Abstract
We obtained a rickettsial isolate from the ovaries of the blacklegged tick, Ixodes scapularis. The isolate (ISO7T) was grown in the Ixodes ricinus embryonic cell line IRE11. We characterized the isolate by transmission electron microscopy and gene sequencing. Phylogenetic analysis of 11 housekeeping genes demonstrated that the isolate fulfils the criteria to be classified as a representative of a novel rickettsial species closely related to ‘Rickettsia monacensis’. These rickettsiae form a clade separate from other species of rickettsiae. Gene sequences indicated that several genes important in rickettsial motility, invasiveness and temperature adaptation were mutated (e.g. sca2, rickA, hsp22, pldA and htrA). We propose the name Rickettsia buchneri sp. nov. for this bacterium that infects the ovaries of the tick I. scapularis to acknowledge the pioneering contributions of Professor Paul Buchner (1886–1978) to research on bacterial symbionts. The type strain of R. buchneri sp. nov. is strain ISO-7T ( = DSM 29016T = ATCC VR-1814T).
Ticks harbour non-pathogenic endosymbiotic bacteria that are transovarially transmitted (Buchner, 1926; Cowdry, 1925; Noda et al., 1997). In his seminal publications, Paul Buchner (Buchner, 1926, 1965) described bacterial endosymbionts within oocytes of Ixodes hexagonus, a tick that parasitizes the European hedgehog. In North America, Ixodes scapularis, the blacklegged tick, is an important vector of bacterial pathogens (Munderloh & Kurtti, 2011), but it has not been associated with transmission of pathogenic rickettsiae. Nevertheless, presumably non-pathogenic endosymbiotic rickettsiae are frequently detected in this tick by microscopy (Magnarelli et al., 1991) and PCR assays (Benson et al., 2004; Moreno et al., 2006; Noda et al., 1997; Weller et al., 1998). Several different names have been given to these rickettsiae, i.e. ‘Rickettsia cooleyi’ (Billings et al., 1998), ‘Rickettsia midichlorii’ (Troughton & Levin, 2007) and the rickettsial endosymbiont of I. scapularis (REIS) (Felsheim et al., 2009). Analysis of data generated by PCR-mediated amplification of selected gene sequences indicate that the I. scapularis rickettsia is a single species that displays limited sequence divergence indicating it is ancestral to the spotted fever group rickettsiae (Gillespie et al., 2012). A draft genome of REIS obtained from the project to sequence the genome of I. scapularis (Gillespie et al., 2012) revealed that REIS was closely related to rickettsiae detected in other Ixodes ticks: Ixodes pacificus in California (Phan et al., 2011), Ixodes ricinus in Europe (Corrain et al., 2012; Madeddu et al., 2012; Schicht et al., 2012; Simser et al., 2002), Ixodes nipponensis in Korea (Lee et al., 2013; Shin et al., 2013) and Ixodes boliviensis in Central America (Troyo et al., 2013).
We have observed rickettsiae, by light and electron microscopy, which are restricted to the ovaries of field collected and laboratory reared I. scapularis females (Munderloh et al., 2005). Here we describe the isolation and cultivation of a rickettsial species from ovaries of I. scapularis in a tick cell line. We examined the isolate, ISO7T (I. scapularis ovary from tick 7), by transmission electron microscopy and confirmed that the culture isolate displayed the ultrastructural features typical of rickettsiae. Pulsed field gel electrophoresis (PFGE) (Baldridge et al., 2010) and PCR data of the plasmids matched those described for REIS (Gillespie et al., 2012). In addition, a draft genome sequence confirmed the identity of isolate ISO7T as REIS. In honour of the first description and analysis of presumed rickettsial endosymbionts by Paul Buchner (Buchner, 1926, 1965), we propose the name Rickettsia buchneri sp. nov. to accommodate isolate ISO-7. A comparative phylogenetic analysis of 11 protein sequences from a draft genome sequence of ISO7T generated by us and a draft genome sequence of ‘R. monacensis’ (NZ_CBUA000000000) isolated from I. ricinus (Simser et al., 2002) confirmed that these two rickettsiae form a clade separate from other rickettsiae, as noted by others (Gillespie et al., 2012; Lee et al., 2013). Preliminary data from our laboratory indicate that this clade will include rickettsial endosymbionts from other Ixodes species as well.
ISO7T (I. scapularis ovary from tick 7) was isolated from a female tick removed from a dog in October 2007 (GPS position; 45° 16′ 07˝ N 93° 04′ 51˝ W, Columbus, MN, USA). The tick was surface disinfected 1 week after collection (Kurtti et al., 1996) and the developing ovaries were extirpated, minced and inoculated into the wells of a 24-well plate previously seeded with tick cell lines IRE11 or ISE6 derived from embryos of I. ricinus (Simser et al., 2002) and I. scapularis (Munderloh et al., 1999), respectively. Infected and uninfected tick cells were cultured in modified Leibovitz’s L15 medium supplemented with fetal bovine serum and tryptose phosphate broth as described elsewhere (Munderloh & Kurtti, 1989; Oliver et al., 2014). The plate was incubated in a humidified candle jar at 26–28 °C. After 3–4 weeks, ovarian fragments and infected cells were transferred to IRE11 or ISE6 cell cultures in 12.5 cm2 flasks (vented caps) and maintained in a humidified candle jar for six subcultures. Initially, ISO7T was sensitive to atmospheric conditions, growing best in a hypoxic and CO2-enriched atmosphere. Once adapted, isolate ISO7T was maintained using ambient air. Monthly subcultures were made by transferring 1 ml of heavily infected cells (>95 % infected) onto a fresh layer of uninfected tick cells in 5 ml of medium in 25 cm2 tissue culture flasks. The isolate did not form plaques but heavily infected cells detached from the substrate. Optimal growth of ISO7T was obtained in line IRE11 incubated at 25–28 °C, temperatures lower than those routinely used with pathogenic rickettsiae. When ISO7T was incubated at 32 °C or higher, rickettsial growth and cross infection of uninfected cells ceased (Fig. 1). ISO7T was able to grow in ISE6 cells, but cell-to-cell spread was considerably slower than in IRE11 cultures, and rickettsial numbers declined when cultures were incubated at more than 25 °C.
Fig. 1.
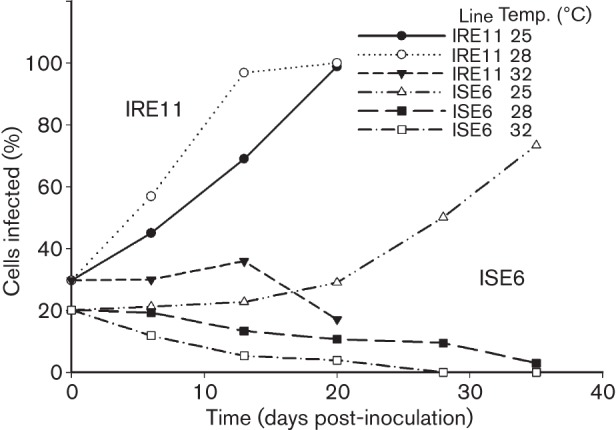
Growth of ISO7T in cell lines IRE11 and ISE6. Infected IRE11 cells were diluted 1 : 5 with uninfected cells and inoculated into replicated cultures. Infection was monitored by preparing cytocentrifuge slides from each culture at selected times for staining with Giemsa stain. Spread of infection was measured by determining the proportion of infected cells at each time point. The values at each time point are the mean of two replicated cultures.
The ultrastructure of ISO7T in IRE11 cells (Fig. 2) was similar to that of rickettsiae found in I. scapularis ovarian cells (Munderloh et al., 2005). Infected cells contained rickettsiae in tightly packed colonies within the cytoplasm only (Fig. 2a, black arrowheads) or dispersed within large vacuoles (Fig. 2a, black arrows), similar to observations reported for Rickettsia peacockii (Balraj et al., 2008; Simser et al., 2001). ISO7T was generally in direct contact with the cytoplasm, but some were being degraded within membrane bound vesicles (presumed lysosomes). In both the ovary and IRE11 cells, rod-shaped rickettsiae were 1.0 to 1.5 µm by 0.4 to 0.5 µm (Fig. 2b). The mottled cytoplasm was surrounded by a narrow periplasmic space of 5 to 10 nm enclosed by a trilaminar cell wall (15–20 nm) (arrow). The inner leaflet was slightly thicker or similar in thickness to the outer leaflet. The microcapsular layer was not prominent and the halo (slime layer) of intracytoplasmic rickettsiae was thin (30 nm or less). ISO7T was never found within host cell nuclei or pseudopodia.
Fig. 2.
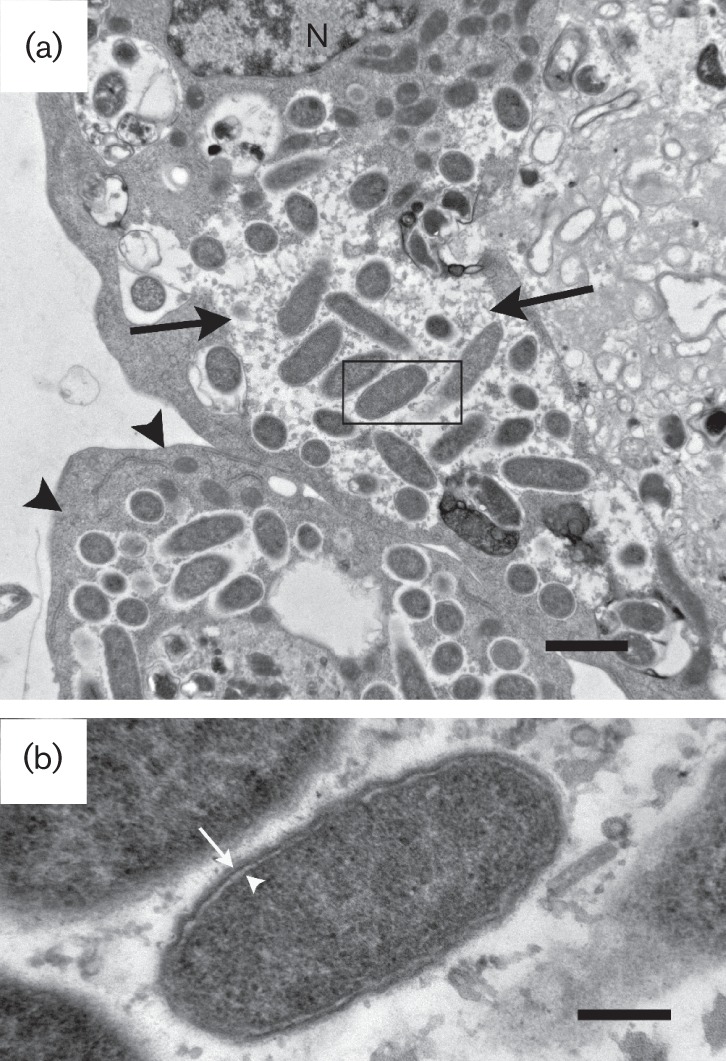
Transmission electron microscopy of IRE11 cells infected with ISO7T. (a) I. ricinus (IRE11) cell with ISO7T in cytoplasm (arrowheads) and in a vacuole (arrows). N, host cell nucleus. Bar, 1 µm. (b) Rickettsiae free in the cytoplasm showing the outer microcapsular layer, cell wall (arrow), electron-lucent periplasmic space and the periplasmic membrane (white arrowhead). Bar, 0.2 µm. The rectangle in (a) delineates the area shown in (b).
The DNA sequences of PCR-products targeting ompA, ompB, 17 kDa and gltA genes, obtained from ovaries of I. scapularis and whole female I. scapularis previously collected in Minnesota, matched sequences of the same genes amplified from the isolate ISO7T and REIS. The DNA sequences of ompA, 17 kDa and gltA were identical to those reported for REIS (Gillespie et al., 2012) but the ompB sequences indicated the gene was not mutated in ISO7T as previously reported for REIS (Gillespie et al., 2012).
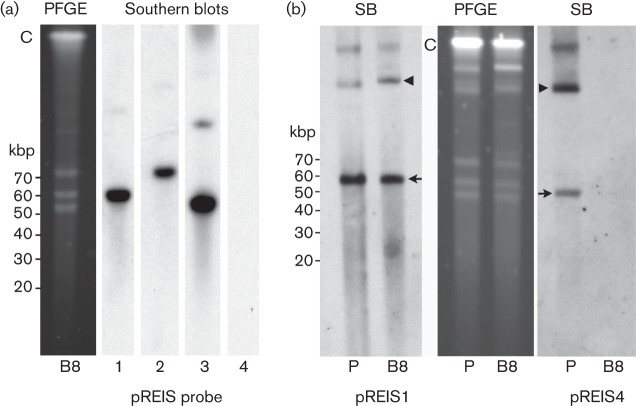
REIS carries four plasmids, pREIS1, pREIS2, pREIS3 and pREIS4 that are 55, 67, 50 and 34 kbp, respectively (Gillespie et al., 2012). PCR using plasmid-specific parA gene primers (Baldridge et al., 2010) demonstrated that each of the four plasmids was present in ISO7T. End point dilution of host-cell-free R. buchneri sp. nov. was used to purify isolate ISO7T in an effort to generate clonal populations instead of the standard rickettsial plaque assay due to the sensitivity of R. buchneri sp. nov. to suboptimal growth conditions. ISO7T plasmids were resolved using PFGE and subjected to Southern blot as previously described (Burkhardt et al., 2011); this enabled comparison of the plasmid profiles from the parental isolate ISO7T and clones derived from it. ISO7T (P) and clone (B8) had similar PFGE profiles with three prominent plasmid bands ranging from 50 to 70 kbp (Fig. 3). Southern blots of gels probed with digoxigenin labelled parA probes specific for pREIS1, pREIS2, pREIS3 and pREIS4 confirmed the presence of all four plasmids in uncloned ISO7T and the absence of pREIS4 in clone B8 (Fig. 3b). These results suggested that pREIS4 was present only in a subpopulation of ISO7T.
Fig. 3.
PFGE and Southern blot (SB) analysis of parental isolate ISO7T and clone B8. Cells were released by passing infected IRE11 cells through a 25 G needle. The suspension was filtered through a sterile 2 µm syringe filter and prepared for PFGE and Southern blot analysis (Burkhardt et al., 2011). (a) B8, PFGE and Southern blots probed with digoxigenin-labelled parA probes specific for plasmid pREIS1, -2, -3 or -4. Note absence of pREIS4. (b) Parental ISO7T (P) and clone B8 PFGE gel and Southern blots. Blots were probed with digoxigenin-labelled parA probes specific for plasmid pREIS1 or pREIS4. Note presence of pREIS4 in the parent (P). Arrows mark putative monomers of each plasmid and arrowheads indicate their conformational isomers. C, chromosomal DNA. Linear DNA marker positions are to the left of panels (a) and (b).
We sequenced the genome of ISO7T clone B8 using DNA extracted from rickettsiae purified from IRE11 cells and compared it to the draft genome sequence of REIS (Gillespie et al., 2012). Genomic DNA was pyrosequenced (454 GS FLX titanium platform; Roche) and sequences assembled using Newbler software (Roche). We predicted potential coding sequences using the annotation pipeline Prokka (http://vicbioinformatics.com/) (Seemann, 2014) together with the Artemis Genome Browser (http://www.sanger.ac.uk/resources/software/artemis/) (Rutherford et al., 2000) and blast to detect split and non-predicted genes. The whole genome shotgun sequence for ISO7T is available in GenBank (JFKF01000000). In summary, the genome assembly of ISO7T B8 comprises 1.66 Mb (1642 ORFs in 207 contigs). Taken together, our PFGE and 454 sequencing data demonstrate that the genome includes the chromosome and four plasmids (pREIS1, pREIS2, pREIS3 and pREIS4). The genome has a DNA G+C content of 32.5 mol%. For comparison, we also sequenced the genome of ‘R. monacensis’ strain IrR/Munich (GenBank, CBUA000000000.1; DSM 29017 available from Leibniz-Institute DSMZ-German Collection of Microorganisms and Cell Cultures).
To analyse the phylogenetic position of R. buchneri sp. nov. (ISO7T) and identify factors germane to its phenotypic characteristics, we compared its draft genome sequence with the genomes of other rickettsial species. The draft genome placed ISO7T into a lineage close to ‘R. monacensis’ and both resided in a clade separate from other rickettsiae. The draft genome sequence also confirmed the absence of pREIS4 in clone B8. Several genes important in rickettsial motility, invasiveness and temperature adaptation were mutated in ISO7T [e.g. sca2 (Kleba et al., 2010), rickA (Simser et al., 2005), hsp22 (Bechah et al., 2010), pldA (Rahman et al., 2013) and htrA (Zhang et al., 2006)]. Additionally, we conducted a neighbour-joining analysis (Saitou & Nei, 1987) of R. buchneri sp. nov. (ISO7T) using the deduced amino acid sequences (concatenated) from 11 housekeeping genes, i.e. htpG, infB, rpoA, rpoB, polA, thrS, groEL, gyrB, recA, dnaE and pnp (Fig. 4 and Table S1, available with the online Supplementary Material). The phylogenetic tree corroborated results obtained using whole genome sequences.
Fig. 4.
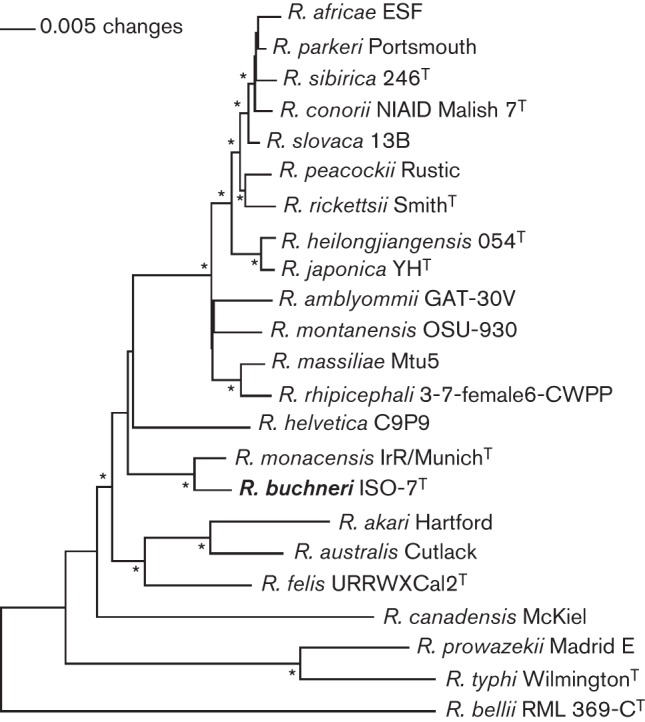
Phylogram showing that ISO7T and ‘R. monacensis’ are closely related and reside in a clade separate from other rickettsiae. Neighbour-joining tree is based on amino acid sequences of 11 concatenated proteins (Table S1). Protein sequences were concatenated in the direction of amine to carboxyl group in the order of proteins HtpG, InfB, RpoA, RpoB, PolA, ThrS, GroEL, GyrB, RecA, DnaE and Pnp. Sequences were aligned using muscle (Edgar, 2004) set at default parameters. The alignments were transferred into the paup* (Swofford, 2002) program as nexus files, and maximum-likelihood and neighbour-joining trees were reconstructed. Trees were rooted by making the outgroup (Rickettsia bellii) paraphyletic with respect to the ingroup. The robustness of clade designations was tested with a full heuristic search and 1000 bootstrap replicates, and nodes with asterisks are supported at values of ≥99 %.
A multigenic approach is used to define new rickettsial species (Duh et al., 2010; Fournier et al., 2003) and improve phylogenetic resolution. The genes commonly analysed are 16S rRNA (Roux & Raoult, 1995), gltA (Roux et al., 1997), ‘geneD’ (Sekeyova et al., 2001), ompA and ompB (Roux & Raoult, 2000). According to the classification scheme proposed by Fournier et al. (2003), ISO7T is a representative of a novel species closely related to ‘Rickettsia monacensis’ that we propose to name Rickettsia buchneri sp. nov. Our proposal is reinforced by the approach of using concatenated gene sequences. Vitorino et al. (2007) have demonstrated that concatenation of nucleotide sequences of eight loci (atpA, recA, virB4, dnaA, dnaK, rrl–rrf internal transcribed spacer, ompA and gltA) gave improved discrimination between rickettsial species. Our phylogenetic placement obtained when concatenating 11 housekeeping genes agrees with that of Gillespie et al. (2012) who concatenated the amino acid sequence of 191 proteins and suggested that REIS was ancestral to spotted fever group rickettsiae (Gillespie et al., 2012).
Description of Rickettsia buchneri sp. nov.
Rickettsia buchneri (buch′ne.ri. N.L. gen. masc. n. buchneri of Buchner, named in honour of Dr Paul Buchner, a German biologist who made pioneering contributions to the identification of non-pathogenic tick endosymbionts that are transovarially transmitted).
An obligate intracellular bacterium found in ovaries of the blacklegged tick I. scapularis. The rickettsiae can be grown in tick cell lines IRE11 and ISE6 at 25–28 °C. The I. ricinus and I. scapularis host cells are grown in a medium formulated for tick cell culture. The ultrastructural appearance of the culture isolate is similar to rickettsiae found in the ovaries of I. scapularis and is typical for the genus Rickettsia. The cell wall and cytoplasmic membrane are separated by a thin periplasmic space. Cells infected with coccobacillary rickettsiae can be seen with Giemsa stain. Grows extensively in the cytoplasm of host cells and causes hypertrophy. To date, R. buchneri has only been isolated from I. scapularis but closely related bacteria are found in other Ixodes ticks. Widely distributed among North American I. scapularis ticks. The draft 1.66 Mb genome sequence indicates that R. buchneri is closely related to ‘R. monacensis’ found in I. ricinus ticks in Europe and forms a clade separate from rickettsiae found in other tick species.
The type strain, ISO7T ( = DSM 29016T = ATCC VR-1814T), was isolated from a partially engorged I. scapularis female collected in Minnesota, USA, October 2007.
Acknowledgements
This research was supported by National Institutes of Health grants R01 AI49424 and R01 AI081690 to U. G. M. (http://www.grants.nih.gov/grants/oer.htm). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Abbreviations:
- PFGE
pulsed field gel electrophoresis
- REIS
rickettsial endosymbiont of Ixodes scapularis
Footnotes
One supplementary table is available with the online Supplementary Material.
References
- Baldridge G. D., Burkhardt N. Y., Labruna M. B., Pacheco R. C., Paddock C. D., Williamson P. C., Billingsley P. M., Felsheim R. F., Kurtti T. J., Munderloh U. G. (2010). Wide dispersal and possible multiple origins of low-copy-number plasmids in rickettsia species associated with blood-feeding arthropods. Appl Environ Microbiol 76, 1718–1731. 10.1128/AEM.02988-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balraj P., El Karkouri K., Vestris G., Espinosa L., Raoult D., Renesto P. (2008). RickA expression is not sufficient to promote actin-based motility of Rickettsia raoultii. PLoS ONE 3, e2582. 10.1371/journal.pone.0002582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechah Y., El Karkouri K., Mediannikov O., Leroy Q., Pelletier N., Robert C., Médigue C., Mege J. L., Raoult D. (2010). Genomic, proteomic, and transcriptomic analysis of virulent and avirulent Rickettsia prowazekii reveals its adaptive mutation capabilities. Genome Res 20, 655–663. 10.1101/gr.103564.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson M. J., Gawronski J. D., Eveleigh D. E., Benson D. R. (2004). Intracellular symbionts and other bacteria associated with deer ticks (Ixodes scapularis) from Nantucket and Wellfleet, Cape Cod, Massachusetts. Appl Environ Microbiol 70, 616–620. 10.1128/AEM.70.1.616-620.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings A. N., Teltow G. J., Weaver S. C., Walker D. H. (1998). Molecular characterization of a novel Rickettsia species from Ixodes scapularis in Texas. Emerg Infect Dis 4, 305–309. 10.3201/eid0402.980221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner P. (1926). Studien an intrazellularen symbionten VI. Zur acarinen-symbiose. Zeitschrift für Morphologie und Oekologie der Tiere 6, 625–644 10.1007/BF00464432 [DOI] [Google Scholar]
- Buchner P. (1965). Endosymbiosis of Animals with Plant Microorganisms. New York: Interscience Publishers. [Google Scholar]
- Burkhardt N. Y., Baldridge G. D., Williamson P. C., Billingsley P. M., Heu C. C., Felsheim R. F., Kurtti T. J., Munderloh U. G. (2011). Development of shuttle vectors for transformation of diverse Rickettsia species. PLoS ONE 6, e29511. 10.1371/journal.pone.0029511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrain R., Drigo M., Fenati M., Menandro M. L., Mondin A., Pasotto D., Martini M. (2012). Study on ticks and tick-borne zoonoses in public parks in Italy. Zoonoses Public Health 59, 468–476. 10.1111/j.1863-2378.2012.01490.x [DOI] [PubMed] [Google Scholar]
- Cowdry E. V. (1925). A group of microorganisms transmitted hereditarily in ticks and apparently unassociated with disease. J Exp Med 41, 817–830. 10.1084/jem.41.6.817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duh D., Punda-Polic V., Avsic-Zupanc T., Bouyer D., Walker D. H., Popov V. L., Jelovsek M., Gracner M., Trilar T. & other authors (2010). Rickettsia hoogstraalii sp. nov., isolated from hard- and soft-bodied ticks. Int J Syst Evol Microbiol 60, 977–984. 10.1099/ijs.0.011049-0 [DOI] [PubMed] [Google Scholar]
- Edgar R. C. (2004). muscle: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32, 1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsheim R. F., Kurtti T. J., Munderloh U. G. (2009). Genome sequence of the endosymbiont Rickettsia peacockii and comparison with virulent Rickettsia rickettsii: identification of virulence factors. PLoS ONE 4, e8361. 10.1371/journal.pone.0008361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier P.-E., Dumler J. S., Greub G., Zhang J., Wu Y., Raoult D. (2003). Gene sequence-based criteria for identification of new rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J Clin Microbiol 41, 5456–5465. 10.1128/JCM.41.12.5456-5465.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. J., Joardar V., Williams K. P., Driscoll T., Hostetler J. B., Nordberg E., Shukla M., Walenz B., Hill C. A. & other authors (2012). A Rickettsia genome overrun by mobile genetic elements provides insight into the acquisition of genes characteristic of an obligate intracellular lifestyle. J Bacteriol 194, 376–394. 10.1128/JB.06244-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleba B., Clark T. R., Lutter E. I., Ellison D. W., Hackstadt T. (2010). Disruption of the Rickettsia rickettsii Sca2 autotransporter inhibits actin-based motility. Infect Immun 78, 2240–2247. 10.1128/IAI.00100-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtti T. J., Munderloh U. G., Hughes C. A. N., Engstrom S. M., Johnson R. C. (1996). Resistance to tick-borne spirochete challenge induced by Borrelia burgdorferi strains that differ in expression of outer surface proteins. Infect Immun 64, 4148–4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.-M., Choi Y.-J., Shin S.-H., Choi M. K., Song H. J., Kim H. C., Klein T. A., Richards A. L., Park K. H., Jang W. J. (2013). Spotted fever group rickettsia closely related to Rickettsia monacensis isolated from ticks in South Jeolla province, Korea. Microbiol Immunol 57, 487–495. [DOI] [PubMed] [Google Scholar]
- Madeddu G., Mancini F., Caddeo A., Ciervo A., Babudieri S., Maida I., Fiori M. L., Rezza G., Mura M. S. (2012). Rickettsia monacensis as cause of Mediterranean spotted fever-like illness, Italy. Emerg Infect Dis 18, 702–704. 10.3201/eid1804.111583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli L. A., Andreadis T. G., Stafford K. C., III, Holland C. J. (1991). Rickettsiae and Borrelia burgdorferi in ixodid ticks. J Clin Microbiol 29, 2798–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno C. X., Moy F., Daniels T. J., Godfrey H. P., Cabello F. C. (2006). Molecular analysis of microbial communities identified in different developmental stages of Ixodes scapularis ticks from Westchester and Dutchess Counties, New York. Environ Microbiol 8, 761–772. 10.1111/j.1462-2920.2005.00955.x [DOI] [PubMed] [Google Scholar]
- Munderloh U. G., Kurtti T. J. (1989). Formulation of medium for tick cell culture. Exp Appl Acarol 7, 219–229. 10.1007/BF01194061 [DOI] [PubMed] [Google Scholar]
- Munderloh U. G., Kurtti T. J. (2011). Emerging and re-emerging tick-borne diseases: new challenges at the interface of human and animal health. In Critical Needs and Gaps in Understanding Prevention, Amelioration, and Resolution of Lyme and Other Tick-Borne Diseases: the Short-Term and Long-Term outcomes: Workshop Report, pp. 376–404 Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Munderloh U. G., Jauron S. D., Fingerle V., Leitritz L., Hayes S. F., Hautman J. M., Nelson C. M., Huberty B. W., Kurtti T. J. & other authors (1999). Invasion and intracellular development of the human granulocytic ehrlichiosis agent in tick cell culture. J Clin Microbiol 37, 2518–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munderloh U. G., Jauron S. D., Kurtti T. J. (2005). The tick: a different kind of host for human pathogens. In Tick-Borne Diseases of Humans, pp. 37–64 Edited by Goodman J. L., Dennis D. T., Sonenshine D. E. Washington, DC: American Society for Microbiology; 10.1128/9781555816490.ch3 [DOI] [Google Scholar]
- Noda H., Munderloh U. G., Kurtti T. J. (1997). Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl Environ Microbiol 63, 3926–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. D., Burkhardt N. Y., Felsheim R. F., Kurtti T. J., Munderloh U. G. (2014). Motility characteristics are altered for Rickettsia bellii transformed to overexpress a heterologous rickA gene. Appl Environ Microbiol 80, 1170–1176. 10.1128/AEM.03352-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan J. N., Lu C. R., Bender W. G., Smoak R. M., III, Zhong J. (2011). Molecular detection and identification of Rickettsia species in Ixodes pacificus in California. Vector Borne Zoonotic Dis 11, 957–961. 10.1089/vbz.2010.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M. S., Gillespie J. J., Kaur S. J., Sears K. T., Ceraul S. M., Beier-Sexton M., Azad A. F. (2013). Rickettsia typhi possesses phospholipase A2 enzymes that are involved in infection of host cells. PLoS Pathog 9, e1003399. 10.1371/journal.ppat.1003399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux V., Raoult D. (1995). Phylogenetic analysis of the genus Rickettsia by 16S rDNA sequencing. Res Microbiol 146, 385–396. 10.1016/0923-2508(96)80284-1 [DOI] [PubMed] [Google Scholar]
- Roux V., Raoult D. (2000). Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int J Syst Evol Microbiol 50, 1449–1455. 10.1099/00207713-50-4-1449 [DOI] [PubMed] [Google Scholar]
- Roux V., Rydkina E., Eremeeva M., Raoult D. (1997). Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the rickettsiae. Int J Syst Bacteriol 47, 252–261. 10.1099/00207713-47-2-252 [DOI] [PubMed] [Google Scholar]
- Rutherford K., Parkhill J., Crook J., Horsnell T., Rice P., Rajandream M. A., Barrell B. (2000). Artemis: sequence visualization and annotation. Bioinformatics 16, 944–945. 10.1093/bioinformatics/16.10.944 [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4, 406–425. [DOI] [PubMed] [Google Scholar]
- Schicht S., Schnieder T., Strube C. (2012). Rickettsia spp. and coinfections with other pathogenic microorganisms in hard ticks from northern Germany. J Med Entomol 49, 766–771. 10.1603/ME11204 [DOI] [PubMed] [Google Scholar]
- Seemann T. (2014). Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069. 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- Sekeyova Z., Roux V., Raoult D. (2001). Phylogeny of Rickettsia spp. inferred by comparing sequences of ‘gene D’, which encodes an intracytoplasmic protein. Int J Syst Evol Microbiol 51, 1353–1360. [DOI] [PubMed] [Google Scholar]
- Shin S.-H., Seo H.-J., Choi Y.-J., Choi M. K., Kim H. C., Klein T. A., Chong S. T., Richards A. L., Park K. H., Jang W. J. (2013). Detection of Rickettsia monacensis from Ixodes nipponensis collected from rodents in Gyeonggi and Gangwon Provinces, Republic of Korea. Exp Appl Acarol 61, 337–347. 10.1007/s10493-013-9699-1 [DOI] [PubMed] [Google Scholar]
- Simser J. A., Palmer A. T., Munderloh U. G., Kurtti T. J. (2001). Isolation of a spotted fever group Rickettsia, Rickettsia peacockii, in a Rocky Mountain wood tick, Dermacentor andersoni, cell line. Appl Environ Microbiol 67, 546–552. 10.1128/AEM.67.2.546-552.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simser J. A., Palmer A. T., Fingerle V., Wilske B., Kurtti T. J., Munderloh U. G. (2002). Rickettsia monacensis sp. nov., a spotted fever group Rickettsia, from ticks (Ixodes ricinus) collected in a European city park. Appl Environ Microbiol 68, 4559–4566. 10.1128/AEM.68.9.4559-4566.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simser J. A., Rahman M. S., Dreher-Lesnick S. M., Azad A. F. (2005). A novel and naturally occurring transposon, ISRpe1 in the Rickettsia peacockii genome disrupting the rickA gene involved in actin-based motility. Mol Microbiol 58, 71–79. 10.1111/j.1365-2958.2005.04806.x [DOI] [PubMed] [Google Scholar]
- Swofford D. L. (2002). paup*: Phylogenetic analysis using parsimony (and other methods), version 4. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Troughton D. R., Levin M. L. (2007). Life cycles of seven ixodid tick species (Acari: Ixodidae) under standardized laboratory conditions. J Med Entomol 44, 732–740. 10.1603/0022-2585(2007)44[732:LCOSIT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Troyo A., Carranza M., Moreira A., Calderon-Arguedas O., Hun L., Taylor L. (2013). Detection of a Rickettsia closely related to R. monacensis in Ixodes boliviensis from Costa Rica. Am Soc Trop Med Hyg Abstract Book Abstract 747 http://www.astmh.org/Meeting_Archives.htm. [Google Scholar]
- Vitorino L., Chelo I. M., Bacellar F., Zé-Zé L. (2007). Rickettsiae phylogeny: a multigenic approach. Microbiology 153, 160–168. 10.1099/mic.0.2006/001149-0 [DOI] [PubMed] [Google Scholar]
- Weller S. J., Baldridge G. D., Munderloh U. G., Noda H., Simser J., Kurtti T. J. (1998). Phylogenetic placement of rickettsiae from the ticks Amblyomma americanum and Ixodes scapularis. J Clin Microbiol 36, 1305–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Z., Hao J. F., Walker D. H., Yu X. J. (2006). A mutation inactivating the methyltransferase gene in avirulent Madrid E strain of Rickettsia prowazekii reverted to wild type in the virulent revertant strain Evir. Vaccine 24, 2317–2323. 10.1016/j.vaccine.2005.11.044 [DOI] [PubMed] [Google Scholar]