Abstract
Background & Aims
The impact of vitamin D supplementation in overweight and obese adults during resistance training on body composition, muscle function, and glucose tolerance was investigated.
Methods
Twenty-three overweight and obese (age: 26.1±4.7 y; BMI: 31.3±3.2 kg/m2; 25-hydroxyvitamin D: 19.3±7.2 ng/mL) adults were recruited for participation in a double-blind, placebo-controlled trial. Participants were randomly divided into vitamin D (VitD, 4000 IU/d; 5 female, 5 male) and placebo (PL; 7 female, 6 male) groups. Both groups completed 12 wks of resistance training. 25-hydroxyvitamin D, parathyroid hormone, body composition, and glucose tolerance were assessed at baseline and 12 wks. Muscle function (strength and power) was assessed at baseline, 4, 8, and 12 wks.
Results
During the intervention, 25-hydroxyvitamin D increased and parathyroid hormone decreased in the VitD group (P<0.05). Peak power was significantly increased at 4 wks in the VitD group only (P<0.05). Regression analysis revealed an inverse association between the change in 25-hydroxyvitamin D with the change in waist-to-hip ratio (R2=0.205, P=0.02). No other improvements were observed with supplementation.
Conclusions
Vitamin D supplementation in overweight and obese adults during resistance training induced an early improvement in peak power, and elevated vitamin D status was associated with reduced waist-to-hip ratio.
Clinical trial registration number
Keywords: 25-hydroxyvitamin D, parathyroid hormone, lean mass, muscular strength, muscular power
Introduction
It is clear that attainment of optimal muscle mass is important to health as greater lean mass is associated with improved insulin sensitivity and reduced risk for obesity.1–2 In addition, improved muscle function is associated with reduced falls, subsequent bone fractures and associated sequelae.3 It has been shown that vitamin D contributes to the improvement of bone health independent of calcium regulation. It is proposed that vitamin D effects on muscle mass may mediate this effect by inducing increased mechanical stress that may improve bone mass.4–5 In addition, risk of acquiring diseases associated with obesity may be lessened with participation in interventions that contribute to gains in skeletal muscle – the primary site for glucose disposal.6 For example, a modest increase in type II muscle led to increased glucose uptake in diet-induced obese rats.2 Conversely, low levels of lower body muscle mass may provoke the development of insulin resistance.7 Therefore, it is critically important to determine factors that will contribute to optimizing the level and function of muscle to improve health consequences, including obesity, diabetes, and osteoporosis, as well as quality of life.
Evidence is accumulating to suggest that optimal vitamin D intake can promote muscle mass accumulation and improve insulin signaling.5,8 Vitamin D status is assessed by serum 25-hydroxyvitamin D (25OHD) levels and is linked to changes in muscle mass, 9–10 and insulin sensitivity.8,11 For example, low vitamin D status has been associated with muscle weakness, muscle wasting (with preferential atrophy of type II muscle fibers), and insulin resistance, 9,12 that may be reversed with supplementation.9 For instance, vitamin D supplementation (4000 IU/day) for six months resulted in significant improvements in insulin sensitivity among vitamin D deficient women with insulin resistance.8 In another study, vitamin D deficient elderly stroke survivors were treated with vitamin D (1000 IU/day) for two years and type II muscle fiber size and number were significantly increased.9 In contrast, during a nine month exercise training intervention for elderly participants, vitamin D supplementation (400 IU/day) did not enhance improvements in muscle function compared to exercise training alone.13 Thus, the potential contribution of vitamin D supplementation to improved muscle growth and function as well as enhanced glucose tolerance requires further investigation.
The purpose of this study was to investigate the impact of vitamin D supplementation on body composition, muscle function, and glucose tolerance in overweight and obese adults during participation in a resistance exercise training program. The hypothesis was that vitamin D supplementation during resistance training would result in greater gains in muscle mass and function as well as improved glucose tolerance compared to exercise training alone.
Methods
Subjects
Participants were recruited during winter months (October to March) and participated in a brief telephone screening interview, followed by a visit for an additional assessment. A total of 34 eligible participants were recruited and 23 completed all the necessary requirements. Of the 11 drop-outs, three were due to injury unrelated to the study; six due to time constraints; one became pregnant; and one perceived discomfort with supplement ingestion (placebo group). Using a difference shown previously 14 of 1.16 kg (SD: 0.68 kg) in lean mass accumulation when vitamin D was consumed during exercise training, compared to a control diet (low calcium, low dairy), an 80% power to measure this difference required a final subject number of 7/group. Using this error, a difference of 0.95 kg between groups can be measured with 10 participants/group. All experimental procedures were approved by the Purdue University Committee on the Use of Human Research Subjects and each participant provided written consent.
Baseline Assessments
During the first visit to the laboratory, participants completed a health history and physical activity assessment by questionnaire.15 Subject’s height, body mass, and waist-to-hip ratio were also obtained. Waist-to-hip ratio was measured by the same trained investigator by recording the circumference of the smallest diameter of the waist (waist circumference) and the circumference of the largest diameter of the hips (hip circumference). Maximal oxygen consumption (VO2max) was estimated following completion of the modified Balke sub-maximal treadmill test.16 Volunteers with physical activity scores in the “low” to “very low” category coupled with VO2max estimations in the “below average” or lower categories were eligible for participation.16
Exclusionary criteria were: use of tanning booths; high baseline vitamin D (>600 IU/day) and calcium (>1000 mg/day) intake; plans to visit sunny/warm destinations during the winter months/study period; history or presence of metabolic disease, type 2 diabetes, eating disorders, gastrointestinal disorders, pregnancy or lactation; use of drugs to treat obesity (last 12 weeks); use of over the counter anti-obesity agents (last 12 weeks); and recent initiation of an exercise program (last four weeks).
Vitamin D Supplementation
The study design was a double-blind, randomized, placebo controlled vitamin D supplementation trial in which all participants initiated a resistance exercise training program. Participants were randomized to consume daily either a 4000 IU vitamin D3 supplement (VitD, five females and five males) or a placebo (PL, seven females and six males) that contained microcrystalline cellulose in identical capsules prepared by Family Pharmacy, West Lafayette, IN. Evidence from a recent benefit-risk assessment of vitamin D supplementation in randomized control trials suggest that the dose of vitamin D supplement necessary to increase serum 25OHD to optimum levels is in the range of 1800 to 4000 IU/day.17
To control for calcium intake all participants were asked to consume one calcium tablet (500 mg/tablet) per day. Participants were provided with single use sunscreen packets and instructed to apply the contents of four packets per day to all skin exposed to the sun. Compliance for pill consumption and sunscreen use was determined by random pill and sunscreen packet counts. Supplemental intakes of vitamin D and calcium were estimated based on individual compliance to pill intake, and total intake was calculated by the sum of the dietary intake from three day dietary records and supplemented intake.
Exercise Training
Subjects were acclimated to the treadmill (LifeFitness Treadmills, Schiller Park, IL) and 8 resistance exercises: leg extension, leg flexion, leg press, hip adduction, hip abduction, chest press, seated row, and lateral pull down (Keiser Equipment, Fresno, CA) during a three session acclimation week.18 For each acclimation day, subjects were asked to walk on the treadmill for five minutes and complete a set of standard stretches as a warm-up. On the first acclimation day, participants were provided with instruction on proper lifting technique and completed an 8 repetition maximum (RM) for the exercises listed above. For the purpose of calculating exercise training resistance settings, 1RM’s were estimated for all participants using the measured 8RM value recorded for each exercise. On the second acclimation day, participants completed two sets of 8 resistance exercises at 50% of estimated 1RM. The third and final acclimation session required the participants to complete a 1RM muscular strength assessment for chest press, leg press, leg curl, and the seated row. Following completion of the 1RM tests, participants were asked to rest for 15 minutes. Thereafter, using only the seated row, the resistance was set for 60% of their measured 1RM and the participants were asked to perform 15 repetitions as quickly as possible while maintaining full range of motion to assess muscular power with the intention of eliciting peak power during the first one to three repetitions. The seated row machine (Keiser A420, Fresno, CA) was interfaced with a computer and displayed instantaneous power values. At least 48 hours recovery was required between acclimation sessions. Muscular strength and power measurements conducted during the final acclimation session were assessed again at the end of the exercise training program using the same protocol.
Following completion of the acclimation week, each participant completed a supervised 12 week (three days/week for a total of 36 sessions) resistance exercise training program. Prior to each training session, participants walked on the treadmill for five minutes and performed light stretching as a warm-up. Following the warm-up, participants performed three sets of 8 resistance exercises (three upper body, five lower body). For the first week of training, participants completed the resistance exercises at 70% of estimated 1RM and 80% of estimated 1RM thereafter. Resistance was subsequently increased (~5 – 10%) based on performance. For the first set, subjects were asked to complete 8 repetitions, and for the second and third sets participants completed the exercise until “momentary muscular failure”, or until 15 repetitions were performed. The 1RM and muscular power assessments were reassessed at 4, 8, and 12 weeks. Compliance to the exercise training protocol was calculated by the percent attendance of the prescribed 36 sessions during the intervention.
Anabolic conditions for muscle growth, during participation in a resistance exercise training program, fluctuate depending on the timing of daily macronutrient intake.19 Thus, to control for the timing of post-exercise caloric intake, all participants consumed a nutrition shake in the presence of an investigator (Gatorade Performance Series: Nutrition Shake) during the hour following each exercise session. The composition of the drink (360 kcal) was 8 g fat, 54 g carbohydrate, 20 g milk protein isolate, vitamin D (100 IU), and calcium (300 mg).
Dietary Assessment
At baseline and at one week prior to completion of the study, subjects were provided with detailed instructions for completing a three-day diet record (two weekdays, one weekend day). Dietary records were analyzed by the same trained nutritionist, using Nutrition Data System for Research, Version 4.04, Food and Nutrient Database 28 (Minneapolis, Minnesota).
Vitamin D Status and Body Composition
Assessments of vitamin D status and body composition were conducted at baseline and following the 12 week intervention. Body composition was assessed using dual energy x-ray absorptiometry (software version 4.3e Lunar Corp, Madison, WI). To minimize the influence of dietary macronutrient content on blood variables, all subjects were instructed to consume the same foods during the 24 hours immediately prior to each blood sampling session. Prior to each blood draw, participants were also asked to refrain from exercise and supplement intake for 72 hours. To control for biological differences during the menstrual cycle, blood sampling and all other measurements collected from female participants at baseline and at 12 weeks were performed during the early follicular phase of their menstrual cycle. Thus, the timing of enrollment was adjusted for female participants to correspond to the early follicular phase. Subjects arrived at the laboratory following an overnight fast (12 hours) and rested for 30 minutes. Thereafter, a venous blood sample was drawn, maintained at room temperature for 30 minutes and separated for serum by centrifugation. Blood drawn into sodium heparin tubes was centrifuged immediately for plasma collection. Serum and plasma samples were frozen at −80°C until further analysis.
Serum 25OHD and parathyroid hormone (PTH) were measured in duplicate by enzyme immunoassay (Heartland Assays Inc, Ames, IA) and human bioactive PTH 1–84 Enzyme Linked Immunosorbant Assay kit from Immutopics, Inc. (San Clemente, CA), respectively. The inter-assay coefficients of variation are 10.5% and 6.7% for 25OHD and PTH, respectively. Serum calcium was assessed by Inductively Coupled Plasma Optimal Emission Spectrometer, Optima 4300, Perkin Elmer Instruments (Boston, MA; Shelton, CT).
Oral Glucose Tolerance Test
Participants completed an oral glucose tolerance test at baseline and following the 12 week intervention. After the initial blood draw, participants were given a beverage containing 75 g of glucose and were instructed to consume the beverage within 10 minutes. Blood samples were collected at 15, 30, 60, and 120 minutes following consumption of the beverage. The area under the glucose curve (AUCglucose) was calculated to assess glucose tolerance, and the homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using the following equation: fasting insulin (μU/ml) × fasting glucose (mmol/l)/22.5.
Serum glucose and plasma insulin were measured in duplicate. Serum glucose was assessed using the Wako Autokit Glucose assay purchased from Wako Diagnostics (Richmond, VA). Plasma insulin was measured using an Enzyme Linked Immunosorbant Assay purchased from ALPCO Diagnostics (Salem, NH).
Statistical Analysis
Descriptive analyses included calculation of mean and standard deviation (SD). Assumptions of normality (Shapiro-Wilkes) and homogeneity of variance (residual plots) were assessed and potential outliers were identified. Non-normal variables were appropriately transformed following a diagnostic analysis using the Box-Cox transformation procedure. Absolute values as well as the change (12 week – baseline) of all dependent variables were assessed by analysis of variance (ANOVA) using the general linear model (GLM). Muscular strength and peak power variables at baseline, 4, 8, and 12 weeks were analyzed by repeated measures ANOVA using the GLM. A Tukey post hoc multiple comparison analysis was used when appropriate. Simple linear regression analyses were introduced to model the effect of serum 25OHD (independent variable) on anthropometric measures (dependent variables). All statistical analyses were carried out with SAS (version 9.1, SAS Institute, Cary, NC) statistical software package. All variables are presented as mean ± SD. Differences were considered significant at P<0.05.
Results
There were no significant differences at baseline in age, height, body mass, BMI, percent body fat, and waist-to-hip ratio between the drop-outs and those who completed the study. Of those who completed the trial, age, height, body mass, lean mass, measures of body fatness (percent body fat, BMI, and fat mass), and waist-to-hip ratio were similar between groups at baseline (Table 1).
Table 1.
Baseline characteristics.
| Placebo (n = 13) | Vitamin D (n = 10) | |
|---|---|---|
| Age (y) | 26.0 ± 4.5 | 26.2 ± 5.1 |
| Height (cm) | 169.4 ± 10.6 | 168.5 ± 9.2 |
| Weight (kg) | 92.3 ± 16.9 | 86.8 ± 10.9 |
| BMI (kg·m−2) | 31.9 ± 3.3 | 30.6 ± 3.1 |
| Body fat (%) | 43.7 ± 5.8 | 41.3 ± 5.1 |
| Fat mass (kg) | 38.8 ± 8.2 | 34.3 ± 4.1 |
| Lean mass (kg) | 50.4 ± 12.0 | 49.2 ± 9.2 |
| Waist-to-hip ratio | 0.94 ± 0.10 | 0.96 ± 0.10 |
No significant differences detected between groups (P>0.05). All values are means values are means ± SDs.
Dietary intake of vitamin D and calcium at baseline were similar between groups. Compliance to the 12 week supplement (vitamin D or placebo) and calcium intervention for both groups was 93% and 88%, respectively. Consequently, total vitamin D intake was significantly higher in the VitD group (VitD: 3859 ± 445 IU/day) compared to the PL group (215 ± 213 IU/day) and total calcium intake was similar between groups (1695 ± 757 mg/day and 1510 ± 613 mg/day, respectively) during the 12 week intervention.
Total energy, protein, carbohydrate and fat intake were not significantly different between groups at baseline or at 12 weeks. In addition, there was no significant main effect of group on the change in total energy, protein, carbohydrate and fat intake (Table 2). For all subjects combined, however, there was a significant effect of the exercise intervention on protein intake (P=0.04) with a trend towards significance for an increase in total energy intake (P=0.09).
Table 2.
| Placebo (n = 13) | Vitamin D (n = 10) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Baseline | 12 weeks | Change | Baseline | 12 weeks | Change | |
| Energy (kcal) | 2348.5 ± 907.0 | 2543.5 ± 1275.6 | 169.7 ± 661.4 | 2160.0 ± 654.1 | 2643.7 ± 579.6 | 466.7 ± 931.5 |
| Carbohydrate (g) | 281.5 ± 98.9 | 295.0 ± 131.5 | 19.1 ± 85.6 | 297.7 ± 98.5 | 324.0 ± 75.8 | 27.6 ± 99.5 |
| Protein (g) | 83.1 ± 27.2 | 95.8 ± 44.0 | 12.2 ± 29.9 | 71.8 ± 20.6 | 94.3 ± 33.8 | 21.9 ± 41.4 |
| Fat (g) | 97.9 ± 62.1 | 107.1 ± 82.0 | 4.5 ± 31.8 | 79.0 ± 25.6 | 110.2 ± 28.0 | 29.1 ± 45.0 |
All values are means ± SDs.
There were no significant main effects of group for baseline, 12 weeks or change in energy, carbohydrate, protein or fat intake.
Vitamin D-related serum biochemical parameters (25OHD, calcium, and PTH) were not significantly different between groups at baseline (Table 3). During the intervention, serum 25OHD increased and PTH decreased in the VitD group compared to baseline levels. In contrast, both 25OHD and PTH levels remained stable during the duration of the study in the PL group.
Table 3.
Serum levels of 25OHD, calcium, PTH, and metabolic characteristics prior to and following the 12-week intervention.
| Placebo (n = 13) | Vitamin D (n = 10) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Baseline | 12 weeks | Change | Baseline | 12 weeks | Change | |
| 25OHD (ng·mL−1) | 18.1 ± 6.5 | 23.5 ± 6.0 | 5.5 ± 7.9 | 20.8 ± 8.3 | 33.4 ± 7.2a | 12.3 ± 8.4 |
| Calcium (mmol·L−1) | 22.0 ± 0.9 | 21.8 ± 0.7 | −0.2 ± 0.7 | 22.5 ± 0.8 | 22.5 ± 0.6 | 0.0 ± 0.8 |
| PTH (pg·mL−1) | 43.2 ± 34.6 | 43.5 ± 29.8 | 0.3 ± 8.9 | 36.1 ± 10.7 | 24.0 ± 12.0a | −11.7 ± 10.6b |
| Fasting Glucose (mmol·L−1) | 5.3 ± 0.4 | 5.2 ± 0.4 | −0.1 ± 0.7 | 5.4 ± 0.5 | 5.1 ± 0.4 | −0.3 ± 0.6 |
| 2h Post-load Glucose (mmol·L−1) | 5.4 ± 1.2 | 5.5 ± 1.1 | 0.4 ± 1.4 | 6.2 ± 1.7 | 6.3 ± 1.7 | −0.1 ± 1.1 |
| Fasting Insulin (pmol·L−1) | 105.8 ± 71.2 | 114.9 ± 46.2 | −2.5 ± 44.1 | 108.5 ± 62.3 | 124.3 ± 69.5 | 13.6 ± 42.2 |
| AUCglucose (mmol·L−1·2 h−1) | 409.9 ± 82.9 | 391.9 ± 56.9 | −18.0 ± 63.4 | 447.7 ± 112.1 | 422.0 ± 95.7 | −25.7 ± 31.1 |
| HOMA-IR | 3.6 ± 2.4 | 3.9 ± 1.5 | −0.2 ± 1.7 | 3.8 ± 2.3 | 4.1 ± 2.3 | 0.2 ± 1.6 |
All values are means ± SDs.
Significantly different from baseline (P<0.05);
Significant group difference (P<0.05).
25OHD, 25-hydroxyvitamin D; PTH, parathyroid hormone; AUCglucose, area under the glucose curve; HOMA-IR, homeostatic model assessment of insulin resistance.
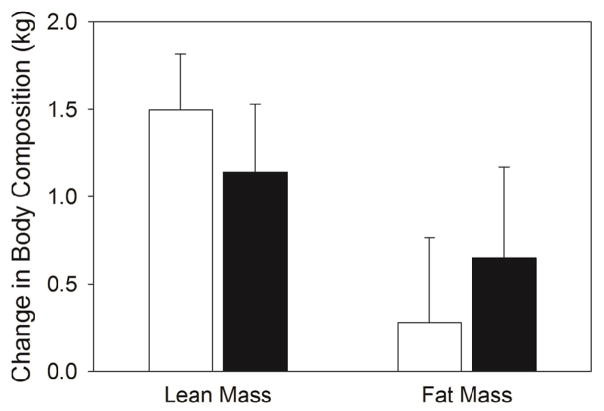
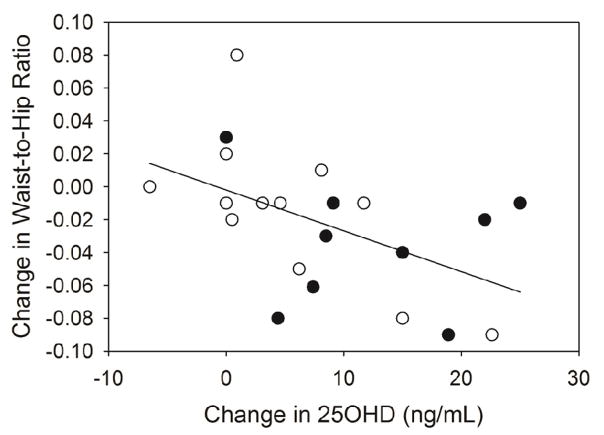
After the 12 week intervention, fat mass and lean mass were not significantly different compared to baseline (Figure 1). When the VitD and PL groups were pooled, however, the change (post – pre) in lean mass accumulation (1.35 kg) was significantly greater than zero (P<0.001). No main effects of group were detected for the change in body mass, BMI, percent body fat, fat mass, lean mass and waist-to-hip ratio during the intervention. Linear regression analysis for the change in serum 25OHD revealed an inverse association with the change in waist-to-hip ratio (R2 = 0.256, F (1, 19) = 6.54, P=0.02) (Figure 2). This was explained primarily by the relationship between the change in serum 25OHD with the change in waist circumference (r = −0.41;P=0.06), rather than the change in hip circumference (r = 0.09; P=0.70). Consistent with these results, there was a trend towards a significant correlation between the change in serum 25OHD and the change in android fat mass estimated by DXA measurements (R2 = 0.16, F(1,19) = 3.58, P=0.07) with no association between the change in serum 25OHD and the change in gynoid fat mass (R2= 0.0005, F(1,19) = 0.01, P = 0.93).
Figure 1.
Absolute change (from baseline) in lean mass and fat mass after the 12 week resistance exercise intervention. All values are mean ± SEM’s. Placebo (□) and vitamin D (■) groups.
Figure 2.
Correlation between the change in waist-to-hip ratio and the change in serum 25OHD at 12 weeks. A significant correlation between change (12 week-baseline) in waist-to-hip ratio and change in serum 25OHD was shown (simple linear regression R2 = 0.205, F (1, 21) = 5.40, P = 0.02). Placebo (○) and vitamin D (●) groups.
There were no significant group differences detected for estimated VO2max at baseline or for the change in estimated VO2max from baseline. As expected, compared to baseline, muscular strength (1RM) significantly increased in both groups for leg press, chest press, and leg curl after 12 weeks of resistance training. There were, however, no significant differences detected between groups for the change in 1RM measurements following the 12 week intervention (Table 4). Furthermore, no significant differences between groups were found for the change in the sum of 1RM’s (leg press, chest press, and leg curl) per kilogram body mass (PL: 0.8 ± 0.3 1RM’s/kg body mass; VitD: 0.8 ± 0.2 1RM’s/kg body mass), or the sum of 1RM’s per kilogram lean mass (PL: 1.4 ± 0.6 1RM’s/kg lean mass; VitD: 1.5 ± 0.5 1RM’s/kg lean mass). At baseline and 12 weeks for PL and VitD combined, percent body fat was negatively correlated with the sum of 1RM’s/kg body mass (r= −0.76, P<0.001; r = −0.84, P < 0.001, respectively). Repeated measures analysis of 1RM for leg press, chest press, and leg curl revealed no main effect of group at any time point (baseline, 4, 8, and 12 weeks).
Table 4.
Muscular strength (1RM) and aerobic fitness prior to and following the 12-week intervention.
| Placebo (n = 13) | Vitamin D (n = 10) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Baseline | 12 weeks | Change | Baseline | 12 weeks | Change | |
| Leg Press (kg) | 222.0 ± 55.0 | 272.2 ± 51.1a | 50.2 ± 25.0 | 229.2 ± 62.8 | 281.4 ± 63.5a | 52.2 ± 20.3 |
| Chest Press (kg) | 43.0 ± 18.2 | 53.1 ± 20.7a | 10.1 ± 3.4 | 46.3 ± 16.9b | 56.2 ± 20.0a,b | 10.0 ± 3.6 |
| Leg Curl (kg) | 63.6 ± 21.2 | 84.5 ± 24.2a | 21.0 ± 8.3 | 63.6 ± 16.0 | 83.3 ± 20.6a | 19.7 ± 8.5 |
| Est. VO2max (mL·kg−1·min−1) | 33.3 ± 5.8 | 39.0 ± 9.3 | 5.7 ± 8.2 | 33.5 ± 3.8 | 38.8 ± 4.3 | 6.0 ± 6.0 |
All values are means ± SDs.
Significantly different from baseline (P<0.05);
Significant group difference (P<0.05).
1RM, one repetition maximum. Est. VO2max, estimated maximal oxygen consumption.
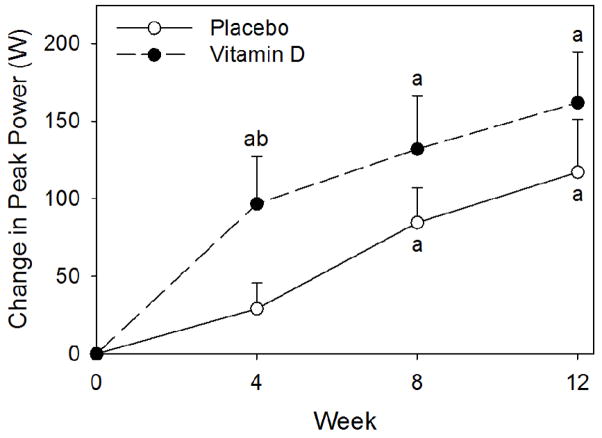
There were no significant differences detected between the groups at baseline for peak power. After four weeks of training, only the VitD group significantly increased peak power and the change (from baseline) was significantly greater compared to the PL group (Figure 3), but at 8 and 12 weeks no differences were detected between groups. Further, no main effect of group was detected for the change (12 weeks – baseline) in peak power per kilogram of upper body lean mass (PL: 3.2 ± 3.3 W/kg upper body lean mass; VitD: 5.0 ± 3.3 W/kg upper body lean mass). Compliance to exercise training sessions for the VitD and PL groups were 96% and 97%, respectively.
Figure 3.
Change from baseline in peak power. All values are mean ± SEM’s. The (a) indicates a significant increase from baseline, P < 0.05. The (b) indicates a significant difference in the change from baseline compared to placebo, P< 0.05. Results from placebo (○) and vitamin D (●) groups.
There was no main effect of group at baseline, after the 12 week intervention, or for the change in fasting glucose, fasting insulin, 2h post-load glucose, AUCglucose, and HOMA-IR (Table 2). There were no main effects of time found for fasting glucose, fasting insulin, 2h post-load glucose, AUCglucose, and HOMA-IR after the 12 week intervention.
Discussion
Associations between vitamin D status and muscle function are well documented in both epidemiological as well as small intervention studies.20 However, the impact of high vitamin D intake on the attainment of muscle mass or enhancement of muscle function is not clear. The results of the current study demonstrated that vitamin D supplementation improved muscular power in healthy overweight and obese individuals within four weeks and that elevated vitamin D status was associated with greater losses in waist circumference, with no additional benefits in lean mass accumulation, muscular strength, or glucose tolerance during participation in a 12 week resistance exercise training program.
The current results support previous findings that indicate a relationship between vitamin D status and waist circumference rather than fat mass.21 The inverse relationship with waist circumference is particularly important as abdominal fat has been implicated as an important factor in the development of Type 2 diabetes. It has been suggested that the opposing actions of these fat depots could potentially weaken any correlation between total fat mass and vitamin D status.21 Furthermore, waist circumference is a surrogate for abdominal obesity, and is a key marker in the assessment of metabolic syndrome as defined by the International Diabetes Federation.22 Waist circumference is also an independent risk factor for cardiovascular disease.23 Therefore, the greater decrease in waist circumference associated with higher vitamin D intake represents a potential reduction in risk for metabolic disease and cardiovascular risk.
There is controversy in the literature regarding the appropriate time frame and dosage of vitamin D supplementation for attaining vitamin D sufficiency.17 According to estimates developed by Heaney24 using a conservative rate of increase for 25OHD, 4000 IU/day should raise serum 25OHD by ~28 ng/mL. However, the 25OHD level of the participants in the current study consuming the vitamin D supplement increased less than this prediction (~13 ng/mL). Furthermore, in the five participants that were vitamin D deficient at baseline serum 25OHD was increased by (16.7 ng/mL) during the 12 weeks of vitamin D supplementation. Compared to thinner individuals serum 25OHD levels do not increase as much in obese adults potentially due to 25OHD sequestration in adipose tissue.25 Thus, it is possible that in overweight and obese individuals, as in the current study, higher levels of vitamin D supplementation may be necessary to achieve levels of 25OHD that would translate into positive changes in muscle growth and function as well as improved glucose tolerance.
Previous studies suggest that vitamin D supplementation may contribute to lean mass accumulation and improved muscle function.5,9 For example, a high intake of vitamin D induced greater lean mass accumulation in rodents.10 Furthermore, results from a one year vitamin D supplementation (2000 IU/day) study in premenarcheal girls revealed an increase in lean mass accumulation, without exercise training, compared to placebo.5 Given that vitamin D supplementation did not augment lean mass accumulation, the current results are not consistent with the aforementioned findings regarding the relationship between 25OHD and lean mass accumulation. A longer supplementation period may be necessary to achieve sufficient levels of 25OHD that influence muscle mass. On the other hand, the prescribed exercise regimen in the current study may have provided a sufficient stimulus to mask potential vitamin D-induced benefits on muscle. Nevertheless, the current results are similar to those of Bunout, et al.13 In this study, older adults consumed a vitamin D supplement (400 IU/day) during participation in an exercise training program for 9 months. No synergistic effects of vitamin D supplementation on muscular strength and physical function were reported.
Peak power production requires the generation of force from anaerobic metabolism that is most efficiently produced from type II muscle fibers. Type II fibers have a limited capacity for aerobic metabolism with greater glycolytic and anaerobic capability.26 Significant increases in type II fiber size and number have been identified in post-stroke patients after vitamin D supplementation and in another study, increased cross sectional area of type II fibers have been reported after only one month of resistance training.9,27 Thus, a potential mechanism for the early increase in peak power is the improvement of type II fiber function. Furthermore, a large portion of the early improvements in muscle that occur with exercise training are attributed to neural adaptations, rather than hypertrophy.28 Dhesi et al.29 reported significant improvements in neuromuscular function six months after a single intramuscular injection of 600,000 IU of vitamin D2, compared to a non-significant decline with placebo. Thus, it is possible that vitamin D-related changes in neuromuscular function partially contributed to the early increase in peak power. After 8 weeks of training, however, there were no significant differences in peak power between groups, suggesting that either vitamin D supplementation had no effect on long term peak power or that improvements in muscle function from exercise training were sufficient to mask the early additive effects of vitamin D supplementation.
In the current study, no improvements were observed in glucose tolerance following vitamin D supplementation during resistance training. Glucose tolerance was determined by fasting glucose and 2 h post-load glucose levels. According to recent American Diabetes Association criteria, the participants in the current study had normal fasting glucose (<5.6 mmol/L) and glucose tolerance (2 h post-load glucose: <7.8 mmol/L).30 In a longer supplementation period (six months) at the same vitamin D dosage as the current study in insulin resistant individuals, von Hurst et al.8 reported a significant decrease in fasting insulin and HOMA-IR. The discrepancy of the current results with those of von Hurst et al.8 may be that overweight and obese individuals with normal glucose tolerance, as in the current study, do not benefit from short-term vitamin D supplementation.
There are several limitations of the current study. Non-exercise placebo and vitamin D supplementation-only groups were not included in the study design which would allow for an understanding of the interaction of exercise with vitamin D supplementation. Furthermore, the compliance to sunscreen use was relatively low (~40%) in both groups. This, however, did not translate into a significant change in vitamin D status within the placebo group after the 12 week intervention, but greater differences induced by smaller alterations in vitamin D status may have been obscured. The choice to examine healthy, overweight and obese participants, with normal fasting glucose, may have limited further gains in serum 25OHD that would potentially translate into improvements in muscle and glucose tolerance.
In conclusion, vitamin D supplementation in overweight and obese adults induced a short-term increase in peak muscular power and the change in vitamin D status was associated with reduced waist-to-hip ratio. Vitamin D supplementation, however, did not augment the effects of 12 weeks of resistance exercise training on lean mass accumulation, muscular strength, or glucose tolerance in the studied population.
Acknowledgments
This work was supported by the Gatorade Sports Science Institute and by the National Institutes of Health, National Cancer Institute R25CA128770 (D. Teegarden) Cancer Prevention Internship Program (Yan Jiang) administered by the Oncological Sciences Center and the Discovery Learning Research Center at Purdue University.
We would like thank our phlebotomist Douglas Maish, as well as Krysta Rickey, Elizabeth Kuhns, Lauren Wagner, and Jessica Harris for assisting with data collection, analysis, and for guiding and motivating participants during training sessions.
Non-standard abbreviations
- 25OHD
25-hydroxyvitamin D
- PTH
Parathyroid hormone
- VO2max
Maximal oxygen consumption
- RM
Repetition maximum
- AUCglucose
Area under the glucose curve
- HOMA-IR
Homeostatic model assessment of insulin resistance
Footnotes
Conference presentation: Carrillo A.E., Flynn M.G., Pinkston C., Markofski M.M., Jiang Y., Donkin S.S., and Teegarden D. (2010). Effects of vitamin D supplementation during exercise training on strength and body composition. Experimental Biology. April 24 – 28, Anaheim, CA, USA.
Conflict of Interest
The authors report no conflict of interest.
AEC, MGF, SSD, and DT contributed to the design of the experiment, collection and analysis of data and writing of the manuscript. CP, MMM, and YJ contributed to collection and analysis of data and writing of the manuscript. All authors read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andres E. Carrillo, Email: aecarrillo@cereteth.gr.
Michael G. Flynn, Email: flynnmg@cofc.edu.
Catherine Pinkston, Email: cburlage@purdue.edu.
Melissa M. Markofski, Email: memarkof@utmb.edu.
Yan Jiang, Email: yanjian@med.umich.edu.
Shawn S. Donkin, Email: sdonkin@purdue.edu.
Dorothy Teegarden, Email: teegarden@purdue.edu.
References
- 1.Lazar MA. Developmental biology. How now, brown fat? Science. 2008;321:1048–9. doi: 10.1126/science.1164094. [DOI] [PubMed] [Google Scholar]
- 2.Izumiya Y, Hopkins T, Morris C, Sato K, Zeng L, Viereck J, Hamilton JA, Ouchi N, LeBrasseur NK, Walsh K. Fast/Glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 2008;7:159–72. doi: 10.1016/j.cmet.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bischoff-Ferrari HA, Dawson-Hughes B. Where do we stand on vitamin D? Bone. 2007;41:S13–9. doi: 10.1016/j.bone.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 5.El-Hajj FG, Nabulsi M, Tamim H, Maalouf J, Salamoun M, Khalife H, Choucair M, Arabi A, Vieth R. Effect of vitamin D replacement on musculoskeletal parameters in school children: a randomized controlled trial. J Clin Endocrinol Metab. 2006;91:405–12. doi: 10.1210/jc.2005-1436. [DOI] [PubMed] [Google Scholar]
- 6.DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992;15:318–68. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen BK. Muscle-to-fat interaction: a two-way street? J Physiol. 2010;588:21. doi: 10.1113/jphysiol.2009.184747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient - a randomised, placebo-controlled trial. Br J Nutr. 2010;103:549–55. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- 9.Sato Y, Iwamoto J, Kanoko T, Satoh K. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovasc Dis. 2005;20:187–92. doi: 10.1159/000087203. [DOI] [PubMed] [Google Scholar]
- 10.Siddiqui SM, Chang E, Li J, Burlage C, Zou M, Buhman KK, Koser S, Donkin SS, Teegarden D. Dietary intervention with vitamin D, calcium, and whey protein reduced fat mass and increased lean mass in rats. Nutr Res. 2008;28:783–90. doi: 10.1016/j.nutres.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980–86. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 12.Liu E, Meigs JB, Pittas AG, McKeown NM, Economos CD, Booth SL, Jacques PF. Plasma 25-hydroxyvitamin d is associated with markers of the insulin resistant phenotype in nondiabetic adults. J Nutr. 2009;139:329–34. doi: 10.3945/jn.108.093831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunout D, Barrera G, Leiva L, Gattas V, de la Maza MP, Avendaño M, Hirsch S. Effects of vitamin D supplementation and exercise training on physical performance in Chilean vitamin D deficient elderly subjects. Exp Gerontol. 2006;41:746–52. doi: 10.1016/j.exger.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 14.White KM, Bauer SJ, Hartz KK, Baldridge M. Changes in body composition with yogurt consumption during resistance training in women. Int J Sport Nutr Exerc Metab. 2009;19:18–33. doi: 10.1123/ijsnem.19.1.18. [DOI] [PubMed] [Google Scholar]
- 15.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3:A118. [PMC free article] [PubMed] [Google Scholar]
- 16.Allen K, Armstrong LE, Balady GJ, Berry MJ, Broeder C, Castellani J, Clark B, Coe DP, Deschenes M, Doyle JA, Franklin B, Fulco CS, Garber CE, Gordon PM, Headley S, Hodgkin JE, Jakicic JM, Kohrt W, McConnell TR, McInnis K, Morey MC, Muza S, Myers J, Nixon PA, Rupp J, Squires R, Stevinson C, Thomas S, Vanlandewijck Y. ACSM’s Guidelines for Exercise Testing and Prescription. 8. Philadelphia: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 17.Bischoff-Ferrari HA, Shao A, Dawson-Hughes B, Hathcock J, Giovannucci E, Willett WC. Benefit-risk assessment of vitamin D supplementation. Osteoporos Int. 2010;21:1121–32. doi: 10.1007/s00198-009-1119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timmerman KL, Flynn MG, Coen PM, Markofski MM, Pence BD. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? J Leukoc Biol. 2008;84:1271–8. doi: 10.1189/jlb.0408244. [DOI] [PubMed] [Google Scholar]
- 19.Cribb PJ, Hayes A. Effects of supplement timing and resistance exercise on skeletal muscle hypertrophy. Med Sci Sports Exerc. 2006;38:1918–25. doi: 10.1249/01.mss.0000233790.08788.3e. [DOI] [PubMed] [Google Scholar]
- 20.Ceglia L. Vitamin D and its role in skeletal muscle. Curr Opin Clin Nutr Metab Care. 2009;12:628–33. doi: 10.1097/MCO.0b013e328331c707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGill AT, Stewart JM, Lithander FE, Strik CM, Poppitt SD. Relationships of low serum vitamin D3 with anthropometry and markers of the metabolic syndrome and diabetes in overweight and obesity. Nutr J. 2008;7:4. doi: 10.1186/1475-2891-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.International Diabetes Federation. [Accessed January 4, 2011];The IDF consensus worldwide definition of the metabolic syndrome. 2006 at http://wwwidforg/webdata/docs/MetS_def_update2006pdf.
- 23.de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. 2007;28:850–6. doi: 10.1093/eurheartj/ehm026. [DOI] [PubMed] [Google Scholar]
- 24.Heaney RP. Barriers to optimizing vitamin D3 intake for the elderly. J Nutr. 2006;136:1123–5. doi: 10.1093/jn/136.4.1123. [DOI] [PubMed] [Google Scholar]
- 25.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–3. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 26.Powers SK, Howley ET. Exercise Physiology: Theory and Application to Fitness and Performance. 7. Boston: McGraw Hill; 2009. [Google Scholar]
- 27.Woolstenhulme MT, Conlee RK, Drummond MJ, Stites AW, Parcell AC. Temporal response of desmin and dystrophin proteins to progressive resistance exercise in human skeletal muscle. J Appl Physiol. 2006;100:1876–82. doi: 10.1152/japplphysiol.01592.2005. [DOI] [PubMed] [Google Scholar]
- 28.Gabriel DA, Kamen G, Frost G. Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sports Med. 2006;36:133–49. doi: 10.2165/00007256-200636020-00004. [DOI] [PubMed] [Google Scholar]
- 29.Dhesi JK, Jackson SH, Bearne LM, Moniz C, Hurley MV, Swift CG, Allain TJ. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing. 2004;33:589–95. doi: 10.1093/ageing/afh209. [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl 1):S62–7. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]