Abstract
The clinical significance of endotoxin detection in blood has been evaluated for a broad range of patient groups in over 40 studies published over 4 decades. The influences of Gram-negative (GN) bacteremia species type and patient inclusion criteria on endotoxemia detection rates in published studies remain unclear. Studies were identified after a literature search and manual reviews of article bibliographies, together with a direct approach to authors of potentially eligible studies for data clarifications. The concordance between GN bacteremia and endotoxemia expressed as the summary diagnostic odds ratios (DORs) was derived for three GN bacteremia categories across eligible studies by using a hierarchical summary receiver operating characteristic (HSROC) method. Forty-two studies met broad inclusion criteria, with between 2 and 173 GN bacteremias in each study. Among all 42 studies, the DORs (95% confidence interval) were 3.2 (1.7 to 6.0) and 5.8 (2.4 to 13.7) in association with GN bacteremias with Escherichia coli and those with Pseudomonas aeruginosa, respectively. Among 12 studies of patients with sepsis, the proportion of endotoxemia positivity (95% confidence interval) among patients with P. aeruginosa bacteremia (69% [57 to 79%]; P = 0.004) or with Proteus bacteremia (76% [51 to 91%]; P = 0.04) was significantly higher than that among patients without GN bacteremia (49% [33 to 64%]), but this was not so for patients bacteremic with E. coli (57% [40 to 73%]; P = 0.55). Among studies of the sepsis patient group, the concordance of endotoxemia with GN bacteremia was surprisingly weak, especially for E. coli GN bacteremia.
INTRODUCTION
The Limulus amebocyte lysis (LAL) assay, which utilizes extracts of blood cells (amebocytes) of the Limulus polyphemus horseshoe crab, is a highly sensitive and specific test available for the detection of endotoxin (lipopolysaccharide [LPS]) (1). This assay is a reliable method for the detection of infection with Gram-negative (GN) bacteria in body fluids other than blood (1). However, the clinical significance of endotoxin detection in blood as both a diagnostic and a prognostic test is unclear, despite over 100 studies published over 4 decades for a broad range of patient groups (2–45). The interpretation of the literature is confounded by a 100-fold increase in assay sensitivity (3) and substantial differences in patient inclusion criteria among the studies in the literature over this period.
Moreover, in the evaluations of endotoxemia therapies over this time period, there has been a substantial and unexplained disconnect between the results of animal models of sepsis and the results of subsequent clinical trials of the same therapies (46).
Five factors have prompted a reappraisal of this question of the clinical significance of endotoxin detection. First, new studies and new data from older studies relating to endotoxemia detection have appeared and need to be incorporated (5–7, 15, 24, 32, 37, 43, 44). Second, the relevance of recently defined structural differences in lipid A, the biologically active component of LPS of different pathogens causing GN bacteremia, for endotoxemia detection needs to be clarified (47). Third, paradoxical observations among animal models of sepsis indicate that the concordance of endotoxemia with GN bacteremia and also with outcome is expected to differ for different GN bacteremia types (48). The detection of endotoxemia is of interest in relation to ongoing efforts to develop rapid detection methods for GN bacteremia and the possible application of emerging endotoxemia therapies (46). Finally, newer statistical methods have enabled a reappraisal over a broad range of assay breakpoints (49).
The purpose here was to reappraise the literature, with particular interest in patients with documented GN bacteremia within those studies that used sepsis criteria for patient inclusion.
MATERIALS AND METHODS
Data sources.
The previously undertaken search of the literature was updated to February 2014. The search strategy was detailed previously (2, 3). In addition, a call for data was issued (50), and additional data were sought by personal communications with authors of potentially relevant publications.
Study selection and data extraction.
The following inclusion criteria were used: (i) the study provided the results of Limulus assays and blood cultures for patients with suspected GN bacteremia; (ii) the study had a minimum sample size of 10 patients. The following exclusion criteria were used: animal models, studies of endotoxemia in settings other than suspected GN bacteremia (e.g., colonoscopy and intraoperative settings), studies using assays other than the Limulus assay, studies that were restricted to specific types of GN bacteremia (e.g., those caused by Neisseria meningitidis), studies which lack specific data for bacteremia or other data, and duplicate studies. Note that some studies may have been excluded for more than one reason. A complete catalog of studies excluded from the analysis was reported previously (2).
The endotoxemia detection data were extracted from each study on a per-patient basis as follows. Patients in each of the following three categories of bacteremia were counted: (i) Escherichia coli, (ii) Pseudomonas aeruginosa, (iii) non-E. coli Enterobacteriaceae. The last category included Klebsiella species, Enterobacter species, Proteus species, and Serratia species. Any patients with Gram-positive bacteremia or fungemia were counted in the “GN bacteremia absent” category. Polymicrobial bacteremias were not included in the analysis. For each study, a breakpoint between endotoxemia positive and negative was determined which, unless otherwise indicated, was usually the sensitivity limit for the internal endotoxin standard of the Limulus assay used in each study. This breakpoint was converted to the units nanograms per milliliters by using the conversion factor of 1 endotoxin unit (EU) = 0.1 ng of endotoxin, where necessary. Those patients with GN bacteremia with endotoxemia detected above versus below the breakpoint were counted as a true positive (TP), versus false negative (FN), respectively. Those patients in the category of GN bacteremia absent above versus below the breakpoint were counted as false positives (FP), versus true negatives (TN), respectively. Two studies (24, 32) provided endotoxemia data that were stratified for two breakpoints; each of these studies was analyzed by entering all study data separately for each breakpoint at half the study weight. For each study, the diagnostic odds ratio (DOR) was calculated as follows: DOR = (TP/FN)/(FP/TN).
Data analysis.
The derivations of the summary statistics for the DOR, sensitivity, and specificity were performed using the metandi command in STATA (release 10.0; STATA Corp., College Station, TX, USA) as previously described (2, 3). This command fits a two-level mixed logistic regression model, with independent binomial distributions for the true positives and true negatives conditional on the sensitivity and specificity in each study and a bivariate normal model for the logit transforms of sensitivity and specificity between studies. The metandi command also generates a plot containing the following: a hierarchical summary receiver operating characteristic (HSROC) curve derived from the individual study results, which were represented as data points proportional to study size, and the sensitivity and specificity, conjointly summarized as a single summary point surrounded by 95% confidence and 95% prediction ellipses.
RESULTS
This analysis included 42 studies published between the years 1970 and 2013 (4–45) (Fig. 1; Table 1). There were 8 studies with data clarifications obtained through personal communication, 10 studies not included in a previous meta-analysis (5–7, 15, 22, 24, 32, 37, 43, 44), and 3 studies not published in English (8, 15, 43). The reported assay sensitivities to the internal endotoxin standard in the studies varied by >100-fold and typically ranged between 0.1 and 10 ng/ml for the 18 studies that used the gelation version of the LAL assay, compared to a range between 0.001 and 0.1 ng/ml for the 24 studies that used the chromogenic version of the LAL assay. There were 21 studies published up to or including 1990 and 21 that were published after 1990. All but one of the studies published after 1990 used the chromogenic version of the LAL assay (Table 1).
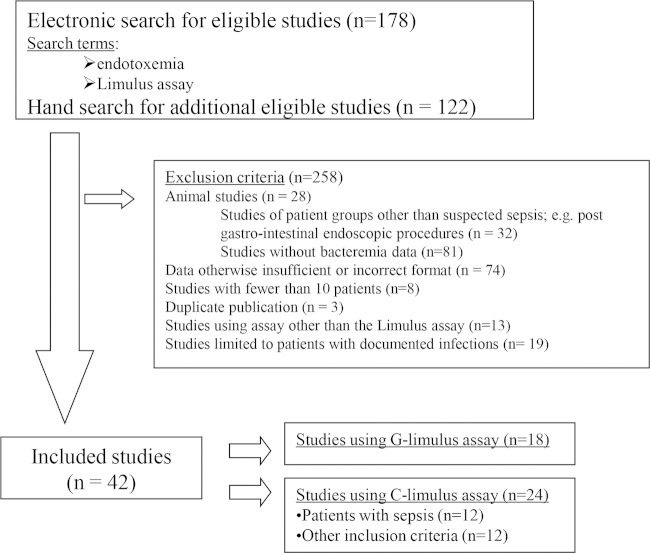
FIG 1.
Flow chart of our literature search strategy and study accrual. C-limulus and G-limulus refer to the chromogenic and gelation versions of the LAL assay, respectively. Note that studies may have been excluded for more than one reason but have been counted only once.
TABLE 1.
Studies analyzed to determine the concordance of endotoxemia with Gram-negative bacteremia
| First author, yr (reference) | LAL versiona | Sensitivity limitb (ng/ml) | Patient population | Total no. of patients |
|---|---|---|---|---|
| Ahmed, 2004 (4) | C | 0.004 | Pediatric | 35 |
| Bailey, 1976 (5) | G | 5 | Surgical | 24 |
| Bion, 1994 (6) | C | 0.02 | Surgical | 52 |
| Byl, 2001 (7) | C | 0.005* | Sepsis syndrome | 23 |
| Clumeck, 1977 (8) | G | 3 | Unrestricted | 46 |
| Cooperstock, 1985 (9) | G | 1 | Pediatric | 37 |
| Danner, 1991 (10)c | C | 0.01 | Sepsis syndrome | 96 |
| Dofferhoff, 1992 (11) | C | 0.005 | Sepsis syndrome | 18 |
| Engervall, 1997 (12) | C | 0.005* | Neutropenic | 22 |
| Feldman, 1974 (13) | G | 1 | Pediatric | 78 |
| Fossard, 1974 (14) | G | 1 | Surgical | 25 |
| Garcia Curiel, 1979 (15) | G | 1 | Shock | 41 |
| Giamarellos-Bourboulis, 1999 (16) | C | 0.1 | Urosepsis | 25 |
| Goldie, 1995 (17)c | C | 0.002 | Sepsis syndrome | 129 |
| Guidet, 1994 (18)c | C | 0.005 | Sepsis syndrome | 81 |
| Hass, 1986 (19) | C | 0.01 | Pediatric | 35 |
| Hynninen, 1995 (20) | C | 0.013 | Neutropenic | 98 |
| Jirillo, 1975 (21) | G | 1 | Pediatric | 10 |
| Kelsey, 1982 (22) | G | 50 | Pediatric | 30 |
| Ketchum, 1997 (23)c | C | 0.005 | Sepsis syndrome | 362 |
| Kritselis, 2013 (24)c,d | C | 0.6 | Sepsis syndrome | 341 |
| Kritselis, 2013 (24)c,d | C | 0.025 | Sepsis syndrome | 341 |
| Lau, 1996 (25) | C | 0.01 | Surgical | 38 |
| Levin, 1970 (26) | G | 5 | Unrestricted | 93 |
| Levin, 1972 (27) | G | 5 | Suspected bacteremia | 217 |
| Martinez, 1973 (28) | G | 5 | Suspected bacteremia | 75 |
| Massignon, 1996 (29)b | C | 0.004 | Sepsis syndrome | 55 |
| McCartney, 1987 (30) | C | NS | Neutropenic | 26 |
| Oberle, 1974 (31) | G | 0.5 | Pediatric | 23 |
| Opal (high), 1999 (32)c,d | C | 0.6 | Sepsis syndrome | 727 |
| Opal (low), 1999 (32)c,d | C | 0.02 | Sepsis syndrome | 727 |
| Pearson, 1985 (33) | G | 0.1 | Unrestricted | 41 |
| Prins, 1995 (34)c | C | 0.04 | Urosepsis | 30 |
| Scheifele, 1985 (35) | G | 0.2 | Pediatric | 43 |
| Shenep, 1988 (36) | G | 0.025 | Pediatric | 20 |
| Strutz, 1999 (37) | C | 0.01 | Sepsis | 28 |
| Stumacher, 1973 (38) | G | 0.5 | Unrestricted | 126 |
| Suyasa, 1995 (39) | G | 0.01 | Unrestricted | 13 |
| Togari, 1983 (40) | G | 0.5 | Pediatric | 10 |
| Van Deventer, 1988 (41) | C | 0.005 | Unrestricted | 433 |
| Van Dissel, 1993 (42) | C | NS | Sepsis syndrome | 14 |
| Watzke, 1987 (43) | C | 0.01 | Unrestricted | 20 |
| Wong, 2013 (44) | C | 0.003 | Neutropenic | 103 |
| Yoshida, 1993 (45)c | C | 0.003 | Neutropenic | 125 |
LAL assay version abbreviations: C, chromogenic; G, gelation.
The Limulus assay sensitivity limit to an internal control endotoxin standard (in ng/ml). For those studies which used EU rather than ng/ml (marked with an asterisk), a conversion based on the formula 1 EU = 100 pg was used. NS, not specified.
Data provided via personal communication with the study author.
There were two studies (24, 32) for which the patients were classified at two breakpoints into subgroups with either high (>660 pg/ml), low (25 to 660 pg/ml), or nondetectable (<25 pg/ml) levels of endotoxemia. There were 12 studies (14 groups) that were limited to patients with either sepsis or septic shock. All of these 12 studies used the chromogenic version of the LAL assay, and 11 of these 12 studies were published after 1990. The other 30 studies examined a diverse range of patient groups, such as pediatric, perioperative, febrile neutropenic oncology, or otherwise-unspecified adult patient groups, and used either version of the LAL assay.
The 42 studies included data for 3,868 patients, with 1,389 patients among the 10 newly included studies (5–7, 15, 22, 24, 32, 37, 43, 44). The number of patients in each study with GN bacteremia was lower among studies published up to and in 1990 (pre-1990, median of 9 patients, with an interquartile range of 3 to 16) versus those published after 1990 (post-1990, median of 15 patients with an interquartile range of 8 to 21). There were 295 E. coli (Fig. 2), 239 non-E. coli Enterobacteriaceae, and 133 P. aeruginosa (Fig. 3) GN bacteremias (Table 2). The concordance between the detection of endotoxemia and GN bacteremia is displayed in the HSROC plots (Fig. 2 and 3), and the summary DORs are shown in Table 3. The DORs were lower among the 12 studies limited to patients with sepsis than were the DORs among all studies.
FIG 2.
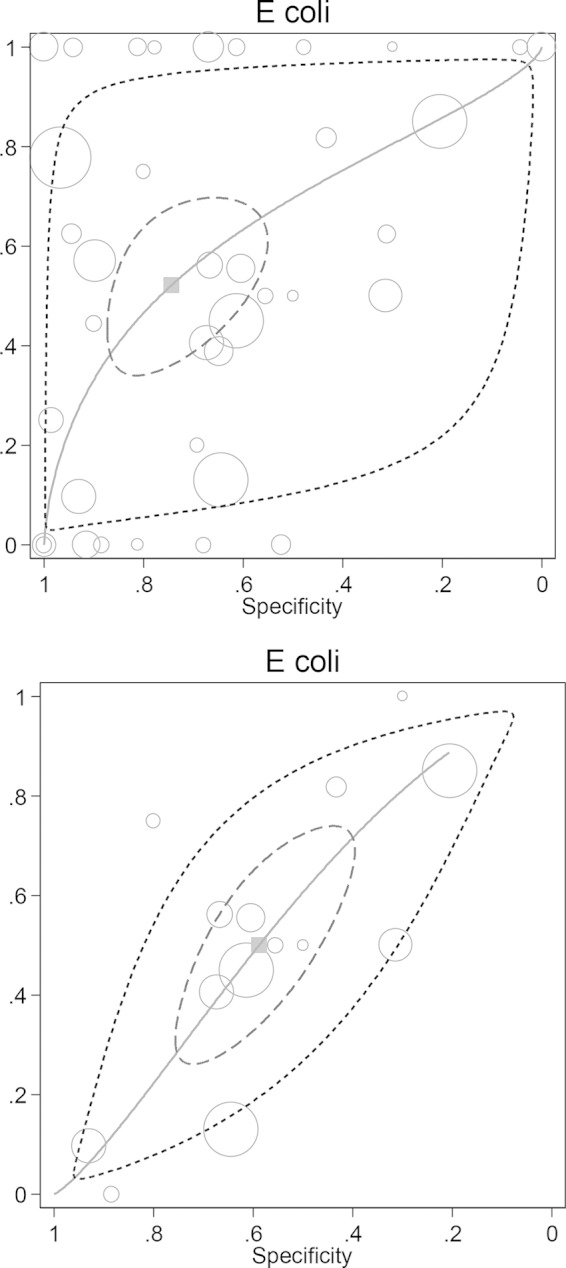
Plot of sensitivity versus specificity from 37 studies for all patients populations (39 groups; top plot) and for patients with sepsis (14 groups; bottom plot) for the detection of endotoxemia using the Limulus assay versus E. coli bacteremia, together with the fitted HSROC curve and the bivariate summary estimate (solid square) for sensitivity and specificity together with the corresponding 95% confidence ellipse (inner broken line) and 95% prediction ellipse (outer dotted line). The symbol size for each study is proportional to the study size.
FIG 3.
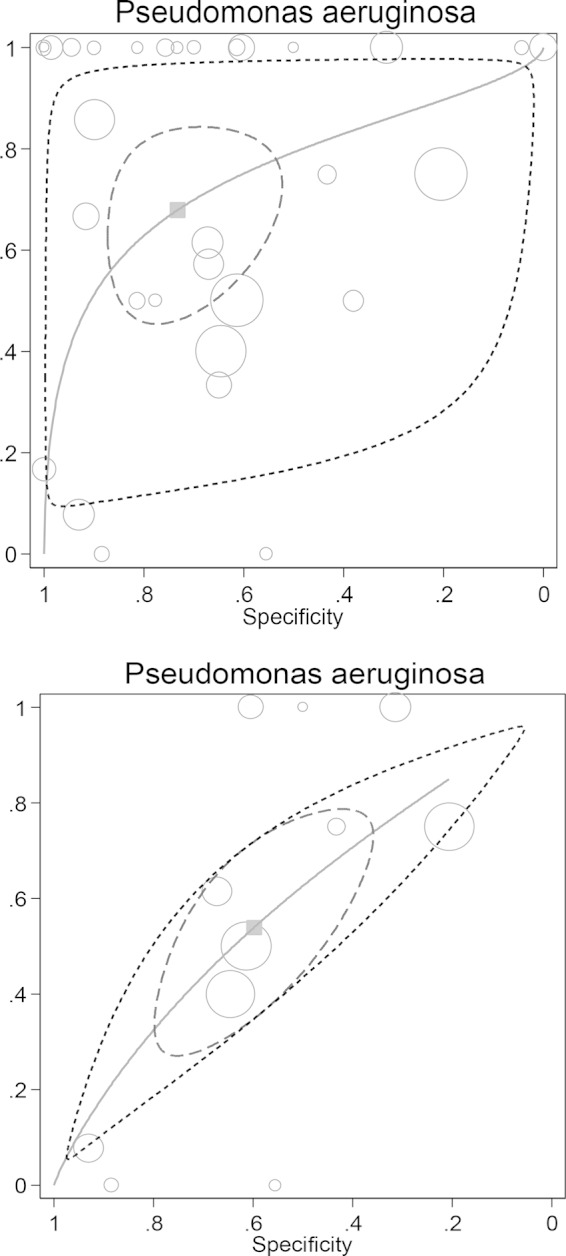
Plot of sensitivity versus specificity for 31 studies for all patient populations (33 groups; top plot) and for patients with sepsis (11 groups; bottom plot) for the detection of endotoxemia using the Limulus assay versus Pseudomonas aeruginosa GN bacteremia together with the fitted HSROC curve and the bivariate summary estimate (solid square) for sensitivity and specificity, together with the corresponding 95% confidence ellipse (inner broken line) and 95% prediction ellipse (outer dotted line). The symbol size for each study is proportional to the study size.
TABLE 2.
Detection rates by species group for each of the studies included in our analysis
| First author, yr (reference) | Detection rate (no. of patients) among species groupa |
|||||||
|---|---|---|---|---|---|---|---|---|
|
E. coli |
Non-E. coli Enterobacteriaceae |
P. aeruginosa |
Non-GN bacteremia |
|||||
| TP | FN | TP | FN | TP | FN | FP | TN | |
| Ahmed, 2004 (4) | 1 | 0 | 0 | 0 | 3 | 0 | 12 | 19 |
| Bailey, 1976 (5) | 1 | 0 | 0 | 0 | 0 | 0 | 12 | 11 |
| Bion, 1994 (6) | 0 | 0 | 0 | 0 | 1 | 1 | 31 | 19 |
| Byl, 2001 (7) | 6 | 2 | 0 | 0 | 0 | 0 | 3 | 12 |
| Clumeck, 1977 (8) | 5 | 3 | 0 | 0 | 2 | 0 | 2 | 34 |
| Cooperstock, 1985 (9) | 3 | 0 | 0 | 0 | 1 | 1 | 6 | 26 |
| Danner, 1991 (10)b | 5 | 4 | 1 | 3 | 2 | 0 | 32 | 49 |
| Dofferhoff, 1992 (11) | 1 | 1 | 1 | 1 | 2 | 0 | 6 | 6 |
| Engervall, 1997 (12) | 1 | 0 | 0 | 1 | 1 | 1 | 4 | 14 |
| Feldman, 1974 (13) | 0 | 9 | 0 | 4 | 2 | 10 | 0 | 53 |
| Fossard, 1974 (14) | 1 | 0 | 0 | 0 | 1 | 0 | 22 | 1 |
| Garcia Curiel, 1979 (15) | 0 | 3 | 1 | 10 | 0 | 1 | 3 | 23 |
| Giamarellos-Bourboulis, 1999 (16)b | 2 | 8 | 1 | 1 | 0 | 0 | 4 | 9 |
| Goldie, 1995 (17)b | 1 | 1 | 3 | 1 | 2 | 0 | 83 | 38 |
| Guidet, 1994 (18)b | 9 | 7 | 5 | 0 | 0 | 0 | 20 | 40 |
| Hass, 1986 (19) | 0 | 0 | 0 | 0 | 2 | 0 | 8 | 25 |
| Hynninen, 1995 (20) | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 96 |
| Jirillo, 1975 (21) | 0 | 0 | 1 | 1 | 0 | 0 | 4 | 4 |
| Kelsey, 1982 (22) | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 28 |
| Ketchum, 1997 (23)b | 3 | 20 | 5 | 22 | 2 | 3 | 109 | 198 |
| Kritselis, 2013 (24)b,c | 6 | 56 | 9 | 45 | 1 | 23 | 13 | 188 |
| Kritselis, 2013 (24)b,c | 25 | 37 | 24 | 30 | 15 | 9 | 66 | 135 |
| Lau, 1996 (25) | 5 | 3 | 1 | 0 | 0 | 0 | 20 | 9 |
| Levin, 1970 (26) | 0 | 2 | 0 | 5 | 2 | 1 | 7 | 76 |
| Levin, 1972 (27) | 4 | 3 | 6 | 10 | 6 | 1 | 19 | 168 |
| Martinez, 1973 (28) | 1 | 3 | 0 | 3 | 1 | 0 | 1 | 66 |
| Massignon, 1996 (29) | 9 | 2 | 3 | 0 | 3 | 1 | 21 | 16 |
| McCartney, 1987 (30) | 0 | 1 | 0 | 0 | 0 | 0 | 8 | 17 |
| Oberle, 1974 (31) | 0 | 0 | 1 | 0 | 2 | 0 | 6 | 14 |
| Opal (high), 1999 (32)b,c | 18 | 21 | 15 | 18 | 16 | 15 | 241 | 383 |
| Opal (low), 1999 (32)b,c | 33 | 6 | 27 | 6 | 24 | 7 | 496 | 128 |
| Pearson, 1985 (33) | 5 | 0 | 1 | 1 | 0 | 0 | 2 | 32 |
| Prins, 1995 (34)b | 4 | 5 | 0 | 0 | 1 | 0 | 2 | 18 |
| Scheifele, 1985 (35) | 0 | 1 | 0 | 0 | 0 | 0 | 20 | 22 |
| Shenep, 1988 (36) | 0 | 1 | 2 | 0 | 1 | 0 | 3 | 13 |
| Strutz, 1999 (37) | 4 | 4 | 1 | 0 | 0 | 1 | 8 | 10 |
| Stumacher, 1973 (38) | 7 | 11 | 10 | 18 | 2 | 4 | 26 | 48 |
| Suyasa, 1995 (39) | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 9 |
| Togari, 1983 (40) | 0 | 0 | 1 | 0 | 0 | 0 | 7 | 2 |
| Van Deventer, 1988 (41) | 7 | 2 | 4 | 0 | 0 | 0 | 14 | 406 |
| Van Dissel, 1993 (42) | 2 | 0 | 2 | 0 | 0 | 0 | 7 | 3 |
| Watzke, 1987 (43) | 0 | 0 | 3 | 1 | 1 | 0 | 4 | 11 |
| Wong, 2013 (44) | 8 | 0 | 5 | 0 | 1 | 0 | 89 | 0 |
| Yoshida, 1993 (45)b | 1 | 0 | 6 | 5 | 4 | 3 | 35 | 71 |
Abbreviations: TP, true positive, the number of patients with endotoxemia and Gram-negative bacteremia; FP, false positive, the number of patients with endotoxemia and without Gram-negative bacteremia; FN, false negative, the number of patients without endotoxemia and with Gram-negative bacteremia; TN, true negative, the number of patients with neither endotoxemia nor Gram-negative bacteremia. Patients with polymicrobial bacteremia were not included in the analysis.
Data provided via personal communication with the study author.
TABLE 3.
Summary data for endotoxemia concordance with GN bacteremia
| GN bacteremia species group | DORa (95% CI), no. of studies |
|
|---|---|---|
| All studies | Studies of sepsisb | |
| E. coli | 3.2 (1.7–6.0), 37 | 1.4 (0.89–2.3), 14 |
| Non-E. coli Enterobacteriaceae | 2.8 (1.5–5.5), 31 | 1.5 (0.82–2.8), 13 |
| Pseudomonas aeruginosa | 5.8 (2.4–13.7), 31 | 1.7 (1.02–3.0), 11 |
DOR = (TP/FN)/(FP/TN), derived using HSROC meta-analysis.
All studies of sepsis used chromogenic versions of the LAL assay, which are typically 100-fold more sensitive than gelation-based versions.
Among the 12 studies limited to patients with sepsis, the proportion of patients without GN bacteremia who were endotoxemia positive was 49% (95% confidence interval [CI], 33 to 64%). In contrast, the proportions of patients with detectable endotoxemia among bacteremic patients for these 12 studies were as follows: E. coli bacteremias, 57% (CI, 40 to 73%; 12 studies); Klebsiella bacteremias, 41% (CI, 29 to 55%; 9 studies); Enterobacter bacteremias, 32% (CI, 10 to 67%; 5 studies); Proteus bacteremias, 76% (CI, 51 to 91%; 3 studies); Serratia bacteremias, 32% (CI, 10 to 67%; 1 study); P. aeruginosa bacteremias, 69% (CI, 57 to 79%; 9 studies). Only in the cases of patients with bacteremia caused by P. aeruginosa (P = 0.004) or Proteus species (P = 0.04), and not those with bacteremia caused by E. coli (P = 0.55) or other Enterobacteriaceae, were the differences compared to those in patients without GN bacteremia statistically significant. Among the 12 studies limited to patients with sepsis, the DORs were marginal, and the DORs in relation to E. coli bacteremias and the category of non-E. coli Enterobacteriaceae bacteremias, but not that in relation to P. aeruginosa, included unity within the respective 95% confidence intervals (Table 3).
DISCUSSION
Endotoxin is present in all GN bacteria. Hence, it might be expected that endotoxemia detection, especially in newer assays, would perform better as a diagnostic marker of GN bacteremia than methods using clinical criteria, which perform poorly (51–53). Moreover, GN bacteremia has a high mortality in association with sepsis despite antibiotic therapy, and observations derived from animal models generally, but not always (48, 54), have implicated a key role for endotoxemia in GN bacteremia pathogenesis. Hence, it might also be anticipated that the concordance between endotoxemia and GN bacteremia would be high in those patients with sepsis. However, the diagnostic and prognostic significance of endotoxemia is complex, and the findings here are somewhat at variance with these expectations.
Endotoxemia is detected in approximately half of those with GN bacteremia, and similarly, GN bacteremia is detected in approximately half of those with endotoxemia. It has been established in previous analyses that the concordance of endotoxemia with GN bacteremia overall is weak regardless of whether a more- or less-sensitive version of the LAL assay is used (3). The questions addressed here were the additional influences of GN bacteremia species type and the use of sepsis criteria for patient inclusion on this concordance and on the endotoxemia detection rates in studies published over the past 4 decades.
There were wide ranges of GN bacteremia types, patient groups, and study sizes among the studies published since the original studies of the Limulus assay for endotoxemia detection in the early 1970s. The findings here indicate that the concordance of endotoxemia with GN bacteremias differs between GN bacteremias of different types. The concordance is surprisingly weak, especially for Enterobacteriaceae GN bacteremia and among studies of patients with sepsis. The concordance between endotoxemia and GN bacteremia found here was higher for P. aeruginosa than for most Enterobacteriaceae. Among studies limited to patients with sepsis, only in the cases of bacteremia with either P. aeruginosa or Proteus spp. was the proportion with endotoxemia found to be significantly above the background detection rate.
Moreover, the prognostic significance of endotoxemia is dependent on the copresence of GN bacteremia and is unequal for GN bacteremias of different types. For example, endotoxemia with E. coli bacteremia has no prognostic significance (55). The somewhat surprising finding here is that the concordance between endotoxemia and GN bacteremia was lower within studies that used sepsis criteria for patient inclusion versus studies that selected patients more broadly, despite the fact that the studies of patients with sepsis all used the more sensitive chromogenic version of the Limulus assay.
This finding is surprising for three reasons. In the application of any assay, the use of a more sensitive version would be expected to achieve a higher true-positive rate (sensitivity). However, this is generally achieved at the cost of a lower true-negative rate (specificity), an expected trade-off (49). Also, given the presumption of a key role of endotoxemia in the mediation of sepsis, a higher concordance between endotoxemia and GN bacteremia would have been expected among studies of patients meeting the criteria for sepsis versus studies of patients more broadly selected. The third reason relates to the complex interrelation between the pathogenesis of bacteremia on the one hand and, on the other hand, the structure-function activity related to how endotoxemia is sensed by the host immune system, which is dependent on the specific lipopolysaccharide structure, and also with regard to how it is detected in the Limulus assay, which is not so dependent.
In this regard, there are key structural differences between the lipid A components of the endotoxin molecule (LPS) of different GN bacteria. Enterobacteriaceae such as E. coli characteristically have a lipid A with a hexa-acyl structure, whereas other lipid A structures are present in non-Enterobacteriaceae species, such as P. aeruginosa (47). The hexa-acyl structure of lipid A is now known to be optimal for the recognition of GN bacteremia by the host immune system via the MD2–Toll-like receptor 4 interaction and the stimulation of cytokine release. The lipid A structure is not critical for sensing by the clotting proteins of the blood cells of the Limulus polyphemus horseshoe crab, from which the LAL assay was derived.
It should be noted that the LAL assay is an assay for endotoxin, not for GN bacteria per se. With an amount of LPS of ∼0.025 pg/CFU from E. coli or P. aeruginosa, it would be expected that there would be 0.25 pg/ml of endotoxin for a bacteremia with 10 viable bacteria per 1 ml of blood (56). Even if this estimated total amount of bacterial cell-bound endotoxin were to be completely available for detection in association with a bacteremia, this would still be below the detection limit of even the most sensitive LAL assay (5 pg/ml). In contrast, but in line with these estimates, in experimental rabbit (57, 58) and canine (59) models of GN bacteremia with either E. coli or Pasteurella sp. bacterial challenge, GN bacteremias at levels of ∼10,000 CFU/ml corresponded to endotoxemia levels of 10 ng/ml (58), 500 ng/ml (57), and 50 EU/ml (∼5 ng/ml) (59). Note, however, that these bacteremia levels are approximately 1,000 times higher than those typically seen in sepsis in humans.
The quantitative relationship between endotoxemia and GN bacteremia is not simple and is subject to influence from several additional bacterial, physicochemical, and patient factors. In particular, the activity of endotoxin is not a uniform gravimetric property for endotoxins of different bacterial origins. Also, the mode of LPS aggregation (60), the interactive effect of plasma (61), and the presence of nonviable bacterial cells and cell fragments that accompany a GN bacteremia (62) influence the relationship. Moreover, differential kinetics of endotoxemia and GN bacteremia are likely each influenced by the presence of virulence factors, which differ for different species of GN bacteria (63).
There are several strengths of this analysis. A broad range of studies have been included, in order to address the impact of factors that cannot be addressed in an animal model of sepsis. The specification of the type of GN bacteremia was a requirement for inclusion in this analysis. The number of studies increased after additional data were sought from authors of potentially eligible studies to enable their inclusion. This resulted in a substantial increase in eligible studies and patient data available, compared to those included in a previous analysis (3). Also, the method of meta-analysis was optimal to enable the inclusion of studies across a broad range of assay breakpoints and studies of various sizes. No single study among those included here would have been sufficiently powered to answer the questions of interest. The findings would not have been achievable using any previously available meta-analytic method. Moreover, there were 12 studies of patient groups with sepsis, and the restricted concordance appeared consistent across this subgroup of studies; hence, the findings appear to be generalizable. The patient group with sepsis is of particular interest, as it would be in this group that any new therapies for either endotoxemia or sepsis would likely be tested.
The influence of several other study parameters, such as study size, study design quality, and method of plasma pretreatment, have been considered elsewhere (2, 3). For example, a disproportionate number of small studies (n < 25) had 100% sensitivity for the detection of endotoxemia (3). Otherwise, these parameters were each found to have a minor impact on concordance compared to the factors identified here.
The findings here are in contrast to paradoxical observations from a controlled model of septic shock in dogs. In the dog, implantation of an intraperitoneal infected clot induces bacteremia with various selected Gram-negative and Gram-positive challenge bacteria, together with cardiovascular changes characteristic of septic shock leading to mortality (48, 54, 59, 65, 66). This model enables the study of quantitative levels of endotoxemia as measured with the chromogenic LAL assay as well as bacteremia. In comparative studies with this model, a relatively avirulent strain of E. coli versus P. aeruginosa (48), Staphylococcus aureus (66), or a more virulent strain of E. coli (65) showed similar quantitative levels of bacteremia, whereas the associated hemodynamic changes and shortened survival times were in each case more severe than those observed in association with the avirulent E. coli strain, as would be expected (48, 65, 66). Surprisingly, despite these expected differences, in each study the levels of endotoxemia were 3-fold (65) to 10-fold (48) lower or even undetectable (46) versus the levels seen after challenge with the avirulent E. coli strain. Interestingly, endotoxins extracted and purified from the virulent and avirulent E. coli strains were equal with respect to potency and endotoxin amount per bacterium; hence, the lack of an association between endotoxemia and disease severity in this experimental model could not be explained on this basis (65).
Moreover, following intraperitoneal challenge with strains of E. coli with (O6:H1:K2) or without (O86:H8) virulence factors for human disease, survival times were shorter and the associated hemodynamic changes were more severe after challenge with the virulent strain, as might be expected. However, there were three paradoxical observations: (i) bacteremia occurred earlier and more frequently after challenge with the avirulent E. coli strain; (ii) levels of endotoxemia were 3-fold higher after challenge with the avirulent E. coli strain; (iii) challenge with heat-killed bacteria at a 10-fold-higher dose was associated with a reversal of the effects on survival and hemodynamic changes seen with live bacterial challenge, as the survival was significantly shortened after challenge with the killed nonvirulent bacteria versus the killed virulent bacteria. Despite this reversal, the levels of endotoxemia were again 3-fold higher after challenge with the killed avirulent versus the killed virulent E. coli strains (65).
Limitations.
There are several limitations of this analysis. Both endotoxemia and GN bacteremia are episodic phenomena, and endotoxemia levels may be increased by antibiotic therapy (64). For example, in one clinical study (10) of 100 patients with sepsis in an intensive care unit (ICU) setting, the cumulative percentage of patients found to have endotoxemia rose from 20% to 40% between 0 and 24 h after study entry. For all but three studies included here, the timing of antibiotic administration in relation to determinations of endotoxemia and bacteremia is unclear.
The studies included in our analysis were published over a period of over 40 years, during which time supportive and antibiotic therapies and underlying patient prognosis factors likely varied. Many relevant patient-specific details, such as age and patient comorbidities, were not available. The findings in this analysis differ slightly from the findings of a previous analysis (3) in which the summary DORs were higher than those derived here. This difference may be a consequence of the studies included in the present analysis being slightly larger in size and mostly more recently published, as well as our analysis having been restricted to bacteremias within three categories of GN bacterial species.
Even with data for 3,868 patients from 42 studies published over the past 4 decades, it is still unclear how substantial the differences are in the proportion of patients with detectable endotoxemia for different GN bacteremia species. The estimates here imply differences that may be as great as 40%. However, given the small numbers for each species, the associated 95% confidence intervals are as wide as 50%.
Another limitation is that the estimations of endotoxemia detection and the detection of bacteremia in the studies here do not take into account bacterial cells that were either nonviable or difficult to grow using current methods, as well as cell fragments associated with a GN bacteremia and how these associations may differ for different specific types of GN bacteremia (62, 63). For example, for Neisseria meningitidis, the concordance of endotoxemia is higher for these fragments than it is for viable bacterial cells (63).
Conclusion.
The concordance between endotoxemia and GN bacteremia differs for different types of GN bacteremia and is marginal among studies using sepsis criteria for patient inclusion.
ACKNOWLEDGMENTS
Work by Charalambos Gogos, Apostolos Armaganidis, and Evangelos J. Giamarellos-Bourboulis in this study was conducted on behalf of the Hellenic Sepsis Study Group (http://www.sepsis.gr).
We thank R. Danner (NIH, Bethesda, MD) (10), K. C. H. Fearon, Royal Infirmary, Edinburgh (17), B. Guidet, Hopital Saint-Antoine, Paris (18), D. Bates, Brigham and Women's Hospital, Boston (23), S. Opal, Alpert Medical School of Brown University, Providence (32), Jan M. Prins, Academic Medical Center, Amsterdam (34), and M. Yoshida, Jichi Medical School, Japan (45) for their clarifications of previously published data.
This research was supported by the Australian Government Department of Health and Ageing through the Rural Clinical Training and Support program.
We declare we have no conflict of interest in the material presented in the manuscript.
REFERENCES
- 1.Hurley JC. 1995. Endotoxemia: methods of detection and clinical correlates. Clin Microbiol Rev 8:268–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurley JC. 2013. Endotoxemia: concordance with Gram-negative bacteremia and association with outcome. Doctor of Medical Science thesis, University of Melbourne, Melbourne, Australia: http://repository.unimelb.edu.au/10187/17991 Accessed 22 October 2014. [Google Scholar]
- 3.Hurley JC. 2009. Does gram-negative bacteremia occur without endotoxaemia? A meta-analysis using hierarchical summary ROC curves. Eur J Clin Microbiol Infect Dis 29:207–215. doi: 10.1007/s10096-009-0841-2. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed T, Azam MA, ArMed Jamil NKM, Hassan F, Ogura N, Tamura H, Yokochi T. 2004. Detection of endotoxin in sera from children hospitalized for treatment of diarrhea in Bangladesh. J Endotoxin Res 10:223–228. doi: 10.1177/09680519040100040401. [DOI] [PubMed] [Google Scholar]
- 5.Bailey ME. 1976. Endotoxin, bile salts and renal function in obstructive jaundice. Br J Surg 63:774–778. doi: 10.1002/bjs.1800631011. [DOI] [PubMed] [Google Scholar]
- 6.Bion JF, Badger I, Crosby HA, Hutchings P, Kong KL, Baker J, Hutton P, McMaster P, Buckels JA, Elliott TS. 1994. Selective decontamination of the digestive tract reduces Gram-negative pulmonary colonization but not systemic endotoxemia in patients undergoing elective liver transplantation. Crit Care Med 22:40–49. [DOI] [PubMed] [Google Scholar]
- 7.Byl B, Clevenbergh P, Kentos A, Jacobs F, Marchant A, Vincent JL, Thys JP. 2001. Ceftazidime- and imipenem-induced endotoxin release during treatment of Gram-negative infections. Eur J Clin Microbiol Infect Dis 20:804–807. doi: 10.1007/s100960100609. [DOI] [PubMed] [Google Scholar]
- 8.Clumeck N, Lauwers S, Kahn A, Mommens M, Butzler JP. 1976. Contribution of the “limulus test” to the diagnosis of endotoxemias and meningitis due to Gram-negative bacteria. Nouv Presse Med 6:1451–1454. (In French.) [PubMed] [Google Scholar]
- 9.Cooperstock M, Riegle L. 1985. Plasma Limulus gelation assay in infants and children: correlation with Gram-negative bacterial infection and evidence for “intestinal endotoxemia.” Prog Clin Biol. Res 189:329–345. [PubMed] [Google Scholar]
- 10.Danner RL, Elin RJ, Hosseini JM, Wesley RA, Reilly JM, Parillo JE. 1991. Endotoxemia in human septic shock. Chest 99:169–175. doi: 10.1378/chest.99.1.169. [DOI] [PubMed] [Google Scholar]
- 11.Dofferhoff AS, Bom VJ, de Vries-Hospers HG, van Ingen J, vd Meer J, Hazenberg BP, Mulder PO, Weits J. 1992. Patterns of cytokines, plasma endotoxin, plasminogen activator inhibitor, and acute-phase proteins during the treatment of severe sepsis in humans. Crit Care Med 20:185–192. doi: 10.1097/00003246-199202000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Engervall P, Granstrom M, Andersson B, Kalin M, Bjorkholm M. 1997. Endotoxemia in febrile patients with hematological malignancies. Relationship of type of bacteremia, clinical findings and serum cytokine pattern. Infection 25:2–7. [DOI] [PubMed] [Google Scholar]
- 13.Feldman S, Pearson TA. 1974. The Limulus test and Gram-negative bacillary sepsis. Am J Dis Child 128:172–174. [DOI] [PubMed] [Google Scholar]
- 14.Fossard DP, Kakkar VV. 1974. The Limulus test in experimental and clinical endotoxaemia. Br J Surg 61:798–804. doi: 10.1002/bjs.1800611013. [DOI] [PubMed] [Google Scholar]
- 15.Garcia Curiel A, Cantos de la Casa A, Martin Martin A, Aleman Aleman C. 1979. Determination of endotoxemia by means of the Limulus test in patients with septicemia. Med Clin (Barc) 72:375–377. (In Spanish.) [PubMed] [Google Scholar]
- 16.Giamarellos-Bourboulis EJ, Perdios J, Lelekis M, Eoconomou E, Tsouroulas P, Giamarellou H. 1999. Impact of cefuroxime administration on endotoxin (LPS) and tumour necrosis factor-α (TNF-α) blood levels in patients suffering from acute pyelonephritis: a preliminary report. Int J Antimicrob Agents 11:115–119. doi: 10.1016/S0924-8579(98)00106-X. [DOI] [PubMed] [Google Scholar]
- 17.Goldie AS, Fearon KC, Ross JA, Barclay GR, Jackson RE, Grant IS, Ramsay G, Blyth AS, Howie JC. 1995. Natural cytokine antagonists and endogenous anti-endotoxin core antibodies in sepsis syndrome. The Sepsis Intervention Group. JAMA 274:172–177. [PubMed] [Google Scholar]
- 18.Guidet B, Barakett V, Vassal T, Petit JC, Offenstadt G. 1994. Endotoxemia and bacteremia in patients with sepsis syndrome in the intensive care unit. Chest 106:1194–1201. doi: 10.1378/chest.106.4.1194. [DOI] [PubMed] [Google Scholar]
- 19.Hass A, Rossberg MI, Hodes HL, Hyatt AC, Hodes DS. 1986. Endotoxin levels in immunocompromised children with fever. J Pediatr 109:265–269. doi: 10.1016/S0022-3476(86)80383-3. [DOI] [PubMed] [Google Scholar]
- 20.Hynninen M, Valtonen M, Vaara M, Markkanen H, Kuusela P, Saxen H, Takkunen O. 1995. Plasma endotoxin and cytokine levels in neutropenic and non-neutropenic bacteremic patients. Eur J Clin Microbiol Infect Dis 14:1039–1045. doi: 10.1007/BF01590936. [DOI] [PubMed] [Google Scholar]
- 21.Jirillo E, Pasquetto N, Marcuccio L, Monno R, De Rinaldis P, Fumarola D. 1975. Endotoxemia detected by Limulus assay in severe malnourished children. Plasma effects on leucocyte migration: preliminary investigations. G Batteriol Virol Immunol 68:174–178. [PubMed] [Google Scholar]
- 22.Kelsey MC, Lipscomb AP, Mowles JM. 1982. Limulus amoebocyte lysate endotoxin test: an aid to the diagnosis in the septic neonate? J Infect 4:69–72. doi: 10.1016/S0163-4453(82)91104-5. [DOI] [PubMed] [Google Scholar]
- 23.Ketchum PA, Parsonnet J, Stotts LS, Novitsky TJ, Schlain B, Bates DW. 1997. Utilization of a chromogenic Limulus amebocyte lysate blood assay in a multi-center study of sepsis. J Endotoxin Res 4:9–16. [Google Scholar]
- 24.Kritselis I, Tzanetakou V, Adamis G, Anthopoulos G, Antoniadou E, Bristianou M, Giamarellos-Bourboulis EJ. 2013. The level of endotoxemia in sepsis varies in relation to the underlying infection: impact on final outcome. Immunol Lett 152:167–172. doi: 10.1016/j.imlet.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Lau JY, Chung SC, Leung JW, Ling TK, Yung MY, Li AK. 1996. Endoscopic drainage aborts endotoxaemia in acute cholangitis. Br J Surg 83:181–184. doi: 10.1002/bjs.1800830210. [DOI] [PubMed] [Google Scholar]
- 26.Levin J, Poore TE, Young NS, Margolis S, Zauber NP, Townes AS, Bell WR. 1972. Gram-negative sepsis: detection of endotoxemia with the limulus test. With studies of associated changes in blood coagulation, serum lipids, and complement. Ann Intern Med 76:1–7. doi: 10.7326/0003-4819-76-1-1. [DOI] [PubMed] [Google Scholar]
- 27.Levin J, Poore TE, Zauber NP, Oser RS. 1970. Detection of endotoxin in the blood of patients with sepsis due to gran-negative bacteria. N Engl J Med 283:1313–1316. doi: 10.1056/NEJM197012102832404. [DOI] [PubMed] [Google Scholar]
- 28.Martinez LA, Quintiliani R, Tilton RC. 1973. Clinical experience on the detection of endotoxemia with the limulus test. J Infect Dis 127:102–105. doi: 10.1093/infdis/127.1.102. [DOI] [PubMed] [Google Scholar]
- 29.Massignon D, Lepape A, Debize G, Remillieux MF, De Pasquale V, Banssillon V, Coeur P, et al. . 1996. Detection of Gram-negative bacteraemia in early sepsis by a quantitative chromogenic and kinetic endotoxin assay. Eur J Clin Invest 26:596–601. doi: 10.1046/j.1365-2362.1996.1810531.x. [DOI] [PubMed] [Google Scholar]
- 30.McCartney AC, Robertson MR, Piotrowicz BI, Lucie NP. 1987. Endotoxaemia, fever and clinical status in immunosuppressed patients: a preliminary study. J Infect 15:201–206. doi: 10.1016/S0163-4453(87)92528-X. [DOI] [PubMed] [Google Scholar]
- 31.Oberle MW, Graham GG, Levin J. 1974. Detection of endotoxemia with the Limulus test: preliminary studies in severely malnourished children. J Pediatr 85:570–573. doi: 10.1016/S0022-3476(74)80473-7. [DOI] [PubMed] [Google Scholar]
- 32.Opal SM, Scannon PJ, Vincent JL, White M, Carroll SF, Palardy JE, Parejo NA, Pribble JP, Lemke JH. 1999. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J Infect Dis 180:1584–1589. doi: 10.1086/315093. [DOI] [PubMed] [Google Scholar]
- 33.Pearson FC, Dubczak J, Weary M, Bruszer G, Donohue G. 1985. Detection of endotoxin in the plasma of patients with Gram-negative bacterial sepsis by the Limulus amoebocyte lysate assay. J Clin Microbiol 21:865–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prins JM, van Agtmael MA, Kuijper EJ, van Deventer SJ, Speelman P. 1995. Antibiotic-induced endotoxin release in patients with Gram-negative urosepsis: a double-blind study comparing imipenem and ceftazidime. J Infect Dis 172:886–891. doi: 10.1093/infdis/172.3.886. [DOI] [PubMed] [Google Scholar]
- 35.Scheifele DW, Olsen EM, Pendray MR. 1985. Endotoxinemia and thrombocytopenia during neonatal necrotizing enterocolitis. Am J Clin Pathol 83:227–229. [DOI] [PubMed] [Google Scholar]
- 36.Shenep JL, Flynn PM, Barrett FF, Stidham GL, Westenkirchner DF. 1988. Serial quantitation of endotoxemia and bacteremia during therapy for Gram-negative bacterial sepsis. J Infect Dis 157:565–568. doi: 10.1093/infdis/157.3.565. [DOI] [PubMed] [Google Scholar]
- 37.Strutz F, Heller G, Krasemann K, Krone B, Muller GA. 1999. Relationship of antibodies to endotoxin core to mortality in medical patients with sepsis syndrome. Intensive Care Med 25:435–444. doi: 10.1007/s001340050877. [DOI] [PubMed] [Google Scholar]
- 38.Stumacher RJ, Kovnat MJ, McCabe WR. 1973. Limitations of the usefulness of the Limulus assay for endotoxin. N Engl J Med 288:1261–1264. doi: 10.1056/NEJM197306142882402. [DOI] [PubMed] [Google Scholar]
- 39.Suyasa IG, Reka IG, Inada K, Suda H, Kojima M, Mushiaki K, Okamoto S, Yoshida M. 1995. Plasma endotoxin in typhoid fever. Kobe J Med Sci 41:175–186. [PubMed] [Google Scholar]
- 40.Togari H, Mikawa M, Iwanaga T, Matsumoto N, Kawase A, Hagisawa M, Ogino T, Goto R, Watanabe I, Kito H. 1983. Endotoxin clearance by exchange blood transfusion in septic shock neonates. Acta Paediatr Scand 72:87–91. doi: 10.1111/j.1651-2227.1983.tb09669.x. [DOI] [PubMed] [Google Scholar]
- 41.van Deventer SJ, Buller HR, ten Cate JW, Sturk A, Pauw W. 1988. Endotoxaemia: an early predictor of septicaemia in febrile patients. Lancet i:605–609. [DOI] [PubMed] [Google Scholar]
- 42.van Dissel JT, van Furth R, Compier BA, Feuth HD, Frolich M. 1993. Survival in selected patients with Gram-negative sepsis after adjunctive therapy with HA-1A. Lancet 341:959–960. doi: 10.1016/0140-6736(93)91250-P. [DOI] [PubMed] [Google Scholar]
- 43.Watzke H, Schwarz HP, Luger A, Stummvoll HK. 1987. Clinical value of endotoxin determination in infection. Comparison of the Limulus amebocyte lysate test with detection of bacterial pathogens. Acta Med Austriaca 14:21–24. (In German.) [PubMed] [Google Scholar]
- 44.Wong M, Barqasho B, Öhrmalm L, Tolfvenstam T, Nowak P. 2013. Microbial translocation contribute to febrile episodes in adults with chemotherapy-induced neutropenia. PLoS One 8:e68056. doi: 10.1371/journal.pone.0068056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshida M, Obayashi T, Tamura H, Tanaka S, Kawai T, Sakamoto S, Miura Y. 1994. Diagnostic and prognostic significance of plasma endotoxin determination in febrile patients with haematological malignancies. Eur J Cancer 30A:145–147. [DOI] [PubMed] [Google Scholar]
- 46.Hurley JC. 2013. Towards clinical applications of anti-endotoxin antibodies: a re-appraisal of the disconnect. Toxins 5:2589–2620. doi: 10.3390/toxins5122589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munford RS. 2008. Sensing Gram-negative bacterial lipopolysaccharides: a human disease determinant? Infect Immun 76:454–465. doi: 10.1128/IAI.00939-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Danner RL, Natanson C, Elin RJ, Hosseini JM, Banks S, MacVittie TJ, Parrillo JE. 1990. Pseudomonas aeruginosa compared with Escherichia coli produces less endotoxemia but more cardiovascular dysfunction and mortality in a canine model of septic shock. Chest 98:1480–1487. doi: 10.1378/chest.98.6.1480. [DOI] [PubMed] [Google Scholar]
- 49.Hurley JC. 2011. Meta-analysis of clinical studies of diagnostic tests: developments in how the receiver operating characteristic “works.” Arch Pathol Lab Med 135:1585–1590. [DOI] [PubMed] [Google Scholar]
- 50.Hurley JC. 2001. Endotoxemia and Gram-negative bacteremia as predictors of outcome in sepsis: a call for data. J Endotoxin Res 7:467. doi: 10.1177/09680519010070060201. [DOI] [PubMed] [Google Scholar]
- 51.Peduzzi P, Shatney C, Sheagren J, Sprung C. 1992. Predictors of bacteremia and Gram-negative bacteremia in patients with sepsis. The Veterans Affairs Systemic Sepsis Cooperative Study Group. Arch Intern Med 152:529–535. [PubMed] [Google Scholar]
- 52.Bates DW, Cook EF, Goldman L, Lee TH. 1990. Predicting bacteremia in hospitalized patients. A prospectively validated model. Ann Intern Med 113:495–500. [DOI] [PubMed] [Google Scholar]
- 53.Bates DW, Sands K, Miller E, Lanken PN, Hibberd PL, Graman PS, Schwartz JS, Kahn K, Snydman DR, Parsonnet J. 1997. Predicting bacteremia in patients with sepsis syndrome. Academic Medical Center Consortium Sepsis Project Working Group. J Infect Dis 176:1538–1551. [DOI] [PubMed] [Google Scholar]
- 54.Hoffman WD, Pollack M, Banks SM, Koev LA, Solomon MA, Danner RL, Koles N, Guelde G, Yatsiv I, Mouginis T. 1994. Distinct functional activities in canine septic shock of monoclonal antibodies specific for the O polysaccharide and core regions of Escherichia coli lipopolysaccharide. J Infect Dis 169:553–561. doi: 10.1093/infdis/169.3.553. [DOI] [PubMed] [Google Scholar]
- 55.Hurley JC, Opal S. 2013. Prognostic value of endotoxemia in patients with Gram-negative bacteremia is bacterial species dependent. J Innate Immun 5:555–564. doi: 10.1159/000347172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yagupsky P, Nolte FS. 1990. Quantitative aspects of septicemia. Clin Microbiol Rev 3:269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corrigan JJ Jr, Kiernat JF. 1975. Effect of heparin in experimental Gram-negative septicemia. J Infect Dis 131:138–143. doi: 10.1093/infdis/131.2.138. [DOI] [PubMed] [Google Scholar]
- 58.Shenep JL, Barton RP, Mogan KA. 1985. Role of antibiotic class in the rate of liberation of endotoxin during therapy for experimental Gram-negative bacterial sepsis. J Infect Dis 151:1012–1018. doi: 10.1093/infdis/151.6.1012. [DOI] [PubMed] [Google Scholar]
- 59.Quezado ZM, Natanson C, Alling DW, Banks SM, Koev CA, Elin RJ, Hosseini JM, Bacher JD, Danner RL, Hoffman WD. 1993. A controlled trial of HA-1A in a canine model of Gram-negative septic shock. JAMA 269:2221–2227. doi: 10.1001/jama.1993.03500170051033. [DOI] [PubMed] [Google Scholar]
- 60.Komuro T, Murai T, Kawasaki H. 1987. Effect of sonication on the dispersion state of lipopolysaccharide and its pyrogenicity in rabbits. Chem Pharm Bull (Tokyo) 35:4946–4952. doi: 10.1248/cpb.35.4946. [DOI] [PubMed] [Google Scholar]
- 61.Hurley JC, Tosolini FA, Louis WJ. 1991. Quantitative Limulus lysate assay for endotoxin and the effect of plasma. J Clin Pathol 44:849–854. doi: 10.1136/jcp.44.10.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sleigh J, Cursons R, La Pine M. 2001. Detection of bacteraemia in critically ill patients using 16S rDNA polymerase chain reaction and DNA sequencing. Intensive Care Med 27:1269–1273. doi: 10.1007/s001340100981. [DOI] [PubMed] [Google Scholar]
- 63.Ovstebo R, Brandtzaeg P, Brusletto B, Haug KB, Lande K, Hoiby EA, Kierulf P. 2004. Use of robotized DNA isolation and real-time PCR to quantify and identify close correlation between levels of Neisseria meningitidis DNA and lipopolysaccharides in plasma and cerebrospinal fluid from patients with systemic meningococcal disease. J Clin Microbiol 42:2980–2987. doi: 10.1128/JCM.42.7.2980-2987.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hurley JC. 1992. Antibiotic-induced release of endotoxin: a reappraisal. Clin Infect Dis 15:840–854. doi: 10.1093/clind/15.5.840. [DOI] [PubMed] [Google Scholar]
- 65.Hoffman WD, Danner RL, Quezado ZM, Banks SM, Elin RJ, Hosseini JM, Natanson C. 1996. Role of endotoxemia in cardiovascular dysfunction and lethality: virulent and nonvirulent Escherichia coli challenges in a canine model of septic shock. Infect Immun 64:406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Natanson C, Danner RL, Elin RJ, Hosseini JM, Peart KW, Banks SM, MacVittie TJ, Walker RI, Parrillo JE. 1989. Role of endotoxemia in cardiovascular dysfunction and mortality. Escherichia coli and Staphylococcus aureus challenges in a canine model of human septic shock. J Clin Invest 83:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]