. Genetic diversity of clinical Pseudomonas aeruginosa isolates in a public hospital in Spain. BMC Microbiol 13:138. doi: 10.1186/1471-2180-13-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koutsogiannou M, Drougka E, Liakopoulos A, Jelastopulu E, Petinaki E, Anastassiou ED, Spiliopoulou I, Christofidou M. 2013. Spread of multidrug-resistant Pseudomonas aeruginosa clones in a university hospital. J Clin Microbiol 51:665–668. doi: 10.1128/JCM.03071-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cholley P, Ka R, Guyeux C, Thouverez M, Guessennd N, Ghebremedhin B, Frank T, Bertrand X, Hocquet D. 2014. Population structure of clinical Pseudomonas aeruginosa from west and central African countries. PLoS One 9:e107008. doi: 10.1371/journal.pone.0107008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spinali S, van Belkum A, Goering RV, Girard V, Welker M, Van Nuenen M, Pincus DH, Arsac M, Durand G. 23 July 2014. Microbial typing by MALDI-TOF MS: do we need guidance for data interpretation? J Clin Microbiol. doi: 10.1128/JCM.01635-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauget M, Nicolas-Chanoine MH, Cabrolier N, Bertrand X, Hocquet D. 2014. Matrix-assisted laser desorption ionization–time of flight mass spectrometry assigns Escherichia coli to the phylogroups A, B1, B2 and D. Int J Med Microbiol 304:977–983. doi: 10.1016/j.ijmm.2014.06.004. [DOI] [PubMed] [Google Scholar]