Abstract
The usefulness of bronchoalveolar lavage (BAL) fluid cellular analysis in non-human immunodeficiency virus (HIV)-infected patients with Pneumocystis jirovecii pneumonia (PCP) has not been adequately evaluated. The objective of this study was to analyze the cellular profiles of BAL fluid and to evaluate their prognostic significance in non-HIV-infected patients with PCP. A 7-year retrospective cohort study of 166 non-HIV-infected adult patients with PCP who underwent BAL was performed in a tertiary care hospital. The median total BAL fluid white blood cell count was 180/μl (interquartile range, 80 to 330) and was unaffected by the severity of PCP. The median percentages of BAL fluid neutrophils, lymphocytes, and alveolar macrophages were 13.1%, 31.7%, and 30.2%, respectively. The median percentage of BAL fluid neutrophils was significantly higher in severe than in mild-to-moderate PCP (20.4% versus 6.0%, P < 0.001), as was the absolute neutrophil count (24/μl versus 13/μl, P = 0.001). The percentage of BAL fluid neutrophils was an independent predictor of 30-day (adjusted odds ratio [aOR], 1.02; 95% confidence interval [CI], 1.01 to 1.03) and 60-day (aOR, 1.02; 95% CI, 1.01 to 1.04) mortalities. The 30-day and 60-day mortalities increased at rates of 15% (P = 0.006) and 21% (P < 0.001) per 10% increment of BAL fluid neutrophil levels, respectively. The degree of BAL fluid pleocytosis was relatively low without regard to the severity of PCP. The percentage of BAL fluid neutrophils can be used as a prognostic marker in non-HIV-infected patients with PCP.
INTRODUCTION
Pneumocystis jirovecii pneumonia (PCP) remains one of the most prevalent and serious opportunistic infections in patients with human immunodeficiency virus (HIV) infection globally (1). Recently, PCP has been reported with increasing frequency in non-HIV-infected patients, particularly in those with underlying immunosuppressive conditions such as hematologic malignancies, solid tumors, collagen vascular diseases, and solid-organ transplants and those receiving immunosuppressive therapies (2, 3). Although clinical outcomes of PCP in HIV-infected patients have improved with the introduction of antiretroviral therapy, mortality rates among other immunocompromised patients with PCP who do not have HIV remain high at 30% to 60% (3–5). Identifying potential prognostic factors in non-HIV-infected patients with PCP could help clinicians make therapeutic decisions, such as choosing the appropriate site of care (intensive care unit versus general ward) or level of patient monitoring.
Diagnosis of PCP relies largely on microscopic examination of clinical specimens. While the diagnostic value of bronchoalveolar lavage (BAL) is recognized and while it is considered a key diagnostic procedure (1), data regarding the profiles of BAL fluid cellular analysis and their prognostic value are limited. A few studies have described the cellular profiles of BAL fluid in HIV-infected patients with PCP, and these suggested that BAL fluid neutrophilia might be associated with poor clinical course and outcome (6–9). Investigations addressing this issue in non-HIV-infected patients with PCP are rare, with small sample sizes (<40 cases) (10, 11). Therefore, we conducted a large cohort study to investigate the prognostic value of BAL fluid cellular analysis in non-HIV-infected patients with PCP.
MATERIALS AND METHODS
Study design, setting, and study population.
This retrospective cohort study was performed at the Asan Medical Center (Seoul, Republic of Korea), a 2,700-bed tertiary care-affiliated teaching hospital with 203 intensive care unit beds and active programs for solid-organ and hematopoietic stem cell transplantation. Adult patients (aged ≥ 16 years) who underwent fiberoptic bronchoscopy with BAL for suspected PCP from February 2007 to April 2014 were identified by examining bronchoscopy logs and laboratory databases. Only the results from the first bronchoscopy in patients who underwent multiple bronchoscopies during a single clinical episode were included. Patients with HIV infection and those who had inadequate results of BAL cellular analysis were excluded from the analysis. The study was approved by the Asan Medical Center Institutional Review Board.
Data collection and definitions.
All medical records were retrospectively reviewed using standardized study protocols. Demographic characteristics, laboratory results, underlying diseases or conditions, patient management, and clinical outcomes were evaluated. On the basis of determinations of partial pressure of arterial oxygen (PaO2) measured while the subject was breathing room air or of the alveolar oxygen-arterial oxygen difference (AaDO2) prior to the first bronchoscopy, patients were grouped into those with mild (PaO2 > 70 mm mercury [Hg] or AaDO2 < 35 mm Hg), moderate (PaO2 ≤ 70 mm Hg or AaDO2 ≥ 35 mm Hg), or severe (PaO2 < 60 mm Hg or AaDO2 ≥ 45 mm Hg) PCP (12). Temporary use of mechanical ventilation (MV) for the purpose of bronchoscopic examination was not considered to be “MV use” to avoid overestimation of MV users. Failure of initial treatment regimen was defined as clinical deterioration during the first 5 days of treatment or lack of improvement after 7 or more days of treatment (13). Patient outcomes were in-hospital, 30-day, and 60-day all-cause mortalities, defined as deaths from all causes occurring during admission or within 30 days and 60 days, respectively, of the initial bronchoscopy that yielded a positive result for P. jirovecii.
Bronchoscopic BAL and BAL fluid processing and analysis.
Fiberoptic bronchoscopy with BAL was performed according to a standardized protocol described previously (14, 15). Briefly, three consecutive aliquots (20, 30, and 30 ml) of sterile saline solution were instilled into the bronchial tree where the abnormality on the chest radiography was most apparent. In the event of bilateral diffuse infiltration, the right middle lobe or lingual segment was chosen. The fluid retrieved after first instillation was discarded, and the fluid that was subsequently retrieved was collected for analysis. A hemocytometer was used to determine total cell count. The Department of Laboratory Medicine of our institution has maintained continuous accreditation by the Accreditation Committee of the College of American Pathologists (CAP) since 1999. According to CAP Laboratory Accreditation Program hematology checklist question HEM.35340, quality control of the hemocytometer is performed after every 8 h of patient testing using a previously assayed patient sample. The amount of BAL fluid corresponding to 103 cells was centrifuged at 500 rpm for 5 min at room temperature and prepared on a microscope slide using a Thermo Shandon Cytospin instrument (Thermo Fisher Scientific Inc., Waltham, MA, USA). The slide was immediately air-dried and stained with Wright-Giemsa stain. Differential cell counting was used to determine percentages of neutrophils, lymphocytes, and alveolar macrophages.
Microbiological evaluation.
From February 2007 to February 2012, direct immunofluorescence assays were performed to detect P. jirovecii in accordance with the manufacturer's instructions, using a commercially available murine monoclonal antibody labeled with fluorescein isothiocyanate, which reacts with human and rodent Pneumocystis cysts and trophozoites (Light Diagnostics Pneumocystis carinii direct fluorescent antibody kit; Millipore, Billerica, MA, USA). From March 2012 to April 2014, we used a commercially available immunocytochemistry assay and mouse monoclonal antibody clone 3F6 (Dako Corporation, Carpinteria, CA, USA), which reacts with an antigenic epitope highly specific for P. jirovecii.
Additional microbiological evaluations were conducted according to standard procedures as previously described (14). Plate assays of BAL fluid specimens were used to quantify conventional bacteria, and for cytomegalovirus (CMV), samples were inoculated into shell vial cultures of human fetal lung fibroblasts (Diagnostic Hybrids, Inc., Athens, OH, USA), and growth was examined on days 1 and 7 by direct immunofluorescence staining.
Statistical analysis.
Categorical variables were analyzed using the chi-square or Fisher's exact test. Normally and non-normally distributed continuous variables were analyzed using Student's t test and the Mann-Whitney U test, respectively. To evaluate the association between the neutrophil increment in BAL fluid and mortality rate, we used the linear-by-linear association test for trend determinations. Risk factors associated with mortality were assessed using univariate and multivariate logistical regression analysis. Variables with P values of less than 0.1 in the univariate analysis were included in the multivariate analysis. The final model was constructed using the stepwise selection procedure. A P value of <0.05 was considered statistically significant. The data were analyzed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Demographics, clinical characteristics, treatments, and outcomes.
During the study period, a total of 209 patients with PCP were identified. Of these, 29 HIV-infected patients and 14 patients who had inadequate results for the BAL fluid cellular analysis were excluded, leaving a total of 166 patients with PCP in the final analysis.
Characteristics of the 166 non-HIV-infected patients with PCP are shown in Table 1. The median age was 55 years (interquartile range [IQR], 42to 64), and 103 patients (62.0%) were men. Hematologic malignancy was the most common underlying disease (34.9%), followed by solid-organ transplantation (30.7%), solid tumor (16.3%), connective-tissue disease (9.6%), and interstitial lung disease (ILD) (9.0%). CMV coinfection was identified in 43 patients (25.9%).
TABLE 1.
Demographics, clinical characteristics, treatments, and outcomes of 166 non-HIV-infected patients with Pneumocystis jirovecii pneumoniaa
| Characteristic | Values for patients with indicated disease status |
P | ||
|---|---|---|---|---|
| All (n = 166) | Mild to moderate (n = 46) | Severe (n = 120) | ||
| Demographics | ||||
| Age (yrs), median (IQR) | 55 (42–64) | 51 (37–60) | 56 (44–64) | 0.04 |
| Male gender | 103 (62.0) | 27 (58.7) | 76 (63.3) | 0.58 |
| Underlying disease | ||||
| Hematologic malignancy | 58 (34.9) | 19 (41.3) | 39 (32.5) | 0.29 |
| Solid-organ transplantation | 51 (30.7) | 18 (39.1) | 33 (27.5) | 0.15 |
| Solid tumor | 27 (16.3) | 6 (13.0) | 21 (17.5) | 0.49 |
| Connective-tissue disease | 16 (9.6) | 3 (6.5) | 13 (10.8) | 0.56 |
| Interstitial lung disease | 15 (9.0) | 4 (8.7) | 11 (9.2) | >0.99 |
| HSCT recipient | 13 (7.8) | 6 (13.0) | 7 (5.8) | 0.19 |
| Immunosuppressive agent use (≤1 mo) | 153 (92.2) | 43 (93.5) | 110 (91.7) | >0.99 |
| Steroid | 71 (42.8) | 15 (32.6) | 56 (46.7) | 0.10 |
| T-cell immunosuppressantb | 58 (34.9) | 19 (41.3) | 39 (32.5) | 0.29 |
| Anticancer agent | 67 (40.4) | 19 (41.3) | 48 (40.0) | 0.88 |
| Pulmonary coidentification | ||||
| Bacteria | 6 (3.6) | 2 (4.4) | 4 (3.3) | 0.67 |
| CMV | 43 (25.9) | 11 (23.9) | 32(26.7) | 0.72 |
| Laboratory findings | ||||
| CRP (mg/dl), median (IQR) | 10.2 (4.8–17.6) | 5.9 (3.1–10.6) | 12.8 (6.1–20.1) | <0.001 |
| LDH (IU/liter), median (IQR) | 461 (341–624) | 370 (320–459) | 490 (378–660) | 0.003 |
| PaO2 (mm Hg), median (IQR) | 82 (64–109) | 101 (80–135) | 74 (61–96) | <0.001 |
| AaDO2 (mm Hg), median (IQR) | 113 (43–196) | 19 (8–31) | 156 (83–226) | <0.001 |
| APACHE II score, median (IQR) | 16 (12–21) | 13 (10–16) | 18 (14–23) | <0.001 |
| Mechanical ventilation at initial presentation | 34 (20.5) | 1 (2.2) | 33 (27.5) | <0.001 |
| Treatment | ||||
| Treatment duration (days), median (IQR) | 18 (14–23) | 15 (14–20) | 19 (14–24) | 0.02 |
| TMP-SMX as initial regimenc | 161/162 (99.4) | 42/43 (97.7) | 119/119 (100) | 0.27 |
| Change to second-line regimenc | 57/162 (35.2) | 11/43 (25.6) | 46/119 (38.7) | 0.12 |
| Due to treatment failure | 42/162 (25.9) | 4/43 (9.3) | 38/119 (31.9) | 0.004 |
| Due to adverse reaction | 17 (10.2) | 7 (15.2) | 10 (8.3) | 0.25 |
| Outcome | ||||
| 30-day all-cause mortality | 36 (21.7) | 2 (4.3) | 34 (28.3) | 0.001 |
| 60-day all-cause mortality | 49 (29.5) | 5 (10.9) | 52 (43.3) | <0.001 |
| In-hospital mortality | 48 (28.9) | 2 (4.3) | 46 (38.3) | <0.001 |
Data are no. (%) of patients unless indicated otherwise. IQR, interquartile range; HSCT, hematopoietic stem cell transplantation; CMV, cytomegalovirus; CRP, C-reactive protein; LDH, lactate dehydrogenase; AaDO2, alveolar-arterial oxygen difference; APACHE, acute physiology and chronic health evaluation; TMP-SMX, trimethoprim-sulfamethoxazole.
The immunosuppressants included cyclosporine, tacrolimus, mycophenolate mofetil, tumor necrosis factor alpha (TNF-α) blockers, specific monoclonal antibodies (such as alemtuzumab), and nucleoside analogues.
A total of 4 patients were excluded from the analysis due to death before treatment initiation.
PCP was classified as mild (n = 39, 23.5%), moderate (n = 7, 4.2%), and severe (n = 120, 72.3%). A comparison of patients with mild-to-moderate PCP to patients with severe PCP is presented in Table 1. Patients with severe PCP were older. There was little difference in terms of underlying disease and immunosuppressive-agent use between the two groups. In addition, the proportions of patients with CMV coinfection were not different in the two groups (23.9% versus 26.7%, P = 0.72). However, laboratory findings, such as median C-reactive protein (CRP), lactate dehydrogenase (LDH), and AaDO2 levels, were significantly higher in patients with severe PCP. A higher acute physiology and chronic health evaluation (APACHE) II score was associated with severe PCP (18 versus 13, P < 0.001). At initial presentation, patients with severe PCP were more likely to receive mechanical ventilation (27.5% versus 2.2%, P < 0.001). The majority (99.4%) of patients received trimethoprim-sulfamethoxazole as an initial treatment regimen. Severe PCP was associated with higher 30-day (28.3% versus 4.3%, P = 0.001) and 60-day (43.3% versus 10.9%, P < 0.001) all-cause mortality than mild-to-moderate PCP.
BAL fluid cellular analysis.
The results of BAL fluid cellular analysis are shown in Table 2. The median total white blood cell (WBC) count was 180/μl (IQR, 80 to 330) and was not associated with severity of PCP. The median percentage of neutrophils was higher in patients with severe PCP (20.4% versus 6.0%, P < 0.001), as was the absolute neutrophil count (24/μl versus 13/μl, P = 0.001). Neutrophil dominance (defined as neutrophils being the most common cell type in BAL fluid WBCs) was also significantly associated with severe PCP (29.2% versus 6.5%, P = 0.002). In contrast, the absolute alveolar macrophage count was lower in patients with severe PCP (50/μl versus 76/μl, P = 0.03). However, the median percentages of CD4+ and CD8+ T cells and the CD4+/CD8+ T cell ratios were similar in the two groups.
TABLE 2.
Cellular profiles of bronchoalveolar lavage fluid in 166 non-HIV-infected patients with Pneumocystis jirovecii pneumoniaa
| Parameter | Values |
P | ||
|---|---|---|---|---|
| All (n = 166) | Mild to moderate (n = 46) | Severe (n = 120) | ||
| Total WBC count (cell/μl), median (IQR) | 180 (80–330) | 230 (130–355) | 180 (80–300) | 0.32 |
| Neutrophil %, median (IQR) | 13.1 (5.3–36.2) | 6.0 (3.4–12.9) | 20.4 (5.8–40.2) | <0.001 |
| Lymphocyte %, median (IQR) | 31.7 (11.8–55.4) | 45.3 (19.9–59.8) | 32.1 (13.0–54.6) | 0.12 |
| Alveolar macrophage %, median (IQR) | 30.2 (18.2–54.2) | 40.7 (22.0–57.1) | 28.3 (15.4–39.8) | 0.01 |
| Neutrophil count (cell/μl), median (IQR) | 22 (6–59) | 13 (4–30) | 24 (7–73) | 0.001 |
| Lymphocyte count (cell/μl), median (IQR) | 44 (12–143) | 68 (29–210) | 49 (8–144) | 0.17 |
| Alveolar macrophage count (cell/μl), median (IQR) | 51 (19–112) | 76 (36–125) | 50 (13–96) | 0.03 |
| Neutrophils dominantb | 38 (22.9) | 3 (6.5) | 35 (29.2) | 0.002 |
| Lymphocytes dominantb | 76 (45.8) | 24 (52.2) | 52 (43.3) | 0.31 |
| Alveolar macrophages dominantb | 52 (31.3) | 19 (41.3) | 33 (27.5) | 0.09 |
| CD4+ T cell %, median (IQR) | 33.4 (23.4–41.3) | 34.4 (23.5–50.8) | 33.0 (23.3–39.8) | 0.21 |
| CD8+ T cell %, median (IQR) | 49.6 (32.1–59.8) | 50.3 (29.4–59.6) | 49.0 (32.7–60.4) | 0.74 |
| CD4+/CD8+ T cell ratio, median (IQR) | 0.7 (0.4–1.1) | 0.7 (0.4–1.5) | 0.7 (0.4–1.0) | 0.74 |
Data are no. (%) of patients unless indicated otherwise. WBC, white blood cell; IQR, interquartile range.
The most common cell type among bronchoalveolar lavage fluid WBCs.
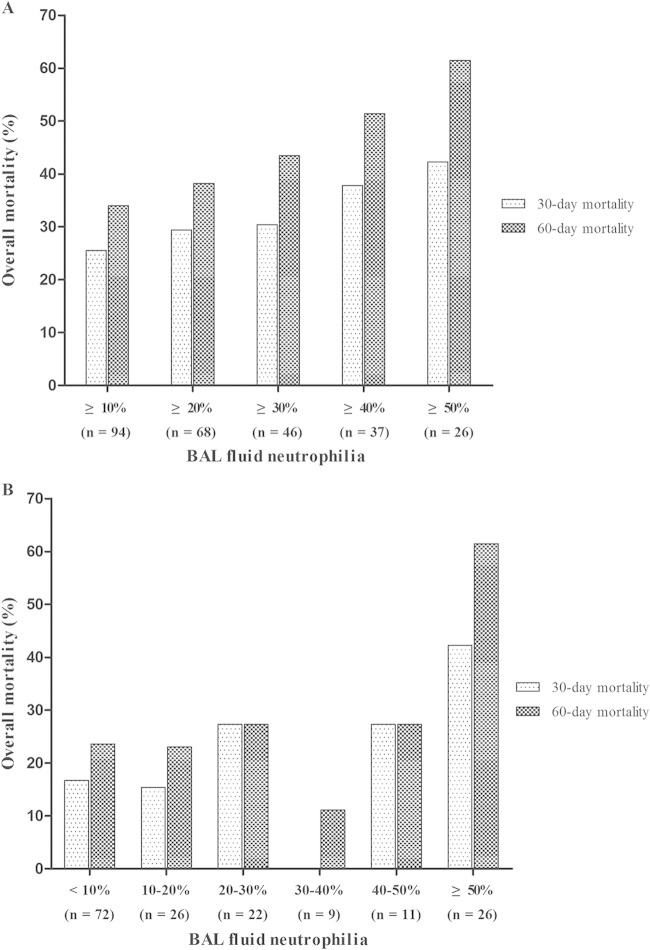
Figure 1 shows the 30-day and 60-day overall mortality rates determined according to the levels of BAL fluid neutrophilia at different cutoff points (Fig. 1A) and over different ranges (Fig. 1B). In Fig. 1A, the total numbers of patients and 30-day and 60-day overall mortality rates, respectively, were as follows: for ≥10% neutrophilia (n = 94), 26% and 34%; for ≥20% neutrophilia (n = 68), 29% and 38%; for ≥30% neutrophilia (n = 46), 30% and 44%; for ≥40% neutrophilia (n = 37), 38% and 51%; and for ≥50% neutrophilia (n = 26), 42% and 62%. Levels of 30-day and 60-day overall mortality increased at rates of 15% (P = 0.006) and 21% (P < 0.001), respectively, per 10% incremental increase of BAL fluid neutrophil levels. As shown in Fig. 1B, the total numbers of patients and the 30-day and 60-day overall mortality rates, respectively, were as follows: for <10% neutrophilia (n = 72), 17% and 24%; for 10% to 20% neutrophilia (n = 26), 15% and 23%; for 20% to 30% neutrophilia (n = 22), 27% and 27%; for 30% to 40% neutrophilia (n = 9), 0% and 11%; for 40% to 50% neutrophilia (n = 11), 27% and 27%; and for ≥50% neutrophilia (n = 16), 42% and 62%. The levels of 30-day and 60-day overall mortality increased at rates of 29% (P < 0.001) and 32% (P < 0.001), respectively, per 10% incremental increase of BAL fluid neutrophil levels.
FIG 1.
The relationship between BAL fluid neutrophilia and 30-day and 60-day all-cause mortalities in 166 non-HIV-infected patients with PCP as a function of different cutoff points (A) and different ranges (B).
Risk factors associated with 30-day and 60-day mortalities.
In the univariate analysis, age, male gender, hematologic malignancy, solid-organ transplantation, CRP and LDH levels, APACHE II score, mechanical ventilation at initial presentation, and percentage of BAL fluid neutrophils were identified as significant variables associated with mortality and were included in the final model. The results of multiple-logistic-regression analysis of predictors for 30-day and 60-day mortalities are summarized in Table 3. Male gender (adjusted OR [aOR], 2.52; 95% confidence interval [CI], 1.10 to 5.75; P = 0.03), solid-organ transplantation (aOR, 0.28; 95% CI, 0.09 to 0.87; P = 0.03), APACHE II score (aOR, 1.08; 95% CI, 1.01 to 1.16; P = 0.02), and percentage of BAL fluid neutrophils (aOR, 1.02; 95% CI, 1.01 to 1.03; P = 0.03) were associated with 30-day all-cause mortality. In addition, solid-organ transplantation (aOR, 0.20; 95% CI, 0.07 to 0.57; P = 0.003), APACHE II score (aOR, 1.09; 95% CI, 1.03 to 1.17; P = 0.01), and percentage of BAL fluid neutrophils (aOR, 1.02; 95% CI, 1.01 to 1.04; P = 0.02) were associated with 60-day all-cause mortality.
TABLE 3.
Multiple logistic-regression analysis of predictors for 30-day and 60-day mortalities in non-HIV-infected patients with Pneumocystis jirovecii pneumoniaa
| Predictor | 30-day mortality |
60-day mortality |
||
|---|---|---|---|---|
| aOR (95% CI) | P value | aOR (95% CI) | P value | |
| Male gender | 2.52 (1.10–5.75) | 0.03 | ||
| Solid-organ transplantation | 0.28 (0.09–0.87) | 0.03 | 0.20 (0.07–0.57) | 0.003 |
| APACHE II score | 1.08 (1.01–1.16) | 0.02 | 1.09 (1.03–1.17) | 0.01 |
| BAL fluid neutrophil % | 1.02 (1.01–1.03) | 0.03 | 1.02 (1.01–1.04) | 0.02 |
aOR, adjusted odds ratio; CI, confidence interval; APACHE, acute physiology and chronic health evaluation; BAL, bronchoalveolar lavage.
DISCUSSION
In the present study, we analyzed the cellular profiles of BAL fluid in 166 non-HIV-infected patients with PCP, and it was, to the best of our knowledge, the largest cohort study to have evaluated their prognostic significance. The degree of BAL fluid pleocytosis was relatively low regardless of the severity of PCP, with a median WBC count of 180/μl (IQR, 80 to 330). Neutrophil dominance was rare in mild-to-moderate cases, whereas it was seen in a third of severe cases. The percentage of BAL fluid neutrophils was significantly associated with 30-day and 60-day all-cause mortalities. This finding suggests that the percentage of BAL fluid neutrophils may be used as a prognostic marker in non-HIV-infected patients with PCP.
It has been suggested that cellular profiles of BAL fluid may provide useful information for the diagnosis of various lung diseases, including under both infectious and noninfectious conditions, when used in conjunction with clinical data and other diagnostic tests (15–19). As typical radiographic features of PCP are diffuse, bilateral, and interstitial infiltrates, clinicians are frequently faced with differential diagnoses of PCP and other diseases, such as atypical bacterial pneumonia, viral pneumonia, and ILD. Recently, several authors of the present study investigated the predictive role of BAL fluid cellular composition in the etiology of pneumonia in critically ill patients and reported that both bacterial pneumonia and viral pneumonia were frequently associated with BAL fluid neutrophilia, although the median percentage of neutrophils (80.5% versus 54.0%) was significantly higher in the bacterial pneumonia group (15). The median total WBC counts for the bacterial pneumonia group and the viral pneumonia group were 2,815/μl (IQR, 645 to 6,163) and 300/μl (IQR, 130 to 500), respectively. Given that the median total WBC count and the percentage of neutrophils in our patients were 180/μl (IQR, 80 to 330) and 13% (IQR, 5% to 36%), respectively, the cellular patterns of BAL fluid seen with non-HIV-infected patients with PCP may be distinguishable from those of bacterial pneumonia or viral pneumonia, although there appears to be an overlap between PCP and viral pneumonia. However, as the authors excluded patients who had received antimicrobial agents for more than 24 h before BAL, the cellular patterns of BAL fluid in partially treated bacterial pneumonia may be altered, making differential diagnosis complicated. In addition, distinguishing PCP from ILD is a diagnostic challenge, because they share many clinical features. ILD consists of a heterogeneous group of disorders, and BAL findings alone are usually nondiagnostic for a specific type of ILD (18, 19). Studies analyzing the cellular profiles of BAL fluid in various ILDs have shown that the total WBC counts were modestly elevated and that the percentages of neutrophils were relatively low (20, 21), presenting a cellular profile similar to those of patients with PCP in our data. Therefore, BAL fluid cellular analysis per se may be insufficient to differentiate PCP from ILD and should be interpreted in conjunction with clinical, radiological, and microbiological data.
The results of this study showed that BAL fluid neutrophilia in non-HIV-infected patients with PCP was associated with severe disease and higher mortality. It is thought that severe pneumocystis pneumonia is characterized by neutrophilic lung inflammation that may result in diffuse alveolar damage, impaired gas exchange, and respiratory failure (1). Limper et al. suggested that poor clinical outcomes were more closely related to the degree of lung inflammation than to the organism burden in PCP (22). This implies that measuring the degree of neutrophilia in BAL fluid may play a role in predicting clinical outcome. A few investigators have suggested that BAL fluid neutrophilia (>5% to 10%) can be a useful marker for predicting poor clinical course and outcome, such as the use of mechanical ventilation and death, in HIV-infected patients with PCP (6–9). Very limited information is available on the prognostic utility of BAL fluid cellular analysis in non-HIV-infected patients with PCP. A French group and a Japanese group, studying 39 and 29 patients, respectively, found that BAL fluid neutrophilia (>15% to 30%) might be a significant prognostic factor associated with fatal outcome (10, 11). In the current study, we included a relatively large number of cases with enhanced statistical power compared to that of previous studies and provided detailed data on the association between neutrophil incremental increases in BAL fluid and mortality rates according to different cutoff points.
Although neutrophilia appears to be a useful marker of lung damage during pneumocystis infection, its role in the pathogenesis of PCP remains unclear. It has been proposed that severe respiratory failure in PCP may be related to neutrophil-mediated inflammatory processes resulting in early-stage acute respiratory distress syndrome (8, 23). However, in a recent animal study, there were no significant differences in terms of pulmonary damage between knockout mice with neutrophil dysfunction and comparable wild-type mice, showing that neutrophils might not be the causative agent of tissue damage (24). Therefore, further research is warranted to determine the exact role of neutrophils in the pathogenesis of PCP.
Through multivariate analysis, we found that solid-organ transplantation was significantly associated with a better outcome. Previous reports have shown that mortality rates vary according to underlying disease or condition among non-HIV-infected patients with PCP (3–5). A Dutch group reported that the overall mortality rate was 35% among 78 HIV-negative patients, with the lowest rate (8%) among 13 kidney transplant recipients (25). In the present study, the 30-day mortality rates in transplantation were low regardless of transplant organ type (kidney, 11.8% [4/34]; liver, 12.5% [1/8]; heart, 0% [0/4]; simultaneous pancreas-kidney, 0% [0/4]; pancreas, 0% [0/1]) compared with those seen with other underlying diseases. Factors that contribute to differences in mortality rates among different disease groups in non-HIV-infected patients with PCP should be further investigated.
This study had several limitations. First, the retrospective design and single-center site were the main limitations. Second, we could not establish the organism burden in our PCP patients because the number of P. jirovecii in BAL fluid samples had not been determined. Third, concomitant bacterial or viral infection in patients with PCP might result in BAL fluid neutrophilia and act as a confounding factor in interpreting our results. However, the median percentages of neutrophils were not different in patients with and without bacterial coinfection (14.5% versus 13.0%, P = 0.42), because there were only 6 patients (3.6%) coinfected with bacteria. In addition, although we found that patients with CMV coinfection had a higher median percentage of neutrophils than patients without CMV coinfection (25.1% versus 10.9%, P = 0.02), the proportions of patients with CMV coinfection were not different in the mild-to-moderate PCP group and the severe PCP group, and the median percentage of neutrophils was higher in patients with severe PCP, regardless of the presence of CMV coinfection (see Table S1 in the supplemental material). Furthermore, CMV coinfection was not associated with increased levels of 30-day and 60-day all-cause mortalities (see Table S2). This suggests that bacterial and CMV coinfections are unlikely to have influenced our findings. Finally, while Fig. 1A shows a sustained increase in the overall mortality rate as the cutoff points increase by 10% increments, the results for some of the ranges of BAL fluid neutrophilia shown in panel B, especially for 30% to 40% neutrophilia, did not follow this trend, possibly because of the small numbers of patients falling in these ranges.
In conclusion, regardless of severity, the degree of BAL fluid pleocytosis is relatively low in PCP. The percentage of BAL fluid neutrophils may be used as a prognostic marker in non-HIV-infected patients with PCP. Our study results suggest that clinicians could make use of BAL fluid cellular profiles to identify potential patients at risk of poor outcome and to make therapeutic decisions, such as choosing the appropriate site of care or the level of patient monitoring.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Asan Institute of Life Sciences (grant 2015-389]. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We declare that we have no potential conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.03494-14.
REFERENCES
- 1.Thomas CF, Limper AH. 2004. Pneumocystis pneumonia. N Engl J Med 350:2487–2498. doi: 10.1056/NEJMra032588. [DOI] [PubMed] [Google Scholar]
- 2.Maini R, Henderson KL, Sheridan EA, Lamagni T, Nichols G, Delpech V, Phin N. 2013. Increasing pneumocystis pneumonia, England, UK, 2000–2010. Emerg Infect Dis 19:386–392. doi: 10.3201/eid1903.121151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roblot F, Godet C, Le Moal G, Garo B, Faouzi Souala M, Dary M, de Gentile L, Gandji JA, Guimard Y, Lacroix C, Roblot P, Becq-Giraudon B. 2002. Analysis of underlying diseases and prognosis factors associated with Pneumocystis carinii pneumonia in immunocompromised HIV-negative patients. Eur J Clin Microbiol Infect Dis 21:523–531. doi: 10.1007/s10096-002-0758-5. [DOI] [PubMed] [Google Scholar]
- 4.Sepkowitz KA. 2002. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis 34:1098–1107. doi: 10.1086/339548. [DOI] [PubMed] [Google Scholar]
- 5.Moon SM, Kim T, Sung H, Kim MN, Kim SH, Choi SH, Jeong JY, Woo JH, Kim YS, Lee SO. 2011. Outcomes of moderate-to-severe Pneumocystis pneumonia treated with adjunctive steroid in non-HIV-infected patients. Antimicrob Agents Chemother 55:4613–4618. doi: 10.1128/AAC.00669-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith RL, El-Sadr WM, Lewis ML. 1988. Correlation of bronchoalveolar lavage cell populations with clinical severity of Pneumocystis carinii pneumonia. Chest 93:60–64. doi: 10.1378/chest.93.1.60. [DOI] [PubMed] [Google Scholar]
- 7.Mason GR, Hashimoto CH, Dickman PS, Foutty LF, Cobb CJ. 1989. Prognostic implications of bronchoalveolar lavage neutrophilia in patients with Pneumocystis carinii pneumonia and AIDS. Am Rev Respir Dis 139:1336–1342. doi: 10.1164/ajrccm/139.6.1336. [DOI] [PubMed] [Google Scholar]
- 8.Azoulay E, Parrot A, Flahault A, Cesari D, Lecomte I, Roux P, Saidi F, Fartoukh M, Bernaudin JF, Cadranel J, Mayaud C. 1999. AIDS-related Pneumocystis carinii pneumonia in the era of adjunctive steroids: implication of BAL neutrophilia. Am J Respir Crit Care Med 160:493–499. doi: 10.1164/ajrccm.160.2.9901019. [DOI] [PubMed] [Google Scholar]
- 9.Bang D, Emborg J, Elkjaer J, Lundgren JD, Benfield TL. 2001. Independent risk of mechanical ventilation for AIDS-related Pneumocystis carinii pneumonia associated with bronchoalveolar lavage neutrophilia. Respir Med 95:661–665. doi: 10.1053/rmed.2001.1119. [DOI] [PubMed] [Google Scholar]
- 10.Zahar JR, Robin M, Azoulay E, Fieux F, Nitenberg G, Schlemmer B. 2002. Pneumocystis carinii pneumonia in critically ill patients with malignancy: a descriptive study. Clin Infect Dis 35:929–934. doi: 10.1086/342338. [DOI] [PubMed] [Google Scholar]
- 11.Tamai K, Tachikawa R, Tomii K, Nagata K, Otsuka K, Nakagawa A, Otsuka K, Matsumoto T, Monden K, Takeshita J, Tanaka K, Kawamura T, Otoshi T, Fujimoto D. 2014. Prognostic value of bronchoalveolar lavage in patients with non-HIV pneumocystis pneumonia. Intern Med 53:1113–1117. doi: 10.2169/internalmedicine.53.0520. [DOI] [PubMed] [Google Scholar]
- 12.Pfaller MA, Anaissie EJ. 2009. Pneumocystis, p 385–401. In Anaissie EJ, McGinnis MR, Pfaller MA (ed), Clinical mycology, 2nd ed Churchill Livingstone, Oxford, United Kingdom. [Google Scholar]
- 13.Smego RA Jr, Nagar S, Maloba B, Popara M. 2001. A meta-analysis of salvage therapy for Pneumocystis carinii pneumonia. Arch Intern Med 161:1529–1533. doi: 10.1001/archinte.161.12.1529. [DOI] [PubMed] [Google Scholar]
- 14.Choi SH, Hong SB, Ko GB, Lee Y, Park HJ, Park SY, Moon SM, Cho OH, Park KH, Chong YP, Kim SH, Huh JW, Sung H, Do KH, Lee SO, Kim MN, Jeong JY, Lim CM, Kim YS, Woo JH, Koh Y. 2012. Viral infection in patients with severe pneumonia requiring intensive care unit admission. Am J Respir Crit Care Med 186:325–332. doi: 10.1164/rccm.201112-2240OC. [DOI] [PubMed] [Google Scholar]
- 15.Choi SH, Hong SB, Hong HL, Kim SH, Huh JW, Sung H, Lee SO, Kim MN, Jeong JY, Lim CM, Kim YS, Woo JH, Koh Y. 2014. Usefulness of cellular analysis of bronchoalveolar lavage fluid for predicting the etiology of pneumonia in critically ill patients. PLoS One 9:e97346. doi: 10.1371/journal.pone.0097346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huh JW, Lim CM, Koh Y, Oh YM, Shim TS, Lee SD, Kim WS, Kim DS, Kim WD, Hong SB. 2008. Diagnostic utility of the soluble triggering receptor expressed on myeloid cells-1 in bronchoalveolar lavage fluid from patients with bilateral lung infiltrates. Crit Care 12:R6. doi: 10.1186/cc6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stolz D, Stulz A, Müller B, Gratwohl A, Tamm M. 2007. BAL neutrophils, serum procalcitonin, and C-reactive protein to predict bacterial infection in the immunocompromised host. Chest 132:504–514. doi: 10.1378/chest.07-0175. [DOI] [PubMed] [Google Scholar]
- 18.Bradley B, Branley HM, Egan JJ, Greaves MS, Hansell DM, Harrison NK, Hirani N, Hubbard R, Lake F, Millar AB, Wallace WA, Wells AU, Whyte MK, Wilsher ML, British Thoracic Society Interstitial Lung Disease Guideline Group British Thoracic Society Standards of Care Committee, Thoracic Society of Australia, New Zealand Thoracic Society, Irish Thoracic Society . 2008. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax 63(Suppl V):v1–v58. doi: 10.1136/thx.2008.101691. [DOI] [PubMed] [Google Scholar]
- 19.Meyer KC, Raghu G, Baughman RP, Brown KK, Costabel U, du Bois RM, Drent M, Haslam PL, Kim DS, Nagai S, Rottoli P, Saltini C, Selman M, Strange C, Wood B; American Thoracic Society Committee on BAL in Interstitial Lung Disease. 2012. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 185:1004–1014. doi: 10.1164/rccm.201202-0320ST. [DOI] [PubMed] [Google Scholar]
- 20.Domagała-Kulawik J, Skirecki T, Maskey-Warzechowska M, Grubek-Jaworska H, Chazan R. 2012. Bronchoalveolar lavage total cell count in interstitial lung diseases—does it matter? Inflammation 35:803–809. doi: 10.1007/s10753-011-9378-5. [DOI] [PubMed] [Google Scholar]
- 21.Welker L, Jörres RA, Costabel U, Magnussen H. 2004. Predictive value of BAL cell differentials in the diagnosis of interstitial lung diseases. Eur Respir J 24:1000–1006. doi: 10.1183/09031936.04.00101303. [DOI] [PubMed] [Google Scholar]
- 22.Limper AH, Offord KP, Smith TF, Martin WJ II. 1989. Pneumocystis carinii pneumonia: differences in lung parasite number and inflammation in patients with and without AIDS. Am Rev Respir Dis 140:1204–1209. doi: 10.1164/ajrccm/140.5.1204. [DOI] [PubMed] [Google Scholar]
- 23.Weiland JE, Davis WB, Holter JF, Mohammed JR, Dorinsky PM, Gadek JE. 1986. Lung neutrophils in the adult respiratory distress syndrome. Am Rev Respir Dis 133:218–225. [DOI] [PubMed] [Google Scholar]
- 24.Swain SD, Wright TW, Degel PM, Gigliotti F, Harmsen AG. 2004. Neither neutrophils nor reactive oxygen species contribute to tissue damage during Pneumocystis pneumonia in mice. Infect Immun 72:5722–5732. doi: 10.1128/IAI.72.10.5722-5732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arend SM, Kroon FP, van't Wout JW. 1995. Pneumocystis carinii pneumonia in patients without AIDS, 1980 through 1993: an analysis of 78 cases. Arch Intern Med 155:2436–2441. doi: 10.1001/archinte.1995.00430220094010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.