Summary
Mitochondria are well appreciated for their role as biosynthetic and bioenergetic organelles. In the past two decades, mitochondria have emerged as signaling organelles that contribute critical decisions about cell proliferation, death and differentiation. Mitochondria not only sustain immune cell phenotypes but also are necessary for establishing immune cell phenotype and their function. Mitochondria can rapidly switch from primarily being catabolic organelles generating ATP to anabolic organelles that generate both ATP and building blocks for macromolecule synthesis. This enables them to fulfill appropriate metabolic demands of different immune cells. Mitochondria have multiple mechanisms that allow them to activate signaling pathways in the cytosol including altering in AMP/ATP ratio, the release of ROS and TCA cycle metabolites, as well as the localization of immune regulatory proteins on the outer mitochondrial membrane. In this Review, we discuss the evidence and mechanisms that mitochondrial dependent signaling controls innate and adaptive immune responses.
Introduction
Over the last 30 years, the molecular events that drive immune responses have been characterized in great detail in numerous cell types. A successful immune response requires a delicate balance of activation and inhibition of distinct signaling pathways in diverse cell types. Classically these signaling pathways are conceptualized as linear phosphorylation-based cascades initiated at the cell surface and transmitted to the nucleus. However increasing data suggest that many of these signaling pathways are highly integrated with cellular metabolism, which not only fuels active cells but also provides guidance for cell fate decisions. These studies have led to the creation of the new field of investigation termed immunometabolism.
For decades we have observed that immune cells transform from a state of relative metabolic quiescence to a highly active metabolic state during the activation phase of an immune response. Predictably, this conversion requires a shift in cellular metabolism from a catabolic to an anabolic metabolic program (Pearce and Pearce, 2013). In a catabolic state, macromolecules are completely degraded and shuttled through energy-generating pathways to produce ATP to maintain cellular homeostasis and allow long-term survival during quiescence. Alternatively, in an anabolic state, cellular metabolism is reorganized to balance a need for ATP with the need for metabolic intermediates that are required for de novo synthesis of macromolecules. Yet our lab and others have observed that during both catabolism and anabolism, metabolic pathways provide more than just ATP and biosynthetic intermediates and importantly provide signaling intermediates that are essential for cellular function. An emerging concept is that metabolism not only sustains diverse immune cell phenotypes as a consequence of alterations in cellular signaling, but metabolism also feeds back and alters signaling to drive immune cell phenotypes. Mitochondria are central hubs of metabolism thus have emerged to be necessary for both the maintenance and establishment of immune cell phenotypes. In this review, we will create a conceptual framework to establish mitochondria as signaling organelles that are critical for innate and adaptive immune responses.
Mitochondria are bioenergetic, biosynthetic and signaling organelles
Historically, the major role of mitochondria is thought to be to the efficient coupling of metabolite oxidation through the tricarboxylic acid (TCA) cycle to ATP production by the electron transport chain (ETC). Fatty acids or pyruvate are oxidized into acetyl-CoA by fatty acid oxidation or pyruvate dehydrogenase (PDH), respectively. Subsequently, the TCA cycle is initiated by the enzyme citrate synthase that catalyzes the condensation reaction of acetyl-CoA with oxaloacetate to generate citrate. The TCA cycle generates reducing equivalents NADH and FADH2 that provide electrons to the electron transport chain. The ETC complexes ultimately transfer electrons to molecular oxygen and concomitantly pump protons across the inner mitochondrial membrane resulting in a generation of a proton-motive force that is utilized to produce ATP by the FoF1 ATP synthase. Mitochondria that fail to generate a mitochondrial membrane potential are targeted for destruction through mitophagy. Mitochondrial oxidation of pyruvate and fatty acids such as palmitate generates 31.5 and 113 ATP, respectively, compared to 2 ATP generated by glycolysis (Mookerjee et al., 2015). Thus mitochondria are the most efficient source of cellular ATP.
An equally important primordial function of mitochondria is the utilization of TCA cycle metabolites for building of macromolecules. For example, citrate can be transported into the cytosol where ATP-citrate lyase (ACLY) converts citrate into acetyl-CoA and oxaloacetate. Cytosolic acetyl-CoA is utilized for protein acetylation as well as de novo fatty acid synthesis (Wellen and Thompson, 2012). Citrate depletion from the TCA cycle for de novo lipid synthesis necessitates replenishment of the TCA cycle (termed anaplerosis) to allow it to continue functioning. Glutamine replenishes the TCA cycle through glutaminolysis, which results in the generation of a-ketoglutarate (Hensley et al., 2013).
These two functions of mitochondria to generate ATP and to support biosynthesis must be carefully balanced to support specific cellular demands. Thus, mitochondria are metabolic hubs within the cell that alter their function to meet cellular needs. Clearly this necessitates that mitochondria receive signals to change their function. But importantly, more and more data suggest that mitochondrial pathways are not just reactive, but also actively provide signals back to the nucleus. This cross talk may coordinate cell fate decisions with metabolic capacity dependent on the cellular environment. Thus we propose that mitochondria are crucial cellular signaling organelles that are integral part of decision making process when cells receives internal and external cues to trigger diverse biological outcomes ranging from metabolic adaptation, proliferation, differentiation and cell death (Chandel, 2014).
There are several known types of signal transduction mechanisms between mitochondria and the rest of the cell. First, anterograde signaling is signal transduction from cytosol to mitochondria. The best example of this is the rapid sequestration of calcium into the mitochondrial matrix in response to elevations in cytosolic calcium (Rizzuto et al., 2012). The influx of calcium into the mitochondria results in activation of multiple enzymes of the TCA cycle and the ETC. Second, retrograde signaling is signal transduction from mitochondria to the cytosol. One of the earliest examples of retrograde signaling was the production of mitochondrial reactive oxygen species (ROS) regulating the activation of the transcription factor hypoxia inducible factor 1 (HIF-1) (Chandel et al., 1998). Recent studies indicate that mitochondrial ROS regulate metabolic adaptation, differentiation and proliferation (Sena and Chandel, 2012). The ETC can produce superoxide, notably from complexes I and III, that can be converted into hydrogen peroxide and released into the cytosol where it can cause thiol oxidation of proteins (Murphy, 2009). There are total of 10 potential sites of ROS generation within mitochondria (Quinlan et al., 2013). Mitochondria can also impact signaling by altering the availability of TCA cycle intermediates acetyl-CoA, succinate, fumarate, and a-ketoglutarate, which can alter protein function (Metallo and Vander Heiden, 2010). Acetyl-CoA is utilized for protein acetylation, α-ketoglutarate is required for function of α-ketoglutarate-dependent dioxygenases family of proteins, which include the prolyl hydroxlylaseas (PHDs) and Jumonji domain-containing histone demethylase (JHDM), and fumarate and succinate are inhibitors of these proteins (Kaelin and McKnight, 2013). PHDs and JHDM are negative regulators of the HIFs and are also sensitive to hydrogen peroxide. As such, the accumulation of fumarate, succinate and hydrogen peroxide can result in inactivation of PHDs and JHDMs resulting in activation of HIFs and hypermethylation of histones. Mitochondrial bioenergetic status can also influence signaling pathways. Notably, the decrease in mitochondrial ATP production typically increases AMP concentrations that cause a shift from an anabolic state to a catabolic state to sustain high ATP/ADP ratio necessary to thermodynamically favor ATP coupled reactions. The increase in AMP/ATP ratio triggers activation of AMP-activated protein kinase (AMPK) that decreases mammalian target of rapamycin (mTOR) activity to diminish anabolic reactions thus reducing ATP demand and activate autophagy to increase metabolic supply by providing nutrients to mitochondria for generation of ATP (Pearce et al., 2013). AMPK activation also promotes fatty acid oxidation while suppressing fatty acid synthesis. Lastly, the outer mitochondrial membrane is known to serve as a signaling platform to align multiple proteins to allow for coordinated interaction and subsequent signaling (West et al., 2011a).
Mitochondrial signaling dictates macrophage polarization and function
Macrophages are commonly distinguished into two lineages, classically activated (M1) and alternatively activated (M2). M1 macrophages display marked production of inflammatory mediators following exposure to pro-inflammatory mediators such as LPS while M2 polarized macrophages display a pro-fibrotic and anti-inflammatory signature in response to the cytokine interleukin-4 (IL-4). Although this classification scheme is imperfect and there almost certainly exists many more macrophage subtypes, it is a useful model for studying how metabolism differs in macrophages with differing functions (Mosser and Edwards, 2008). M1 polarized macrophages exhibit robust glycolysis even in the presence of ample oxygen and decreased oxygen consumption compared to unpolarized macrophages suggesting minimal reliance on mitochondrial metabolism and a dependence on glycolytic ATP production compared with the M0 unpolarized macrophages (Haschemi et al., 2012; Huang et al., 2014). In contrast, M2 polarized macrophages demonstrate an increase in oxygen consumption. The importance of these difference in mitochondrial metabolism between M1 and M2 in vivo is supported by the finding that mice deficient in NDUFS4, a subunit of complex I of the ETC, exhibit an enhanced M1 polarization and diminished M2 polarization (Jin et al., 2014).
The induction of M2 polarization is driven by IL-4 stimulation of signal transducer and activator of transcription 6 (STAT6) resulting in PPARγ-coactivator-1β (PGC-1β) induction of mitochondrial biogenesis and fatty acid oxidation (Vats et al., 2006). The carnitine palmitoyltransferase-1 (CPT1) inhibitor etomoxir, which inhibits fatty acid translocation into the mitochondria, is sufficient to inhibit expression of classic M2 genes indicating that the increase in mitochondrial metabolism is not simply an effect of STAT6 activation needed for sustaining the M2 phenotype, but is in fact a driver of M2 macrophage activation and function. Furthermore, the loss of the transcription factors of the peroxisome proliferator-activated receptor (PPAR) family, transcription factors known to activate oxidative metabolism in numerous tissues, leads to a deficit in M2 polarization (Kang et al., 2008; Odegaard et al., 2007, 2008). In contrast, overexpression of PGC-1β promotes M2 polarization that could be reversed following pharmacologic blockade of fatty acid oxidation or mitochondrial ATP production (Vats et al., 2006). These data are consistent with the observation that M2 macrophages require AMPK, a stimulator of fatty acid oxidation, for proper activation in vivo (Mounier et al., 2013) (Carroll et al., 2013). Interestingly, the potential source of fatty acids required for M2 polarization is internal lysosomal stores. Thus, M2 macrophages require cell autonomous lysosomal based lipolysis to increased internal fatty acids to fuel the enhanced mitochondrial metabolism (Huang et al., 2014). Going forward it will be important to specifically ablate fatty acid oxidation or lysosomal dependent lipolysis in macrophages to confirm in vivo significance lysosomal dependent lipolysis and fatty acid oxidation in establishing and maintaining the M2 phenotype.
A critical question that remains unanswered is what are the advantages of conducting enhanced glycolysis and mitochondrial metabolism in establishing the M1 and M2 phenotype, respectively? A clue may come from the observation that M1 macrophages require glucose-dependent metabolism for anabolic functions while the role of mitochondria is restricted to signaling organelles in response to microorganism-derived pathogen-associated molecular patterns (PAMPs) and endogenous tissue injury derived damage-associated molecular patterns (DAMPs).
Mitochondrial signaling is necessary for responses to activators of innate immune signaling
Pathogen-associated molecular patterns (PAMPs) and damage-associate molecular patterns (DAMPs) bind to specific receptors including RIG-I-like receptors (RLRs), NOD-like receptors (NLRs), Toll-like receptors (TLR), to generate cytokines that are essential for eliminating pathogens or repairing tissue damage. Interestingly, mitochondrial DNA and N-formyl peptides represent two sources of mitochondrial DAMPs that activate pattern recognition receptors (PRR). N-formyl-methionine is the initiating residue for both mitochondria and bacterial protein synthesis. Bacterial N-formyl peptides serve as PAMPs by activating G-protein-coupled formyl peptide receptors (FPRs) (Rabiet et al., 2007), and mitochondrial N-formyl peptides act as DAMPs through activation of the receptor FPR-1 to stimulate cytokine secretion (Carp, 1982; Zhang et al., 2010). Mitochondrial DNA is similar to bacterial DNA in that both share hypomethylated CpG motifs, which activate Toll-like receptor 9 (TLR9) (West et al., 2011a). Direct injection of mitochondrial (but not nuclear) DNA into mouse joints induces a pro-inflammatory response (Collins et al., 2004), and systemic injection of mitochondrial DNA induces lung and liver inflammation (Zhang et al., 2010). Mitochondrial DNA is also released systemically during trauma injury to induce inflammation (Zhang et al., 2010). Thus, mitochondrial DAMPS drive hyperactivation of innate immunity in an absence of an infection by a microorganisms i.e. sterile inflammation. In the next section we review the evidence for mitochondria-dependent signaling in regulating responses to both DAMPs and PAMPs.
Initial studies implicating mitochondria as signaling organelles in innate immunity came from the observations that LPS through toll-like receptor 4 (TLR4) and tumor necrosis factor-α (TNF-α) through TNF receptor associated factors (TRAFs) activate inflammatory cytokines through the generation of mitochondrial generated ROS (Chandel et al., 2000, 2001). More recent studies have shown that decreasing mitochondrial ROS diminishes multiple TLR-initiated pathways and bactericidal activity of macrophages (West et al., 2011b). TLR1, 2 and 4 activation results in mitochondrial translocation of TRAF6 that interacts with ECSIT, a protein that has been implicated in mitochondrial respiratory complex I assembly, leading to increased mitochondrial ROS that aid in the destruction of phagocytosed bacteria. It is not clear how ECSIT regulates mitochondrial ROS production upon TLR stimulation. Furthermore, patients with tumor necrosis factor receptor-associated periodic syndrome (TRAPS) have heightened responsiveness to LPS due to increased mitochondrial ROS production that promotes inflammation (Bulua et al., 2011).
Aside from ROS, the TCA cycle intermediate succinate has also been implicated in LPS-induced inflammatory cytokine signaling. In the TCA cycle, succinate is produced from succinyl-CoA and subsequently converted to fumarate by succinate dehydrogenase. Succinate dehydrogenase is the only TCA enzyme that also functions as an electron carrier in the electron transport chain, which may position it to also modulate ROS signaling (Mills and O'Neill, 2014). In LPS-activated macrophages, increases in succinate concentrations stabilize HIF-1α through inhibition of PHDs (Tannahill et al., 2013), an effect that has previously been reported to occur in tumors (Selak et al., 2005). This stabilization of HIF-1α induces the expression of the pro-inflammatory cytokine IL-1β. In addition to directly activating HIF-1 through PHD inactivation, succinate may also increase mROS production, which is known to activate HIF-1α in macrophages (Wang et al., 2010). Interestingly, a recent study demonstrated that succinate build up results in increased reverse electron transport and ROS production from complex I of the ETC (Chouchani et al., 2014). Going forward it will be important to decipher the mechanism by which LPS results in accumulation of succinate and whether succinate activation of IL-1β expression requires mitochondrial ROS. Citrate is another TCA cycle intermediate implicated in LPS activation of pro-inflammatory gene expression. It is known that it is exported from the mitochondria to the cytosol and converted to acetyl-CoA, however the precise mechanism by which it thereby alters cytokine production is unclear (Infantino et al., 2011).
In addition to TLRs, other pattern recognition receptors are known to depend on mitochondrial ROS signaling including nuclear oligomerization domain- (NOD-) like receptors (NLRs). Upon activation, NLRs form multi-subunit protein complexes termed inflammasomes that activate caspase-1 resulting in proteolytic cleavage and maturation of the pro-inflammatory cytokine IL-1β (Schroder and Tschopp, 2010). Diverse PAMPs and DAMPs such as lipopolysaccharide (LPS), asbestos, ATP, and uric acid lead to NLRP3 activation through increase in ROS (Cruz et al., 2007; Dostert et al., 2008). Specifically, pharmacological manipulations resulting in diminished mitochondrial ROS decrease NLRP3 inflammasome activation, but not other inflammasome subsets (Zhou et al., 2011). Furthermore, pharmacologic or genetic blockade of autophagy, which increases mitochondrial ROS concentrations, enhances inflammasome activation (Saitoh et al., 2008; Zhou et al., 2011). The release of mitochondrial reactive oxygen species (ROS) leads to lysosomal membrane permeabilization necessary for proper NLRP3 activation (Heid et al., 2013). A consequence of NLRP3 activation is the induction of mitochondrial damage with concomitant block in mitophagy to remove damaged mitochondria (Yu et al., 2014). Apart from mitochondrial ROS, release of mitochondrial DNA (mtDNA) into the cytosol was found to enhance NLRP3 activation (Nakahira et al., 2011). Later it was shown that oxidized mtDNA is actually required to activate NLRP3 (Shimada et al., 2012). This finding is provocative because it suggests that mtDNA is released from mitochondria without release of cytochrome c and induction of cell death. Further study of this observation is likely to reveal new mitochondrial transport mechanisms. Another recent finding linking mitochondria to NLRP3 is the observation that cardiolipin on the mitochondrial outer membrane directly binds to NLRP3 resulting in its activation (Iyer et al., 2013). Importantly, although many studies suggest an important role of mitochondrial ROS, cardiolipin and DNA for in regulating NLRP3 activation, direct genetic evidence is still lacking in vivo.
It is important to note that mitochondrial ROS are necessary for optimal activation of NLRP3 inflammasome as other key activators such as influx of calcium and potassium efflux are also major regulators of NLRP3 inflammasome (Gurung et al., 2014). Calcium influx also contributes to mitochondrial damage which might increase mitochondrial ROS and release of mitochondrial DNA to amplify NLRP3 inflammasome activation (Murakami et al., 2012). The specific mechanisms by which diverse PAMPs and DAMPs increase mitochondrial ROS and by which mitochondrial ROS and ions such as calcium cooperate to optimally activate NLRP3 have yet to be delineated.
An emerging theme in the past decade is that the mitochondrial outer membrane serves as a signaling platform for innate immune responses. The most studied example of this is a class of PRRs that respond to viral infection known as the retinoic-acid-inducible protein I (RIG-I) like receptor family (RLRs). The three members of RLRs are RIG-I, MDA5 and DHX58 that function in antiviral immunity by sensing viral 5′-triphosphorylated and uncapped single- or double-stranded RNA resulting in the production of type I interferons and pro-inflammatory cytokines (Reikine et al., 2014). A breakthrough in establishing the role for mitochondria in RLR-activated antiviral immunity was the identification of the RLR mitochondrial adaptor protein MAVS (mitochondrial antiviral-signaling protein)(Kawai et al., 2005; Meylan et al., 2005; Seth et al., 2005; Xu et al., 2005) localization to the outer mitochondrial membrane is indispensable for its function. MAVS contains a C-terminal transmembrane domain, which targets the protein to the outer membrane of mitochondria. Subsequent studies demonstrated that MAVS does not bind to free mitochondria but to mitochondrial associated membrane (MAM), which physically connects ER specialized domain to outer mitochondrial membrane (Horner et al., 2011). MAM provides a mitochondrial–ER inter-organelle communication that regulates stress and metabolic signaling (Hayashi et al., 2009). Mice deficient in MAVS are impaired in their ability to produce type I IFNs thus are highly susceptible to RNA virus infection (Sun et al., 2006). RIG-I and MDA5 interact with MAVS through mutual caspase activation and recruitment domains (CARDs) upon recognition of viral RNA. Subsequently, RIG-I interaction with MAVS induces prion-like aggregates of MAVS on the outer mitochondrial membrane leads to activation of downstream pathways such as interferon regulatory factor 3 (IRF3), MAP kinases, and nuclear factor-κB (NF-κB) (Hou et al., 2011).
It is important to note that MAVS is also localized to the membranes of peroxisomes, which is necessary for the rapid early but transient expression of antiviral genes called interferon-stimulated genes (ISGs) (Dixit et al., 2010). Furthermore, mitochondrial MAVS induces both IFN-b and IFN-λ while peroxisomal MAVS induces IFN-λ in a interferon regulatory factor 1 (IRF1) dependent manner (Odendall et al., 2014). Thus, maximal antiviral response requires the coordination of both mitochondrial and peroxisomal MAVS. Peroxisomes like mitochondria carry out oxidation of fatty acids and generate ROS (Lodhi and Semenkovich, 2014). Peroxisomes also exchange proteins with mitochondria (Camões et al., 2009). Given the shared roles in ROS and lipid metabolism between the two organelles, we speculate that MAVS recruitment to mitochondrial and peroxisomal membranes could be related to lipid and/or ROS metabolism. Indeed peroxisomal lipid synthesis can regulate immune cells as highlighted by the recent finding that loss of PexRAP, a peroxisomal enzyme required for ether lipid synthesis, results in neutropenia (Lodhi et al., 2015).
MAVS activation is also regulated by mitochondrial dynamics. Healthy mitochondria displaying robust mitochondrial membrane potential are in a fused network whereas fission is indicative of damaged depolarized mitochondria. Multiple studies have demonstrated that mitochondrial fusion promotes, whereas mitochondrial fission inhibits RLR signaling (Castanier et al., 2010; Yasukawa et al., 2009; Zhao et al., 2012). Further strengthening this link, mitochondrial membrane potential alone is required for proper induction of antiviral signaling (Koshiba et al., 2011). It is unclear why mitochondrial dynamics dramatically influences antiviral signaling. Moreover, the central question as to why mitochondrial localization is necessary for MAVS to propagate RIG-I signaling remains unanswered. A speculative idea is that initiation of MAVS aggregation requires a lipid or protein component in the outer mitochondrial membrane. Alternatively, mitochondria may release protein, lipids, metabolites or ROS necessary for optimal MAVS dependent antiviral signaling. Indeed, mitochondrial ROS have been shown to enhance MAVS mediated antiviral signaling (Tal et al., 2009; Zhao et al., 2012). Interestingly, MAVS is also necessary for NLRP3 activation (Subramanian et al., 2014). Future experiments will have to delineate the mechanisms by which outer mitochondrial membrane as a signaling platform controls innate immune responses.
The induction of type I IFNs can also be invoked by release of mitochondrial DNA. Two recent studies demonstrate that Bak- and Bax-mediated mitochondrial damage in the absence of activating the downstream apoptotic caspases triggers the release of mitochondrial DNA (mtDNA) which cyclic GMP-AMP synthase (cGAS)/STING-mediated cytosolic DNA sensing pathway (Rongvaux et al., 2014; White et al., 2014). DNA binding to cGAS catalyzes the production of cyclic GMP-AMP dinucleotide (cGAMP), which binds to and activates STING resulting in induction of type I IFN transcription via the Tbk1-Irf3 signaling axis (Barber, 2014). STING localizes to endoplasmic reticulum (ER)–mitochondrial contact sites (Ishikawa and Barber, 2008; Zhong et al., 2008). It remains to be tested whether there are physiological and pathological conditions where mitochondrial DNA is released without mitochondrial damage to increase IFN-β expression. There is precedent that mitochondria can transport proteins and lipids through small vesicular carriers (Sugiura et al., 2014).
Aside from macrophages, other important antigen presenting cells are dendritic cells (DCs). Upon exposure to antigen, DCs rapidly increased their phagocytic capacity while simultaneously elevating expression of major histocompatibility complex I (MHC I) and MHC II. This process, termed DC maturation, also promotes migration of DCs to the T cell zones of the secondary immune organs and is required for proper activation and control of an adaptive immune response (Joffre et al., 2009). The activation of this program using TLR stimulation results in a robust increase in glycolytic flux in dendritic cells (Everts et al., 2012; Krawczyk et al., 2010). This alteration in metabolism is required to meet the increased bioenergetic and biosynthetic demands of an activated DC, specifically by funneling metabolites into pathways for lipid and protein synthesis (Everts et al., 2014). Other studies have correlated increased intracellular lipid concentrations in DCs with enhanced antigen presentation and polarization of T cells towards inflammatory lineages suggesting that the rate of de novo fatty acid synthesis may regulate the immunogenicity of dendritic cells (Ibrahim et al., 2012). Emerging data has recently demonstrated that dendritic cells can be grouped into distinct subsets that display unique characteristics; similar to macrophages and T cells. As with macrophages, DCs likely exist in numerous different subsets however for simplicity that are classically designated as either an immunogenic or tolerant subset. Immunogenic DCs display high phagocytic activity and MHC expression after activation, and are thought to drive inflammatory T cell responses (Everts et al., 2014). In contrast, tolerogenic DCs are characterized by a resistance to maturation along with the expression of immune-modulatory factors which corresponds to an increased T regulatory cell (Treg) response (Pulendran et al., 2010). New data suggests tolerogenic DCs display a high levels of fatty acid oxidation and low levels of glycolysis reminiscent of the anti-inflammatory M2 macrophage (Cook et al., 2012; Ferreira et al., 2012; Szanto et al., 2010) DCs lacking PPAR-γ show increased immunogenicity while simultaneously failing to induce tolerogenic T cell responses (Klotz et al., 2007), further promoting a link between fatty acid oxidation and a suppressive immune phenotype,. Future studies need to further explore how metabolism differs in different subsets of DCs, and whether alterations in metabolism specifically in these cells is necessary to activate the transcriptional networks that establish these subsets.
Mitochondrial signaling controls adaptive immunity
T cells respond to antigens thus are central orchestrators of adaptive immune responses. During infection, naïve T cells (TN) challenged with an antigen rapidly proliferate into effector T cells (TE). The majority of TE cells undergo cell death with a few long-lived memory T cells (TM) after the infection diminishes. TM cells can be reactivated into rapidly expanding into TE cells if a similar infection occurs to quickly curtail the infection. There also exist active immunosuppressive cells termed regulatory T cells (Tregs) that suppress proliferation and function of effector T cells. These T cells subtypes have different metabolic demands and functions therefore exhibit diverse metabolic profiles. Aberrant T cell function results in a myriad of pathologies including auto-immune diseases.
Mitochondrial ROS regulate T cell activation
Antigen stimulation of the T cell receptor (TCR) along with engagement of co-stimulatory molecules on naïve T cells shifts them from a quiescent catabolic state in which nutrients are utilized to generate ATP required for cellular survival to a robust anabolic state where nutrients feed into metabolic pathways that generate macromolecules necessary for cell proliferation. Classical studies demonstrate that the anabolism of activated T cells is supported by large increases in glucose consumption that feed into multiple pathways. More recent studies indicate that glutamine is also an important fuel source, which supports mitochondrial metabolism through glutaminolysis (Carr et al., 2010; Sinclair et al., 2013). The transcription factor myc is necessary for this increased mitochondrial flux (Wang et al., 2011). Pharmacologic inhibition of mitochondrial oxidative phosphorylation or glycolysis in vitro diminishes T cell proliferation indicating that mitochondrial metabolism and glycolysis support T cell proliferation (Chang et al., 2013; Sena et al., 2013). However, glycolysis but not mitochondrial metabolism is dispensable for T cell activation and production of the cytokine IL-2 prior to proliferation (Sena et al., 2013). In fact, mitochondrial metabolism was found to be required for T cell activation through generation of mitochondrial ROS necessary for optimal activity of NFAT, NF-KB and proximal TCR signaling (Gill and Levine, 2013; Kaminski et al., 2010; Kamiński et al., 2012; Sena et al., 2013). It is known that mitochondrial localization to the immune synapse is required for T cell activation (Contento et al., 2010; Martín-Cófreces et al., 2014; Quintana et al., 2007), likely for efficiency of both calcium and ROS signals. Future studies will more clearly define the mitochondrial ROS molecular target in T cell activation. The notion that mitochondrial metabolism is necessary for T cell activation is further supported by the observations that chronically activated T cells isolated from mouse model of lupus are dependent on mitochondrial metabolism and peripheral blood lymphocytes from patients with lupus have increased mitochondrial metabolism and ROS production (Gergely et al., 2002; Wahl et al., 2010).
Differential metabolic pathways regulate CD4+ T cell differentiation
Once activated, T cells differentiate into different effector T cell (Te) subsets ranging from pro-inflammatory T helper 1 (Th1), Th17 and Th22 cells to suppressive regulatory T (Tregs) cells to curtail infection. Traditionally, these subsets have been classified by specific transcription factor activation. Emerging data indicate that these different T cell subsets have distinctive metabolic phenotypes that can promote T cell subset differentiation. Perhaps the best-studied subsets are Treg and Th17 cells, which have different metabolic profiles that are essential to establish their phenotype. Tregs have elevated levels of oxidative phosphorylation and decreased glycolytic flux compared to Th17 cells (Michalek et al., 2011).
This increased mitochondrial metabolism in Tregs was found to be due to increased AMPK-dependent fatty acid oxidation (MacIver et al., 2011; Michalek et al., 2011). Pharmacologically attenuating fatty acid oxidation by etomoxir impaired Treg differentiation but did not affect other CD4 helper subsets in vitro. By contrast, Th17 cells engage in de novo fatty acid synthesis that is necessary for the Th17 phenotype. Pharmacologic and genetic inhibition of the enzyme acetyl-CoA carboxylase 1 (ACC1), the enzyme that catalyzes the first step of de novo fatty acid synthesis, impaired Th17 cell differentiation and promoted Tregs in vitro and in vivo as well as attenuated EAE in mice (Berod et al., 2014). It is presently not clear why fatty acid oxidation versus synthesis appears to be a checkpoint in the T cell fate decision between Treg and Th17 cells.
Increased glycolytic metabolism in Th17 cells also appears to be important to maintaining their Th17 lineage state. Pharmacological inhibition of glucose metabolism by administering 2-deoxyglucose attenuated Th17 cell development and interestingly promoted Treg cell development and diminished pathology in a Th17-dependent experimental autoimmune encephalomyelitis (EAE) (Shi et al., 2011). The increase in glycolysis observed in Th17 cells is due to an increase in HIF-1, and mice with T cells deficient in HIF-1α display diminished Th17 cells, increased Treg cells, and resistance to EAE (Dang et al., 2011; Shi et al., 2011). Further evidence comes from the observation that the HIF-1 target PDHK1 is expressed in Th17 cells but not in Treg cells, and diminishing PDHK1, the negative regulator of pyruvate dehydrogenase (PDH), suppressed Th17 formation and increased Treg formation in vitro (Gerriets et al., 2014). PDH is necessary for converting pyruvate to acetyl-CoA in mitochondrial matrix and increasing PDHK1 reduces PDH activity thus limiting mitochondrial acetyl-CoA availability. These data are supported in vivo by the observation that pharmacologic inhibition of PDHK by dichloracetate (DCA) in mice diminishes Th17 and promotes Tregs resulting inhibition of Th17 dependent colitis and EAE pathologies.
It is not fully understood why diminishing mitochondrial metabolism and/or enhancing glycolysis increases Th17 concomitantly decreases Tregs. It is possible that the decrease in mitochondrial metabolism concomitantly with an increase in glycolysis diminishes ROS through unknown mechanisms to promote Th17 differentiation. It is important to note that the ROS regulation of Th17/Treg axis has yet to be confirmed genetically. This is imperative since pharmacological inhibition of these pathways can affect multiple cell types and conclusion drawn from these studies can be difficult to interpret. For example, unexpectedly fatty acid oxidation inhibition by etomoxir reduces disease severity of EAE (Shriver and Manchester, 2011). Based on in vitro findings, fatty acid oxidation inhibition is predicted to decrease Tregs cells thus exacerbating EAE. Nevertheless, collectively these emerging metabolic studies suggest that mitochondrial metabolism function dictates the different inflammatory and suppressive CD4+ T helper lineages.
Mitochondrial metabolism regulates CD8+ memory T cell formation
During the resolution phase of an infection, the majority of CD8+ Te cells undergo cell death with the survival of a few long-lived CD8+ memory T cells (Tm). Re-infection with a pathogen containing similar antigens allow these Tm cells to be reactivated and rapidly expand into T cells to quickly control the infection. Tm cells are not rapidly proliferating and thus do not have high anabolic requirements. They efficiently catabolize nutrients to generate ATP to maintain long-term cell survival. Thus, Tm cells have a contrasting metabolic profile compared to Tm cells. Tm cells display increased mitochondrial number and spare respiratory capacity, which is fueled by fatty acid oxidation to generate copious amount of ATP (Pearce et al., 2009; van der Windt et al., 2012). Fatty acid oxidation generates almost 3 times more ATP than glucose oxidation by mitochondria, thus it is robust mechanism to generate ATP. The source of fatty acids in Tm cells is not extracellular, but rather internal lysosomal stores. Tm cells display reduced surface expression of CD36, necessary for fatty acid uptake, compared with Te cells and engage in de novo lipogenesis and store lipids in lysosomes (O'Sullivan et al., 2014). Tm cells utilize lysosomal acid lipase (LAL) in Tm cells to liberate free fatty acids from storage for the robust fatty acid oxidation in the mitochondria. Thus, Tm cells appear to utilize a “futile cycle” whereby fatty acid synthesis occurs concurrently with fatty acid oxidation, which is required for proper maintenance of memory T cells. The biochemical basis as to how and why Tm cells conduct concurrent fatty acid synthesis and fatty acid oxidation is not fully understood, as most cells utilize regulatory mechanisms that prevent this inefficiency. One possibility is that the availability of fatty acids from the extracellular environment in vivo might differ depending on the tissues where they reside therefore Tm cells continuously store lipids that can be utilized for fatty acid oxidation. The importance of fatty acid oxidation for Tm cells is bolstered by the observations that diminishing or enhancing AMPK, a positive regulator of fatty acid oxidation, decreases or increases formation of memory T cells (MacIver et al., 2011; Pearce et al., 2009; Rolf et al., 2013; Tamás et al., 2010). Further evidence supporting the importance of mitochondrial metabolism comes from the observation that increasing or decreasing glycolytic flux, which reciprocally modulates mitochondrial metabolism, results in enhanced or diminished generation of memory T cells, respectively (Sukumar et al., 2013). Although there is mounting evidence that mitochondrial metabolism maintains the memory T cell phenotype, it remains unknown why this is and whether mitochondria participate in cell signaling necessary to establish the memory T cell phenotype.
Similar to T cells, B cells also undergo major transitions in their metabolic profiles as they transition from naïve quiescent cells to anabolic proliferative cells. Upon activation, B cells greatly enhance glucose and glutamine metabolism during clonal expansion comparable to T cells (Doughty et al., 2006; Garcia-Manteiga et al., 2011; Le et al., 2012). Along with these changes in metabolism, B cell receptor (BCR) activation is regulated by ROS (Capasso et al., 2010; Singh et al., 2005). Specifically, BCR ligation stimulates calcium release into the cytoplasm that promotes ROS production and inactivation of receptor-coupled phosphates, allowing for activation of downstream signaling pathways. Depending on the magnitude of the ROS pulse, there is increased duration of BCR signaling and enhanced activation of downstream signaling pathways (Singh et al., 2005). Initially, the cytosolic NADPH oxidases were surmised to be source of ROS for B cell function. However, new data from primary B cells suggests that early ROS production by NADPH oxidases is dispensable for BCR signaling (Richards and Clark, 2009; Wheeler and Defranco, 2012). Instead, long term elevations in ROS levels, potentially driven by increased production of mitochondrial ROS, are required for downstream BCR signaling and cellular proliferation in response to BCR cross-linking (Wheeler and Defranco, 2012). Along with ROS signaling, new studies also suggest that other mitochondrial-derived molecules have important roles in B cell activation and effector function. Recent studies have suggested that plasma cells produce increased levels of phospholipids when compared to naïve B cells, and plasma cell differentiation is dependent on mitochondrial citrate conversion to acetyl-CoA and oxaloacetate in the cytoplasm (Dufort et al., 2014; Fagone et al., 2007). Although the evidence is incomplete, these new studies indicate that mitochondrial metabolism is altered during B cell activation and plasma cell development. Future studies will delineate whether mitochondria metabolism is essential in driving differential B cell function in a manner similar to T cells.
Concluding remarks
The mammalian immune response is extremely complex, requiring the coordination of multiple organ systems and a large variety of cells within those organs. These immune cells are so effective because they are able to rapidly respond to the stress of infection through activation and often proliferation, differentiation, and finally cell death. Furthermore, during an immune response, immune cells must decide to participate as an effector cell promoting inflammation or a suppressor cell ensuring adequate control of the inflammation. Within the capacity of an effector cell, various cells acquire more specific abilities necessary to fighting specific types of infections be it viral, bacterial, or parasitic. Thus, a rapid change in cell fate is absolutely essential to immunity. While decades of study have shown that direct signaling from the cell surface to the nucleus is critical to these cell fate decisions, more recent work has shown that these signaling pathways often include detours that transmit through metabolic machinery to ensure cooperation of metabolism in the cell fate decision (Pearce et al., 2013) It is becoming clear that cellular metabolism plays a highly active role in determining cellular destiny in the immune system—in other words, we are learning that the immune cell is what it eats.
In this review, we highlight the mitochondrion as a central hub of immune cell regulation. We outline that in immune cells, mitochondria participate in signaling through ROS production, metabolite availability, and by physically acting as scaffolding for protein interaction. Mitochondrial signals appear to be necessary for the immune cell to fulfill its specific role in the immune response in both innate and adaptive settings to a variety of intruders. Ironically, this organelle of bacterial origin has established itself as a major conductor of the defense of the organism from invaders such as bacteria.
The studies we have discussed open several avenues for future investigation. First, we are lacking important mechanistic detail regarding the role of mitochondria in immune cells. Some questions include: what are the critical mitochondrial ROS targets in immune cell activation? How do the peroxisomes, ER, and mitochondria communicate to optimally regulate immune responses? Does availability of TCA cycle intermediates like acetyl-CoA, alpha-ketoglutarate, and citrate alter immune cell activation similar to succinate? If so, how? Secondly, several of the mechanisms discussed in this review are lacking in vivo evidence. Future study should use genetics and targeted small molecules to show that mitochondria are indeed important in the setting of a complex organismal immune response.
Lastly, the essential question remains whether targeting mitochondrial metabolism in humans will allow for modulation of the immune response in disease. We are very interested in determining the specific metabolic fuels and enzymes that sustain mitochondrial function in the immune response, as identification of these major crutches of mitochondrial function within immune cells may allow for development of small molecules for immune therapy. For example, the anti-diabetic drug metformin is being utilized an immunomodulator for tuberculosis (Singhal et al., 2014). It is not clear what the target of metformin in immune cells is but the anti-tumor effects of metformin are through inhibiting mitochondrial complex I (Wheaton et al., 2014). Moreover, Bz-423, a small-molecule inhibitor of the mitochondrial ATP-synthase, arrested established graft versus host disease (GVHD) in several bone marrow transplant models without affecting hematopoietic engraftment or lymphocyte reconstitution (Gatza et al., 2011). Beyond pharmaceuticals, modulation of total calorie availability as well as type of calorie availability and subsequent support of specific cellular metabolic pathways will also prove to be a useful method of immune modulation.
Figure 1. Mitochondria are essential metabolic and signaling organelles.
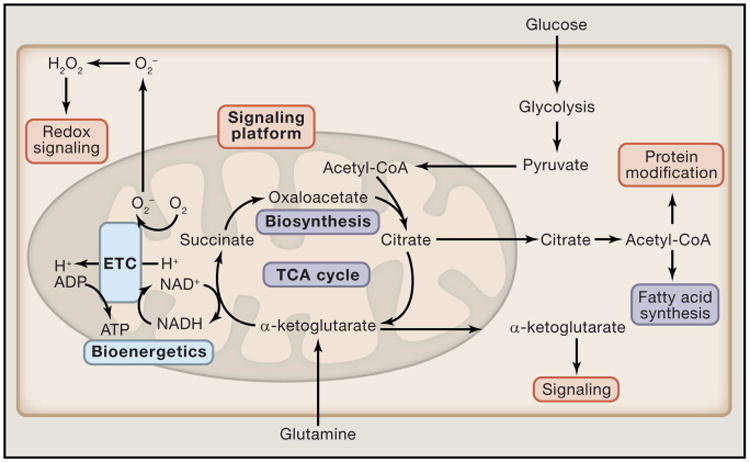
Cytosolic metabolic pathways funnel into the mitochondria where they constantly replenish the TCA cycle. Depending on the cellular metabolic state, TCA cycle intermediates can be further oxidized to generate ATP (Blue) or they can be shuttled out of the mitochondria into subsidiary pathways to generate cellular building blocks such as fatty acids (purple). Finally, TCA cycle metabolites (Orange) in addition to other byproducts of mitochondrial metabolism, such as ROS, function as important signaling molecules which control cellular functions.
Figure 2. Mitochondria are critical to activation of the immune response.
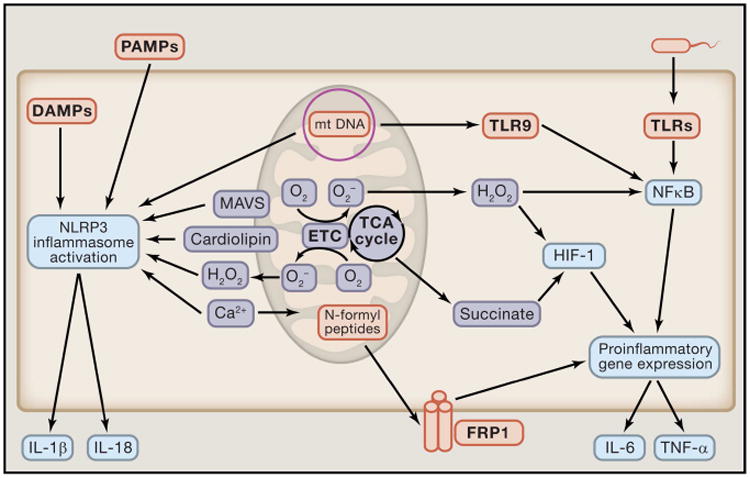
Mitochondrial components such as mitochondrial DNA (mtDNA) and N-formyl peptides can act as damage-associated molecular patterns (DAMPs). Specifically, mtDNA can activate the NLRP3 inflammasome and toll-like receptor-9 (TLR-9) to induce an inflammatory response, while N-formyl peptides activate pro-inflammatory gene expression through the N-formyl peptide receptor-1 (FRP1). In addition to production and presentation of DAMPs, mitochondrial metabolism and signaling further promote the induction of inflammation to pathogens. Mitochondrial derived metabolites such as mROS and succinate enhance pro-inflammatory gene expression, and mROS also can function directly as an anti-microbial effector molecule and NLRP3 activator. Additionally, mitochondrial localized proteins and lipids, MAVS and cardiolipin respectively, are required for proper activation of the NRLP3 inflammasome. Finally, cellular calcium flux is linked to NLRP3 activation and mitochondrial function and signaling.
Figure 3. The mitochondria are essential for the proper induction of antiviral signaling.
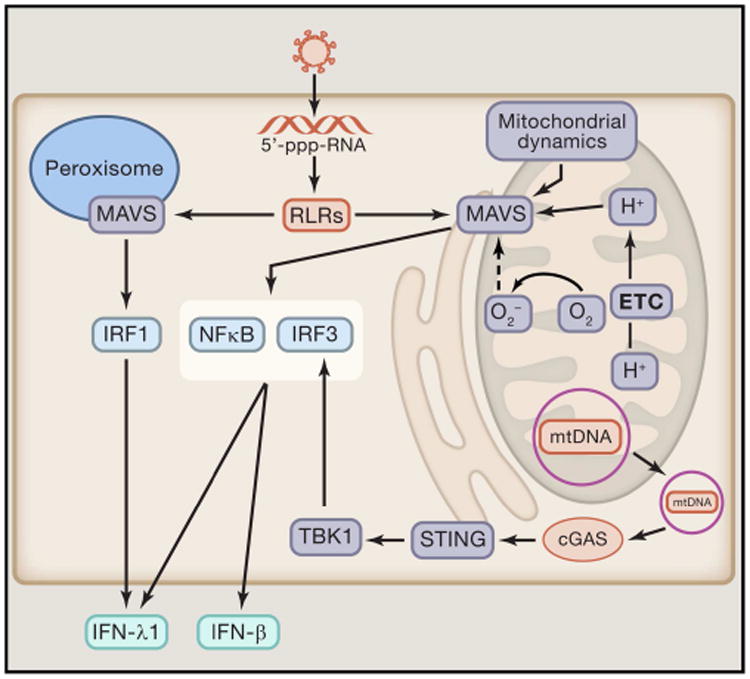
The mitochondrial antiviral signaling protein (MAVS) is required for proper induction of RLR mediated activation of antiviral immunity. Importantly, mitochondrial dynamics, membrane potential, and ROS production are critical regulators of MAVS signaling. MAVS also localizes to the peroxisome, an organelle with a well described role in fatty acid oxidation and H2O2 generation. Stimulation of peroxisomal MAVS signaling induces IFN-λ1 production through IRF1 activation, while mitochondrial localized MAVS drives IFN-β and IFN-λ1 production. Along with MAVS, the mitochondrial also function as an activator of the stimulator of interferon genes protein (STING). Specifically, mtDNA translocated to the cytoplasm activates cyclic GMP-AMP synthase (cGAS) which triggers STING signaling which further enhances antiviral
Figure 4. Mitochondrial signaling is required for T cell activation.
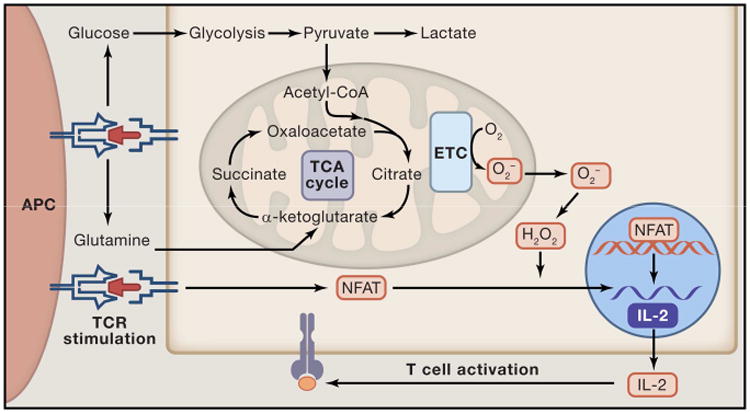
Upon binding of the TCR with MHC, numerous signaling cascades are activated. One of the activated pathways MYC activates an anabolic metabolic program that increases uptake of glucose and glutamine and allows for the cells to meet the increased metabolic demands of proliferation and induction of an adaptive immune response (Blue). Importantly, in this model metabolism works to sustain cellular activity required for a proper immune response. However, the mitochondria also alter cellular signaling upon ligation of the TCR. Specifically, mitochondrial ROS production following TCR stimulation is required for proper activation of NFAT and IL-2 production (Orange). In this way, the mitochondrial signaling and metabolism are required for proper T cell activation.
Figure 5. CD8+ effector T cells display a distinct mitochondrial metabolic profile compared to CD8+ memory T cells.
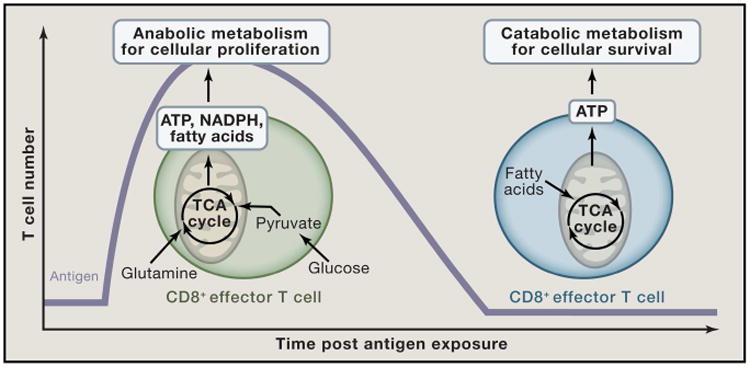
Upon T cell activation, effector T cells rapidly increase uptake of glucose and glutamine which is utilized to produce the ATP and cellular building blocks (NADPH and fatty acids) required for production of clonal T cells. The mitochondria in effector T cells functions as an anabolic hub where TCA cycle intermediates are shuttled into the cytoplasm to promote production of increase cellular biomass. In contrast, memory T cells utilize fatty acid catabolism to efficiently generate ATP to fuel cellular survival.
Acknowledgments
We apologize to investigators whose work we could not highlight due to space limitations. This work is supported by R01CA123067, RO1HL12206201 and 5P01HL071643 to NSC and T32HL076139 to SEW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barber GN. STING-dependent cytosolic DNA sensing pathways. Trends Immunol. 2014;35:88–93. doi: 10.1016/j.it.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Berod L, Friedrich C, Nandan A, Freitag J, Hagemann S, Harmrolfs K, Sandouk A, Hesse C, Castro CN, Bähre H, et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat Med. 2014;20:1327–1333. doi: 10.1038/nm.3704. [DOI] [PubMed] [Google Scholar]
- Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) J Exp Med. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camões F, Bonekamp NA, Delille HK, Schrader M. Organelle dynamics and dysfunction: A closer link between peroxisomes and mitochondria. J Inherit Metab Dis. 2009;32:163–180. doi: 10.1007/s10545-008-1018-3. [DOI] [PubMed] [Google Scholar]
- Capasso M, Bhamrah MK, Henley T, Boyd RS, Langlais C, Cain K, Dinsdale D, Pulford K, Khan M, Musset B, et al. HVCN1 modulates BCR signal strength via regulation of BCR-dependent generation of reactive oxygen species. Nat Immunol. 2010;11:265–272. doi: 10.1038/ni.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H. Mitochondrial N-formylmethionyl proteins as chemoattractants for neutrophils. J Exp Med. 1982;155:264–275. doi: 10.1084/jem.155.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr EL, Kelman A, Wu GS, Gopaul R, Senkevitch E, Aghvanyan A, Turay AM, Frauwirth KA. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol. 2010;185:1037–1044. doi: 10.4049/jimmunol.0903586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KC, Viollet B, Suttles J. AMPKα1 deficiency amplifies proinflammatory myeloid APC activity and CD40 signaling. J Leukoc Biol. 2013;94:1113–1121. doi: 10.1189/jlb.0313157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanier C, Garcin D, Vazquez A, Arnoult D. Mitochondrial dynamics regulate the RIG-I-like receptor antiviral pathway. EMBO Rep. 2010;11:133–138. doi: 10.1038/embor.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel NS. Mitochondria as signaling organelles. BMC Biol. 2014;12:34. doi: 10.1186/1741-7007-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel NS, Trzyna WC, McClintock DS, Schumacker PT. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J Immunol. 2000;165:1013–1021. doi: 10.4049/jimmunol.165.2.1013. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Schumacker PT, Arch RH. Reactive oxygen species are downstream products of TRAF-mediated signal transduction. J Biol Chem. 2001;276:42728–42736. doi: 10.1074/jbc.M103074200. [DOI] [PubMed] [Google Scholar]
- Chang CH, Curtis JD, Maggi LB, Faubert B, Villarino AV, O'Sullivan D, Huang SCC, van der Windt GJW, Blagih J, Qiu J, et al. Posttranscriptional Control of T Cell Effector Function by Aerobic Glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouchani ET, Pell VR, Gaude E, Aksentijević D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord ENJ, Smith AC, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014 doi: 10.1038/nature13909. advance on. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LV, Hajizadeh S, Holme E, Jonsson IM, Tarkowski A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J Leukoc Biol. 2004;75:995–1000. doi: 10.1189/jlb.0703328. [DOI] [PubMed] [Google Scholar]
- Contento RL, Campello S, Trovato AE, Magrini E, Anselmi F, Viola A. Adhesion shapes T cells for prompt and sustained T-cell receptor signalling. EMBO J. 2010;29:4035–4047. doi: 10.1038/emboj.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PC, Jones LH, Jenkins SJ, Wynn TA, Allen JE, MacDonald AS. Alternatively activated dendritic cells regulate CD4+ T-cell polarization in vitro and in vivo. Proc Natl Acad Sci U S A. 2012;109:9977–9982. doi: 10.1073/pnas.1121231109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz CM, Rinna A, Forman HJ, Ventura ALM, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit E, Boulant S, Zhang Y, Lee ASY, Odendall C, Shum B, Hacohen N, Chen ZJ, Whelan SP, Fransen M, et al. Peroxisomes are signaling platforms for antiviral innate immunity. Cell. 2010;141:668–681. doi: 10.1016/j.cell.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty CA, Bleiman BF, Wagner DJ, Dufort FJ, Mataraza JM, Roberts MF, Chiles TC. Antigen receptor-mediated changes in glucose metabolism in B lymphocytes: role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth. Blood. 2006;107:4458–4465. doi: 10.1182/blood-2005-12-4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufort FJ, Gumina MR, Ta NL, Tao Y, Heyse SA, Scott DA, Richardson AD, Seyfried TN, Chiles TC. Glucose-dependent de novo lipogenesis in B lymphocytes: a requirement for atp-citrate lyase in lipopolysaccharide-induced differentiation. J Biol Chem. 2014;289:7011–7024. doi: 10.1074/jbc.M114.551051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts B, Amiel E, van der Windt GJW, Freitas TC, Chott R, Yarasheski KE, Pearce EL, Pearce EJ. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood. 2012;120:1422–1431. doi: 10.1182/blood-2012-03-419747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts B, Amiel E, Huang SCC, Smith AM, Chang CH, Lam WY, Redmann V, Freitas TC, Blagih J, van der Windt GJW, et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKε supports the anabolic demands of dendritic cell activation. Nat Immunol. 2014;15:323–332. doi: 10.1038/ni.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagone P, Sriburi R, Ward-Chapman C, Frank M, Wang J, Gunter C, Brewer JW, Jackowski S. Phospholipid biosynthesis program underlying membrane expansion during B-lymphocyte differentiation. J Biol Chem. 2007;282:7591–7605. doi: 10.1074/jbc.M608175200. [DOI] [PubMed] [Google Scholar]
- Ferreira GB, Kleijwegt FS, Waelkens E, Lage K, Nikolic T, Hansen DA, Workman CT, Roep BO, Overbergh L, Mathieu C. Differential protein pathways in 1,25-dihydroxyvitamin d(3) and dexamethasone modulated tolerogenic human dendritic cells. J Proteome Res. 2012;11:941–971. doi: 10.1021/pr200724e. [DOI] [PubMed] [Google Scholar]
- Garcia-Manteiga JM, Mari S, Godejohann M, Spraul M, Napoli C, Cenci S, Musco G, Sitia R. Metabolomics of B to plasma cell differentiation. J Proteome Res. 2011;10:4165–4176. doi: 10.1021/pr200328f. [DOI] [PubMed] [Google Scholar]
- Gatza E, Wahl DR, Opipari AW, Sundberg TB, Reddy P, Liu C, Glick GD, Ferrara JLM. Manipulating the bioenergetics of alloreactive T cells causes their selective apoptosis and arrests graft-versus-host disease. Sci Transl Med. 2011;3:67ra8. doi: 10.1126/scitranslmed.3001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergely P, Niland B, Gonchoroff N, Pullmann R, Phillips PE, Perl A. Persistent Mitochondrial Hyperpolarization, Increased Reactive Oxygen Intermediate Production, and Cytoplasmic Alkalinization Characterize Altered IL-10 Signaling in Patients with Systemic Lupus Erythematosus. J Immunol. 2002;169:1092–1101. doi: 10.4049/jimmunol.169.2.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerriets VA, Kishton RJ, Nichols AG, Macintyre AN, Inoue M, Ilkayeva O, Winter PS, Liu X, Priyadharshini B, Slawinska ME, et al. Metabolic programming and PDHK1 control CD4+T cell subsets and inflammation. J Clin Invest. 2014;125 doi: 10.1172/JCI76012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill T, Levine AD. Mitochondria-derived hydrogen peroxide selectively enhances T cell receptor-initiated signal transduction. J Biol Chem. 2013;288:26246–26255. doi: 10.1074/jbc.M113.476895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P, Lukens JR, Kanneganti TD. Mitochondria: diversity in the regulation of the NLRP3 inflammasome. Trends Mol Med. 2014 doi: 10.1016/j.molmed.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haschemi A, Kosma P, Gille L, Evans CR, Burant CF, Starkl P, Knapp B, Haas R, Schmid JA, Jandl C, et al. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab. 2012;15:813–826. doi: 10.1016/j.cmet.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heid ME, Keyel PA, Kamga C, Shiva S, Watkins SC, Salter RD. Mitochondrial reactive oxygen species induces NLRP3-dependent lysosomal damage and inflammasome activation. J Immunol. 2013;191:5230–5238. doi: 10.4049/jimmunol.1301490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest. 2013;123:3678–3684. doi: 10.1172/JCI69600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner SM, Liu HM, Park HS, Briley J, Gale M. Mitochondrial-associated endoplasmic reticulum membranes (MAM) form innate immune synapses and are targeted by hepatitis C virus. Proc Natl Acad Sci U S A. 2011;108:14590–14595. doi: 10.1073/pnas.1110133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SCC, Everts B, Ivanova Y, O'Sullivan D, Nascimento M, Smith AM, Beatty W, Love-Gregory L, Lam WY, O'Neill CM, et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014 doi: 10.1038/ni.2956. advance on. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim J, Nguyen AH, Rehman A, Ochi A, Jamal M, Graffeo CS, Henning JR, Zambirinis CP, Fallon NC, Barilla R, et al. Dendritic cell populations with different concentrations of lipid regulate tolerance and immunity in mouse and human liver. Gastroenterology. 2012;143:1061–1072. doi: 10.1053/j.gastro.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infantino V, Convertini P, Cucci L, Panaro MA, Di Noia MA, Calvello R, Palmieri F, Iacobazzi V. The mitochondrial citrate carrier: a new player in inflammation. Biochem J. 2011;438:433–436. doi: 10.1042/BJ20111275. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, Sadler JJ, Knepper-Adrian V, Han R, Qiao L, et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity. 2013;39:311–323. doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Wei W, Yang M, Du Y, Wan Y. Mitochondrial Complex I Activity Suppresses Inflammation and Enhances Bone Resorption by Shifting Macrophage-Osteoclast Polarization. Cell Metab. 2014;20:483–498. doi: 10.1016/j.cmet.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffre O, Nolte MA, Spörri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009;227:234–247. doi: 10.1111/j.1600-065X.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- Kaelin WG, McKnight SL. Influence of metabolism on epigenetics and disease. Cell. 2013;153:56–69. doi: 10.1016/j.cell.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski MM, Sauer SW, Klemke CD, Süss D, Okun JG, Krammer PH, Gülow K. Mitochondrial reactive oxygen species control T cell activation by regulating IL-2 and IL-4 expression: mechanism of ciprofloxacin-mediated immunosuppression. J Immunol. 2010;184:4827–4841. doi: 10.4049/jimmunol.0901662. [DOI] [PubMed] [Google Scholar]
- Kamiński MM, Sauer SW, Kamiński M, Opp S, Ruppert T, Grigaravičius P, Grudnik P, Gröne HJ, Krammer PH, Gülow K. T cell activation is driven by an ADP-dependent glucokinase linking enhanced glycolysis with mitochondrial reactive oxygen species generation. Cell Rep. 2012;2:1300–1315. doi: 10.1016/j.celrep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, Lee CH. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- Klotz L, Dani I, Edenhofer F, Nolden L, Evert B, Paul B, Kolanus W, Klockgether T, Knolle P, Diehl L. Peroxisome proliferator-activated receptor gamma control of dendritic cell function contributes to development of CD4+ T cell anergy. J Immunol. 2007;178:2122–2131. doi: 10.4049/jimmunol.178.4.2122. [DOI] [PubMed] [Google Scholar]
- Koshiba T, Yasukawa K, Yanagi Y, Kawabata S. Mitochondrial membrane potential is required for MAVS-mediated antiviral signaling. Sci Signal. 2011;4:ra7. doi: 10.1126/scisignal.2001147. [DOI] [PubMed] [Google Scholar]
- Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J, Tsukamoto T, Rojas CJ, Slusher BS, Zhang H, et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012;15:110–121. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodhi IJ, Semenkovich CF. Peroxisomes: a nexus for lipid metabolism and cellular signaling. Cell Metab. 2014;19:380–392. doi: 10.1016/j.cmet.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodhi IJ, Wei X, Yin L, Feng C, Adak S, Abou-Ezzi G, Hsu FF, Link DC, Semenkovich CF. Peroxisomal lipid synthesis regulates inflammation by sustaining neutrophil membrane phospholipid composition and viability. Cell Metab. 2015;21:51–64. doi: 10.1016/j.cmet.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIver NJ, Blagih J, Saucillo DC, Tonelli L, Griss T, Rathmell JC, Jones RG. The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J Immunol. 2011;187:4187–4198. doi: 10.4049/jimmunol.1100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Cófreces NB, Baixauli F, Sánchez-Madrid F. Immune synapse: conductor of orchestrated organelle movement. Trends Cell Biol. 2014;24:61–72. doi: 10.1016/j.tcb.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metallo CM, Vander Heiden MG. Metabolism strikes back: metabolic flux regulates cell signaling. Genes Dev. 2010;24:2717–2722. doi: 10.1101/gad.2010510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- Michalek RD, Gerriets Va, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan Sa, Nichols AG, Rathmell JC. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E, O'Neill LAJ. Succinate: a metabolic signal in inflammation. Trends Cell Biol. 2014;24:313–320. doi: 10.1016/j.tcb.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Mookerjee SA, Goncalves RLS, Gerencser AA, Nicholls DG, Brand MD. The contributions of respiration and glycolysis to extracellular acid production. Biochim. Biophys Acta - Bioenerg. 2015;1847:171–181. doi: 10.1016/j.bbabio.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier R, Théret M, Arnold L, Cuvellier S, Bultot L, Göransson O, Sanz N, Ferry A, Sakamoto K, Foretz M, et al. AMPKα1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab. 2013;18:251–264. doi: 10.1016/j.cmet.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, Horng T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A. 2012;109:11282–11287. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan D, van der Windt GJW, Huang SCC, Curtis JD, Chang CH, Buck MD, Qiu J, Smith AM, Lam WY, DiPlato LM, et al. Memory CD8(+) T Cells Use Cell-Intrinsic Lipolysis to Support the Metabolic Programming Necessary for Development. Immunity. 2014;41:75–88. doi: 10.1016/j.immuni.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odendall C, Dixit E, Stavru F, Bierne H, Franz KM, Durbin AF, Boulant S, Gehrke L, Cossart P, Kagan JC. Diverse intracellular pathogens activate type III interferon expression from peroxisomes. Nat Immunol. 2014;15:717–726. doi: 10.1038/ni.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce T(H)2 and tolerogenic responses. Nat Immunol. 2010;11:647–655. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Orr AL, Brand MD. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 2013;1:304–312. doi: 10.1016/j.redox.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana A, Schwindling C, Wenning AS, Becherer U, Rettig J, Schwarz EC, Hoth M. T cell activation requires mitochondrial translocation to the immunological synapse. Proc Natl Acad Sci U S A. 2007;104:14418–14423. doi: 10.1073/pnas.0703126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiet MJ, Huet E, Boulay F. The N-formyl peptide receptors and the anaphylatoxin C5a receptors: an overview. Biochimie. 2007;89:1089–1106. doi: 10.1016/j.biochi.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reikine S, Nguyen JB, Modis Y. Pattern Recognition and Signaling Mechanisms of RIG-I and MDA5. Front Immunol. 2014;5:342. doi: 10.3389/fimmu.2014.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards SM, Clark EA. BCR-induced superoxide negatively regulates B-cell proliferation and T-cell-independent type 2 Ab responses. Eur J Immunol. 2009;39:3395–3403. doi: 10.1002/eji.200939587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- Rolf J, Zarrouk M, Finlay DK, Foretz M, Viollet B, Cantrell DA. AMPKα1: a glucose sensor that controls CD8 T-cell memory. Eur J Immunol. 2013;43:889–896. doi: 10.1002/eji.201243008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongvaux A, Jackson R, Harman CCD, Li T, West AP, de Zoete MR, Wu Y, Yordy B, Lakhani SA, Kuan CY, et al. Apoptotic Caspases Prevent the Induction of Type I Interferons by Mitochondrial DNA. Cell. 2014;159:1563–1577. doi: 10.1016/j.cell.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Sena LA, Chandel NS. Physiological Roles of Mitochondrial Reactive Oxygen Species. Mol Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, et al. Mitochondria Are Required for Antigen-Specific T Cell Activation through Reactive Oxygen Species Signaling. Immunity. 2013;38:225–236. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriver LP, Manchester M. Inhibition of fatty acid metabolism ameliorates disease activity in an animal model of multiple sclerosis. Sci Rep. 2011;1:79. doi: 10.1038/srep00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair LV, Rolf J, Emslie E, Shi YB, Taylor PM, Cantrell DA. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat Immunol. 2013;14:500–508. doi: 10.1038/ni.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh DK, Kumar D, Siddiqui Z, Basu SK, Kumar V, Rao KVS. The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell. 2005;121:281–293. doi: 10.1016/j.cell.2005.02.036. [DOI] [PubMed] [Google Scholar]
- Singhal A, Jie L, Kumar P, Hong GS, Leow MKS, Paleja B, Tsenova L, Kurepina N, Chen J, Zolezzi F, et al. Metformin as adjunct antituberculosis therapy. Sci Transl Med. 2014;6:263ra159–ra263ra159. doi: 10.1126/scitranslmed.3009885. [DOI] [PubMed] [Google Scholar]
- Subramanian N, Natarajan K, Clatworthy M, Wang Z, Germain R. Mitochondria play a central role in NLRP3 inflammasome activation (349.1) FASEB J. 2014;28:349.1. [Google Scholar]
- Sugiura A, McLelland GL, Fon EA, McBride HM. A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J. 2014;33:2142–2156. doi: 10.15252/embj.201488104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z, Roychoudhuri R, Palmer DC, Muranski P, Karoly ED, et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest. 2013;123:4479–4488. doi: 10.1172/JCI69589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Sun L, Liu HH, Chen X, Seth RB, Forman J, Chen ZJ. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24:633–642. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Szanto A, Balint BL, Nagy ZS, Barta E, Dezso B, Pap A, Szeles L, Poliska S, Oros M, Evans RM, et al. STAT6 transcription factor is a facilitator of the nuclear receptor PPARγ-regulated gene expression in macrophages and dendritic cells. Immunity. 2010;33:699–712. doi: 10.1016/j.immuni.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal MC, Sasai M, Lee HK, Yordy B, Shadel GS, Iwasaki A. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc Natl Acad Sci U S A. 2009;106:2770–2775. doi: 10.1073/pnas.0807694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamás P, Macintyre A, Finlay D, Clarke R, Feijoo-Carnero C, Ashworth A, Cantrell D. LKB1 is essential for the proliferation of T-cell progenitors and mature peripheral T cells. Eur J Immunol. 2010;40:242–253. doi: 10.1002/eji.200939677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013 doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl DR, Petersen B, Warner R, Richardson BC, Glick GD, Opipari AW. Characterization of the metabolic phenotype of chronically activated lymphocytes. Lupus. 2010;19:1492–1501. doi: 10.1177/0961203310373109. [DOI] [PubMed] [Google Scholar]
- Wang D, Malo D, Hekimi S. Elevated mitochondrial reactive oxygen species generation affects the immune response via hypoxia-inducible factor-1alpha in long-lived Mclk1+/- mouse mutants. J Immunol. 2010;184:582–590. doi: 10.4049/jimmunol.0902352. [DOI] [PubMed] [Google Scholar]
- Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger J, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Thompson CB. A two-way street: reciprocal regulation of metabolism and signalling. Nat Rev Mol Cell Biol. 2012;13:270–276. doi: 10.1038/nrm3305. [DOI] [PubMed] [Google Scholar]
- West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011a;11:389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011b;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton WW, Weinberg SE, Hamanaka RB, Soberanes S, Sullivan LB, Anso E, Glasauer A, Dufour E, Mutlu GM, Budigner GS, et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife. 2014;3:e02242. doi: 10.7554/eLife.02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ML, Defranco AL. Prolonged production of reactive oxygen species in response to B cell receptor stimulation promotes B cell activation and proliferation. J Immunol. 2012;189:4405–4416. doi: 10.4049/jimmunol.1201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MJ, McArthur K, Metcalf D, Lane RM, Cambier JC, Herold MJ, van Delft MF, Bedoui S, Lessene G, Ritchie ME, et al. Apoptotic Caspases Suppress mtDNA-Induced STING-Mediated Type I IFN Production. Cell. 2014;159:1549–1562. doi: 10.1016/j.cell.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Windt GJW, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell. 2005;19:727–740. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Yasukawa K, Oshiumi H, Takeda M, Ishihara N, Yanagi Y, Seya T, Kawabata S, Koshiba T. Mitofusin 2 inhibits mitochondrial antiviral signaling. Sci Signal. 2009;2:ra47. doi: 10.1126/scisignal.2000287. [DOI] [PubMed] [Google Scholar]
- Yu J, Nagasu H, Murakami T, Hoang H, Broderick L, Hoffman HM, Horng T. Inflammasome activation leads to Caspase-1-dependent mitochondrial damage and block of mitophagy. Proc Natl Acad Sci U S A. 2014;111:15514–15519. doi: 10.1073/pnas.1414859111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Sun X, Nie X, Sun L, Tang TS, Chen D, Sun Q. COX5B regulates MAVS-mediated antiviral signaling through interaction with ATG5 and repressing ROS production. PLoS Pathog. 2012;8:e1003086. doi: 10.1371/journal.ppat.1003086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong B, Yang Y, Li S, Wang YY, Li Y, Diao F, Lei C, He X, Zhang L, Tien P, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]


