Abstract
Streptococcus gallolyticus subsp. pasteurianus, previously known as Streptococcus bovis biotype II.2, is an uncommon pathogen in neonates. Nevertheless, it can cause severe neonatal sepsis and meningitis often clinically indistinguishable from those caused by group B streptococci and has been associated with considerable morbidity. We report the first known cases of S. gallolyticus subsp. pasteurianus infection in twin infants.
CASE REPORTS
Twin B, a 3-week-old diamniotic, dichorionic twin male at Texas Children's Hospital Pavilion for Women, had a 1-day history of pale color, lethargy, poor feeding, and increased irritability. He was born at 32 weeks of gestation, weighing 2,120 g, to a 26-year-old primigravida mother via Cesarean section because of severe preeclampsia. The mother had an unknown group B streptococcal (GBS) status and did not receive antibiotic prophylaxis during delivery. Immediately following his birth, the patient developed respiratory distress requiring intubation and surfactant. He was transported to the neonatal intensive care unit (NICU) for further management and continued to improve in the NICU until day of life (DOL) 24, when he was found to have pale color, loose stool, respiratory distress, and hypoxemia. Within the next few hours, he also experienced mild hypothermia. A full sepsis workup was initiated, and empirical parenteral vancomycin (15 mg/kg of body weight every 12 h), gentamicin (4 mg/kg every 24 h), and ceftazidime (40 mg/kg every 8 h) were initiated. Approximately 6 h after the onset of his symptoms, the child developed acute respiratory failure, requiring intubation, and refractory hypotension, requiring dopamine infusion. Two hours later, he developed new-onset seizures that progressed to epilepticus status. The chest radiograph revealed coarse markings within the lungs, with superimposed mild volume loss in the right upper lobe.
Initial laboratory studies are presented in Table 1. The cerebrospinal fluid (CSF) white blood cell (WBC) differential was not available. Degenerated inflammatory cells, including monocytes and neutrophils, were reported. The CSF Gram stain revealed Gram-positive cocci in clusters, with many WBCs. (The initial Gram stain from the original specimen was reported as “in clusters,” likely due to the appearance of very small clusters [1 to 3 cocci] along with pairs.) However, when the Gram staining was performed from colonies on solid media, Gram-positive cocci in pairs were found, consistent with what occurs with Streptococcus species.
TABLE 1.
Blood and cerebrospinal fluid results
| Parameter (reference range) | Twin B on: |
Twin A on DOL 24 | |
|---|---|---|---|
| DOL 24 | DOL 30 | ||
| White blood cell count (6 × 103–19 × 103/μl) | 1,310 | 21,030 | |
| % neutrophils (32–67) | 9.5 | 33.9 | |
| % in bands (0–8) | 7.6 | 17.8 | |
| % lymphocytes (25–37) | 60 | 39.8 | |
| % monocytes (0–9) | 1.9 | 5.1 | |
| Hemoglobin concn (13–22 g/dl) | 10.3 | 11.7 | |
| Platelet count (150 ×103–450 ×103/μl) | 312 | 414 | |
| C-reactive protein level (<1 mg/dl) | 3.9 | 11.6 | |
| CSF WBC counta (0–25/μl) | 61 | 3,693 | 8 |
| CSF red blood cell count (0–2/μl) | 84 | 30,250 | 1,445 |
| CSF glucose concn (24–63 mg/dl) | <20 | <20 | 27 |
| CSF protein concn (<150 mg/dl) | 404 | 846 | 115 |
| CSF Gram stain result | Many Gram+ cocci in pairs | Rare Gram+ cocci in pairs | No organisms seen |
| CSF culture result | Streptococcus gallolyticus subsp. pasteurianus | Negative | Negative |
| Blood culture resultb | Streptococcus gallolyticus subsp. pasteurianus | Streptococcus gallolyticus subsp. pasteurianus | |
A CSF WBC differential was not available.
Repeat blood cultures on DOL 25 and 26 were negative for both patients.
The peripheral, aerobic blood culture was positive for Gram-positive cocci in pairs and chains at 8 h in the VersaTREK microbial detection system (TREK Diagnostic Systems, Cleveland, OH). The initial CSF culture for twin B revealed abundant gamma-hemolytic streptococci, not enterococci, identified as Streptococcus gallolyticus subsp. pasteurianus with 99% probability and excellent identification (confidence level) by the GP identification card on the Vitek 2 platform (software version 4.01; bioMérieux, USA). The urine culture was negative. Parenteral ampicillin (75 mg/kg every 6 h) was initiated, and all other antibiotics were discontinued.
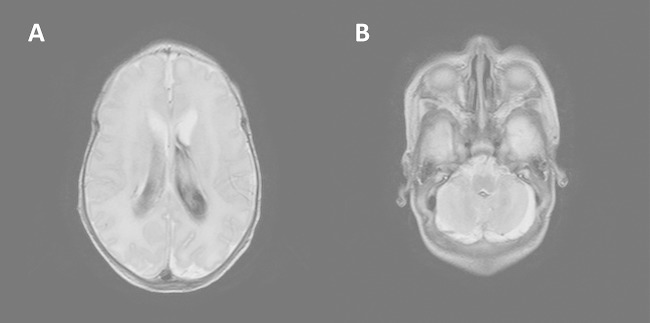
Over the next few days, twin B's clinical course continued to deteriorate. He experienced continuous seizures accompanied by myoclonus, with abnormal electroencephalographic studies. He was given multiple blood products for worsening anemia and thrombocytopenia and electrolyte supplementation for moderate hyponatremia and hyperkalemia. A head ultrasound obtained on the first day of illness showed normal anatomy; however, a magnetic resonance image of the brain with and without contrast taken 4 days after onset demonstrated moderate lateral, third- and fourth-ventricular hemorrhage with secondary mild dilatation of the lateral and third ventricles and without evidence of acute infarction, parenchymal abscess, or empyema (Fig. 1). Repeat head ultrasound 2 days later showed grade 3 intraventricular hemorrhage, which then remained stable, and the infant gradually improved without further complications for the rest of his admission. Repeat blood cultures after 24 h and 48 h of antibiotic treatment were negative. A repeat CSF culture after 6 days of antibiotic treatment showed Gram-positive cocci by Gram staining, but the culture was negative. Twin B was treated with ampicillin for a total of 14 days from CSF clearance.
FIG 1.
Magnetic resonance imaging of the brain, with contrast. (A) Moderate lateral ventricular hemorrhage. Lateral ventricles are mildly enlarged. (B) Hemorrhage in the cisterna magna inferior to the cerebellar vermis.
Twin A, the 3-week-old sister of twin B, was born weighing 1,474 g and exhibited similar symptoms of respiratory distress immediately after delivery. Resuscitation included positive-pressure ventilation and continuous positive airway pressure, but no intubation or surfactant was required. On DOL 24, she experienced intermittent tachypnea and underwent a full sepsis workup due to the severe infection in twin B. The results of subsequent laboratory studies are presented in Table 1. Initial CSF studies showed colorless, hazy cerebrospinal fluid, and the CSF Gram stain revealed no organisms and was culture negative. The blood culture was positive for Gram-positive cocci in pairs after 11 h. Twin A remained hemodynamically stable despite positive blood cultures and was treated with intravenous ampicillin (75 mg/kg every 6 h). She completed a 10-day course and was discharged upon completion of therapy.
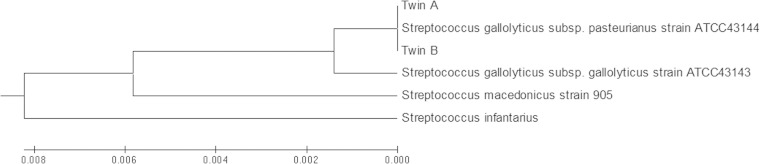
Further analysis of the organisms growing in the blood culture from twin A and blood and CSF cultures from twin B were identified as Streptococcus gallolyticus using pyrosequencing of three hypervariable regions (V1, V3, and V6) of the 16S rRNA gene (1). Briefly, extracted bacterial DNA (MagNA Pure liquid chromatography [LC] total nucleic acid isolation kit; Roche Applied Science, IN) was used as the template in PCRs with universal primers targeting the conserved regions directly flanking the variable regions. The resulting sequence was searched against the Ribosomal Database Project (RDP) maintained by Michigan State University and the SmartGene Integrated Database Networking System (IDNS) (SmartGene, Inc., Raleigh, NC) (2, 3). The 16S rRNA gene was amplified by PCR using primers 8F and 1541R (1), and the products were sequenced at SeqWright Inc. (Houston, TX). The sequences were compared to the corresponding region of published 16S sequences of related species and subspecies, deposited in GenBank, using MEGA 5.1 (4). The neighbor-joining tree (Fig. 2) shows an alignment of the twin isolates with S. gallolyticus subsp. pasteurianus, and a 100% sequence match was observed by nucleotide BLAST analysis alignment with ATCC 43144 (5–8; http://blast.ncbi.nlm.nih.gov/).
FIG 2.
Neighbor-joining tree of the 16S rRNA partial gene sequences (1,433 bp) of the clinical isolates from twins A and B in comparison to corresponding sequences of strains representing related species/subspecies available in GenBank. The neighbor-joining tree was constructed using MEGA5.1. The following comparator strains were downloaded from GenBank: Streptococcus gallolyticus subsp. pasteurianus ATCC 43144, NCBI reference sequence NR_102811.1 (7); Streptococcus gallolyticus subsp. gallolyticus ATCC 43143, GenBank accession number AF104114.1 (6); Streptococcus infantarius, NCBI reference sequence NR_028761.1 (8); and Streptococcus macedonicus, GenBank accession number EU163501.1 (5).
Analysis of the twins' isolates by pulsed-field gel electrophoresis using previously described methods and digestion with SmaI revealed that the twins' isolates were identical, suggesting that the twins were infected by the same strain (9, 10; data not shown). Antimicrobial susceptibility testing by Etest using Clinical and Laboratory Standards Institute (CLSI) guidelines (29) demonstrated susceptibility to ampicillin (MIC, 0.125 μg/ml), penicillin (MIC, 0.125 μg/ml), cefotaxime (MIC, 0.25 μg/ml), and vancomycin (MIC, 0.5 to 1 μg/ml) for all the isolates.
Streptococcus gallolyticus subsp. pasteurianus is a highly unusual pathogen in neonates. Only a few case reports exist. This is the first report of infection by this organism in neonatal twins. The pathogen has been associated with considerable morbidity and, in some cases, mortality over the last 4 decades. The strain was previously recognized as Streptococcus bovis biotype II/2, but its taxonomy has been revised. Originating as one of the six DNA groups of the S. bovis/S. equinus complex (11), it was later subdivided into biotype II based on its inability to ferment mannitol (11) and then into biotype II/2 based on phenotypic testing and sequencing of its sodA gene (12). In the last 10 years, biochemical tests have further differentiated biotypes I and II into distinct subspecies, and new species nomenclature has replaced the old biotype classification (11). The three species formerly known as S. bovis biotype II.2, S. waius, and S. macedonicus were grouped into one DNA cluster but show limited DNA-DNA relatedness and sufficient divergence in their 16S rRNA genes sequences to be separable (5, 12, 13). Reclassified as S. gallolyticus subsp. pasteurianus, S. gallolyticus subsp. gallolyticus, and S. gallolyticus subsp. macedonicus, the three subspecies differ biochemically (12, 14). S. gallolyticus subsp. pasteurianus can be differentiated from other subspecies based on beta-galactosidase (BGAL) and beta-glucuronidase (BGAR) tests, by which S. gallolyticus subsp. pasteurianus is positive while S. gallolyticus subsp. gallolyticus and S. gallolyticus subsp. macedonicus are negative. While the Vitek platform results are commonly reported only at the species level by clinical laboratories, a study of Gram-positive bacteria using the Vitek 2 GP identification card showed 100% accuracy in the identification of three Streptococcus gallolyticus subsp. pasteurianus isolates; none of the other 214 isolates of the Streptococcaceae family, including 4 subsp. gallolyticus isolates, were identified as this species (15). The Vitek biochemical panel includes the BGAL and BGAR biochemical tests. The present case reports show a 100% 16S rRNA alignment of the two neonatal isolates to the previously confirmed strain Streptococcus gallolyticus subsp. pasteurianus ATCC 43144 and positive BGAL and BGAR biochemical tests, confirming the identity of the isolated pathogens and further supporting the previous notion that distinct differences between subspecies can be reliably detected by Vitek and confirmed by 16S sequencing (5, 13, 15).
While S. bovis (biotype I), part of a normal colonic microbiota, has long been associated with colonic neoplasia and bacterial endocarditis in adults, the clinical significance of S. bovis biotype II, particularly within the neonatal population, has been less well established (16). Over the last 2 decades, however, a small, but growing, pool of literature has described invasive infections in neonates and infants. Most of these cases have been sporadic and document bacterial sepsis with or without accompanying meningitis in neonates less than 24 h to 2 months of age with severe disease rarely described in those older than 1 week of age (13, 17). Almost all of the patients presented with gastrointestinal symptoms and responded well to a 2-week course of a penicillin derivative, with minimal complications (13). In recent years, similar infections in adults have been reported, and some have even documented relative penicillin and gentamicin resistance, making careful evaluation and awareness of this pathogen imperative (16, 18–20).
As with patterns of early- and late-onset GBS disease, invasive S. gallolyticus subsp. pasteurianus infection presumably occurs either via an ascending route (early-onset) or via vaginal delivery or postnatally from maternal or caretaker contact, infected breast milk, or nosocomial sources (21). Fikar and Levy reported a neonate with S. bovis meningitis whose mother had the same organism growing from rectal and vaginal cultures (22). In 2006, the first outbreak of S. gallolyticus subsp. pasteurianus bloodstream infections was reported, and it involved five preterm infants in a university hospital neonatal intensive care unit in France, implicating hand carriage and poor sterile procedures (23). The exact pathophysiology of S. bovis group meningitis is unclear, though recent studies have suggested the gastrointestinal tract as a possible source. Noble demonstrated that S. bovis was isolated more frequently from the stool samples of neonates (5/21, 23.8%) than from adults (2/39, 5%) (24), while Gerber et al. reported a high prevalence of abdominal symptoms (5/7, 71%) in infants with systemic S. bovis infection without isolation of the pathogen from the stool (25). More recently, Takahashi et al. isolated identical isolates of S. gallolyticus subsp. pasteurianus from blood, CSF, and stool, suggesting translocation of previously commensal bacteria, with initiation of bacteremia and subsequent systemic dissemination and infiltration into CSF as a possible mechanism (26). In our case, no further investigation into the source of the organism was pursued.
Twin gestation is a known risk factor for the development of invasive GBS disease, but its role in other neonatal infections remains unclear. Within the former process, the relative risk of invasive GBS infection in the twin of an affected neonate has been reported to be as high as 25-fold (27). Prematurity and precipitous labor further increase susceptibility to infection (28). While studies of neonatal S. gallolyticus subsp. pasteurianus infections are limited, we hypothesize that the pathogeneses of its disease are similar. Furthermore, the extreme variability of the clinical presentations of these twins raises important questions regarding management of neonatal infection in twins. The neonates in our case not only differed in their presentations, with prominent respiratory rather than gastrointestinal symptoms, but also demonstrated two extremes of possible sequelae in the disease course. While one twin was mildly symptomatic, the other twin faced an unusually severe course for a late-onset infection, including overwhelming sepsis with serious neurologic damage, a complication that has rarely been reported. One previous report regarding S. gallolyticus subsp. pasteurianus meningitis included two infants with seizure-like activity but none with neurologic sequelae (13).
In summary, these cases broaden the clinical diversity of neonatal infections in twins and also highlight the importance of a thorough evaluation of both twins in the setting of suspected sepsis, regardless of the presence of symptoms (28).
ACKNOWLEDGMENTS
We have no conflicts of interest or source of funding to declare.
REFERENCES
- 1.Luna RA, Fasciano LR, Jones SC, Boyanton BL Jr, Ton TT, Versalovic J. 2007. DNA pyrosequencing-based bacterial pathogen identification in a pediatric hospital setting. J Clin Microbiol 45:2985–2992. doi: 10.1128/JCM.00630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cole JR, Chai B, Farris RJ, Wang Q, Kulam-Syed-Mohideen AS, McGarrell DM, Bandela AM, Cardenas E, Garrity GM, Tiedje JM. 2007. The ribosomal database project (RDP-II): introducing myRDP space and quality controlled public data. Nucleic Acids Res 35:D169–D172. doi: 10.1093/nar/gkl889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simmon KE, Croft AC, Petti CA. 2006. Application of SmartGene IDNS software to partial 16S rRNA gene sequences for a diverse group of bacteria in a clinical laboratory. J Clin Microbiol 44:4400–4406. doi: 10.1128/JCM.01364-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck M, Frodl R, Funke G. 2008. Comprehensive study of strains previously designated Streptococcus bovis consecutively isolated from human blood cultures and emended description of Streptococcus gallolyticus and Streptococcus infantarius subsp. coli. J Clin Microbiol 46:2966–2972. doi: 10.1128/JCM.00078-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitehead TR, Cotta MA. 2000. Development of molecular methods for identification of Streptococcus bovis from human and ruminal origins. FEMS Microbiol Lett 182:237–240. doi: 10.1111/j.1574-6968.2000.tb08901.x. [DOI] [PubMed] [Google Scholar]
- 7.Lin IH, Liu TT, Teng YT, Wu HL, Liu YM, Wu KM, Chang CH, Hsu MT. 2011. Sequencing and comparative genome analysis of two pathogenic Streptococcus gallolyticus subspecies: genome plasticity, adaptation and virulence. PLoS One 6:e20519. doi: 10.1371/journal.pone.0020519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlegel L, Grimont F, Collins MD, Regnault B, Grimont PA, Bouvet A. 2000. Streptococcus infantarius sp. nov., Streptococcus infantarius subsp. infantarius subsp. nov. and Streptococcus infantarius subsp. coli subsp. nov., isolated from humans and food. Int J Syst Evol Microbiol 50:1425–1434. doi: 10.1099/00207713-50-4-1425. [DOI] [PubMed] [Google Scholar]
- 9.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33:2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez BE, Hulten KG, Lamberth L, Kaplan SL, Mason EO. 2006. Streptococcus pneumoniae serogroups 15 and 33. Pediatr Infect Dis J 25:301–305. doi: 10.1097/01.inf.0000207484.52850.38. [DOI] [PubMed] [Google Scholar]
- 11.Poyart C, Quesne G, Trieu-Cuot P. 2002. Taxonomic dissection of the Streptococcus bovis group by analysis of manganese-dependent superoxide dismutase gene (sodA) sequences: reclassification of ‘Streptococcus infantarius subsp. coli’ as Streptococcus lutetiensis sp. nov. and of Streptococcus bovis biotype II 2 as Streptococcus pasteurianus sp. nov. Int J Syst Evol Microbiol 52:1247–1255. doi: 10.1099/ijs.0.02044-0. [DOI] [PubMed] [Google Scholar]
- 12.Schlegel L, Grimont F, Ageron E, Grimont PA, Bouvet A. 2003. Reappraisal of the taxonomy of the Streptococcus bovis/Streptococcus equinus complex and related species: description of Streptococcus gallolyticus subsp. gallolyticus, subsp. nov., S. gallolyticus subsp. macedonicus nov. and S. gallolyticus subsp. pasteurianus subsp. nov. Int J Syst Evol Microbiol 53:631–645. doi: 10.1099/ijs.0.02361-0. [DOI] [PubMed] [Google Scholar]
- 13.Klatte JM, Clarridge JE III, Bratcher D, Selvarangan R. 2012. A longitudinal case series description of meningitis due to Streptococcus gallolyticus subsp. pasteurianus in infants. J Clin Microbiol 50:57–60. doi: 10.1128/JCM.05635-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong S, Park JY, Han SH, Lee Y, Yong D, Lee K, Chong Y. 2011. First isolation of Streptococcus gallolyticus subsp. pasteurianus from a Korean patient with severe septic shock. Korean J Clin Microbiol 14:144–147. doi: 10.5145/KJCM.2011.14.4.144. [DOI] [Google Scholar]
- 15.Funke G, Funke-Kissling P. 2005. Performance of the new VITEK 2 GP card for identification of medically relevant gram-positive cocci in a routine clinical laboratory. J Clin Microbiol 43:84–88. doi: 10.1128/JCM.43.1.84-88.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sturt AS, Yang L, Sandhu K, Pei Z, Cassai N, Blaser MJ. 2010. Streptococcus gallolyticus subspecies pasteurianus (biotype II/2), a newly reported cause of adult meningitis. J Clin Microbiol 48:2247–2249. doi: 10.1128/JCM.00081-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gavin PJ, Thomson RB Jr, Horng SJ, Yogev R. 2003. Neonatal sepsis caused by Streptococcus bovis variant (biotype II/2): report of a case and review. J Clin Microbiol 41:3433–3435. doi: 10.1128/JCM.41.7.3433-3435.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan A. 2009. Relative penicillin resistance in Streptococcus bovis. A case of neonatal meningitis. J Paediatr Child Health 45:474–475. doi: 10.1111/j.1440-1754.2009.01541.x. [DOI] [PubMed] [Google Scholar]
- 19.Chow VC, Hawkey PM, Chan EW, Chin ML, Au TK, Fung DK, Chan RC. 2007. High-level gentamicin resistance mediated by a Tn4001-like transposon in 7 non-clonal hospital isolates of Streptococcus pasteurianus, Antimicrob Agents Chemother 51:2508–2513. doi: 10.1128/AAC.00603-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okumura A, Takahashi H, Ogawa A, Kuno K, Watanabe K. 2002. Streptococcus bovis meningitis in an otherwise healthy infant. Clin Pediatr 41:523–524. doi: 10.1177/000992280204100711. [DOI] [PubMed] [Google Scholar]
- 21.Doran KS, Benoit VM, Gertz RE, Beall B, Nizet V. 2002. Late-onset group B streptococcal infection in twins: insight to disease pathogenesis. J Perinatol 22:326–330. doi: 10.1038/sj.jp.7210675. [DOI] [PubMed] [Google Scholar]
- 22.Fikar CR, Levy J. 1979. Streptococcus bovis meningitis in a neonate. Am J Dis Child 133:1149–1150. [DOI] [PubMed] [Google Scholar]
- 23.Floret N, Bailly P, Thouverez M, Blanchot C, Alez-Martin D, Menget A, Thiriez G, Hoen B, Talon D, Bertrand X. 2010. A cluster of bloodstream infections caused by Streptococcus gallolyticus subsp. pasteurianus that involved 5 preterm neonates in a university hospital during a 2-month period. Infect Control Hosp Epidemiol 31:194–196. doi: 10.1086/650380. [DOI] [PubMed] [Google Scholar]
- 24.Noble CJ. 1978. Carriage of group D streptococci in the human bowel. J Clin Pathol 31:1182–1186. doi: 10.1136/jcp.31.12.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerber JS, Glas M, Frank G, Shah SS. 2006. Streptococcus bovis infection in young infants. Pediatr Infect Dis J 25:1069–1073. doi: 10.1097/01.inf.0000240334.91713.48. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi Y, Tanaka J, Okusu K, Ichimura S, Hishiki H. 2014. Streptococcus gallolyticus subsp. pasteurianus meningitis in an infant. Pediatr Int 56:282–285. doi: 10.1111/ped.12254. [DOI] [PubMed] [Google Scholar]
- 27.Pai JR, Tremlett CH, Clarke P. 2010. Late-onset sepsis in a preterm twin may harbinger life-threatening sepsis for the asymptomatic co-twin. Pediatr Infect Dis J 29:381–382. doi: 10.1097/INF.0b013e3181c42545. [DOI] [PubMed] [Google Scholar]
- 28.Moylett EH, Fernandez M, Rench MA, Hickman ME, Baker CJ. 2000. A 5-year review of recurrent group B streptococcal disease: lessons from twin infants. Clin Infect Dis 30:282–287. doi: 10.1086/313655. [DOI] [PubMed] [Google Scholar]
- 29.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial susceptibility testing; 23rd informational supplement. CLSI M100-S23. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]