Abstract
Disease severity in patients with pulmonary tuberculosis is associated with mycobacterial sputum load. To ascertain whether reduced sputum production during treatment is a useful clinical sign of improvement, we analyzed the mycobacterial loads of 5,552 sputum samples collected from 439 newly diagnosed sputum smear-positive tuberculosis patients who participated in six 14-day studies of antituberculosis treatment. Sputum volumes were categorized as low (<6 ml), medium (6 to 10 ml), or large (>10 ml), and mycobacterial load was measured by the time to positivity in liquid culture and the CFU counts on solid culture. The association of sputum volume with mycobacterial load was estimated with multiple linear regression models adjusted for repeated measures. The predictor variables were sputum volume category, treatment day, specific study , and the interaction of sputum volume category and treatment day. Mycobacterial load was significantly associated only with the day on treatment and sputum volume, which tended to decrease with ongoing treatment. With the volume held constant, each day on treatment decreased the log CFU by 0.082 (P < 0.001) and increased the time to positivity (TTP) by 1.04 h (P < 0.001). From low to medium and from medium to large sputum volumes, the log CFU/ml increased by 0.265 (P < 0.003) and 0.490 (P < 0.001), respectively, and the TTP decreased by 1.17 h (P < 0.001) and 1.30 h (P < 0.001), respectively, for a given day of treatment. The variability of the sputum load measurements increased with the day of treatment and lower sputum volumes. The significant association of sputum volume and mycobacterial load validates decreasing sputum production as a clinical sign of improvement during early antituberculosis treatment.
INTRODUCTION
Tuberculosis (TB) remains a major cause of morbidity and mortality worldwide. In 2012, the World Health Organization (WHO) estimated that 8.5 million new TB cases and 1.3 million TB deaths occurred globally (1). In 2008, the incidence of TB in South Africa was 960 cases per 100,000 people, with an annual increase of 6.4% from 1998 to 2008 (2). TB is most commonly diagnosed by the identification of Mycobacterium tuberculosis in expectorated spot sputum specimens, either by sputum smear microscopy or by the identification of mycobacterial antigens with PCR (3). The viable sputum mycobacterial load can be measured by counting CFU on agar plates or by the time to positivity (TTP) in culture with liquid medium (4–6). Greater initial spot sputum mycobacterial burden has been linked to more extensive radiological lung involvement, worse treatment outcomes, and a higher risk of relapse (7–9), and more recently, greater sputum volume has been linked to worse treatment outcomes in HIV-infected TB patients (10).
The decrease in the sputum mycobacterial burden in pooled 16-h sputum samples collected overnight is an established measure of early antituberculosis drug effects over the first 2 weeks of treatment (11), but few studies have assessed the association of sputum volumes with measurements of mycobacterial load. In a trial reported in 1950, before effective treatments became available, it was documented that hospitalized patients treated with a placebo had stable sputum volumes and percentages of positive smears over 14 weeks, whereas para-aminosalicylic acid-treated patients experienced reductions in both (12). Yoon, Lee, and Yim (13) found that purulent or blood-tinged sputum, as well as a larger volume of early morning and spot sputum samples predicted smear positivity.
TB patients frequently report a prompt reduction in productive coughs upon the initiation of treatment. This is commonly accepted as a clinical sign of improvement. The purpose of this study was to substantiate this association by investigating whether decreased sputum volume is associated with a reduced sputum mycobacterial load during early antituberculosis treatment.
MATERIALS AND METHODS
Study population and specimens.
We studied the sputum samples from 6 consecutive 14-day early bactericidal activity (EBA) studies conducted between 2008 and 2012. The locations, procedures, and relevant participation criteria were identical for all the studies. The subjects were recruited from outpatient clinics in Cape Town, South Africa, and were enrolled if they were aged 18 to 65 years, had ≥1+ smear-positive sputum on auramine microscopy (International Union Against Tuberculosis and Lung Disease [IUATLD]/WHO scale) (14), and were without major underlying medical conditions. Spontaneously expectorated sputum samples were collected over a period of 16 h overnight, refrigerated, and sent to a single laboratory in Cape Town under controlled conditions.
Laboratory methods.
During the entire study period, the laboratory supplied the clinical sites with identical, transparent, and wide-mouthed collection containers of 125 ml in volume and with a screw top (Scientific Group, Vorna Valley, South Africa). Upon submission to the laboratory, two technologists trained on sample reception were in charge of estimating the sputum volumes by comparing the containers to reference containers filled with standard volumes of water. Sputum volume was categorized arbitrarily as <6 ml, 6 to 10 ml, or >10 ml. Discrepancies were resolved by consensus between the two technologists. All studies employed the same standardized laboratory methodology for processing. The sputum samples were homogenized using magnetic stirring and the addition of 0.1% dithiothreitol (Sputasol; Oxoid, Cambridge, United Kingdom) for digestion. All samples were assessed for mycobacterial load by both CFU count and TTP.
For CFU quantification, 10-fold serial dilutions were inoculated onto two halves of two 7H11 agar biplates. The plates were then incubated at 37°C for a period of 3 weeks, and the CFU were counted using the dilution with counts between 20 and 200. After calculating an average of the duplicate CFU counts and correcting for the dilution factor, the results were reported as the CFU per ml of sputum.
For TTP determination, digested sputum was decontaminated for 15 min at room temperature by using sodium hydroxide at a final concentration of 1% (MycoPrep; Becton Dickinson, Sparks, MD). The specimen was then neutralized using phosphate-buffered saline (pH 6.8) (Becton Dickinson) and concentrated through centrifugation (15 min at 3,000 × g and 4°C). The supernatant was then decanted and the pellet resuspended to a volume of 2 ml using phosphate-buffered saline. The resuspended pellet was then used to inoculate duplicate mycobacterial growth indicator tubes (MGITs) (Becton Dickinson) that were enriched with oleic acid-albumin-dextrose-catalase [OADC]; Becton Dickinson) and polymyxin B, amphotericin B, nalidixic acid, trimethoprim, and azlocillin (PANTA) (Becton Dickinson). The MGITs were then incubated at 37°C in the Bactec MGIT 960 instrument (Becton Dickinson), which monitors the cultures and automatically records the TTP once the culture is flagged as positive.
Data collection.
The data obtained included individual patient, specific study, sputum volume, treatment day, TTP, and CFU. Negative cultures were excluded for the CFU counts. For TTP determination, negative cultures were censored at 42 days (the maximum length of culture incubation), and this value was used for analysis.
Statistical methods.
The data from all six studies were combined for statistical analysis. Due to the similar inclusion and exclusion criteria, identical source population, and the single laboratory that processed all samples with identical methods, we considered the population not to be clinically heterogeneous. Because the studies were conducted in sequence and, generally, groups within a study were given variations of the same treatment, we adjusted for study but not for individual treatment groups. Neither CFU nor TTP were normally distributed and were both log10 transformed. The means, standard deviations, and coefficients of variation of the log TTP and log CFU were reported by volume category. Simple linear regression analysis was used to determine the effect of time on log TTP and log CFU within the different volume categories.
Two multiple linear regression models were used to estimate the association between sputum volume and the log TTP and log CFU. In order to take into account the correlation of observations within a single participant (cluster), we adjusted the models for the effect of repeated measures per patient by using robust standard errors based on patient number as the cluster variable. The predictor variables included in the initial models were the same for both log TTP and log CFU. They included volume, time on treatment (in days), study code (A to F), and the interaction between volume and time. The final adjusted models were obtained using a backward stepwise approach, and variables with a P value of <0.1 were retained. All statistical analyses were performed using the Stata software (version 12).
Ethical approval.
The protocol for this study was reviewed and approved by the ethics committee of the University of Stellenbosch, Stellenbosch, South Africa (reference S12/11/310). The study was carried out between June and December 2013.
RESULTS
Studies, participants, and specimens.
In 6 EBA studies (59 to 90 participants per study) and 30 treatment groups (8 to 25 participants per group), the 439 participants produced a total of 5,552 sputum samples (4 to 16 per participant, and 470 to 1,421 per study). Table 1 shows the distribution, means, and standard deviations of 5,067 valid CFU and 5,344 valid TTP measurements. We excluded 8.7% and 3.7% of the CFU and TTP results, respectively, for contamination (4.6% and 1.7%, respectively) and negative or missing results (4.1% and 2.0%, respectively).
TABLE 1.
Specimens and mycobacterial load measurements
| Study | No. of patients | No. of samples | Mean no. (SD) of samples per patient | log CFU/ml sputum |
log TTPa |
||||
|---|---|---|---|---|---|---|---|---|---|
| Valid no. (%) of specimens | Mean | SD | Valid no. (%) of specimens | Mean | SD | ||||
| A | 68 | 745 | 11 (0.3) | 646 (86.7) | 4.5 | 1.8 | 716 (96.1) | 2.2 | 0.2 |
| B | 85 | 1,310 | 15 (2.2) | 1,209 (92.3) | 5.1 | 1.5 | 1,283 (97.9) | 2.2 | 0.2 |
| C | 69 | 752 | 11 (0.8) | 714 (94.9) | 5.3 | 1.3 | 735 (97.7) | 2.1 | 0.1 |
| D | 59 | 470 | 8 (0.2) | 426 (90.6) | 5.1 | 1.4 | 456 (97.0) | 2.2 | 0.2 |
| E | 68 | 854 | 12 (1.7) | 825 (96.6) | 5.8 | 1.1 | 771 (90.3) | 2.1 | 0.2 |
| F | 90 | 1,421 | 15 (1.5) | 1,247 (87.8) | 5.3 | 1.5 | 1,383 (97.3) | 2.1 | 0.2 |
| Total | 439 | 5,552 | 13 (3.1) | 5,067 (91.3) | 5.2 | 1.5 | 5,344 (96.3) | 2.2 | 0.2 |
TTP, time to culture positivity.
Sputum volumes.
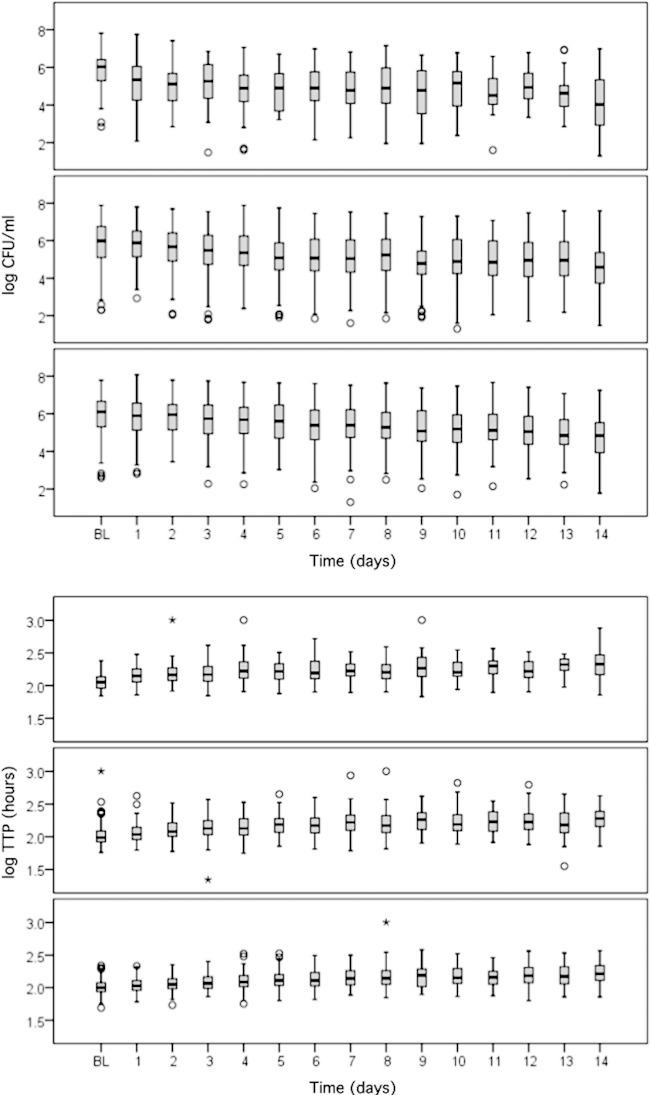
The specimen volume categories, with the distribution over time, mean values, standard deviations, and coefficients of variation are shown in Table 2. The sputum volumes tended to decrease over time, and the variabilities of both measurements increased as the sample volumes decreased. In all volume categories, considerably less variability was observed with TTP, which had a 3- to 4-fold smaller coefficient of variation than that for CFU. A statistically significant decrease in log CFU and an increase in log TTP (Fig. 1A and B) were observed in all volume categories over time (P < 0.001).
TABLE 2.
Sputum volume and mycobacterial load measurements
| Vol (ml) | No. (%) of samples ata: |
Log CFU/ml sputum |
Log TTP (h)b |
TTP (h) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Day 0 | Day 6 | Day 14 | Mean | SD | CV | Mean | SD | CV | Median | IQRc | |
| <6 | 655 (12.2) | 75 (8.6) | 56 (13.0) | 66 (15.7) | 4.7 | 1.7 | 0.359 | 2.2 | 0.2 | 0.094 | 166 | 127–232 |
| 6–10 | 2,325 (43.3) | 363 (41.8) | 196 (45.5) | 190 (45.1) | 5.1 | 1.5 | 0.301 | 2.2 | 0.2 | 0.089 | 140 | 105–197 |
| >10 | 2,392 (44.5) | 431 (49.6) | 179 (41.5) | 165 (39.2) | 5.4 | 1.4 | 0.253 | 2.1 | 0.2 | 0.077 | 123 | 100–161 |
| Total | 5,372 (100) | 869 (100) | 431 (100) | 421 (100) | 5.0 | 1.5 | 0.301 | 2.2 | 0.2 | 0.080 | 134 | 104–186 |
Days 0 (baseline), 6, and 14 are given as examples.
TTP, time to culture positivity; CV, coefficient of variation.
IQR, interquartile range.
FIG 1.
Box plots of log CFU (top) and log TTP (bottom) for treatment days 0 to 14. The volume categories are <6 ml (top), 6 to 10 ml (middle), and >10 ml (bottom). In each volume category, there was a statistically significant decrease in log CFU and increase in log TTP over time (both P < 0.001). BL, baseline.
Statistical modeling.
Among all the outcomes tested, only volume and days on treatment showed significant associations with log CFU and log TTP (Tables 3 and 4). All outcomes were highly significant. The mean log CFU decreased, on average, by 0.082 log CFU for each day on treatment, with volume being held constant (P < 0.001). From <6 ml to between 6 and 10 ml, and 6 to 10 ml to >10 ml of sputum volume, the log CFU increased by 0.265 and 0.490, respectively. Correspondingly, the non-log-transformed TTP increased by 1.04 h per day on treatment, with the volume held constant (P < 0.001). As the specimen volumes increased from <6 ml to between 6 and 10 ml, and from 6 to 10 ml to >10 ml, the TTP decreased by 1.172 h and 1.297 h, respectively.
TABLE 3.
Linear regression model for log CFU, adjusting for clustering of repeated measures in a single participant using robust standard errorsa
| Log CFU | Coefficient | SE | P | 95% CIb |
|---|---|---|---|---|
| Constantc | 5.560 | 0.099 | <0.001 | 5.366 to 5.754 |
| Day on treatment | −0.082d | 0.005 | <0.001 | −0.091 to −0.072 |
| Vol (ml)e | ||||
| 6–10 | 0.265 | 0.090 | 0.003 | 0.088 to 0.442 |
| >10 | 0.490 | 0.108 | <0.001 | 0.278 to 0.701 |
n = 5,067; number of clusters, 435; F (3.434) = 113.38; P < 0.001; R2 = 0.1235; root mean squared error (MSE), 1.1028.
95% CI, 95% confidence interval.
The constant represents the expected log CFU in the smallest volume category (<6 ml) when the time on treatment is zero.
Each day of increase in treatment duration decreases the log CFU by 0.082.
For the expected log CFU when the sputum volume changes from <6 ml to a higher volume category, add the corresponding volume coefficient.
TABLE 4.
Linear regression model for log TTP, adjusting for clustering of repeated measures in a single participant, using robust standard errorsa
| Log TTP | Coefficient | SE | P | 95% CIb |
|---|---|---|---|---|
| Constantc | 2.120 | 0.016 | <0.001 | 2.089 to 2.151 |
| Day on treatment | 0.017d | 0.001 | <0.001 | 0.015 to 0.018 |
| Vol (ml)e | ||||
| 6–10 | −0.069 | 0.015 | <0.001 | −0.098 to −0.040 |
| >10 | −0.113 | 0.017 | <0.001 | −0.148 to −0.079 |
n = 5,372; number of clusters, 439; F (3.438) = 191.52; P < 0.001; R2 = 0.2174; root mean squared error (MSE), 0.1655. TTP, time to culture positivity.
95% CI, 95% confidence interval.
The constant represents the expected log TTP in the smallest volume category (<6 ml) when the time on treatment is zero.
Each day of increase in treatment duration increases the log TTP by 0.017.
For the expected log TPP when the sputum volume changes from <6 ml to a higher volume category, add the corresponding volume coefficient.
DISCUSSION
We found that the volume of sputum expectorated overnight by pulmonary TB patients was positively associated with the mycobacterial sputum load. The sputum volumes became smaller over the first 14 days of treatment and exhibited increased TTP and decreased CFU. This validates the clinical observation that patients reporting diminishing sputum production are responding to treatment, as evidenced by a decrease in the mycobacterial sputum load.
The decrease in mycobacterial load depicted in the prolongation of TTP and decreased CFU is an expected response to treatment, as is the reduction in productive cough. This study exemplifies a well-known dilemma in clinical trials of antituberculosis treatments. Sputum, as the substrate of the main endpoint measurement of trials, becomes smaller in volume over time, and the variation in its mycobacterial load measurements increases. This makes significant differences between treatments harder to detect with ongoing treatment duration, particularly when measured with CFU, for which we found a much larger variation than that for TTP. It has been observed that a longer daily collection period increases sputum volume and leads to an increase in the precision of CFU (15), and that EBAs obtained from pooled sputum specimens that usually have higher volumes (>10 ml) have a lower standard error than estimates obtained from spot sputum samples with volumes of <5 ml (5).
The strength of this study is the large number of aggregated observations from large EBA studies, which increases the precision due to the large sample size. The studies were combined on the basis that all participants were from the same source population and that each of the studies have comparable eligibility criteria. Furthermore, all samples were analyzed by the same laboratory using standardized protocols. However, the studies differed in their treatment groups. We were blinded as to which treatment arm received which type and dosage of treatment, but the diversity in treatments and the small sample size in each treatment arm might be limitations of this study. Also, only a minority of patients were on treatments as efficacious as that of the currently used combination therapy. Standard treatment (body weight-adjusted isoniazid, rifampin, pyrazinamide, and ethambutol) increases the TTP by approximately 13 h per day and decreases the CFU by 0.177 log CFU over the first 14 days of treatment (16). The overall mean change in mycobacterial load was only about half that in our analysis (46% for CFU and 52% for non-log-transformed TTP). Our data might thus still underestimate the drop in sputum volumes and the increases in load measurement variation that might be observed if fully effective treatments are studied.
In conclusion, reduced sputum volume predicts a decrease in mycobacterial sputum load in patients with pulmonary TB. Clinicians can interpret a reduced productive cough in the first 14 days of therapy as a valid clinical sign of effective treatment.
ACKNOWLEDGMENTS
We thank all patients for their participation in the study.
We declare no conflicts of interest.
REFERENCES
- 1.World Health Organization. 2013. Global tuberculosis report 2013. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf. [Google Scholar]
- 2.Claassens M, van Schalkwyk C, den Haan L, Floyd S, Dunbar R, van Helden P, Godfrey-Faussett P, Ayles H, Borgdorff M, Enarson D, Beyers N. 2013. High prevalence of tuberculosis and insufficient case detection in two communities in the Western Cape, South Africa. PLoS One 8:e58689. doi: 10.1371/journal.pone.0058689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson ML. 2013. Rapid diagnosis of Mycobacterium tuberculosis infection and drug susceptibility testing. Arch Pathol Lab Med 137:812–819. doi: 10.5858/arpa.2011-0578-RA. [DOI] [PubMed] [Google Scholar]
- 4.Bark CM, Gitta P, Ogwang S, Nsereko M, Thiel BA, Boom WH, Eisenach KD, Joloba ML, Johnson JL. 2013. Comparison of time to positive and colony counting in an early bactericidal activity study of anti-tuberculosis treatment. Int J Tuberc Lung Dis 17:1448–1451. doi: 10.5588/ijtld.13.0063. [DOI] [PubMed] [Google Scholar]
- 5.Donald PR, Diacon AH. 2008. The early bactericidal activity of anti-tuberculosis drugs: a literature review. Tuberculosis (Edinb) 88(Suppl 1):S75–S83. doi: 10.1016/S1472-9792(08)70038-6. [DOI] [PubMed] [Google Scholar]
- 6.Diacon AH, Maritz JS, Venter A, van Helden PD, Dawson R, Donald PR. 2012. Time to liquid culture positivity can substitute for colony counting on agar plates in early bactericidal activity studies of antituberculosis agents. Clin Microbiol Infect 18:711–717. doi: 10.1111/j.1469-0691.2011.03626.x. [DOI] [PubMed] [Google Scholar]
- 7.Hesseling AC, Walzl G, Enarson DA, Carroll NM, Duncan K, Lukey PT, Lombard C, Donald PR, Lawrence KA, Gie RP, van Helden PD, Beyers N. 2010. Baseline sputum time to detection predicts month two culture conversion and relapse in non-HIV-infected patients. Int J Tuberc Lung Dis 14:560–570. [PubMed] [Google Scholar]
- 8.Weiner M, Prihoda TJ, Burman W, Johnson JL, Goldberg S, Padayatchi N, Duran P, Engle M, Muzanye G, Mugerwa RD, Sturm AW. 2010. Evaluation of time to detection of Mycobacterium tuberculosis in broth culture as a determinant for end points in treatment trials. J Clin Microbiol 48:4370–4376. doi: 10.1128/JCM.00757-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epstein MD, Schluger NW, Davidow AL, Bonk S, Rom WN, Hanna B. 1998. Time to detection of Mycobacterium tuberculosis in sputum culture correlates with outcome in patients receiving treatment for pulmonary tuberculosis. Chest 113:379–386. doi: 10.1378/chest.113.2.379. [DOI] [PubMed] [Google Scholar]
- 10.Pierre C, Jones-Lopez E, Cabral H, Horsburgh CR. 2013. Sputum volume production predicts clinical outcome in HIV-infected tuberculosis patients in Kampala, Uganda; abstr 1130 IDWeek 2013, 2 to 6 October 2013, San Francisco, CA. [Google Scholar]
- 11.Diacon A, Maritz JS, Donald P. 2011. Early bactericidal activity of antituberculosis agents, p 213–219. In Donald PR, Van Helden PD (ed), Antituberculosis chemotherapy, vol 40 Karger, Basel, Switzerland. [Google Scholar]
- 12.The Therapeutic Trials Committee of the Swedish National Association against Tuberculosis. 1950. Para-aminosalicylic acid treatment in pulmonary tuberculosis. Am Rev Tuberc 61:597–612. [PubMed] [Google Scholar]
- 13.Yoon SH, Lee NK, Yim JJ. 2012. Impact of sputum gross appearance and volume on smear positivity of pulmonary tuberculosis: a prospective cohort study. BMC Infect Dis 12:172. doi: 10.1186/1471-2334-12-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Union Against Tuberculosis and Lung Disease. 2013. Laboratory diagnosis of tuberculosis by sputum microscopy: the handbook. International Union Against Tuberculosis and Lung Disease, Paris, France: http://www.theunion.org/what-we-do/publications/technical/english/The-Handbook_lo-res_130930.pdf. [Google Scholar]
- 15.Hafner R, Cohn JA, Wright DJ, Dunlap NE, Egorin MJ, Enama ME, Muth K, Peloquin CA, Mor N, Heifets LB. 1997. Early bactericidal activity of isoniazid in pulmonary tuberculosis. Optimization of methodology. The DATRI 008 Study Group. Am J Respir Crit Care Med 156:918–923. doi: 10.1164/ajrccm.156.3.9612016. [DOI] [PubMed] [Google Scholar]
- 16.Diacon AH, Dawson R, du Bois J, Narunsky K, Venter A, Donald PR, van Niekerk C, Erondu N, Ginsberg AM, Becker P, Spigelman MK. 2012. Phase II dose-ranging trial of the early bactericidal activity of PA-824. Antimicrob Agents Chemother 56:3027–3031. doi: 10.1128/AAC.06125-11. [DOI] [PMC free article] [PubMed] [Google Scholar]