Abstract
Saline stress is one of the most important abiotic factors limiting the growth and development of plants and associated microorganisms. While the impact of salinity on associations of arbuscular fungi is relatively well understood, knowledge of the ectomycorrhizal (EM) fungi of trees growing on saline land is limited. The main objective of this study was to determine the density and diversity of EM fungi associated with three tree species, Salix alba, Salix caprea and Betula pendula, growing in saline soil during two seasons, autumn and spring. The site was located in central Poland, and the increased salinity of the soil was of anthropogenic origin from soda production. The degree of EM colonisation of fine root tips varied between 9 and 34 % and depended on the tree species of interest (S. caprea < S. alba < B. pendula) and season (spring < autumn). Moreover, the ectomycorrhizal colonisation of B. pendula was positively correlated with pH and CaCO3, while for S. caprea and S. alba, colonisation was associated with most of the other soil parameters investigated; e.g. salinity, Corg and N. Analysis of EM fungi revealed four to five different morphotypes per each season: Tomentella sp. Sa-A, Hebeloma collariatum Sc-A, Geopora sp. Sc-A, Helotiales sp. Bp-A in the autumn and Tomentella sp. Sa-S, Tomentella sp. Sc-S and three morphotypes from the families Thelephoraceae and Pyronemataceae in the spring. In conclusion, the density of EM is related to the level of salinity (ECe), season and tree species. Tomentella spp., Hebeloma sp., Geopora sp. and Helotiales sp. are groups of species highly adapted to saline conditions.
Keywords: Salinity, Diversity, Ectomycorrhizal fungi, Willow, Birch
Introduction
Symbiosis of ectomycorrhizal (EM) fungi is a commonly known type of plant association responsible for the proper growth and development of trees under abiotic stress conditions (Hrynkiewicz and Baum 2012a). Well-adapted ectomycorrhizae can increase the availability of nutrients and water to the host plant in adverse soil conditions (e.g. poor and drought-affected soils), decrease the direct negative impact of soil contamination (e.g. heavy metals such as Zn, Cu, Mn, Ni and Cr) and unfavourable pH and increase the resistance to disease from pathogenic fungi and bacteria (Dell 2002; Paradi and Baar 2006). The density and diversity of certain types of EM fungi in abiotic stress conditions can significantly depend on the tree species, seasonal changes and type of biotic and/or abiotic stress (Hrynkiewicz et al. 2008, 2010a, b). High soil salinity is an abiotic factor that may have a negative impact on EM associations, and research into understanding its influence is on-going.
Soil salinity is a serious problem for plant growth and development and may lead to a reduction in the abundance and metabolic activity of microorganisms (Silva Maganhotto de Souza and Fay 2012; Milosević et al. 2012; Szymańska et al. 2013). According to the Food and Agriculture Organization (FAO 2008), saline soils represent more than 6 % of the world's land and is one of the fastest processes causing land degradation (Kõljalg et al. 2000). This is the consequence of the destruction of the soil structure and therefore a reduction in oxygen, as a result of the accumulation of water-soluble salts in the soil (mainly the accumulation of Na+ but also K+, Mg2+, Ca2+, Cl−, SO4 2−, CO3 2− and HCO3 − ions) (Silva Maganhotto de Souza and Fay 2012). Previous studies focused primarily on halotolerant plants and associated halophilic/halotolerant bacteria and endomycorrhizal fungi (Evelin et al. 2009; Gago et al. 2011; Silva and Fay 2012; Milosević et al. 2012; Szymańska et al. 2013). It is already known that arbuscular mycorrhizal (AM) fungi can promote the growth of crops in saline agricultural soils (e.g. effect of fertilisation) (e.g. Aggarwal et al. 2012). Meanwhile, much less attention has been paid to associations of EM fungi and plants growing at saline sites (Dell 2002; Bois et al. 2006; Jimenez-Casas and Zwiazek 2013).
Salix and Betula spp. are pioneer tree species that naturally grow in harsh soil conditions (Varga et al. 2009; Hrynkiewicz et al. 2009a, 2010a, b) and are the only tree species naturally growing in the saline area analysed in this study. The extraordinary properties of these trees to unfavourable soil conditions could be the result of the ease and simplicity in the development of EM symbiosis on the roots of these species. We hypothesised that EM fungi, characterised by a high tolerance to unfavourable abiotic conditions, can be a key factor in the protection of the host plants growing in saline soils (i) and that EM community structure may depend on the season and correlate with salt level and other soil parameters (ii).
The aim of our research was to determine the density and diversity of EM fungi associated with different tree species (S. alba, S. caprea and B. pendula) growing in saline soil in relation to seasonality and changes in salt concentration and other soil parameters.
Materials and Methods
Site Description and Sampling
This study was carried out in autumn 2012 and spring 2013 in Inowrocław-Mątwy (N 52° 48, E 18° 15) in the central part of Poland. This region has a continental climate with a mean annual temperature of +8.3 °C and a mean annual precipitation of 494 mm. The area of our research (ca 100 ha) included a saline meadow located near a soda factory (Soda Poland CIECH SA). It is the only company in Poland and the second company in the European market that produces dense and light soda ash. The factory has been in operation since 1879. Local land degradation was a result of the improper storage of industrial wastes from soda production (Hulisz and Piernik 2013). The only trees naturally growing in this area are Salix alba, Salix caprea and Betula pendula (~20 years old, growing at a distance 20–30 m). The soils degraded by the technogenically induced salinisation process in Inowrocław-Matwy are classified as Mollic Technosoils (Calcaric) (Hulisz and Piernik 2013).
Root and soil samples (20 × 20 cm, 20 cm deep) of the trees were collected in two seasons: autumn 2012 (A) and spring 2013 (S). In each season, nine root and soil samples were collected from three plants. In total, 18 root and soil samples were taken (two seasons, three plant species, each variant in three replications).
Due to the fact that in the study area, naturally occurred only in three trees (as described above), we were not able to collect a larger number of natural samples/reps. However, in view of the pioneering nature of research on abundance and diversity of ectomycorrhizae at saline areas and the relatively high sensitivity of trees that naturally colonise the saline lands, our findings may help to initiate further research, which confirm the possibilities of creating mycorrhizal association under high salinity.
Soil Description
Rhizosphere soil (soil closely adjacent to the plant roots) of each sample was gently separated from the roots and analysed. Conventional physico-chemical analysis of the soil rhizosphere was conducted to determine the impact of soil on the abundance and diversity of EM fungi. Within the basic analysis, the concentrations of organic matter, organic carbon and calcium carbonate were determined according to methods described by Bednarek (2005). The total nitrogen, phosphorus soluble in 1 % citric acid solution (Pca), pH-H2O and pH-KCl levels, the salinity of a saturated paste (expressed as electrical conductivity (ECe)) and the concentration of major anions (Cl−, SO4 2−, HCO3 −) and cations (K+, Ca2+, Na+, Fe2+) were determined based on methods described by van Reeuvijk (2002) (Table 3).
Table 3.
Results of two-factorial ANOVA: MS effect, F value and P level for density of EM fungi observed for the two seasons (autumn 2012, spring 2013) and three tree species (S. alba, S. caprea, B. pendula)
| Parameter | MS effect | F | p level |
|---|---|---|---|
| (1) Season | 4418.1 | 47.01 | 0.000* |
| (2) Tree | 15,606.5 | 166.04 | 0.000* |
| (3) Season × tree | 404.2 | 4.30 | 0.014* |
| Error | 94.0 |
Determination of Density and Diversity of EM Morphotypes
Analysis was performed according to Hrynkiewicz et al. (2012b). Shortly, root samples with soil were soaked in de-ionised water overnight. Then, the roots were gently separated and washed using de-ionised water before microscopic examination (Carl Zeiss, Jena, Germany). From each of the 18 soil samples, from six to ten root fragments (10 × 10 cm for each sample from autumn and S. caprea roots from spring; 6 × 10 cm roots of S. alba and 7 × 10 cm roots of B. pendula from spring) were randomly chosen on a grid for microscopic quantification of EM colonisation of fine root tips. The number of living non-colonised root tips vs. visually colonised EM root tips was counted using the formula: root tips × 100 %/total numbers of root tips (Agerer 1991). In total, 17,513 root tips were scanned. A minimum of 443 to 819 root tips per sample and 1035 to 5309 roots per tree species and season were investigated. All colonised root tips collected from each sample were used separately for analysis of EM fungal species diversity. In total, 914 EM root tips were collected. A minimum of 11 to 16 EM root tips per sample and of 99 to 213 EM root tips per tree species (S. caprea, S. alba, B. pendula) and season (autumn 2012 and spring 2013) were investigated. The morpho-anatomical EM fungal types were distinguished by macroscopic characteristics of the fungal mantle, colour, surface appearance, presence of emanating hyphae and hyphal strands, as well as microscopic features such as mantle type and hyphal connections (Agerer 1987–2002). In total, nine different EM morphotypes were identified from all samples. Two to five root tips per morphotype found in each analysed sample were separately frozen in Eppendorf tubes and stored at −20 °C for molecular analysis.
Molecular Analysis
DNA was extracted from the EM root tips using the plant DNAeasy Plant Mini Kit (Qiagen) according to the protocol. The fungal taxa were identified based on the internal transcribed spacer (ITS) region of the rDNA. To amplify this region, the fungal specific primers ITS1F and ITS4 were used (Gardes and Bruns 1993). The PCR analysis and the DNA sequencing were conducted according to Hrynkiewicz et al. (2009a, b). The forward and reverse sequences were assembled and edited using Sequencher 5.1 (Gene Codes 20). The identification process required a minimum of 98 % similarity to the investigated sequence, with reference sequences deposited in GenBank and/or the UNITE nucleotide database. All DNA sequences determined in this research will be submitted to GenBank, and the accession numbers will be presented in Table 4.
Table 4.
Tukey's test for testing all pairwise comparisons (season and tree species)
| Tukey post hoc comparison | |
|---|---|
| (1) Season | |
| Autumn 2012 | 23.621 b |
| Spring 2013 | 16.053 a |
| (2) Tree | |
| S. alba | 19.388 b |
| S. caprea | 12.003 a |
| B. pendula | 31.347 c |
Significant differences are marked by different letters
Statistical Analysis
Differences in the abundance of EM fungi for all research variants (including different tree species and seasons) were investigated by ANOVA with Tukey's test as a post hoc comparison using Statistica software (Statistica ver. 7, Statsoft). The relation between the level of root colonisation of three tree species (S. alba, S. caprea, B. pendula) and soil properties during two seasons (autumn 2012 and spring 2013) was analysed by redundancy analysis (RDA). The same analysis was used to assess the relation between root colonisation by EM fungi and rhizosphere soil properties. The relation between the most important variable of chemical rhizosphere soil parameters of the tree species at the saline site during two seasons was analysed by discriminant analysis (canonical variate analysis (CVA)). The relative importance and statistical significance of each environmental factor in the ordination model were tested by a forward selection procedure and Monte Carlo permutation test. All ordination methods were applied with the use of the Canoco 4.5 package (ter Braak and Smilauer 2002).
Results
Rhizosphere Soil Parameters
The physico-chemical parameters of soil closely adjacent to the roots differed significantly between the tree species (B. pendula, S. alba, S. caprea) and between the seasons (autumn 2012 and spring 2013) (Table 1). In general, the highest average percentages of organic matter (OM), organic carbon (Corg) and total nitrogen (Ntot) were observed in the rhizosphere soil of S. alba, while significantly lower values were noted in the rhizospheres of B. pendula and S. caprea. Otherwise, the highest levels of C/N, pH and CaCO3 were observed in the rhizosphere soil of B. pendula, with significantly lower values observed in the case of S. caprea and S. alba (Table 1). Significant differences in soil parameters between the two seasons were tree-specific (significant increase marked with an arrow).
Table 1.
Physico-chemical soil parameters (mean and standard deviation) in autumn 2012 and in spring 2013
| Variable | Tree species | Autumn 2012 | Spring 2013 |
|---|---|---|---|
| Org. matter (MO) (g kg−1) | Salix alba | 20.51 c (0.79) | 26.62 c [↑] (1.29) |
| Salix caprea | 9.53 b [↑] (0.60) | 7.01 a (0.59) | |
| Betula pendula | 9.33 (0.61) a | 10.2867 (0.75) b | |
| C org. (g kg−1) | S. alba | 20.51 b [↑] (0.95) | 10.67 b (1.58) |
| S. caprea | 9.53 a [↑] (0.03) | 4.44 a (0.13) | |
| B. pendula | 9.33 a [↑] (0.10) | 3.81 a (0.20) | |
| N total (g kg−1) | S. alba | 0.73 a (0.04) | 0.90 c [↑] (0.04) |
| S. caprea | 0.41 a [↑] (0.01) | 0.20 a (0.00) | |
| B. pendula | 0.20 a (0.0099) | 0.26 b [↑] (0.0074) | |
| C/N | S. alba | 14.64 b (0.45) | 17.76 a [↑] (0.99) |
| S. caprea | 10.87 a (0.24) | 17.61 a [↑] (0.86) | |
| B. pendula | 18.78 c (1.27) | 17.91 a (0.25) | |
| pH-H2O | S. alba | 7.73 a [↑] (0.11) | 7.45 a (0.05) |
| S. caprea | 8.10 b (0.00) | 8.07 b (0.06) | |
| B. pendula | 8.50 c [↑] (0.00) | 7.87 c (0.06) | |
| pH-1 M KCl | S. alba | 7.57 a (0.15) | 7.35 b (0.05) |
| S. caprea | 7.80 b (0.00) | 7.73 a (0.06) | |
| B. pendula | 8.00 c (0.00) | 7.70 a (0.00) | |
| CaCO3 (g kg−1) | S. alba | 5.07 a (0.11) | 10.65 b [↑] (0.15) |
| S. caprea | 19.57 b [↑] (0.76) | 10.50 a (0.20) | |
| B. pendula | 46.03 c (1.62) | 43.23 c (2.16) | |
| Na+ (mg l−1) | S. alba | 101.8 (2.7) | 433.7 (11.5) |
| S. caprea | 33.0 (0.9) | 257.7 (6.8) | |
| B. pendula | 89.7 (2.4) | 280.2 (7.4) | |
| Ca2+ (mg l−1) | S. alba | 287.5 (7.6) | 385.1 (11.6) |
| S. caprea | 60.8 (2.7) | 197.0 (8.6) | |
| B. pendula | 168.2 (7.0) | 89.9 (3.5) | |
| K+ (mg l−1) | S. alba | 205.7 (7.4) | 174.3 (6.1) |
| S. caprea | 33.1 (1.3) | 53.2 (1.9) | |
| B. pendula | 48.1 (2.1) | 33.9 (1.0) | |
| Mg2+ (mg l−1) | S. alba | 34.9 (0.7) | 62.4 (1.9) |
| S. caprea | 8.77 (0.3) | 23.8 (1.0) | |
| B. pendula | 15.9 (0.7) | 14.0 (0.5) | |
| Fe2+ (mg l−1) | S. alba | 1.22 (0.0) | 0.87 (0.0) |
| S. caprea | 0.98 (0.0) | 0.99 (0.0) | |
| B. pendula | 1.25 (0.0) | 1.13 (0.0) | |
| Cl− (mg l−1) | S. alba | 716.5 (29.3) | 1600 (80.2) |
| S. caprea | 160.0 (4.4) | 628.0 (31.3) | |
| B. pendula | 401.0 (7.8) | 590.0 (12.1) | |
| SO4 2− (mg l−1) | S. alba | 283.9 (6.9) | 69.8 (3.4) |
| S. caprea | 53.4 (1.7) | 290.1 (9.6) | |
| B. pendula | 146.4 (6.6) | 109.6 (2.4) | |
| HCO3 − (mg l−1) | S. alba | 16.3 (1.0) | 11.4 (0.3) |
| S. caprea | 8.1 (0.2) | 9.8 (0.6) | |
| B. pendula | 12.2 (0.3) | 8.9 (0.3) |
The content of the components in a saturated extract. The data represent the mean of nine replicates ± SD. The mean values of each parameter within the given column marked with the same letter do not differ significantly (p < 0.05)
“[↑]” significantly higher level of rhizosphere soil parameter observed between the seasons
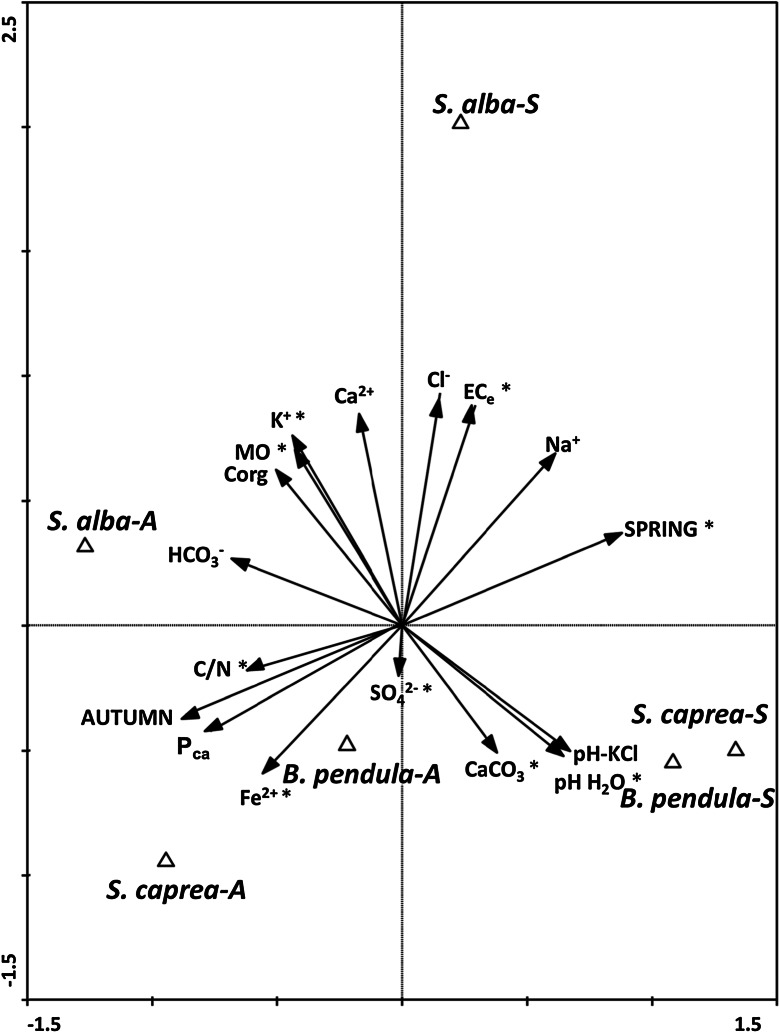
The level of salinity (ECe) in the rhizosphere soils ranged from 0.5 to 5.0 (dS m−1) and was the highest in the rhizosphere of S. alba (2.8 and 5.0 dS m−1 for autumn and spring, respectively) and the lowest in the rhizosphere of B. pendula (0.5 and 2.0 dS m−1 for autumn and spring, respectively). The salinity of the rhizosphere of S. caprea was at the average level (1.4 and 2.4 dS m−1 for autumn and spring, respectively). According to soil scale salinity (Jackson 1958), the rhizosphere samples belong to slightly saline soils (with the exception of B. pendula in autumn, when the salinity was <2 dS m−1 - non-saline soil). Analysis of soil nutrient concentrations revealed the highest levels of the parameters Ca2+, Mg2+, K+, Na+, Cl-, HCO3− and SO4 2− (mg l−1) for S. alba; Fe2+ (mg l−1) and Pca (mg kg−1) for S. caprea; and the lowest in the rhizosphere soil of B. pendula. The level of soil nutrient concentrations (similar to the level of salinity) was in general higher during the spring than the autumn. The CCA diagram based on chemical soil properties in the rhizosphere soil (18 parameters in total) under the three tree species (S. alba, S. caprea, B. pendula) at a saline site during two seasons (autumn and spring) (Fig. 1) allowed the selection of nine factors (MO, C/N, CaCO3, ECe, K+, Fe2+, Pca, spring) that are significant in the differentiation of soil properties. CCA analysis showed that axis 1 significantly contributed to the explained variance (p < 0.05), including the seasons. The soil parameters analysed in the autumn samples were positively related to the C/N, K+, Fe2+ and Pca content of the soil, while the spring soil properties were associated with ECe, Na2+, pH and CaCO3 levels.
Fig. 1.
Canonical variate analysis: diagrams with axes 1 and 2 for 15 chemical soil parameters (MO, C org, C/N, pH-H2O, pH-1 M KCl, CaCO3, ECe, Ca2+, K+, Na+, Fe2+, HCO3 −, Cl−, SO4 2−, Pca) of three tree species (S. alba, S. caprea, B. pendula) at a saline site during two seasons (autumn 2012 and spring 2013). *p ≤ 0.05, significant factors
Effect of Tree Species, Seasons and Soil Parameters on EM Colonisation in Saline Soil
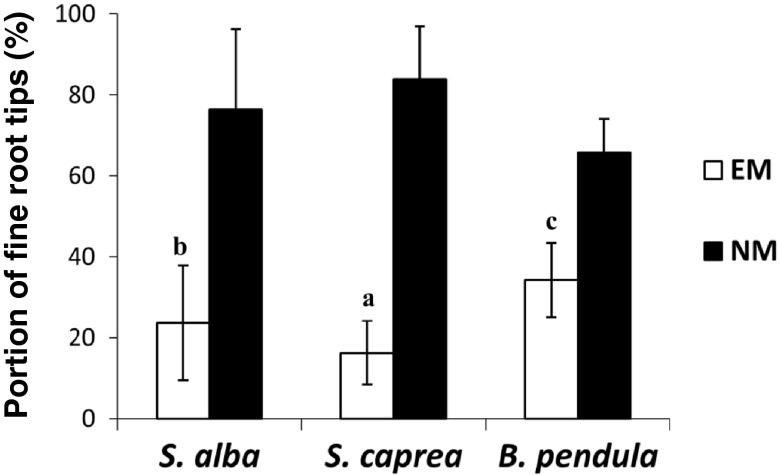
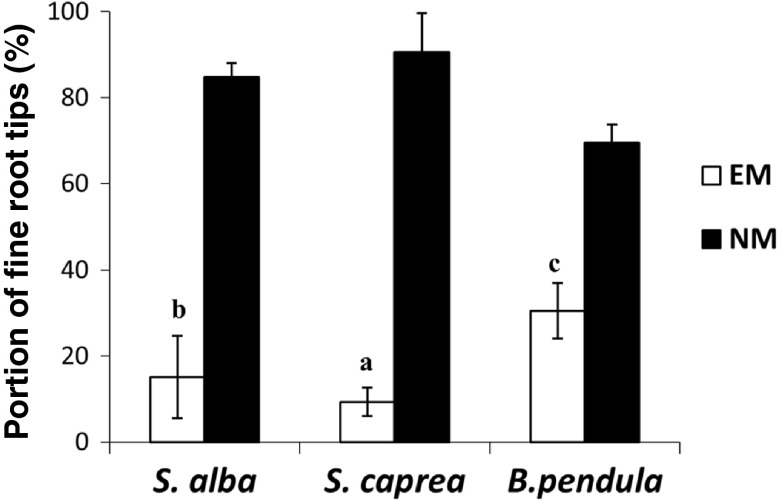
The EM colonisation of fine root tips in all treatments ranged from 16 to 34 % in the autumn and from 9 to 30 % in the spring (Figs. 2 and 3). In general, the highest portions of the EM roots were observed for B. pendula and the lowest for S. caprea (B. pendula > S. alba > S. caprea). The frequency of EM fine root tips in the total number of investigated fine root tips ranged from 30 to 34 % for B. pendula, 15 to 23 % for S. alba and 9 to 16 % for S. caprea in the spring and autumn, respectively (Figs. 2 and 3). The results of two-factorial ANOVA analysis and Tukey's test confirmed a significant effect of all variants analysed during the experiment: season, tree species and season × tree species on EM colonisation of fine root tips in saline soil (Tables 2, 3 and 4). The frequency of non-mycorrhizal fine root tips was always larger in the spring than in the autumn.
Fig. 2.
Ectomycorrhizal (EM) and non-mycorrhizal (NM) fine root tips (%, mean ± standard deviation) under S. alba, S. caprea and B. pendula in autumn 2012
Fig. 3.
Ectomycorrhizal (EM) and non-mycorrhizal (NM) fine root tips (%, mean ± standard deviation) under S. alba, S. caprea and B. pendula in spring 2013
Table 2.
Molecularly identified EM fungi on Salix alba, Salix caprea, and Betula pendula fine roots during autumn 2012 and spring 2013
| Tree species and season | T bp | Closest BLAST match (accession numbers—NCBI* and/or UNITE**) | % similarity | Classified as | EM density |
|---|---|---|---|---|---|
| Autumn 2012 | |||||
| S. alba | 637 | Tomentella isolate [EU668202 and UDB002428]* (Bidartondo and Read 2008); Tomentella stuposa [UDB011637]** | 617/630 (97 %) and 587/595 (98 %) 623/639 (97 %) | Tomentella sp. Sa-A [KP745605] | 24.63 % |
| S. caprea | 699 | Hebeloma collariatum [JQ724066]* | 693/696 (99 %) | H. collariatum Sc-A [KP745607] | 3.88 % |
| H. collariatum [UDB015489]** | 668/674 (99 %) | ||||
| 636 | Geopora sp. [JQ724044]* (Hrynkiewicz et al. 2012b) | 565/636 (89 %) | Geopora sp. Sc-A [KP745606] | 11.82 % | |
| Geopora arenicola [UDB017620]** | 368/406 (90 %) | ||||
| B. pendula | 563 | Uncultured fungus genomic [FN397282]* (Napoli et al. 2010) | 563/566 (99 %) | Helotiales sp. Bp- [KP745604] | 27.18 % |
| Ectomycorrhizal fungus [JX043062]* (Karst et al. 2013) | 542/552 (98 %) | ||||
| Helotiales sp. [JN859267]* (Knapp et al. 2012) | 528/541 (98 %) | ||||
| Spring 2013 | |||||
| S. alba | 874 | Thelephoraceae [EF218829]* (Twieg et al. 2007) | 831/874 (95 %) | Tomentella sp. Sa-S [KP745608] | 10.82 % |
| Thelephoraceae [AJ893343]* (Tedersoo et al. 2006b) | 800/831 (96 %) | ||||
| Tomentella sp. [UDB018687]** | 832/866 (96 %) | ||||
| T. ellisii [UDB016490]** | 818/855 (95 %) | ||||
| S. caprea | 623 | Tomentella ellisii [DQ068971]* (Menkis et al. 2005) | 598/614 (97 %) | Tomentella sp. Sc-S [KP745609] | 7.54 % |
| Tomentella sp. [UDB018687]** | 583/614 (94 %) | ||||
| T. ellisii [UDB016490]** | 569/603 (94 %) | ||||
| B. pendula | – | Colour, brownish; rhizomorphs, not observed; mantle, plectemchymatic B; cystidia, lacking; surface, smooth; emanating hyphae, scarce | – | Thelephoraceae B.p_1S | 6.95 % |
| – | Colour, brown; rhizomorphs, not observed; mantle, plectemchymatic B; cystidia, lacking; surface, smooth; and emanating hyphae, lacking | – | Pyronemataceae B.p_2S | 5.20 % | |
| – | Colour, gold-brown; rhizomorph,: not observed;, mantle, plectemchymatic A; cystidia, lacking; surface: smooth; emanating hyphae: abundant with clamps | – | Thelephoraceae B.p_3S | 1.97 % | |
Abbreviations: B.p Betula pendula, S.c Salix caprea, S.a Salix alba
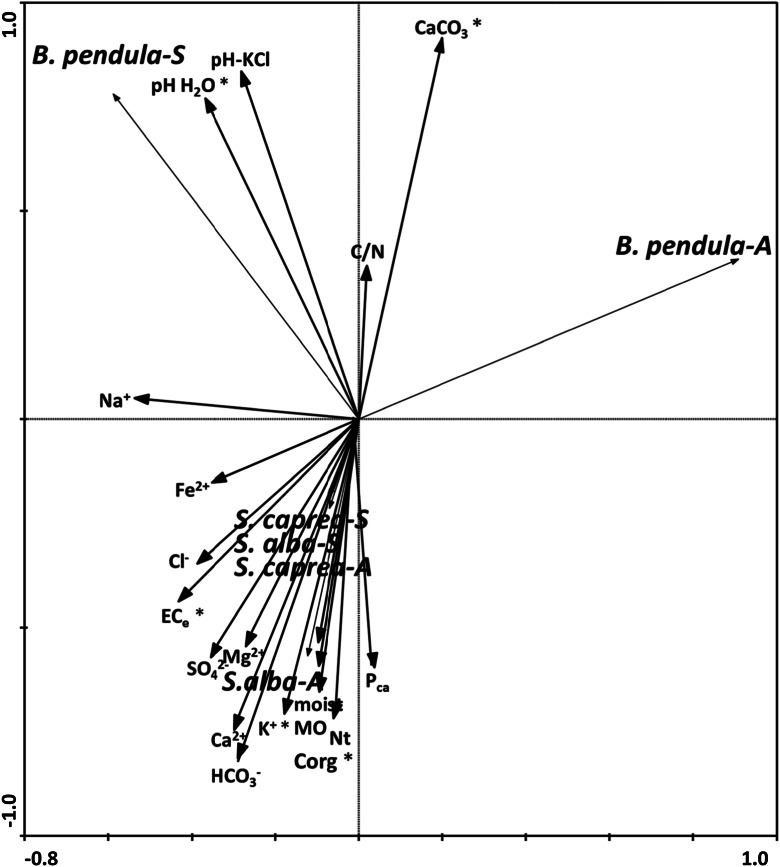
The RDA diagram based on the level of colonisation of EM fungi of the tree species, seasons and chemical soil properties in the rhizosphere is shown in Fig. 4. The analysis allowed the selection of five factors (from 18 analysed) significant in differentiation of the density of EM fungi. The CaCO3 and related pH-H2O level ratio significantly contributed to explaining the density of EM fungi colonising B. pendula (both seasons), while the organic carbon, ECe and K+ significantly contributed to explaining the amount of EM fungi associated with S. alba and S. caprea (both seasons) (Fig. 4).
Fig. 4.
Redundancy analysis, diagrams with axes 1 and 2 for level of EM colonisation of fine roots of three tree species (S. alba, S. caprea, B. pendula) during two seasons (autumn and spring) and soil properties in the rhizosphere. *p ≤ 0.05, significant factors
Diversity of EM Fungi in Relation to Different Tree Species, Seasons and Soil Parameters
Nine EM morphotypes were found in the investigated treatments, two on S. alba (one in each season), three on S. caprea (two in autumn and one in spring) and four on B. pendula (one in autumn and three in spring). One EM fungus was identified to the species level (Hebeloma collariatum Sc-A), five to the genus level (Tomentella sp. Sa-A, Sa-S, Sc-S, Geopora sp. Sc-A, Helotialaes Bp-A) and three morphotypes, which were identified on the basis of morpho-anatomical properties (Bp_1S, Bp_2S, Bp_3S). The number of different EM morphotypes per each variant of the experiment (three tree species and two seasons, six variants in total) ranged from one to three. The number of different morphotypes was higher in the spring than in the autumn (five and four, respectively). EM formation by Thelephoraceae (Tomentella sp. Sa-A, Sa-S and Sc-S) was observed for S. alba (both seasons) and S. caprea (spring) and by Pyronemataceae (Geopora sp. Sc-A, Helotiales sp. Bp-A) for S. caprea and B. pendula (in both cases only in the autumn). A representative of Cortinariaceae (H. collariatum Sc-A) was observed only on the roots of S. caprea (in autumn). Three morphotypes that were not identified molecularly were classified on the basis of their morphological and anatomical properties (Bp_1S, Bp_2S; Bp_3S, see Table 2).
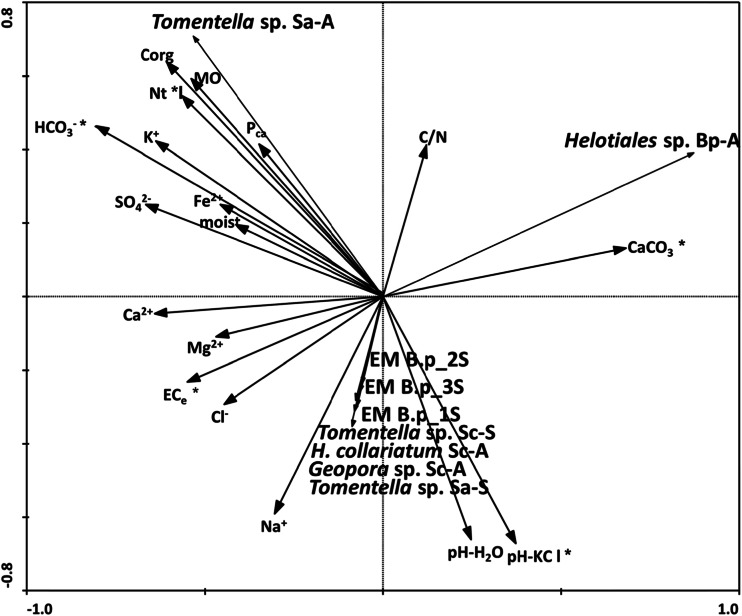
The RDA diagram based on 18 rhizosphere soil properties (similar to the case of RDA analysis made for EM density) and the frequency of EM morphotypes (nine in total) is shown in Fig. 5. Most of the EM types (H. collariatum Sc-A, Geopora sp. Sc-A, Tomentella sp. Sa-S, Tomentella sp. Sc-S, B.p_1S, B.p_2S, B.p_3S) were positively related with pH and ECe level (significant effect). The other two EM morphotypes, associated with S. alba during the autumn (Tomentella sp. Sa-A) and with B. pendula during the autumn (Helotiales sp. Bp-A) were positively related to HCO3 −, Ntot and CaCO3 levels in the soil, respectively (Fig. 5).
Fig. 5.
Redundancy analysis, diagrams with axes 1 and 2 for soil properties in the rhizosphere and level of EM colonisation of fine roots of three tree species (S. alba, S. caprea, B. pendula) during two seasons (autumn and spring). *p ≤ 0.05, significant factors
Discussion
Well-adapted EM fungi can be fundamental for the growth and survival of the host plant in harsh soil conditions (Hrynkiewicz and Baum 2012a; Timling et al. 2012). The presence of EM fungi naturally associated with the roots of tree species (S. alba, S. caprea, B. pendula) growing in unfavourable saline soil supports our initial assumption and confirms their important role in the acclimatisation of hosts under saline stress. The level of EM colonisation (9–34 %) of fine root trees growing in saline conditions was similar to the EM colonisation observed at other disturbed test sites; e.g. S. caprea growing in a heavy metal-contaminated site: 3–36 % (Hrynkiewicz et al. 2008), mycorrhizal formation on roots from three willow clones (Salix spp.) on fly ash: approximately 3 %. The levels were, however, much lower compared with S. repens growing in unfavourable dune ecosystems: 62–87 % (van der Heijden et al. 1999), S. caprea at a heavy metal polluted site: 36–50 % (Regvar et al. 2010) or Salix polaris in the subarctic tundra: 35–64 % (Hrynkiewicz et al. 2009a). Ruotsalainen et al. (2009), who investigated EM colonisation along three environmental gradients (two natural and one with human-induced pollution) within the Kola Peninsula (NW Russia), revealed that total EM colonisation of mountain birch (Betula pubescens ssp. czerepanovii) ranged between 30 and 60 % and did not show stress-related patterns. Our results are in agreement with the observation of Ishida et al. (2009), who noted poor EM colonisation in Mongolian willow in alkaline-saline soil, where some of the root systems were not colonised by EM fungi at all, and more than 57.7 % of the fine root tips were not colonised by EM fungi. The relatively low EM colonisation of fine root tips level observed in this work corresponds to the observation that EM colonisation is problematic in some primary successional sites (Allen et al. 1992) and is regulated mostly by the magnitude of soil disturbance. For instance, very poor EM colonisation was observed on the roots of birch (B. pubescens) seedlings grown in soil from eroded sites in Iceland—an average number of EM roots less than 50 (Oddsdottir et al. 2010)—or in devastated volcanic deserts (Allen et al. 1992). Collecting the post-production waste products of the soda factory in ponds called “white seas” (beginning in 1882) led to a strong degradation of the test site analysed in this study. As a result, a strongly degraded area of approximately 135 ha is currently not used (Hulisz and Piernik 2013). The only herbaceous plants colonising areas with the highest levels of salinity are typical halophytes; e.g. Salicornia europaea and Aster tripolium. The trees analysed in this study are pioneer species that naturally colonise the periphery of the former settlers, suggesting that they have high tolerance to salt conditions. We suppose that the relatively low frequencies of ectomycorrhizae observed on the roots of S. alba and S. caprea are related mostly to the highest levels of salinity (ECe, 1.4–5.0 dS m−1) and concentrations of ions (e.g. Na+, Cl−, Ca2+, K+) in the rhizosphere of willow species compared with B. pendula (ECe, 0.53–1.97). Hyperosmotic stress in fungi is associated with inhibition of cell wall extension and cellular expansion, leading to a reduction in growth. Moreover, an excess of Na+ and/or Cl− ions in fungal cells may alter enzymatic activity and protein and nucleic acid metabolism (Bois et al. 2006), thus decreasing the potential of EM fungi for successful colonisation of the plant roots. This statement may be supported by the observation that significantly lower EM colonisation of fine root tips occurred in the tree species during the spring, correlated with higher salt concentrations in this season. Soil salinity varies from season to season due to variations in hydrological parameters; e.g. the amount of rainwater and groundwater level. Ferjani et al. (2013) reported that the highest value of electrical conductivity (ECe) reached during the irrigation season (7.7 dS m−1) was decreased to 3.1 dS m−1 following the fall rains. In Polish climatic conditions, due to percolative type of the water regime, salt accumulation does not take occur, unlike arid and semi-arid climates. Therefore, the salinity level of the analysed soils was closely linked to the groundwater level (Hulisz and Piernik 2013). The greatest intensity of the salinisation process by capillary rise and evapotranspiration occurs when groundwater is present in a zone called the critical depth, where fluctuations of the groundwater level are relatively small (Hulisz and Piernik 2013). However, the variability of soil salinity at the microscale not only results from the saline water supply but also is favoured by the microrelief and some soil properties (organic matter content and texture—expressed as saturation percentage) (Hulisz and Piernik 2013). The high organic matter content allows for high ion accumulation and therefore higher salinity levels.
Many studies have shown that a high level of P in the soil has a negative effect on the formation of ectomycorrhizae (see reviews by Wallander 1995). In the rhizosphere of S. alba and S. caprea, with a significantly lower EM colonisation of fine root tips level, we have noted higher concentrations of P compared with B. pendula. Reductions in EM colonisation of fine root tips by Salix viminalis and B. pendula seedlings under elevated P levels was observed by Jones et al. (1991) and Newton and Pigott (1991), respectively. Puttsepp et al. (2004) reported that a higher abundance of EM fungi is associated with a lower pH, lower levels of K and P and higher levels of N and organic matter content. Because the highest density observed on the roots of B. pendula in our work was correlated with lower levels of K (33.1–33.9 mg/l) and Pca (155–212 mg kg−1) compared with S. alba and S. caprea, but at the same time represented higher pH (7.87–8.50 in H2O, 7.70–8.00 in KCl), lower levels of N (0.2033–0.2617 %) and organic matter (9.3267–10.2867 %) compared with willow samples, we suggest that differences in the level of salt concentration have a large impact on EM density in saline soils.
Seasonal variability and accompanying changes in the weather can affect populations of EM fungi associated with the host plant (Walker et al. 2008). The necessity for studying systematic seasonal patterns of EM fungi is important because of impending global climate change. In our study, we observed a significantly higher abundance of EM fungi in the autumn (16–34 %) when the salinity of rhizosphere soil was definitely lower, compared with (9–30 %) the spring. The same tendency for willow (S. viminalis) and poplar (Populus nigra × maximowiczii) cultivated as short rotation coppice (SRC) was observed by Hrynkiewicz et al. (2010a): an average of 55 and 25.5 % in autumn and spring, respectively. However, S. caprea growing in heavy metal stress conditions revealed a higher density of EM fungi in the spring (23–36 %) than in the autumn (3–30 %) (Hrynkiewicz et al. 2008). The pattern of EM fungi frequency associated with Alnus acuminata (Betulaceae) occurring at two natural forests was not correlated with the seasons (EM colonisation of fine root tips range from 30.3 to 94 %) (Becerraa et al. 2005).
Our studies describe for the first time the density of EM fungi at saline areas, but there are many reports describing the role in alleviation of salt stress in plants by arbuscular mycorrhizal (AM) fungi (e.g. Evelin et al. 2009; Aggarwal et al. 2012). Salinity may directly and/or indirectly affect the development and functioning of AM fungi; e.g. by inhibition of spore germination and hyphal growth (Campagnac and Khasa 2014). Füzy et al. (2008) revealed that AM colonisation of salt aster (A. tripolium) and sea plantain (Plantago maritima) was greatest in late spring to early summer and had a second peak later in the autumn. Arbuscule formation and overall mycorrhizal colonisation appeared to be inversely correlated with the intensity of rainfall and suggest that, in addition to seasonality, drought may play an important role in governing AM activity in saline habitats. Furthermore, proper management of AM symbiosis has the potential to improve the profitability and sustainability of salt tolerance by host plants by minimising the movement of Na+ to the shoot and in general improving water and nutrient uptake (Aggarwal et al. 2012). It is quite probable that EM fungi possess the same properties, so that in higher salt stress conditions, these processes would intensify. Improved plant productivity of Populus spp. under salt stress conditions associated with proper EM fungi was emphasised by Luo et al. (2009). Comparative metabolite and transcriptome analysis in EM and non-EM roots of grey poplar revealed higher levels of myoinositole, abscisic and salicic acid, and K+ and Na+ in EM roots (Luo et al. 2009).
The diversity of EM fungi, similar to EM density, can be affected by numerous biotic (e.g. coexistence with bacteria, host genotype) and abiotic (e.g. heavy metal contamination, nutrient deficiency, pH) factors (Chai et al. 2013). Many authors emphasise the limiting effect of abiotic stress on the diversity of EM fungi compared with their total density (Paradi and Baar 2006; Hrynkiewicz et al. 2008). In this study, the Salix and Betula species growing in saline soil harboured nine different morphotypes of EM fungi. Four and five different morphotypes were observed during the autumn and spring, respectively, and the number of morphotypes associated with three different tree species (S. alba, S. caprea, B. pendula) depended on two seasons: three morphotypes on the roots of B. pendula during the spring, two morphotypes on S. caprea during the autumn and one EM morphotype per each other variant of the experiment. Those numbers of morphotypes are in line with the numbers reported for other Salix spp.; e.g. 11 EM fungal species associated with Salix linearistipularis growing in alkaline-saline soil (Ishida et al. 2009), 12 EM fungal partners colonising the root tips of S. alba growing in three riparian edge forests (Paradi and Baar 2006), 11 EM fungal partners identified from Salix clones in SRC in Estonia (Puttsepp et al. 2004), 14 EM fungal partners of S. caprea identified at heavy metal contaminated sites (Hrynkiewicz et al. 2008) and 7 fungal taxa identified from S. viminalis grown in SRC at an arable site in Germany (Hrynkiewicz et al. 2010a). Despite significant differences in the parameters of the soil, we have not observed a clear influence of seasonality on the diversity of EM fungi in our study. However, such observations have been made in other cases; e.g. oak seedlings (Walker et al. 2008). The EM fungal taxa were dominated by Basidomycetes (six of nine identified morphotypes): Tomentella sp. Sa-A, Sa-S and Sc-S, H. collariatum Sc-A, Thelephoraceae Bp-1S, Bp-2S with only three representatives (three of nine identified morphotypes) of Ascomycotes: Geopora sp. Sc-A and Helotiales sp. Bp-A and Pyrenomataceae Bp-3S. The dominance of EM fungal taxa from Basidiomycetes to Ascomycetes is in line with our earlier observations (Hrynkiewicz et al. 2008, 2009a) and the results of other authors (e.g. Timling et al. 2012). Fungal species from the family Thelephoraceae (Tomentella sp.) were observed on the roots of all tree species and in both seasons, while two representatives of Ascomycetes (Geopora sp. and Helotiales sp.) were characteristic mostly of autumn.
Thelephorales (Tomentella sp. Sa-A, Sa-S and Sc-S) were observed on the roots of all of the investigated tree species (on the roots of B. pendula identified on the basis of morpho-anatomical features—Thelephoraceae) and constituted the most numerous group of EM symbionts along with the highest level of EM colonisation of fine root tips (49.94 % in general, 100 % of Sa-A and Sa-S, 49.19 % of the EM fine roots for B. pendula). This agreed with the observed dominance of Thelephoraceae as EM fungal partners of S. caprea at heavy metal contaminated sites (Hrynkiewicz et al. 2008), S. alba in riparian edge forests (Paradi and Baar 2006) and S. viminalis grown in SRC at an arable site in Germany (Hrynkiewicz et al. 2010a). The characteristic colour of all tomentelloid EM fungi results from the incorporation of melanin, a natural dark pigment and common fungal wall component known to act as a protective interface between fungal metabolism and biotic and abiotic environmental stressors (Bell and Wheeler 1986; Butler and Day 1998; Kõljalg et al. 2000). An ecological role for melanin in EM fungi is still lacking; however, it is known that melanin in the cell wall of EM fungi can contribute to their heavy metal tolerance (Gadd 1993) and consequently enhance their competitiveness and establishment in polluted environments (Regvar et al. 2010). Bois et al. (2006) observed that excretion of yellowish phenolic-like compounds (e.g. extracellular melanin) by Suillus tomentosus increased with increasing NaCl concentrations present in the medium. Droplets of the same colour appeared on the surface of the mycelium. The authors suggest that the production and exudation of metabolites could be used for external osmotic adjustment to avoid the need of internal adjustment by the accumulation of Na and/or Cl (Bois et al. 2006). Geopora sp. were found on the roots of willow clones growing in unfavourable soil conditions; e.g. two Salix viminalis clones and a S. viminalis x caprea hybrid clone growing in fly ash (Hrynkiewicz et al. 2009a), and on S. linearistipularis roots in alkaline-saline soil (Ishida et al. 2009). This fungal taxa belongs to the subphylum of Pezizomycotina and the family Pyronemataceae. Pezizales species are often the dominant members of EM fungal communities in early succession ecosystems and following disturbances (Gehring et al. 1998). Thick-walled chlamydospores and ascospores may be reasons for their ability to persist under unfavourable environmental conditions (Tedersoo et al. 2006a). Pezizalean species have been discovered as EM symbionts in locally disturbed patches in forests with high pH and low organic matter (Petersen 1985; Dissing 2000), which might explain why the observed morphotype was able to survive in saline soil with a relatively high pH level (7.5–8.5) and formed an ectomycorrhizae with the willow clone in our experiment.
Helotiales sp., from the same subphylum as Geopora sp.—Pezizomycotina, was observed only in one season (autumn) on the roots of B. pendula. Helotiales is an order of Ascomycota, where many EM lineages await discovery (Tedersoo et al. 2009b). Because they are more commonly described as root endophytes and occasionally detected as ericoid mycorrhizal fungi, they most likely form other types of root-associated biotrophic relations with plants or represent secondary colonisers of EM root tips (Tedersoo et al. 2009b). Thus far, records of any sexual structures are lacking for seven out of eight helotialean lineages that strongly hamper their formal description, isolation in pure culture, and subsequent manipulative studies (Tedersoo et al. 2010). However, a yellowish EM morphotype with an ascomycete mantle anatomy, consistently identified as a member of Helotiales, was found on Picea abies (Tedersoo et al. 2008b), and eight species of Helotiales formed ectomycorrhizae with Australian hosts Tedersoo et al. (2008a, 2009a). These species were grouped into four well-supported lineages that are distantly related to the EM taxa in the Northern Hemisphere and to any root endophytes (Tedersoo et al. 2009b). Further investigations should help to clarify the unclear position of this taxa. The presence of this fungal strain in unfavourable saline conditions indicates their important role in plant-fungus interactions under abiotic stress.
The only representatives of the family Agaricales—H. collariatum Sc-A—was observed only once on the roots of S. caprea. Hebeloma spp. belong to the early-stage fungi (e.g. Mason et al. 1983) and are well-known colonisers of EM plants in disturbed or primary habitats (Jumpponen and Trappe 1998; Nara et al. 2003), which suggests that the EM fungus community is at an early successional stage with a relatively low number of fungal taxa. Relatively high colonisation with Hebeloma spp. was identified on S. caprea in heavy metal contaminated sites in Germany (Hrynkiewicz et al. 2008) and on S. alba in riparian edge forests in the Netherlands (Paradi and Baar 2006). Because EM root tips colonised by H. collariatum were identified only in the autumn in the present study (on the roots of S. caprea), we speculate that this genus can belong to periodic symbionts present in saline soils.
Conclusions
The number of EM was different and depended on the tree species and season. As a major parameter, influencing the abundance of EM associated with the investigated tree species was salinity rather than level of P, K and other studied components. EM fungal density and diversity was low; however, the identified EM fungi, Tomentella sp., Hebeloma sp., Geopora sp. and Helotiales sp., can be classified to the group of species highly adapted to saline conditions.
Acknowledgements
This investigation was conducted in the frame of COST action FA1103 and financially supported by a grant from the National Science Centre (Poland) (DEC-2012/07/B/NZ9/01801).
References
- Agerer, R. (Ed.) (1987–2002) Colour Atlas of Ectomycorrhizae, first to twelfth ed. Schwa¨ bisch Gmu¨ nd, Einhorn.
- Agerer R. Characterization of ectomycorrhiza. In: Norris JR, Read DJ, Varma AK, editors. Techniques for the study of mycorrhiza. London: Academic; 1991. pp. 50–51. [Google Scholar]
- Aggarwal A, Kadian N, Karishma, Tanwar KNA, Gupta KK. Arbuscular mycorrhizal symbiosis and alleviation of salinity stress. Journal of Applied and Natural Science. 2012;4:144–155. [Google Scholar]
- Allen EB, Allen MF, Helm DJ, Trappe JM, Molina JM, Rincon E. Patterns and regulation of mycorrhizal plant and fungal diversity. Plant and Soil. 1992;170:47–62. doi: 10.1007/BF02183054. [DOI] [Google Scholar]
- Becerraa A, Pritschb K, Arrigoc N, Palmac M, Bartolonid N. Ectomycorrhizal colonization of Alnus acuminata Kunth in northwestern Argentina in relation to season and soil parameters. Annals of Forest Science. 2005;62:325–332. doi: 10.1051/forest:2005027. [DOI] [Google Scholar]
- Bednarek, R. (2005). Laboratoryjne badania gleb. In: Bednarek R, Dziadowiec H, Pokojska U, Prusinkiewicz Z (Badania ekologiczno-gleboznawcze, pp (100–111). Warszawa: Wydawnictwo Naukowe PWN.
- Bell AA, Wheeler MH. Biosynthesis and functions of fungal melanins. Annual Review of Phytopathology. 1986;24:411–451. doi: 10.1146/annurev.py.24.090186.002211. [DOI] [Google Scholar]
- Bidartondo MI, Read DJ. Fungal specificity bottlenecks during orchid germination and development. Molecular Ecology. 2008;17(16):3707–3716. doi: 10.1111/j.1365-294X.2008.03848.x. [DOI] [PubMed] [Google Scholar]
- Bois G, Bertrand A, Piché Y, Fung M, Khasa DP. Growth, compatible solute and salt accumulation of five mycorrhizal fungal species grown over a range of NaCl concentrations. Mycorrhiza. 2006;16:99–109. doi: 10.1007/s00572-005-0020-y. [DOI] [PubMed] [Google Scholar]
- Butler MJ, Day AW. Fungal melanins: a review. Canadian Journal of Microbiology. 1998;44:1115–1136. doi: 10.1139/w98-119. [DOI] [Google Scholar]
- Campagnac E, Khasa DP. Relationship between genetic variability in Rhizophagus irregularis and tolerance to saline conditions. Mycorrhiza. 2014;24:121–129. doi: 10.1007/s00572-013-0517-8. [DOI] [PubMed] [Google Scholar]
- Chai DD, Guo SJ, Sun XB, Qin TT. The major factors affecting ectomycorrhizal fungi diversity in the forest ecosystem. Advance Journal of Food Science and Technology. 2013;5(7):879–890. [Google Scholar]
- Dell B. Role of mycorrhizal fungi in ecosystems. Chiang Mai University Journal. 2002;1(1):47–60. [Google Scholar]
- Dissing H. Pezizales. In: Hansen L, Knudsen H, editors. Nordic macromycetes, vol. I. Ascomycetes. Copenhagen: Nordsvamp; 2000. pp. 55–128. [Google Scholar]
- Evelin H, Kapoor R, Giri B. Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Annals of Botany. 2009;104:1263–1280. doi: 10.1093/aob/mcp251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (2008). FAO Land and Plant Nutrition Management Service, Available from: http://www.fao.org/ag/agl/agll/spush.
- Ferjani N, Morri M, Daghari I. Estimation of root-zone salinity using saltmod in the irrigated area of Kalaât El Andalous (Tunisia) Journal of Agricultural Science and Technology. 2013;15:1461–1477. [Google Scholar]
- Füzy A, la Biró B, Tótha T, Hildebrandtb U, Bothec H. Drought, but not salinity, determines the apparent effectiveness of halophytes colonized by arbuscular mycorrhizal fungi. Journal of Plant Physiology. 2008;165:1181–1192. doi: 10.1016/j.jplph.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Gadd GM. Interactions of fungi with toxic metals. New Phytologist. 1993;124:25–60. doi: 10.1111/j.1469-8137.1993.tb03796.x. [DOI] [Google Scholar]
- Gago C, Sousa AR, Juliao M, Miguel G, Antunes DC, Panagopoulos T. Sustainable use of energy in the storage of halophytes used for food. International Journal of Energy and Environment. 2011;5:592–599. [Google Scholar]
- Gardes M, Bruns TD. ITS primers with enhanced specificity of basidiomycetes: application to the identification of mycorrhizae and rusts. Molecular Ecology. 1993;2:113–118. doi: 10.1111/j.1365-294X.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- Gehring CA, Theimer TC, Whitham TG, Keim P. Ectomycorrhizal fungal community structure of pinyon pines growing in two environmental extremes. Ecology. 1998;79:1562–1572. doi: 10.1890/0012-9658(1998)079[1562:EFCSOP]2.0.CO;2. [DOI] [Google Scholar]
- Hrynkiewicz, K., & Baum, C. (2012a). Chapter 2: The potential of rhizosphere microorganisms to promote the plant growth in disturbed soils. In: Environmental Protection Strategies for Sustainable Development, Strategies for Sustainability (Malik A., Grohmann E. Red.) (pp. 35–64). Springer Science + Business Media B.V., (ISBN: 978-94-007-1590-5).
- Hrynkiewicz K, Haug I, Baum C. Ectomycorrhizal community structure under willows at former ore mining sites. European Journal of Soil Biology. 2008;44:37–44. doi: 10.1016/j.ejsobi.2007.10.004. [DOI] [Google Scholar]
- Hrynkiewicz K, Baum C, Leinweber P. Mycorrhizal community structure, microbial biomass P and phosphatase activities under Salix polaris as influenced by nutrient availability. European Journal of Soil Biology. 2009;45:168–175. doi: 10.1016/j.ejsobi.2008.09.008. [DOI] [Google Scholar]
- Hrynkiewicz K, Baum C, Niedojadło J, Dahm H. Promotion of mycorrhiza formation and growth of willows by the bacterial strain Sphingomonas sp. 23L on fly ash. Biology and Fertility of Soils. 2009;45:385–394. doi: 10.1007/s00374-008-0346-7. [DOI] [Google Scholar]
- Hrynkiewicz K, Baum C, Leinweber P, Weih M, Dimitriou I. The significance of rotation periods for mycorrhiza formation in Short Rotation Coppice. Forest Ecology and Management. 2010;260:1943–1949. doi: 10.1016/j.foreco.2010.08.020. [DOI] [Google Scholar]
- Hrynkiewicz K, Ciesielska A, Haug I, Baum C. Ectomycorrhiza formation and willow growth promotion as affected by associated bacteria: role of microbial metabolites and use of C sources. Biology and Fertility of Soils. 2010;46:139–150. doi: 10.1007/s00374-009-0419-2. [DOI] [Google Scholar]
- Hrynkiewicz K, Toljander YK, Baum C, Fransson PM, Taylor AF, Weih M. Correspondence of ectomycorrhizal diversity and colonisation of willows (Salix spp.) grown in short rotation coppice on arable sites and adjacent natural stands. Mycorrhiza. 2012;22(8):603–613. doi: 10.1007/s00572-012-0437-z. [DOI] [PubMed] [Google Scholar]
- Hulisz P, Piernik A. Soils affected by soda industry in Inowrocław. In: Charzyński P, Hulisz P, Bednarek R, editors. Technogenic soils of Poland. Toruń: Polish Society of Soil Science; 2013. pp. 125–140. [Google Scholar]
- Ishida TA, Nara K, Ma S, Takano T, Liu S. Ectomycorrhizal fungal community in alkaline-saline soil in northeastern China. Mycorrhiza. 2009;19:329–335. doi: 10.1007/s00572-008-0219-9. [DOI] [PubMed] [Google Scholar]
- Jackson ML. Soil chemical analysis. London: Constable Ldt; 1958. [Google Scholar]
- Jimenez-Casas M, Zwiazek J. Effects of branch pruning and seedling size on total transpiration and tissue Na and Cl accumulation in Pinus leiophylla seedlings exposed to salinity. Forest Science. 2013;59:407–415. doi: 10.5849/forsci.11-117. [DOI] [Google Scholar]
- Jones MD, Durall DM, Tinker PB. Fluxes of carbon and phosphorus between symbionts in willow ectomycorrhizas and their changes with time. New Phytologist. 1991;119:99–106. doi: 10.1111/j.1469-8137.1991.tb01012.x. [DOI] [PubMed] [Google Scholar]
- Jumpponen A, Trappe JM. Dark septate endophytes: a review of facultative biotrophic root-colonizing fungi. New Phytologist. 1998;140:295–310. doi: 10.1046/j.1469-8137.1998.00265.x. [DOI] [PubMed] [Google Scholar]
- Karst J, Piculell B, Brigham C, Booth M, Hoeksema J. Fungal communities in soils along a vegetative ecotone. Mycologia. 2013;105:61–70. doi: 10.3852/12-042. [DOI] [PubMed] [Google Scholar]
- Knapp DG, Pintye A, Kovacs GM. The dark side is not fastidious—dark septate endophytic fungi of native and invasive plants of semiarid sandy areas. PLoS ONE. 2012;7:1–8. doi: 10.1371/journal.pone.0032570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kõljalg, U., Dahlberg, A., Taylor, A. F. S., Larsson, E., Hallenberg, N., Stenlid, J., Larsson, K. H., Fransson, P. M., Karén, O. (2000). Diversity and abundance of resuspinate thelephoroid fungi as ectomycorrhizal symbionts in Swedish boreal forest. Molecular Ecology, 9(121), 1985-1996. [DOI] [PubMed]
- Luo ZB, Janz D, Jiang X, Gobel C, Wildhagen H, Tan Y, Rennenberg H, Feussner I, Polle A. Upgrading root physiology for stress tolerance by ectomycorrhizas: insights from metabolite and transcriptional profiling into reprogramming for stress anticipation. Plant Physiology. 2009;151:1902–1917. doi: 10.1104/pp.109.143735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason PA, Wilson J, Last FT, Walker C. The concept of succession in relation to the spread of sheathing mycorrhizal fungi on inoculated tree seedlings growing in unsterilized soils. Plant and Soil. 1983;71:247–256. doi: 10.1007/BF02182659. [DOI] [Google Scholar]
- Menkis A, Vasiliauskas R, Taylor AF, Stenlid J, Finlay R. Fungal communities in mycorrhizal roots of conifer seedlings in forest nurseries under different cultivation systems, assessed by morphotyping, direct sequencing and mycelial isolation. Mycorrhiza. 2005;16(1):33–41. doi: 10.1007/s00572-005-0011-z. [DOI] [PubMed] [Google Scholar]
- Milosević N, Marinković JB, Tintor B. Mitigating abiotic stress in crop plants by microorganisms. Zbornik Matice Srpske Za Prirodne Nauke. 2012;123:17–26. doi: 10.2298/ZMSPN1223017M. [DOI] [Google Scholar]
- Napoli C, Mello A, Borra A, Vizzini A, Sourzat P, Bonfante P. Tuber melanosporum, when dominant, affects fungal dynamics in truffle grounds. New Phytologist. 2010;185(1):237–247. doi: 10.1111/j.1469-8137.2009.03053.x. [DOI] [PubMed] [Google Scholar]
- Nara K, Nakaya H, Wu B, Zhou Z, Hogetsu T. Underground primary succession of ectomycorrhizal fungi in volcanic desert on Mount Fuji. New Phytologist. 2003;159:743–756. doi: 10.1046/j.1469-8137.2003.00844.x. [DOI] [PubMed] [Google Scholar]
- Newton AC, Pigott CD. Mineral nutrition and mycorrhizal infection of seedling oak and birch. II. The effect of fertilization on growth, mineral uptake and ectomycorrhizal infection. New Phytologist. 1991;117:45–52. doi: 10.1111/j.1469-8137.1991.tb00943.x. [DOI] [Google Scholar]
- Oddsdottir ES, Eilenbergc J, Senb R, Harding S, Halldorssona G. Early reduction of Otiorhynchus spp. larval root herbivory on Betula pubescens by beneficial soil fungi. Applied Soil Ecology. 2010;45:168–174. doi: 10.1016/j.apsoil.2010.03.009. [DOI] [Google Scholar]
- Paradi I, Baar J. Mycorrhizal fungal diversity in willow forests of different age along the river Waal, The Netherlands. Forest Ecology and Management. 2006;237:366–372. doi: 10.1016/j.foreco.2006.09.059. [DOI] [Google Scholar]
- Petersen PM. The ecology of Danish soil-inhabiting Pezizales with emphasis on edaphic conditions. Opera Botanica. 1985;77:1–38. [Google Scholar]
- Puttsepp U, Rosling A, Taylor AFS. Ectomycorrhizal fungal communities associated with Salix viminalis L. and S. dasyclados Wimm. clones in a short-rotation forestry plantation. Forest Ecology and Management. 2004;196:413–424. doi: 10.1016/j.foreco.2004.04.003. [DOI] [Google Scholar]
- Regvar M, Likar M, Piltaver A, Kugonic N, Smith JE. Fungal community structure under goat willows (Salix caprea L.) growing at metal polluted site: the potential of screening in a model phytostabilisation study. Plant and Soil. 2010;330:345–356. doi: 10.1007/s11104-009-0207-7. [DOI] [Google Scholar]
- Ruotsalainen AL, Markkola AM, Kozlov MV. Mycorrhizal colonization of mountain birch (Betula pubescens ssp. czerepanovii) along three environmental gradients: does life in harsh environments alter plant–fungal relationships? Environmental Monitoring and Assessment. 2009;148:215–232. doi: 10.1007/s10661-007-0152-y. [DOI] [PubMed] [Google Scholar]
- Silva C. M. M. S. & Fay, E. F. (2012). Effect of salinity on soil microorganisms. In: M. C. Hernandez-Soriano (Ed.), Soil Health and Land Use Management, InTech. doi:10.5772/2516.
- StatSoft 1995-STATISTICA for Windows-Tulsa, StatSoft, Inc.
- Szymańska S, Piernik A, Hrynkiewicz K. Metabolic potential of microorganisms associated with the halophyte Aster tripolium L. in saline soils. Ecological Questions. 2013;18:9–19. doi: 10.12775/ecoq-2013-0001. [DOI] [Google Scholar]
- Tedersoo L, Hansen K, Perry BA, Kjøller R. Molecular and morphological diversity of Pezizalean ectomycorrhiza. New Phytologist. 2006;170:581–596. doi: 10.1111/j.1469-8137.2006.01678.x. [DOI] [PubMed] [Google Scholar]
- Tedersoo L, Suva T, Larsson E, Kõljalg U. Diversity and community structure of ectomycorrhizal fungi in a wooded meadow. Mycological Research. 2006;110:734–748. doi: 10.1016/j.mycres.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Tedersoo L, Jairus T, Horton BM, Abarenkov K, Suvi T, Saar I, Kõljalg U. Strong host preference of ectomycorrhizal fungi in a Tasmanian wet sclerophyll forest as revealed by DNA barcoding and taxon-specific primers. New Phytologist. 2008;180:479–490. doi: 10.1111/j.1469-8137.2008.02561.x. [DOI] [PubMed] [Google Scholar]
- Tedersoo L, Suvi T, Jairus T, Kõljalg U. Forest microsite effects on community composition of ectomycorrhizal fungi on seedlings of Picea abies and Betula pendula. Environmental Microbiology. 2008;10:1189–1201. doi: 10.1111/j.1462-2920.2007.01535.x. [DOI] [PubMed] [Google Scholar]
- Tedersoo L, Paertel K, Jairus T, Gates G, Poldmaa K, Tamm H. Ascomycetes associated with ectomycorrhizas: molecular diversity and ecology with particular reference to the Helotiales. Environmental Microbiology. 2009;11:3166–3178. doi: 10.1111/j.1462-2920.2009.02020.x. [DOI] [PubMed] [Google Scholar]
- Tedersoo L, Suvi T, Jairus T, Ostonen I, Polme S. Revisiting ectomycorrhizal fungi of the genus Alnus: differential host specificity, diversity and determinants of the fungal community. New Phytologist. 2009;182:727–735. doi: 10.1111/j.1469-8137.2009.02792.x. [DOI] [PubMed] [Google Scholar]
- Tedersoo L, May TW, Smith ME. Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza. 2010;20:217–263. doi: 10.1007/s00572-009-0274-x. [DOI] [PubMed] [Google Scholar]
- ter Braak CJF, Smilauer P. CANOCO Reference manual and CanoDraw for Windows User's guide: software for canonical community ordination (version 4.5) Ithaca: Microcomputer Power; 2002. [Google Scholar]
- Timling I, Dahlberg A, Walker DA, Gardes M, Charcosset JY, Welker JM, Taylor DL. Distribution and drivers of ectomycorrhizal fungal communities across the North American Arctic. Ecosphere. 2012;3:111–125. doi: 10.1890/ES12-00217.1. [DOI] [Google Scholar]
- Twieg BD, Durall DM, Simard SW. Ectomycorrhizal fungal succession in mixed temperate forests. New Phytologist. 2007;176(2):437–447. doi: 10.1111/j.1469-8137.2007.02173.x. [DOI] [PubMed] [Google Scholar]
- van der Heijden EW, Vries FW, Kuyper TW. Mycorrhizal associations of Salix repens L. communities in succession of dune ecosystems. I. Above-ground and below-ground views of ectomycorrhizal fungi in relation to soil chemistry. Canadian Journal of Botany. 1999;77:1821–1832. doi: 10.1139/cjb-77-12-1821. [DOI] [Google Scholar]
- van Reeuvijk, L. P. (2002). Procedures for soil analysis (pp. 4-1–4-2, 6-1–6-2, 13-1, 13-3, 13-4–13-5). Wageningen: ISRIC.
- Varga, C., Marian, M., Mihaly-Cozmuta, L., Mihaly-Cozmuta, A., Mihalescu, L. (2009). Evaluation of the phytoremediation potential of the Salix caprea in tailing ponds Tom. XVI/1 (pp. 141–149). Analele Universitatii din Oradea, Fascicula Biologie.
- Walker JF, Miller OK, Jr, Horton JL. Seasonal dynamics of ectomycorrhizal fungus assemblages on oak seedlings in the southeastern Appalachian Mountains. Mycorrhiza. 2008;18:123–132. doi: 10.1007/s00572-008-0163-8. [DOI] [PubMed] [Google Scholar]
- Wallander H. A new hypothesis to explain allocation of dry matter between mycorrhizal fungi and pine seedlings in relation to nutrient supply. Plant and Soil. 1995;168–169:243–248. doi: 10.1007/BF00029334. [DOI] [Google Scholar]