The authors' findings suggest that in majority minority communities with large cancer centers, racial/ethnic disparities in times to biopsy and treatment initiation can be reduced.
Abstract
Purpose:
Recent studies from large nationwide cancer databases have consistently shown that Hispanic women with breast cancer have delays in treatment initiation compared with non-Hispanic white women. However, time to treatment initiation has not been studied in a community where Hispanics are the majority.
Patients and Methods:
We conducted a retrospective, observational study of 362 female patients with breast cancer treated at a large National Cancer Institute (NCI) –designated cancer center with a largely Hispanic population. We examined the relationship between race/ethnicity and time from mammogram to biopsy as well as time from biopsy to treatment initiation using Kaplan-Meier analyses and multivariable Cox proportional hazards regression.
Results:
Half of the female patients with breast cancer were of Hispanic descent (50.0%; n = 181). Hispanic patients were more likely to be obese, have an Eastern Cooperative Oncology Group functional status ≥ 1, and have higher histologic grade disease (all P ≤ .05); no differences in American Joint Committee on Cancer stage at diagnosis were observed. After comprehensive adjustment for demographic and clinical characteristics, we found no significant differences between Hispanic versus non-Hispanic white patients in time from mammogram to biopsy (hazard ratio [HR], 0.91; 95% CI, 0.68 to 1.21) or time from biopsy to treatment (HR, 1.13; 95% CI, 0.69 to 1.88).
Conclusion:
Hispanic women and Non-Hispanic white women with breast cancer treated at an NCI-designated cancer center had similar times to biopsy and treatment initiation. These findings suggest that in majority minority communities with large cancer centers, racial disparities can be reduced. With a growing Hispanic population throughout the United States, future studies should examine the long-term impact on improved breast cancer survival in this population.
Introduction
Among the more than 230,000 women diagnosed with breast cancer in the United States each year,1 previous studies have shown that black and Hispanic women are more likely to be diagnosed at advance stages, experience significant treatment delays, and have worse survival rates compared with non-Hispanic whites.2–7 These racial/ethnic disparities in care are multifactorial in origin and have been attributed not only to differences in tumor biology across populations but also to poor access to care, lack of financial resources, and structural barriers within the health system.6,8–11 On a national level, population-based studies have demonstrated that blacks, Mexicans, and Puerto Ricans are 20% to 50% more likely to receive or elect a first course of therapy that did not meet National Comprehensive Cancer Network standards.3 Furthermore, studies have identified that younger black women experience longer treatment delays than white women.8 These disparities have played a significant role in the 20% to 200% greater risk of cancer mortality among racial/ethnic minorities at a population level.3 Recently, several studies have investigated how access to high-quality facilities may mitigate disparities in cancer care and outcomes among ethnic and racial minorities. Specifically, these studies suggest that the probability of delays in receipt of timely adjuvant therapy for older breast cancer survivors belonging to racial/ethnic minorities may be attenuated by the hospitals that treat these patients.12 Additional studies of patients with cancer have more broadly identified that access to hospitals with established quality improvement programs may ameliorate racial/ethnic disparities in care and outcomes.13,14
A majority of these prior studies of racial/ethnic disparities in access to quality cancer care and outcomes have focused on geographic regions where non-Hispanic whites are the racial/ethnic majority. With the dramatic increase in the Hispanic population over the past decade and the continuing trend, many areas of the United States are now or will be composed of a majority of traditionally minority populations.15 For example, in San Antonio, Texas, Hispanics comprised 59% of the population in 2012,16 with the Hispanic culture and Spanish language integral parts of the heritage of San Antonio. As more areas transform into majority minority communities as a result of changing US demographics, it is possible to disentangle race/ethnicity from other structural barriers (eg, socioeconomic status, access to care, infrastructure, resource distribution, availability of quality care) that may play a stronger role in cancer outcomes. To ensure optimal cancer treatment and outcomes in the general breast cancer population moving forward, it will be critical to understand reasons for persistent disparities experienced by these vulnerable populations to identify modifiable factors that may reduce inequities in treatment and ultimately survival among racial/ethnic minorities.9
Previous studies provide sparse information on treatment patterns for patients with breast cancer, particularly regarding delays in treatment, in a community where Hispanics are the majority population. Understanding these treatment patterns can shed light on whether previous patterns of racial/ethnic disparities in breast cancer care are mitigated when placed in the context of a community where Hispanics contribute significant cultural influence and also make up a large proportion of the health care workforce. Therefore, we conducted a retrospective, observational study of female patients with breast cancer treated at a large National Cancer Institute (NCI) –designated cancer center with a largely Hispanic population to examine whether there was a relationship between race/ethnicity and treatment delays. We hypothesized that Hispanic women in a majority minority region would have no significant delays in treatment initiation when compared with non-Hispanic white women in the same community.
Patients and Methods
Study Design and Patients
Data were collected retrospectively through medical record abstraction/review for female patients with breast cancer at a large NCI-designated cancer center located in an area with a majority Hispanic population in San Antonio, Texas. To be considered for medical record abstraction, patients had to be female, age ≥ 18 years, and diagnosed with breast cancer between 2009 and 2011 and had to have received treatment at our cancer center. Information about patients' age, race/ethnicity, health conditions, cancer, and treatment characteristics were abstracted. A total of 362 medical records were abstracted. The institutional review board of our institution approved the project before data collection began.
Measures
Time to treatment.
Time to treatment was assessed in two ways: one, receiving a biopsy within 30, 60, or 90 days of mammogram; and two, initiating treatment within 30, 60, or 90 days of biopsy. Dates when patients received mammograms and biopsies and when treatment was initiated (ie, month, day, year) were abstracted from the medical records. Patients receiving a biopsy or initiating treatment after 30, 60, or 90 days were censored for the analysis.
Race/ethnicity.
Information about patients' race/ethnicity was included in the medical record abstraction and was based on patient self-assessment or assessment by clinic staff at the time of enrollment at the cancer center. Possible categorizations were: white, Hispanic, black, American Indian, Asian/Pacific Islander, and other. For our analysis, we grouped patients into the following categories: non-Hispanic white, Hispanic, and other/unknown.
Covariates.
The following patient characteristics were included in the analysis: patient age at enrollment in years, body-mass index (BMI; underweight/normal weight, overweight, or obese), diabetes at enrollment (yes or no), and hypertension at enrollment (yes or no). In addition, the following cancer/treatment characteristics were included: American Joint Committee on Cancer (AJCC) stage at diagnosis (I, II, III, or IV),17 Eastern Cooperative Oncology Group (ECOG) functional status at time of diagnosis (0, fully active; ≥ 1, limited in some way), estrogen receptor (ER) status (positive or negative), progesterone receptor (PR) status (positive or negative), human epidermal growth factor receptor 2 (HER2) status (positive or negative), histologic grade (1, 2, or 3), and Ki-67 in percent at time of diagnosis (< 25%, 25% to 75%, or > 75%). They were chosen to account for clinical and cancer characteristics that might affect the choice of primary therapy and, by extension, potentially influence time to treatment. On the basis of the zip code reported for each patient, we linked this information to the American Community Survey (2008 to 2012; 5-year estimates) to obtain additional sociodemographic information not available in the medical records.18 Neighborhood-level characteristics included: percentage within the zip code living below the poverty level, percentage uninsured, and percentage of the population age ≥ 25 years without a high-school degree/General Educational Development.
Statistical Analyses
We used χ2 tests and unadjusted and adjusted Cox proportional hazards models to examine the association between patient characteristics and time to treatment in 30-day intervals. Percentages by race/ethnicity and two-sided P values are listed for χ2 tests in Table 1. We estimated separate models for time from mammogram to biopsy and time from biopsy to treatment initiation in 30-day intervals (ie, at 30, 60, and 90 days). We also estimated Kaplan-Meier curves stratified by race/ethnicity for time to biopsy (Appendix Fig A1A, online only) and time to treatment initiation (Appendix Fig A1B, online only) and report log-rank test P values. The unadjusted models included race/ethnicity alone, whereas the adjusted models included race/ethnicity, age in years, stage at diagnosis, BMI, ECOG functional status, ER status, PR status, HER2 status, Ki-67, percentage living below poverty line, percentage uninsured, and percentage age ≥ 25 years without a high-school degree. Hazard ratios (HRs) and associated 95% CIs are listed in Tables 2 and 3. We conducted sensitivity analyses by excluding patients with cancer of other/unknown race/ethnicity from our analysis. Our major findings remained unchanged. All analyses were conducted using SAS software (version 9.3; SAS Institute, Cary, NC).
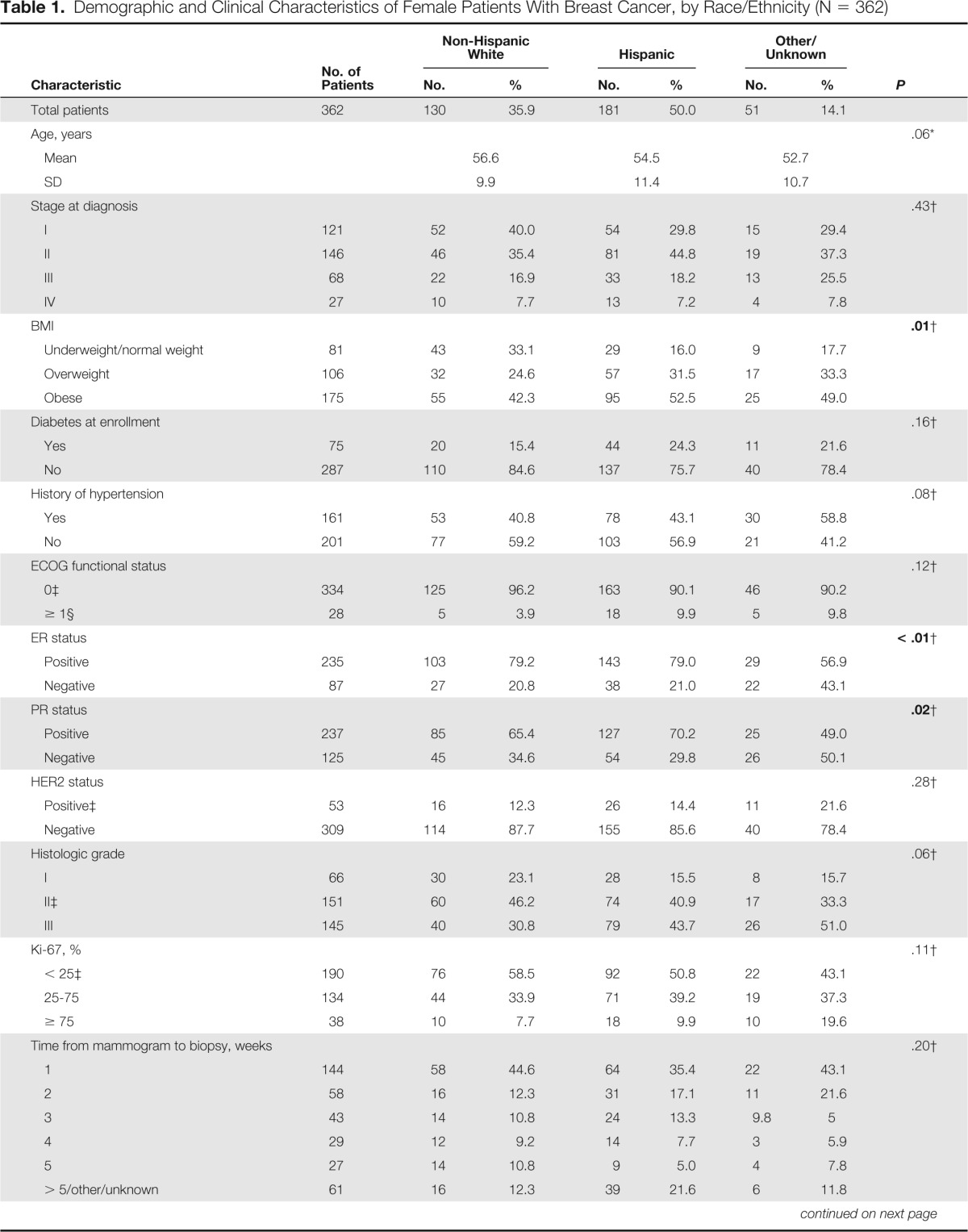
Table 1.
Demographic and Clinical Characteristics of Female Patients With Breast Cancer, by Race/Ethnicity (N = 362)
| Characteristic | No. of Patients | Non-Hispanic White |
Hispanic |
Other/Unknown |
P | |||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||
| Total patients | 362 | 130 | 35.9 | 181 | 50.0 | 51 | 14.1 | |
| Age, years | .06* | |||||||
| Mean | 56.6 | 54.5 | 52.7 | |||||
| SD | 9.9 | 11.4 | 10.7 | |||||
| Stage at diagnosis | .43† | |||||||
| I | 121 | 52 | 40.0 | 54 | 29.8 | 15 | 29.4 | |
| II | 146 | 46 | 35.4 | 81 | 44.8 | 19 | 37.3 | |
| III | 68 | 22 | 16.9 | 33 | 18.2 | 13 | 25.5 | |
| IV | 27 | 10 | 7.7 | 13 | 7.2 | 4 | 7.8 | |
| BMI | .01† | |||||||
| Underweight/normal weight | 81 | 43 | 33.1 | 29 | 16.0 | 9 | 17.7 | |
| Overweight | 106 | 32 | 24.6 | 57 | 31.5 | 17 | 33.3 | |
| Obese | 175 | 55 | 42.3 | 95 | 52.5 | 25 | 49.0 | |
| Diabetes at enrollment | .16† | |||||||
| Yes | 75 | 20 | 15.4 | 44 | 24.3 | 11 | 21.6 | |
| No | 287 | 110 | 84.6 | 137 | 75.7 | 40 | 78.4 | |
| History of hypertension | .08† | |||||||
| Yes | 161 | 53 | 40.8 | 78 | 43.1 | 30 | 58.8 | |
| No | 201 | 77 | 59.2 | 103 | 56.9 | 21 | 41.2 | |
| ECOG functional status | .12† | |||||||
| 0‡ | 334 | 125 | 96.2 | 163 | 90.1 | 46 | 90.2 | |
| ≥ 1§ | 28 | 5 | 3.9 | 18 | 9.9 | 5 | 9.8 | |
| ER status | < .01† | |||||||
| Positive | 235 | 103 | 79.2 | 143 | 79.0 | 29 | 56.9 | |
| Negative | 87 | 27 | 20.8 | 38 | 21.0 | 22 | 43.1 | |
| PR status | .02† | |||||||
| Positive | 237 | 85 | 65.4 | 127 | 70.2 | 25 | 49.0 | |
| Negative | 125 | 45 | 34.6 | 54 | 29.8 | 26 | 50.1 | |
| HER2 status | .28† | |||||||
| Positive‡ | 53 | 16 | 12.3 | 26 | 14.4 | 11 | 21.6 | |
| Negative | 309 | 114 | 87.7 | 155 | 85.6 | 40 | 78.4 | |
| Histologic grade | .06† | |||||||
| I | 66 | 30 | 23.1 | 28 | 15.5 | 8 | 15.7 | |
| II‡ | 151 | 60 | 46.2 | 74 | 40.9 | 17 | 33.3 | |
| III | 145 | 40 | 30.8 | 79 | 43.7 | 26 | 51.0 | |
| Ki-67, % | .11† | |||||||
| < 25‡ | 190 | 76 | 58.5 | 92 | 50.8 | 22 | 43.1 | |
| 25-75 | 134 | 44 | 33.9 | 71 | 39.2 | 19 | 37.3 | |
| ≥ 75 | 38 | 10 | 7.7 | 18 | 9.9 | 10 | 19.6 | |
| Time from mammogram to biopsy, weeks | .20† | |||||||
| 1 | 144 | 58 | 44.6 | 64 | 35.4 | 22 | 43.1 | |
| 2 | 58 | 16 | 12.3 | 31 | 17.1 | 11 | 21.6 | |
| 3 | 43 | 14 | 10.8 | 24 | 13.3 | 9.8 | 5 | |
| 4 | 29 | 12 | 9.2 | 14 | 7.7 | 3 | 5.9 | |
| 5 | 27 | 14 | 10.8 | 9 | 5.0 | 4 | 7.8 | |
| > 5/other/unknown | 61 | 16 | 12.3 | 39 | 21.6 | 6 | 11.8 | |
| Time from biopsy to treatment initiation, weeks | .22† | |||||||
| 1-2 | 18 | 8 | 6.2 | 9 | 5.0 | 1 | 2.0 | |
| 3-4 | 59 | 22 | 16.9 | 28 | 15.5 | 9 | 17.7 | |
| 5-6 | 97 | 33 | 15.4 | 45 | 24.9 | 19 | 37.3 | |
| 7-8 | 84 | 31 | 23.9 | 43 | 23.8 | 10 | 19.6 | |
| 9-10 | 35 | 17 | 13.1 | 12 | 6.6 | 6 | 11.8 | |
| > 10/other/unknown | 69 | 19 | 14.6 | 44 | 24.3 | 6 | 11.8 | |
| Neighborhood socioeconomic status | ||||||||
| Percentage in patient's zip code living below poverty level | < .01* | |||||||
| Mean | 17.3 | 23.0 | 19.4 | |||||
| SD | 9.8 | 9.9 | 10.3 | |||||
| Percentage in patient's zip code who are uninsured | < .01* | |||||||
| Mean | 19.8 | 24.2 | 20.8 | |||||
| SD | 7.2 | 6.5 | 6.7 | |||||
| Percentage of population in patient's zip zode age ≥ 25 years without ≥ high-school degree/GED | < .01* | |||||||
| Mean | 18.0 | 26.4 | 18.8 | |||||
| SD | 12.2 | 13.6 | 11.4 | |||||
NOTE. Percentages may not add to 100 because of rounding. Bold font indicates significance (P < .05).
Abbreviations: BMI, body-mass index; ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; GED, general educational development; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor; SD, standard deviation.
Analysis of variance.
χ2 test.
Category includes missing values: ECOG functional status, n = 2; HER2 status, n = 2; Ki-67, n = 9; and histologic grade, n = 2.
Only five individuals had ECOG functional status ≥ 2.
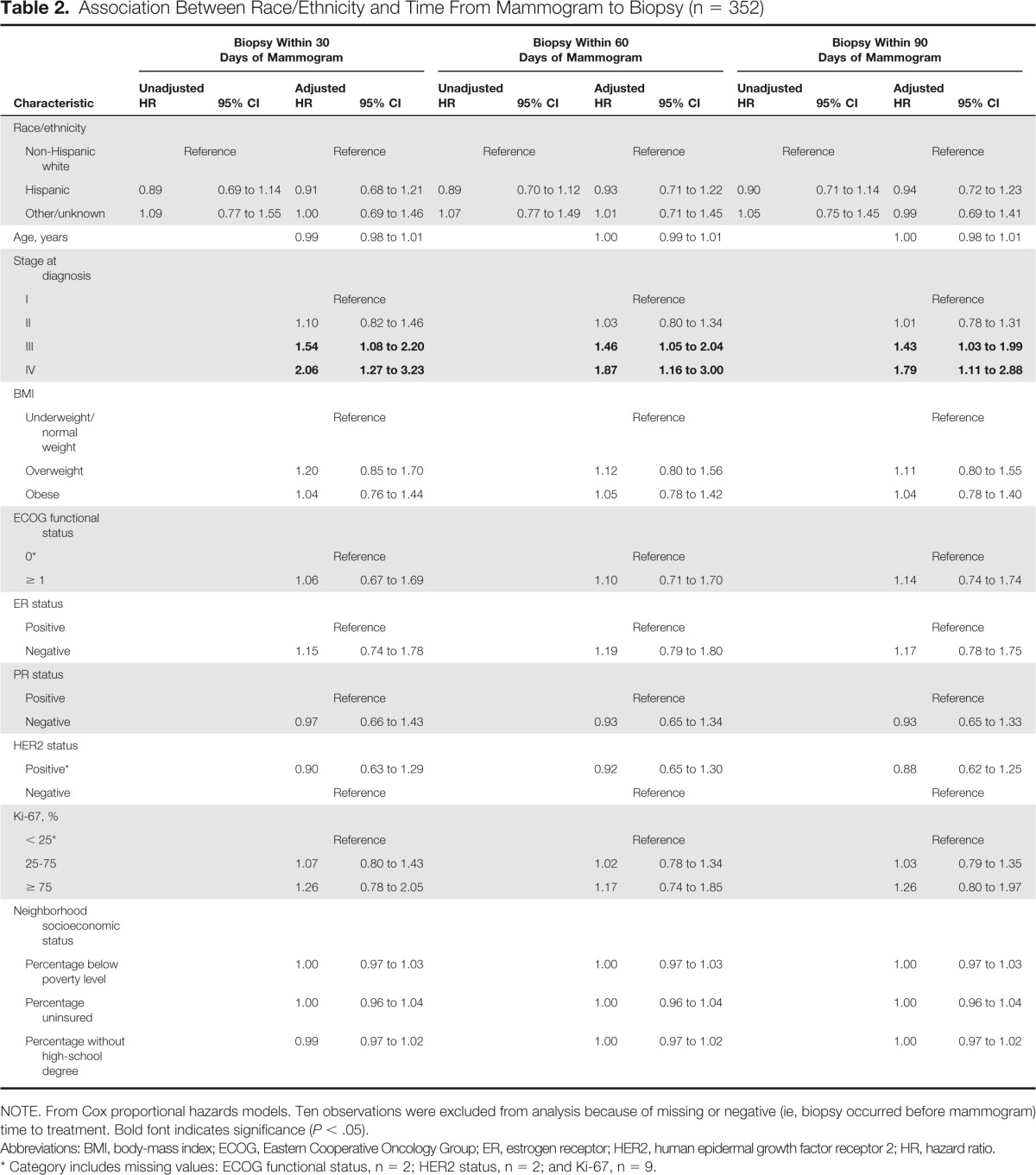
Table 2.
Association Between Race/Ethnicity and Time From Mammogram to Biopsy (n = 352)
| Characteristic | Biopsy Within 30 Days of Mammogram |
Biopsy Within 60 Days of Mammogram |
Biopsy Within 90 Days of Mammogram |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted HR | 95% CI | Adjusted HR | 95% CI | Unadjusted HR | 95% CI | Adjusted HR | 95% CI | Unadjusted HR | 95% CI | Adjusted HR | 95% CI | |
| Race/ethnicity | ||||||||||||
| Non-Hispanic white | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| Hispanic | 0.89 | 0.69 to 1.14 | 0.91 | 0.68 to 1.21 | 0.89 | 0.70 to 1.12 | 0.93 | 0.71 to 1.22 | 0.90 | 0.71 to 1.14 | 0.94 | 0.72 to 1.23 |
| Other/unknown | 1.09 | 0.77 to 1.55 | 1.00 | 0.69 to 1.46 | 1.07 | 0.77 to 1.49 | 1.01 | 0.71 to 1.45 | 1.05 | 0.75 to 1.45 | 0.99 | 0.69 to 1.41 |
| Age, years | 0.99 | 0.98 to 1.01 | 1.00 | 0.99 to 1.01 | 1.00 | 0.98 to 1.01 | ||||||
| Stage at diagnosis | ||||||||||||
| I | Reference | Reference | Reference | |||||||||
| II | 1.10 | 0.82 to 1.46 | 1.03 | 0.80 to 1.34 | 1.01 | 0.78 to 1.31 | ||||||
| III | 1.54 | 1.08 to 2.20 | 1.46 | 1.05 to 2.04 | 1.43 | 1.03 to 1.99 | ||||||
| IV | 2.06 | 1.27 to 3.23 | 1.87 | 1.16 to 3.00 | 1.79 | 1.11 to 2.88 | ||||||
| BMI | ||||||||||||
| Underweight/normal weight | Reference | Reference | Reference | |||||||||
| Overweight | 1.20 | 0.85 to 1.70 | 1.12 | 0.80 to 1.56 | 1.11 | 0.80 to 1.55 | ||||||
| Obese | 1.04 | 0.76 to 1.44 | 1.05 | 0.78 to 1.42 | 1.04 | 0.78 to 1.40 | ||||||
| ECOG functional status | ||||||||||||
| 0* | Reference | Reference | Reference | |||||||||
| ≥ 1 | 1.06 | 0.67 to 1.69 | 1.10 | 0.71 to 1.70 | 1.14 | 0.74 to 1.74 | ||||||
| ER status | ||||||||||||
| Positive | Reference | Reference | Reference | |||||||||
| Negative | 1.15 | 0.74 to 1.78 | 1.19 | 0.79 to 1.80 | 1.17 | 0.78 to 1.75 | ||||||
| PR status | ||||||||||||
| Positive | Reference | Reference | Reference | |||||||||
| Negative | 0.97 | 0.66 to 1.43 | 0.93 | 0.65 to 1.34 | 0.93 | 0.65 to 1.33 | ||||||
| HER2 status | ||||||||||||
| Positive* | 0.90 | 0.63 to 1.29 | 0.92 | 0.65 to 1.30 | 0.88 | 0.62 to 1.25 | ||||||
| Negative | Reference | Reference | Reference | |||||||||
| Ki-67, % | ||||||||||||
| < 25* | Reference | Reference | Reference | |||||||||
| 25-75 | 1.07 | 0.80 to 1.43 | 1.02 | 0.78 to 1.34 | 1.03 | 0.79 to 1.35 | ||||||
| ≥ 75 | 1.26 | 0.78 to 2.05 | 1.17 | 0.74 to 1.85 | 1.26 | 0.80 to 1.97 | ||||||
| Neighborhood socioeconomic status | ||||||||||||
| Percentage below poverty level | 1.00 | 0.97 to 1.03 | 1.00 | 0.97 to 1.03 | 1.00 | 0.97 to 1.03 | ||||||
| Percentage uninsured | 1.00 | 0.96 to 1.04 | 1.00 | 0.96 to 1.04 | 1.00 | 0.96 to 1.04 | ||||||
| Percentage without high-school degree | 0.99 | 0.97 to 1.02 | 1.00 | 0.97 to 1.02 | 1.00 | 0.97 to 1.02 | ||||||
NOTE. From Cox proportional hazards models. Ten observations were excluded from analysis because of missing or negative (ie, biopsy occurred before mammogram) time to treatment. Bold font indicates significance (P < .05).
Abbreviations: BMI, body-mass index; ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR, hazard ratio.
Category includes missing values: ECOG functional status, n = 2; HER2 status, n = 2; and Ki-67, n = 9.
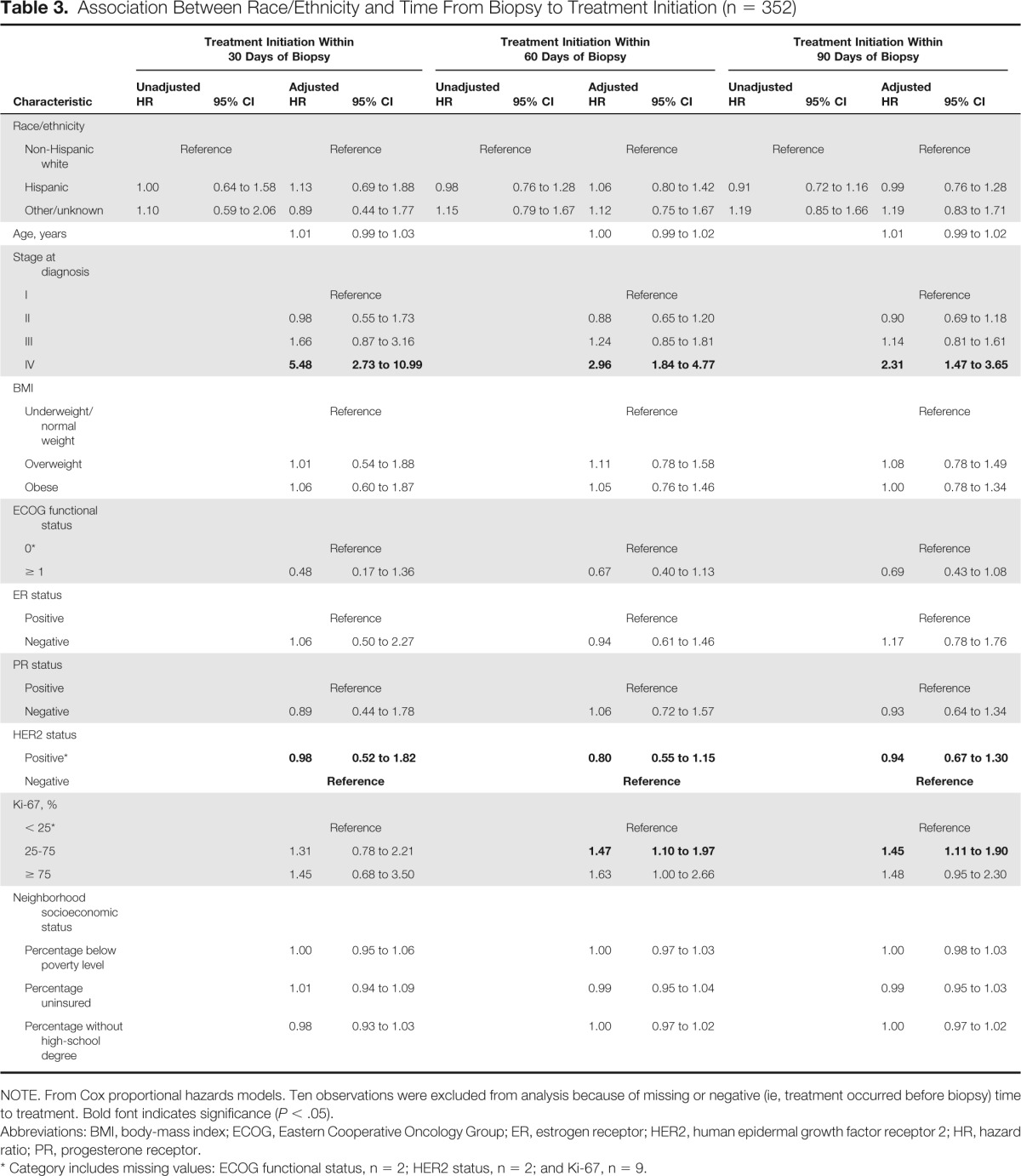
Table 3.
Association Between Race/Ethnicity and Time From Biopsy to Treatment Initiation (n = 352)
| Characteristic | Treatment Initiation Within 30 Days of Biopsy |
Treatment Initiation Within 60 Days of Biopsy |
Treatment Initiation Within 90 Days of Biopsy |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted HR | 95% CI | Adjusted HR | 95% CI | Unadjusted HR | 95% CI | Adjusted HR | 95% CI | Unadjusted HR | 95% CI | Adjusted HR | 95% CI | |
| Race/ethnicity | ||||||||||||
| Non-Hispanic white | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| Hispanic | 1.00 | 0.64 to 1.58 | 1.13 | 0.69 to 1.88 | 0.98 | 0.76 to 1.28 | 1.06 | 0.80 to 1.42 | 0.91 | 0.72 to 1.16 | 0.99 | 0.76 to 1.28 |
| Other/unknown | 1.10 | 0.59 to 2.06 | 0.89 | 0.44 to 1.77 | 1.15 | 0.79 to 1.67 | 1.12 | 0.75 to 1.67 | 1.19 | 0.85 to 1.66 | 1.19 | 0.83 to 1.71 |
| Age, years | 1.01 | 0.99 to 1.03 | 1.00 | 0.99 to 1.02 | 1.01 | 0.99 to 1.02 | ||||||
| Stage at diagnosis | ||||||||||||
| I | Reference | Reference | Reference | |||||||||
| II | 0.98 | 0.55 to 1.73 | 0.88 | 0.65 to 1.20 | 0.90 | 0.69 to 1.18 | ||||||
| III | 1.66 | 0.87 to 3.16 | 1.24 | 0.85 to 1.81 | 1.14 | 0.81 to 1.61 | ||||||
| IV | 5.48 | 2.73 to 10.99 | 2.96 | 1.84 to 4.77 | 2.31 | 1.47 to 3.65 | ||||||
| BMI | ||||||||||||
| Underweight/normal weight | Reference | Reference | Reference | |||||||||
| Overweight | 1.01 | 0.54 to 1.88 | 1.11 | 0.78 to 1.58 | 1.08 | 0.78 to 1.49 | ||||||
| Obese | 1.06 | 0.60 to 1.87 | 1.05 | 0.76 to 1.46 | 1.00 | 0.78 to 1.34 | ||||||
| ECOG functional status | ||||||||||||
| 0* | Reference | Reference | Reference | |||||||||
| ≥ 1 | 0.48 | 0.17 to 1.36 | 0.67 | 0.40 to 1.13 | 0.69 | 0.43 to 1.08 | ||||||
| ER status | ||||||||||||
| Positive | Reference | Reference | Reference | |||||||||
| Negative | 1.06 | 0.50 to 2.27 | 0.94 | 0.61 to 1.46 | 1.17 | 0.78 to 1.76 | ||||||
| PR status | ||||||||||||
| Positive | Reference | Reference | Reference | |||||||||
| Negative | 0.89 | 0.44 to 1.78 | 1.06 | 0.72 to 1.57 | 0.93 | 0.64 to 1.34 | ||||||
| HER2 status | ||||||||||||
| Positive* | 0.98 | 0.52 to 1.82 | 0.80 | 0.55 to 1.15 | 0.94 | 0.67 to 1.30 | ||||||
| Negative | Reference | Reference | Reference | |||||||||
| Ki-67, % | ||||||||||||
| < 25* | Reference | Reference | Reference | |||||||||
| 25-75 | 1.31 | 0.78 to 2.21 | 1.47 | 1.10 to 1.97 | 1.45 | 1.11 to 1.90 | ||||||
| ≥ 75 | 1.45 | 0.68 to 3.50 | 1.63 | 1.00 to 2.66 | 1.48 | 0.95 to 2.30 | ||||||
| Neighborhood socioeconomic status | ||||||||||||
| Percentage below poverty level | 1.00 | 0.95 to 1.06 | 1.00 | 0.97 to 1.03 | 1.00 | 0.98 to 1.03 | ||||||
| Percentage uninsured | 1.01 | 0.94 to 1.09 | 0.99 | 0.95 to 1.04 | 0.99 | 0.95 to 1.03 | ||||||
| Percentage without high-school degree | 0.98 | 0.93 to 1.03 | 1.00 | 0.97 to 1.02 | 1.00 | 0.97 to 1.02 | ||||||
NOTE. From Cox proportional hazards models. Ten observations were excluded from analysis because of missing or negative (ie, treatment occurred before biopsy) time to treatment. Bold font indicates significance (P < .05).
Abbreviations: BMI, body-mass index; ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; PR, progesterone receptor.
Category includes missing values: ECOG functional status, n = 2; HER2 status, n = 2; and Ki-67, n = 9.
Results
Demographic and cancer/treatment characteristics by race/ethnicity are listed in Table 1. Fifty percent of the female patients with breast cancer in our sample were Hispanic; 35.9% were non-Hispanic white; 14.1% were of other (ie, black, American Indian, Asian/Pacific Islander) or unknown race/ethnicity. Significant differences were observed for BMI, ER status, and PR status by race ethnicity (all P ≤ .05). Hispanic and other patients with cancer were more likely to be overweight or obese than non-Hispanic white patients. Patients with cancer of other/unknown race/ethnicity were more likely to have negative ER or PR status than Hispanics or non-Hispanic white women. In addition, when examining Hispanics compared with non-Hispanic whites only, Hispanics were more likely to have an ECOG functional status ≥ 1 and were more likely to have a high histologic grade of breast cancer at diagnosis (P ≤ .05; data not shown). Additionally, Hispanic patients were more likely to live in zip codes with higher poverty levels, higher uninsured rates, and higher proportions of the population without a high-school degree compared with non-Hispanic whites and those of other races/ethnicities. No significant differences by race/ethnicity were detected for age at enrollment, stage at diagnosis, rate of diabetes or hypertension, HER2 status, or percent Ki-67.
Time From Mammogram to Biopsy
The median time from mammogram to biopsy for all patients was 11 days (Appendix Fig A1A, online only). However, no significant differences were observed in the time from abnormal mammogram to biopsy between non-Hispanic white, Hispanic, and other patients with breast cancer (log-rank P = .52). We then examined the relationship between race/ethnicity and time from mammogram to biopsy (within 30, 60, or 90 days after mammogram; Table 2). Unadjusted Cox proportional hazards models continued to demonstrate that race/ethnicity was not associated with whether patients with breast cancer had received a biopsy within 30, 60, or 90 days of the mammogram. After adjusting for age, cancer/treatment characteristics, and sociodemographics, race/ethnicity remained nonsignificant. We continued to find no significant differences between race/ethnicity and time from mammogram to biopsy. However, patients diagnosed with later-stage breast cancer were more likely to receive a biopsy sooner than those with earlier-stage disease (AJCC stage III or IV v I; P < .05).
Time From Biopsy to Treatment Initiation
The median time from biopsy to treatment initiation in our population of patients with breast cancer was 42 days (Appendix Fig A1B, online only). Again, no significant differences between race/ethnicity and time from biopsy to treatment initiation were observed (log-rank P = .27). The Cox proportional hazards models summarized in Table 3 continued to indicate that there were no significant differences in time to treatment initiation among non-Hispanic white, Hispanic, and other patients with breast cancer after adjusting for patient age, cancer/treatment characteristics, and sociodemographics. Specifically, race/ethnicity did not significantly affect the risk of patients with cancer not initiating treatment within 30, 60, or 90 days of biopsy. However, patients with AJCC stage IV cancer were significantly more likely to have initiated treatment within 30, 60, or 90 days after biopsy (P < .05; ie, they were more likely to initiate treatment sooner). In addition, patients with breast cancer with Ki-67 percentages between 25% and 75% were significantly more likely to have initiated treatment within 60 or 90 days of biopsy (P < .05).
Discussion
In our study of patients with breast cancer treated in a culturally diverse southern Texas NCI-designated cancer center, we found that Hispanic women and non-Hispanic white women had similar times to treatment initiation. Specifically, we found no significant differences between time from abnormal mammogram to biopsy or from biopsy to treatment initiation in this population. Overall, this study sheds light on how previous patterns of racial/ethnic disparities in breast cancer care may be mitigated when placed in the context of a community where individuals both have access to an NCI-designated cancer center and are part of a majority Hispanic community that contributes significant cultural influence and also makes up a large proportion of the health care workforce. These findings suggest that in settings with a majority minority population and patients who are treated at cancer centers, racial/ethnic disparities may be attenuated.
Our findings are in contrast to previous studies of treatment delays in cancer survivors across racial/ethnic groups, which have predominately used population-based samples of patients with cancer. Work by Katz et al19 identified that Hispanic women in the Los Angeles–SEER registry have experienced delays across the treatment continuum, including later stages at diagnosis, treatment delays (for primary Spanish speakers),20 and higher rates of worry about treatment.21 Furthermore, in a population-based study of Medicare-eligible breast cancer survivors with AJCC stage I to III disease, Freedman et al12 found that black women had higher odds of experiencing treatment delays compared with non-Hispanic white women. Another study by McGee et al8 examining breast cancer treatment delays in the population-based Carolina Breast Cancer Study found that younger African American women experienced greater delays in treatment initiation compared with white women. Additionally, Smith et al7 reported that young patients with breast cancer who were Hispanic or African American and had no or public health insurance and lower levels of socioeconomic status were more likely to experience a longer time to treatment, which led to worse survival, particularly among African American women. Diagnosis and treatment delays as well as worse outcomes by race/ethnicity have also been identified in cancer survivors more broadly. Li et al3 identified that racial/ethnic minorities were more likely to present with later-stage disease than non-Hispanic whites. As a result, these patients had a 20% to 200% greater likelihood of mortality after diagnosis.3 Our findings of breast cancer treatment in a majority Hispanic population indicate no differences in stage at diagnosis or treatment delays by race/ethnicity, including time from mammogram to biopsy and time from biopsy to treatment. These treatment similarities by race/ethnicity continued throughout the study period, despite slightly higher poverty (patients with breast cancer, 17.9% v United States, 14.9%) and uninsured levels (20.2% v 14.3%) and lower levels of education (no high-school degree, 20% v 14.7%) compared with the country more broadly (Appendix Table A1, online only).18 However, we note that our median time from biopsy to treatment of 42 days may be longer than some previously reported treatment data. Although McLaughlin et al22 identified a median treatment interval of 22 days in a population-based cohort of low-income women in North Carolina, the majority of individuals had received treatment within 60 days, which was also true for our study (73.2%). Furthermore, median time to treatment was 27 days in the National Cancer Database among a predominately non-Hispanic white sample; however, 12% had still not received treatment within 60 days.23 Despite a longer time to treatment compared with some settings, our treatment times were similar to those identified in a set of ethnically diverse public and private hospitals in Atlanta, Georgia.24 Future studies should examine how these treatment similarities identified in our study extend to longer-term outcomes in majority Hispanic populations, including quality of life and survival.
These findings provide significant additional insights into the relationship between race/ethnicity and treatment delays in two main ways. First, our population of breast cancer survivors is part of a majority Hispanic community that provides significant cultural context for appropriate, culturally sensitive (ie, with regard to language spoken and social norms) patient-provider interactions. Specifically, a large proportion of our cancer center faculty and staff (40%) are Hispanic (with remaining faculty/staff composed of 44% non-Hispanic whites, 4% blacks, 8% Asians, and 4% other), many of whom are bilingual—both factors that may contribute to improved patient-provider communication and may help reduce barriers to access and ultimately treatment delays in this population.25,26 Our patients were treated at an NCI-designated cancer center, which has historically provided higher-quality care and improved outcomes compared with other types of treatment facilities.14 Previous studies of patients with cancer have identified that access to quality-driven hospitals may ameliorate racial/ethnic disparities in care and outcomes. Specifically, Onega et al14 examined cancer mortality among non-Hispanic blacks and non-Hispanic whites in a population-based study of Medicare-eligible patients with lung, breast, colorectal, or prostate cancer. Although they identified higher cancer mortality in non-Hispanic blacks compared with whites overall, there were no significant differences in mortality by race/ethnicity among those treated at NCI-designated cancer centers. Parsons et al13 also examined differences in operative outcomes for racial/ethnic minorities treated at participating American College of Surgeons National Quality Improvement Program facilities (ie, quality-seeking facilities with well-defined outcomes collection and reporting mechanisms), finding that racial/ethnic minorities treated for cancer at such hospitals had short-term outcomes similar to those of non-Hispanic whites. Even among other population-based studies identifying disparities in treatment initiation, researchers have pointed toward the important contribution that hospital/facility characteristics may play in mitigating racial/ethnic disparities.2,12 Therefore, ensuring that care is delivered in a coordinated, continuous, and culturally sensitive fashion is critical to reducing racial/ethnic disparities in treatment delays and ultimately improving survival.
We acknowledge the following limitations. First, our study is based on medical record abstractions for patients receiving treatment from physicians at an NCI-designated cancer center and may not be representative of all patients with breast cancer in the area. However, the study population was similar in regard to the sociodemographics of Texas. Second, we used census data to approximate the socioeconomic characteristics of our population, including insurance (because of poor data quality in medical records), income, and education of residents in the patient's zip code. Although there is potential for creating bias in socioeconomic effects when using aggregate data to approximate individual characteristics,27,28 this method has been shown to provide a valid approach to overcoming the absence of socioeconomic data in most medical records.29 Furthermore, we reduced this potential bias by using the smallest available geographic unit (ie, zip code) when obtaining socioeconomic characteristics.30 Third, on the basis of available data, we were unable to assess whether there were racial/ethnic disparities in receipt of all recommended care or whether breast cancer mortality varied among these patients. Fourth, because of the small sample size, we were not able to examine disparities in treatment for other racial/ethnic groups, such as blacks, separately. However, we did not see differences in time to treatment between Hispanics, non-Hispanic whites, and those of other races (which included blacks). Although these limitations may apply, this research sheds light on treatment delays in breast cancer survivors in an ethnically diverse population.
In conclusion, in the United States, Hispanic and other minority women have faced considerable challenges in overcoming disparities in health care for breast cancer. In our population of female patients with breast cancer treated at an NCI-designated cancer center with a majority Hispanic population, we found no significant differences in stage at diagnosis or time from abnormal mammogram to initiation of treatment. These findings highlight the importance of receiving cancer care at an NCI-designated cancer center as well as receiving adequate care in a culturally sensitive setting and suggest that in majority minority communities with large cancer centers, racial disparities may be attenuated. With a growing Hispanic population throughout the United States, future studies should examine the long-term impact on improved breast cancer survival in this population and identify whether these findings can also be replicated in other majority minority settings.
Acknowledgment
Supported by National Cancer Institute Cancer Prevention and Control Career Development Award No. K07CA175063 (H.M.P.). Presented at the ASCO Quality Care Symposium, San Diego, CA, November 1-2, 2013.
Appendix
Table A1.
Zip Code–Level Socioeconomic Characteristics of Patients With Breast Cancer Treated at NCI-Designated Cancer Center in Texas Compared With State of Texas and United States
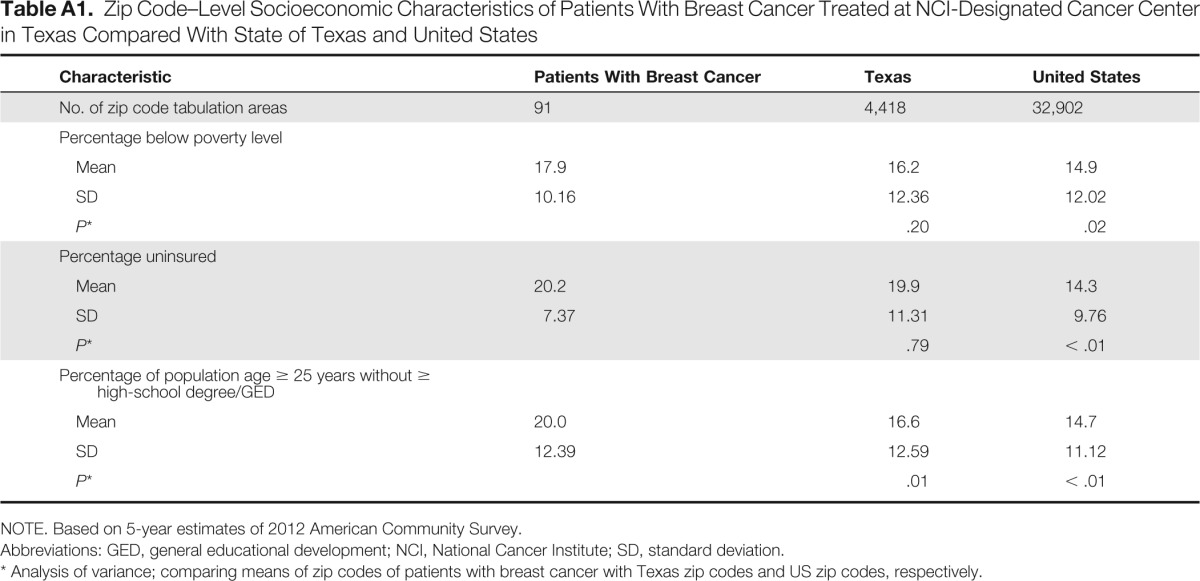
| Characteristic | Patients With Breast Cancer | Texas | United States |
|---|---|---|---|
| No. of zip code tabulation areas | 91 | 4,418 | 32,902 |
| Percentage below poverty level | |||
| Mean | 17.9 | 16.2 | 14.9 |
| SD | 10.16 | 12.36 | 12.02 |
| P* | .20 | .02 | |
| Percentage uninsured | |||
| Mean | 20.2 | 19.9 | 14.3 |
| SD | 7.37 | 11.31 | 9.76 |
| P* | .79 | < .01 | |
| Percentage of population age ≥ 25 years without ≥ high-school degree/GED | |||
| Mean | 20.0 | 16.6 | 14.7 |
| SD | 12.39 | 12.59 | 11.12 |
| P* | .01 | < .01 |
NOTE. Based on 5-year estimates of 2012 American Community Survey.
Abbreviations: GED, general educational development; NCI, National Cancer Institute; SD, standard deviation.
Analysis of variance; comparing means of zip codes of patients with breast cancer with Texas zip codes and US zip codes, respectively.
Figure A1.
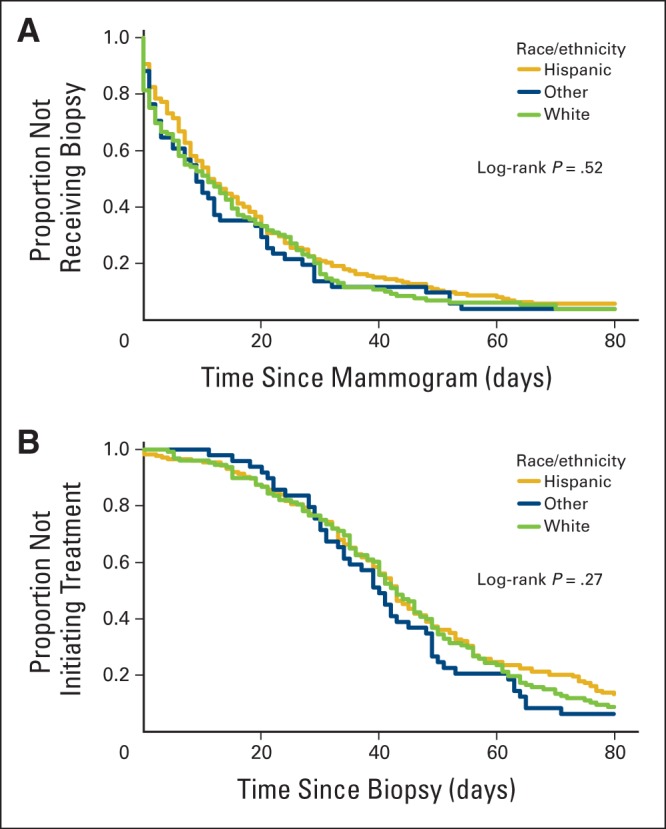
Kaplan-Meier curves stratified by race/ethnicity for (A) time from mammogram to biopsy and (B) time from biopsy to treatment initiation.
Authors' Disclosures of Potential Conflicts of Interest
Disclosures provided by the authors are available with this article at jop.ascopubs.org.
Author Contributions
Conception and design: Helen M. Parsons, Kate I. Lathrop, Susanne Schmidt, Anand Karnad
Financial support: Helen M. Parsons
Administrative support: Helen M. Parsons
Collection and assembly of data: Kate I. Lathrop, Marcela Mazo-Canola, Jessica Trevino-Jones, Heather Speck
Data analysis and interpretation: Helen M. Parsons, Kate I. Lathrop, Susanne Schmidt
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Breast Cancer Treatment Delays in a Majority Minority Community: Is There a Difference?
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jop.ascopubs.org/site/misc/ifc.xhtml.
Helen M. Parsons
No relationship to disclose
Kate I. Lathrop
No relationship to disclose
Susanne Schmidt
No relationship to disclose
Marcela Mazo-Canola
No relationship to disclose
Jessica Trevino-Jones
No relationship to disclose
Heather Speck
No relationship to disclose
Anand Karnad
No relationship to disclose
References
- 1.National Cancer Institute. Breast Cancer. http://www.cancer.gov/cancertopics/types/breast.
- 2.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 3.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163:49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 4.Wheeler SB, Reeder-Hayes KE, Carey LA. Disparities in breast cancer treatment and outcomes: Biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18:986–993. doi: 10.1634/theoncologist.2013-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman RA, He Y, Winer EP, et al. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol. 2009;27:713–719. doi: 10.1200/JCO.2008.17.9234. [DOI] [PubMed] [Google Scholar]
- 6.Morris AM, Rhoads KF, Stain SC, et al. Understanding racial disparities in cancer treatment and outcomes. J Am Coll Surg. 2010;211:105–113. doi: 10.1016/j.jamcollsurg.2010.02.051. [DOI] [PubMed] [Google Scholar]
- 7.Smith EC, Ziogas A, Anton-Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg. 2013;148:516–523. doi: 10.1001/jamasurg.2013.1680. [DOI] [PubMed] [Google Scholar]
- 8.McGee SA, Durham DD, Tse CK, et al. Determinants of breast cancer treatment delay differ for African American and white women. Cancer Epidemiol Biomarkers Prev. 2013;22:1227–1238. doi: 10.1158/1055-9965.EPI-12-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freedman RA, Winer EP. Reducing disparities in breast cancer care: A daunting but essential responsibility. J Natl Cancer Inst. 2008;100:1661–1663. doi: 10.1093/jnci/djn412. [DOI] [PubMed] [Google Scholar]
- 10.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94:490–496. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 11.Esnaola NF, Hall BL, Hosokawa PW, et al. Race and surgical outcomes: It is not all black and white. Ann Surg. 2008;248:647–655. doi: 10.1097/SLA.0b013e31818a159a. [DOI] [PubMed] [Google Scholar]
- 12.Freedman RA, He Y, Winer EP, et al. Racial/ethnic differences in receipt of timely adjuvant therapy for older women with breast cancer: Are delays influenced by the hospitals where patients obtain surgical care? Health Serv Res. 2013;48:1669–1683. doi: 10.1111/1475-6773.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsons HM, Habermann EB, Stain SC, et al. What happens to racial and ethnic minorities after cancer surgery at American College of Surgeons National Surgical Quality Improvement Program hospitals? J Am Coll Surg. 2012;214:539–547. doi: 10.1016/j.jamcollsurg.2011.12.024. discussion 547-549. [DOI] [PubMed] [Google Scholar]
- 14.Onega T, Duell EJ, Shi X, et al. Race versus place of service in mortality among Medicare beneficiaries with cancer. Cancer. 2010;116:2698–2706. doi: 10.1002/cncr.25097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Census Bureau. Projections Show a Slower Growing, Older, More Diverse Nation a Half Century From Now. http://www.census.gov/newsroom/releases/archives/population/cb12-243.html.
- 16.US Census Bureau. American Fact Finder: ACS Demographic and Housing Estimates. http://factfinder2.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_12_5YR_DP05.
- 17.Byrd DR, Compton CC, et al., editors. Breast in Edge SB. AJCC Cancer Staging Manual (ed 7) New York, NY: Springer; 2010. pp. 347–376. [Google Scholar]
- 18.US Census Bureau. American Fact Finder: Download Center. http://factfinder2.census.gov/faces/nav/jsf/pages/download_center.xhtml.
- 19.Lantz PM, Mujahid M, Schwartz K, et al. The influence of race, ethnicity, and individual socioeconomic factors on breast cancer stage at diagnosis. Am J Public Health. 2006;96:2173–2178. doi: 10.2105/AJPH.2005.072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz SJ, Lantz PM, Paredes Y, et al. Breast cancer treatment experiences of Latinas in Los Angeles County. Am J Public Health. 2005;95:2225–2230. doi: 10.2105/AJPH.2004.057950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janz NK, Hawley ST, Mujahid MS, et al. Correlates of worry about recurrence in a multiethnic population-based sample of women with breast cancer. Cancer. 2011;117:1827–1836. doi: 10.1002/cncr.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLaughlin JM, Anderson RT, Ferketich AK, et al. Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. J Clin Oncol. 2012;30:4493–4500. doi: 10.1200/JCO.2012.39.7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fedewa SA, Edge SB, Stewart AK, et al. Race and ethnicity are associated with delays in breast cancer treatment (2003-2006) J Health Care Poor Underserved. 2011;22:128–41. doi: 10.1353/hpu.2011.0006. [DOI] [PubMed] [Google Scholar]
- 24.Mosunjac M, Park J, Strauss A, et al. Time to treatment for patients receiving BCS in a public and a private university hospital in Atlanta. Breast J. 2012;18:163–167. doi: 10.1111/j.1524-4741.2011.01205.x. [DOI] [PubMed] [Google Scholar]
- 25.Brach C, Fraser I. Can cultural competency reduce racial and ethnic health disparities? A review and conceptual model. Med Care Res Rev. 2000;57(suppl 1):181–217. doi: 10.1177/1077558700057001S09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weech-Maldonado R, Morales LS, Elliott M, et al. Race/ethnicity, language, and patients' assessments of care in Medicaid managed care. Health Serv Res. 2003;38:789–808. doi: 10.1111/1475-6773.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Openshaw S. Ecological fallacies and the analysis of areal census data. Environ Plan A. 1984;16:17–31. doi: 10.1068/a160017. [DOI] [PubMed] [Google Scholar]
- 28.Geronimus T, Bound J, Neidert L. On the validity of using census geocode characteristics to proxy individual socioeconomic characteristics. J Am Stat Assoc. 1996;91:529. [Google Scholar]
- 29.Krieger N. Overcoming the absence of socioeconomic data in medical records: Validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soobader M, LeClere FB, Hadden W, et al. Using aggregate geographic data to proxy individual socioeconomic status: Does size matter? Am J Public Health. 2001;91:632–636. doi: 10.2105/ajph.91.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]


