Abstract
Cell microenvironment has a critical role determining cell fate and modulating cell responses to injuries. Hyaluronan (HA) is a ubiquitous extracellular matrix glycosaminoglycan that can be considered a signaling molecule. In fact, interacting with several cell surface receptors can deeply shape cell behavior. In vascular biology, HA triggers smooth muscle cells (SMCs) dedifferentiation which contributes to vessel wall thickening. Furthermore, HA is able to modulate inflammation by altering the adhesive properties of endothelial cells. In hyperglycemic conditions, HA accumulates in vessels and can contribute to the diabetic complications at micro- and macrovasculature. Due to the pivotal role in favoring atherogenesis and neointima formation after injuries, HA could be a new target for cardiovascular pathologies. This review will focus on the recent findings regarding the regulation of HA synthesis in human vascular SMCs. In particular, the effects of the intracellular HA substrates availability, adenosine monophosphate-activated protein kinase (AMPK), and protein O-GlcNAcylation on the main HA synthetic enzyme (i.e., HAS2) will be discussed.
1. Introduction
Cardiovascular pathologies are the major cause of death in western countries, and their impact is increasing due to rising rates of obesity and diabetes [1]. Diabetes is the most widespread metabolic disorder and its medical and socioeconomic burden is caused by the associated complications that are mostly at macrovascular and microvascular level, leading to retinopathy, neuropathy, and nephropathy, as a consequence of accelerated atherogenesis [2, 3]. Limited success of pharmacological and invasive-surgical (i.e., angioplasty and bypass grafting) treatments may be a result of the incomplete understanding of the biological mechanisms which control and contribute to the development of atherosclerosis. At biochemical level, during hyperglycemic conditions, several alterations have been described in different pathways as polyol, hexosamine, protein kinase C, and advanced glycation end-product (AGE) metabolisms [2].
The development of atherosclerosis is coupled to dramatic alterations of the extracellular matrix (ECM), which provides critical support for vascular tissue acting as a scaffold for maintaining the organization of vascular cells into blood vessels, for blood vessel stabilization, and for cell proliferation, migration and survival [4–6]. ECM is a complex milieu of macromolecules that influences the activities of the cells, including cell differentiation, migration, and proliferation by specific cell-matrix interactions [7]. Hyaluronan (HA) is a ubiquitous ECM component with a multitude of functions [8]. HA is a linear polymer belonging to the family of glycosaminoglycans (GAGs), which comprises the major fraction of carbohydrates in ECM. HA is present in low amounts in normal blood vessels but increases dramatically in vascular diseases [9–11].
In this review, we will discuss the new regulatory mechanisms that link HA synthesis, atherosclerosis, and diabetes.
2. Hyaluronan
HA is a linear GAG that is composed of repeating units of D-glucuronic acid (GlcUA) and N-acetylglucosamine (GlcNAc) linked together through alternating β-1,4 and β-1,3 glycosidic bonds. This disaccharide can be repeated several thousand times without any other chemical modification (i.e., sulfation, acetylation, and epimerization) that are typical of the other GAGs [12]. Differently from the other GAGs, HA is not covalently bound to any core protein of proteoglycans, although HA can interact with other ECM molecules as versican, aggrecan, and tumor necrosis factor- (TNF-) stimulated gene 6 (TSG-6) via particular domains (i.e., link domain) [13]. HA is a very multifunctional GAG and HA properties and effects on cells depend on the length of the polysaccharide chains. In tissues, HA molecular mass can range from 500,000 to 10,000,000 Da [13].
HA appeared late during evolution and it is present only from chordate, probably with the aim of modulating the immune system and cells motility [14, 15]. Interestingly, some pathological bacteria (i.e., Streptococcus equisimilis, Streptococcus pyogenes, and Pasteurella multocida) possess the operon that permits both the synthesis of precursors and HA polymerization. This HA stealth or capsule makes the bacteria not easily identifiable by antibodies or attacked by phagocytes.
HA has been considered a mere space filling molecule for a long time, able to modulate tissue hydration. More recently, HA was shown to have other peculiar properties. For instance, high molecular weight HA has typically anti-inflammatory and antiangiogenic properties and inhibits cell proliferation. On the other hand, low molecular weight HA shows opposite characteristics, favoring inflammation and promoting cell growth [16]. These effects are often mediated by several cell surface receptors, including CD44, receptor for HA-mediated motility (RHAMM), lymphatic vessel endothelial receptor 1 (Lyve-1), HA receptor for endocytosis (HARE), and Toll-like receptors 4 and 2 (TLR4-2), all of them able to trigger different intracellular signaling cascades [17]. Moreover, chemical modifications of HA with TSG-6 and bikunin alter the properties of high molecular weight HA [18].
At least three different mechanisms are known to produce low molecular weight HA. High molecular weight HA fragmentation can be achieved either by chemical agents, as free radicals and oxidative stress [19], or by the action of specific degrading enzymes (i.e., hyaluronidases) that chop HA in the extracellular space and, further, continue the degradative process inside the cells [20]. The third mechanism involves the synthetic process. Normally, cells synthesize high molecular weight HA, but metabolic alterations or dysfunctions in the synthetic enzymes could influence the length of the polysaccharide.
HA synthesis is catalyzed by a family of three HA synthases (HAS1, HAS2, and HAS3) that are multipass transmembrane enzymes. HASes use cytosolic UDP-GlcUA and UDP-GlcNAc and are able to extrude the nascent polysaccharide chain through the plasma membrane into the ECM [21]. These HAS isoenzymes have different kinetic properties; in fact HAS3 produces shorter HA chains (ranging from <2 × 105 Da to 3 × 105 Da) with respect to HAS1 and HAS2 that synthesize larger polymers (up to 2 × 106 Da) [22, 23]. An extremely high molecular mass HA of about 12 MDa is produced by naked mole rats (Heterocephalus glaber), which display exceptional longevity, with a maximum lifespan exceeding 30 years [24]. This very long HA protects naked mole rat from tumors and is produced by a HAS2 enzyme with critical substitutions in the catalytic domain [24]. HAS2 is also the predominant isoform in mammals and HAS2 knockout mice die early in gestation due to heart defects, whereas HAS1 or HAS3 null mice are normal and fertile [25, 26]. Recently, in dermal fibroblasts, HAS1 was found to be activated by hyperglycemic conditions and by proinflammatory cytokines [27], suggesting a role during nutrients abundance.
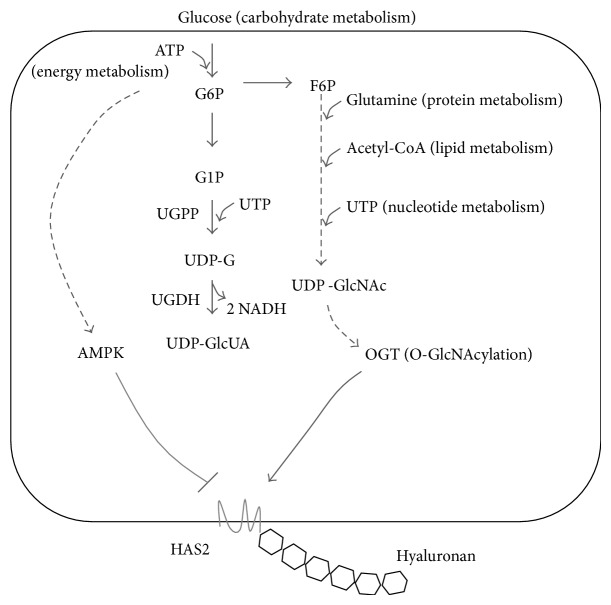
UDP-sugar precursors of HA synthesis are produced in the cytoplasm by two different pathways (Figure 1) [28]. UDP-GlcUA derives from glucose-1-phosphate which is linked to UDP forming UDP-glucose in the irreversible reaction catalyzed by UDP-glucose pyrophosphorylase. UDP-glucose is then oxidized to UDP-GlcUA by the peculiar enzyme UDP-glucose dehydrogenase that catalyzes the double oxidation of the C6 hydroxyl group in the carboxylic group forming two NADH. UDP-GlcNAc can be formed starting from glucose or by glucosamine through the hexosamine biosynthetic pathway (Figure 1).
Figure 1.
HA precursors biosynthesis and main HAS2 regulation in SMCs. Glucose enters in the cells and is phosphorylated by ATP. Glucose 6 phosphate (G6P) can be converted into glucose 1 phosphate (G1P), UDP-glucose (UDP-G), and UDP-glucuronic acid (UDP-GlcUA) by the enzymatic reactions catalyzed by UDP-G pyrophosphorylase (UGPP) and UDP-G dehydrogenase (UGDH). This latter reaction produces 2 NADH. G6P can enter in the hexosamine biosynthetic pathway, which starts from fructose 6 phosphate (F6P) and, in several steps, produces UDP-N-acetylglucosamine (UDP-GlcNAc). These steps depend on carbohydrates, energy, proteins, lipids, and nucleotides metabolisms making UDP-GlcNAc a master nutrient sensor. AMPK in condition of ATP depletion (or activation by metformin) inhibits HAS2 by threonine 110 phosphorylation. An increment of UDP-GlcNAc induces O-GlcNAcylation of serine 221 of HAS2 by OGT. This glycosylation strongly stabilizes HAS2 protein avoiding its degradation.
It is noteworthy that cytoplasmic concentration of UDP-sugars can fluctuate in function of synthetic enzymatic activities and nutrients availability (i.e., glucose) [2, 28, 29]. Therefore, HASes can work using saturating or subsaturating concentration of substrates. This can greatly influence the length of the secreted polysaccharides, as previously demonstrated using purified bacterial HAS [30, 31]. In contrast to HA, the other GAGs are synthesized inside the Golgi apparatus and the high affinity UDP-sugar transporters ensure a high concentration of precursors independently from nutrients availability [32].
3. Role of HA and ECM in Vascular Diseases
Vascular diseases are pathological conditions of arteries that are triggered by endothelial cell dysfunction. Because of factors like pathogens, oxidized LDL particles, and other inflammatory stimuli, endothelial cells become activated and start to synthesize proinflammatory molecules (i.e., cytokines and chemokines) and express adhesion molecules on their surface. This enhances the recruitment of circulating immune cells (i.e., monocytes and lymphocytes) that infiltrate in the vessel wall. Because of endothelial cytokines and immune cell infiltration, SMCs start to proliferate and migrate towards the blood vessel lumen. Moreover, SMCs secrete several ECM molecules (i.e., HA and versican) and EMC degrading enzymes (i.e., matrix metalloproteinases) leading to the thickening of the vessel wall. Atherosclerotic plaque consists of proliferating SMCs, macrophages, and various types of lymphocytes that can obstruct blood flow, leading to diminished amounts of oxygen and nutrients to the surrounding tissues. Eventually, plaque may also rupture causing the formation of clots [33, 34].
HA and the proteoglycan versican are greatly involved in vascular remodeling [11, 35]. Versican is a proteoglycan that interacts with HA forming large aggregates within the blood vessels ECM. Via several domains, versican can mediate binding to cytokines, enzymes (like ADAMTS4), lipoproteins, other extracellular matrix molecules, and signaling receptors [36, 37]. HA/versican are increased in human restenotic lesions that are formed after balloon angioplasty, in pseudoaneurysms of the human temporal artery, in advanced human atherosclerotic plaques, and in plaque thrombus interface, suggesting possible roles in the thrombotic processes [38, 39]. Other proteoglycans are known to modulate vascular ECM as the small leucine-rich repeat proteoglycan biglycan, decorin, and osteoglycin [40–42] even if these molecules do not directly interact with HA.
Vessel thickening is associated with proliferating, migrating, and dedifferentiated arterial SMCs, suggesting a role for these ECM molecules in controlling smooth muscle behavior [43]. Interestingly, also endothelial cells can synthesize HA after proinflammatory stimuli, altering adhesive capacity and recruiting of immune cells [44, 45]. The critical proatherosclerotic properties of HA are demonstrated in several manners. Transgenic HAS2 mice showed an accelerated neointima formation after injury [46] whereas the inhibition of HA synthesis (by using 4-methylumbelliferone) reduced neointima formation [47]. In vitro experiments, 4-methylumbelliferone blocked SMC proliferation, migration, and induced apoptosis [48]. Moreover, the rescuing with high molecular weight HA restored cell viability by inhibiting cell death [49]. CD44 knockout mice, lacking the main HA receptor, were protected against atherosclerosis [50].
As aging is one of the major risk factors for the insurgence of vascular pathologies [51], it is not surprising that many works report the augment of HA content in aged vessels [52–56] and that senescent human SMCs enhance HA synthesis in vitro [57].
Although the causes of atherosclerosis are still debated, the critical role of oxidized low density lipoproteins (ox-LDL) is well accepted [58]. SMCs treated with oxLDL, but not modified LDL, dramatically induced HA secretion in vitro as well as cell proliferation and migration. Interestingly, the blocking of scavenger receptor LOX-1 [59] reduced HA synthesis and inhibits cell migration [60].
These evidences indicate the role of HA in promoting atherosclerosis. A better understanding of the regulatory mechanisms of its production could be useful to limit HA synthesis in order to counteract vessel thickening.
4. HA Synthesis Regulation by Substrates
One of the major points of regulation of HA synthesis is on HASes [61]. First of all, HASes have to reach the plasma membrane and, therefore, are synthesized as part of the secretory pathway. What happens to HASes proteins during ER and Golgi trafficking is not known but it is known that they can be active in intracellular vesicles [62, 63]. This can explain the presence of intracellular HA that seems unrelated to lysosomal turnover [64]. Proinflammatory cytokines increase HASes activity in intracellular compartments leading to the formation of particular filamentous HA structures called HA cables [62]. These cables that emerge from perinuclear structures have the capability to efficiently bind immune cells contributing to inflammation [65, 66] and therefore it could be of great importance to correlate these cables with TSG6-bikunin modified HA [18].
The availability of precursors is also important for controlling HA synthesis since UDP-glucose pyrophosphorylase and dehydrogenase are known to be necessary for sustaining HA production [28]. Although these two enzymes have critical functions in glycogen biosynthesis and in detoxification, little is known about their regulation. In aged SMCs, the increased HA secretion is associated with high levels of both UDP-glucose dehydrogenase and HASes mRNAs [57]. Interestingly, the other GAGs seem not influenced by UDP-GlcUA availability. Therefore, HASes and UDP glucose dehydrogenase could be regulated in a similar manner.
The other HA precursor, UDP-GlcNAc, is the most abundant UDP-sugar within the cells and its concentration greatly depends on the nutrients availability [29]. In fact, hexosamine biosynthetic pathway integrates carbohydrates, lipids, amino acids, and nucleotides metabolisms and is considered one of the most important nutrient sensors in the cells [67]. HA synthesis is influenced by UDP-GlcNAc in at least three aspects. The first regards the substrate availability as all GAGs seem to be altered by UDP-GlcNAc [68]. UDP-GlcNAc controls UDP-N-acetylgalactosamine availability by the action of the UDP-galactose 4-epimerase enzyme [69]. In this way, UPD-GlcNAc regulates also GAGs containing N-acetylgalactosamine.
Secondly, UDP-GlcNAc concentration regulates the activity of the O-GlcNAc transferase (OGT) [29]. OGT is the critical enzyme that catalyzes the transfer of the UDP-GlcNAc to serine or threonine residues of nucleocytoplasmic proteins. This intracellular glycosylation is named O-GlcNAcylation [70]. Although OGT can be regulated posttranslationally [71], this enzyme possesses low affinity for its substrate [72]. Therefore, only when UDP-GlcNAc increases, OGT starts to modify proteins by O-GlcNAcylation. Many critical proteins are regulated by O-GlcNAcylation and HAS2 is among them [68]. O-GlcNAcylation greatly stabilizes HAS2 in the membrane, leading to an increased HA synthesis. Interestingly, as OGT is a nucleocytoplasmic protein, O-GlcNAcylation regulates only HA synthesis without affecting other GAGs synthetic enzymes in the Golgi.
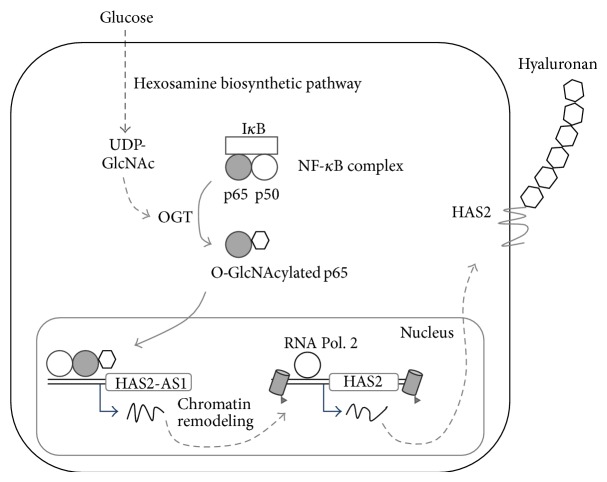
Thirdly, UDP-GlcNAc controls HAS2 expression via OGT, NF-κB, and HAS2-AS1 [73]. The latter is the natural antisense transcript (a particular type of long noncoding RNA) for HAS2 transcribed using the opposite strand of HAS2 locus on chromosome 8. HAS2 and HAS2-AS1 RNA molecules share about 200 base pairs and can form RNA:RNA duplex that stabilizes HAS2 transcript and favors HA synthesis [74]. However, RNA stabilization is not involved in the increase of HAS2 expression due to UDP-GlcNAc augment. Recent findings revealed that OGT triggers HAS2-AS1 transcription which, in turn, is necessary to enhance HAS2 transcription (Figure 2) [73]. As long noncoding RNAs modulate epigenetic modifications, such as acetylation and methylation [75], HAS2-AS1 could represent a new element able to regulate HA synthesis via epigenetic modifications. Interestingly, NF-κB subunit p65 is associated to HAS2-AS1 promoter but not to HAS2 promoter, suggesting the critical role of such noncoding RNA in the regulation of inflammatory properties of HA [73].
Figure 2.
Nuclear control of HAS2 transcription by O-GlcNAcylation. In condition of glucose abundance, OGT modifies p65 by means of O-GlcNAcylation. Glycosylated p65 induces the transcription of HAS2-AS1 that, in turn, enhances HAS2 transcription. The mechanism through which HAS2-AS1 drives HAS2 transcription is complex and partially unknown. Natural antisense RNA can bind enzymes involved in epigenetic modifications. Recent evidences highlight that HAS2-AS1 is able to open chromatin structure around HAS2 promoter enhancing RNA polymerase 2 and other factors accessibility.
5. AMPK and HA
Metabolism has a crucial role to control HA synthesis via substrate availability while a special role is played by cell energy content [28]. HA is a very high energy consuming molecule. The synthesis of an averaged size HA chain, which contains ten thousand disaccharides, represents considerable energy expenditure for the cell. To form a single chain, almost fifty thousand ATP equivalents, twenty thousand NAD cofactors, and ten thousand acetyl-CoA groups are required, in addition to the monosaccharide components and amino groups [76].
Adenosine monophosphate-activated protein kinase (AMPK) has a pivotal role in regulating energy homeostasis in eukaryotic cells [77]. In response to a decrease in cellular ATP levels, AMPK leads to a reduction in the rate of anabolic pathways (ATP-utilizing) and an increase in the rate of catabolic pathways (ATP-producing) [78]. This regulation is due to the phosphorylation of several key enzymes, including HAS2 [79].
In response to low ATP, AMPK inhibits specifically HA synthesis in vascular SMCs [79]. The phosphorylation of HAS2 threonine 110 blocks the HA synthetic process, whereas HAS1 and HAS3 are not AMPK substrates [79]. AMPK activation is known to protect from neointima formation [80, 81] and one of the mechanisms in vivo could be HA synthesis inhibition.
6. HA and Diabetes
Macro- and microangiopathies are the main complications of diabetes. Because of the tight connection between metabolism and HA synthesis, it is possible that HA and diabetes are linked. In serum of diabetic patients, HA amounts and HA staining in vessels are known to be elevated [82, 83]. Similar results were found in a porcine model of diabetes [84] and in SMCs grown in high glucose medium (mimicking diabetes) [85]. Also, nephropathies are associated with diabetes. Indeed, rat mesangial cells are known to increase HA production in hyperglycemic conditions and recruit immune cells in a HA-dependent manner [86–88]. Interestingly, recent evidences found that HA is involved in inflammation of pancreatic islets, highlighting a potential role for HA in the pathogenesis of type 1 diabetes [89, 90].
HA is also involved in diabetic ulcers favoring the healing process [91]. Diabetic foot makes up 50% of all nontraumatic amputations [92]. Peripheral neuropathy and vascular disease are thought to be major factors causing chronic foot ulcerations [93]. The use of HA or of engineered HA scaffolds (mainly composed of HA benzyl esters) with cultured expanded autologous fibroblasts and keratinocytes enhance the healing process by supporting cells proliferation and migration but also providing tissue hydration [94].
From a biochemical point of view, there are several manners in which the enhanced glucose availability induces HA synthesis. Although the effects on HA synthesis of AGEs are not known, it is known that such compounds can induce fragmentation of high molecular weight HA [95], favoring a proinflammatory response via TLR4-2. Moreover, it is also known that high molecular weight HA protects against the proinflammatory effects of AGEs [96].
Protein kinase C (PKC) isoforms dependent on diacylglycerol are known to be activated in cultured microvascular cells of diabetic animals [2]. This is due to the increased levels of DAG in hyperglycemic conditions. As it is well known that PKC activators enhanced HA synthesis [97], it is clear that in diabetic conditions PKC is a plausible cause of HA accumulation [63].
In hyperglycemic conditions, the excess of glucose is known to enter in the hexosamine biosynthetic pathway, leading to an increase of UDP-GlcNAc [29]. As discussed above, this induces a strong HA synthesis activation, as well as the alteration of HAS2 expression [68, 73]. O-GlcNAcylation is also increased in hyperglycemia [98]. Moreover, several proteins (i.e., HAS2 and endothelial nitric oxide synthase) [99] and transcription factors (SP1 and YY1) are regulated by this type of posttranslational modification [100].
Diabetes insurgence depends on a variety of factors while nutrients and lifestyle have a crucial role. High-fat diet is known to be linked with type 2 diabetes [101, 102] and recently it was discovered that rodents fed a high-fat diet leaded to accumulation of HA in skeletal muscle, which contributes to insulin resistance [103].
Nutrients can alter gene expression through epigenetics [104]. Epigenetics plays a critical role in both type 1 and type 2 diabetes [105] and can be involved in the so-called “metabolic memory” [106, 107]. Metabolic memory theory foresees that early hyperglycemic environment is remembered in the target organs (i.e., eye, kidney, heart, blood vessels, and extremities) via epigenetic modifications and that such modifications could persist for years also during positive antidiabetic therapies. As the incidence of diabetic complications is not directly linked to the blood glucose concentration [108], metabolic memory could have a critical role in this issue. As HA and other ECM components synthesis can be controlled by epigenetic modification [109], cell microenvironment could be critical for metabolic memory effects.
Moreover, AMPK is strictly related to diabetes [77, 110]. Although it is not so clear whether metformin, a well-known hypoglycemic drug [111, 112], directly or indirectly activates AMPK, it is known that it specifically reduces the synthesis of HA in SMCs [79] and hyperinsulinemia [113]. Although still debated, metformin could have vasoprotective and antitumoral effects [113–115], which could derive from reducing HA production.
7. Conclusions
ECM remodeling is emerging to have a pivotal role in several pathologies contributing to vascular diseases onset and progression. HA can have a multitude of effects on the vascular cells behavior. Several new mechanisms are recently discovered to regulate HA metabolism, all of them linked to glucose availability. A deeper understanding of such mechanisms will permit the identification of potential new pharmacological targets for the treatment of vascular pathologies.
Acknowledgments
This work was supported by FAR, and EU grant IRSES INFLAMA to Alberto Passi. The authors acknowledge the Ph.D. School in Biological and Medical Sciences for Ilaria Caon and Maria Luisa D'Angelo fellowships.
Abbreviations
- AGE:
Advanced glycation end-products
- ECM:
Extracellular matrix
- HA:
Hyaluronan
- SMCs:
Smooth muscle cells
- UDP-GlcNAc:
Uridine diphosphate N-acetylglucosamine
- HAS:
Hyaluronan synthase
- AMPK:
Adenosine monophosphate activated protein kinase
- OGT:
O-GlcNAc transferase
- oxLDL:
Oxidized low density lipoproteins
- RHAMM:
Receptor for hyaluronan-mediated motility
- HARE:
Hyaluronan receptor for endocytosis
- TLR:
Toll-like receptor
- PKC:
Protein kinase C.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Roger V. L., Go A. S., Lloyd-Jones D. M., et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 3.Nigro J., Osman N., Dart A. M., Little P. J. Insulin resistance and atherosclerosis. Endocrine Reviews. 2006;27(3):242–259. doi: 10.1210/er.2005-0007. [DOI] [PubMed] [Google Scholar]
- 4.Davis G. E., Senger D. R. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circulation Research. 2005;97(11):1093–1107. doi: 10.1161/01.res.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 5.Ross R. Atherosclerosis—an inflammatory disease. The New England Journal of Medicine. 1999;340(2):115–126. doi: 10.1056/nejm199901143400207. [DOI] [PubMed] [Google Scholar]
- 6.Vigetti D., Moretto P., Viola M., et al. Matrix metalloproteinase 2 and tissue inhibitors of metalloproteinases regulate human aortic smooth muscle cell migration during in vitro aging. The FASEB Journal. 2006;20(8):1118–1130. doi: 10.1096/fj.05-4504com. [DOI] [PubMed] [Google Scholar]
- 7.Gilkes D. M., Semenza G. L., Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nature Reviews Cancer. 2014;14(6):430–439. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laurent T. C., Fraser J. R. E. Hyaluronan. The FASEB Journal. 1992;6(7):2397–2404. [PubMed] [Google Scholar]
- 9.Vigetti D., Viola M., Karousou E., et al. Vascular pathology and the role of hyaluronan. TheScientificWorldJournal. 2008;8:1116–1118. doi: 10.1100/tsw.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karousou E. G., Viola M., Genasetti A., et al. Application of polyacrylamide gel electrophoresis of fluorophore-labeled saccharides for analysis of hyaluronan and chondroitin sulfate in human and animal tissues and cell cultures. Biomedical Chromatography. 2005;19(10):761–765. doi: 10.1002/bmc.511. [DOI] [PubMed] [Google Scholar]
- 11.Wight T. N. Arterial remodeling in vascular disease: a key role for hyaluronan and versican. Frontiers in Bioscience. 2008;13(13):4933–4937. doi: 10.2741/3052. [DOI] [PubMed] [Google Scholar]
- 12.Jiang D., Liang J., Noble P. W. Hyaluronan in tissue injury and repair. Annual Review of Cell and Developmental Biology. 2007;23:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 13.Jiang D., Liang J., Noble P. W. Hyaluronan as an immune regulator in human diseases. Physiological Reviews. 2011;91(1):221–264. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stern R., Jedrzejas M. J. Hyaluronidases: their genomics, structures, and mechanisms of action. Chemical Reviews. 2006;106(3):818–839. doi: 10.1021/cr050247k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Csoka A. B., Stern R. Hypotheses on the evolution of hyaluronan: a highly ironic acid. Glycobiology. 2013;23(4):398–411. doi: 10.1093/glycob/cws218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stern R., Asari A. A., Sugahara K. N. Hyaluronan fragments: an information-rich system. European Journal of Cell Biology. 2006;85(8):699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Vigetti D., Karousou E., Viola M., Deleonibus S., de Luca G., Passi A. Hyaluronan: biosynthesis and signaling. Biochimica et Biophysica Acta—General Subjects. 2014;1840(8):2452–2459. doi: 10.1016/j.bbagen.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Day A. J., de la Motte C. A. Hyaluronan cross-linking: a protective mechanism in inflammation? Trends in Immunology. 2005;26(12):637–643. doi: 10.1016/j.it.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Gao F., Koenitzer J. R., Tobolewski J. M., et al. Extracellular superoxide dismutase inhibits inflammation by preventing oxidative fragmentation of hyaluronan. The Journal of Biological Chemistry. 2008;283(10):6058–6066. doi: 10.1074/jbc.m709273200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vigetti D., Passi A. Hyaluronan synthases posttranslational regulation in cancer. Advances in Cancer Research. 2014;123:95–119. doi: 10.1016/B978-0-12-800092-2.00004-6. [DOI] [PubMed] [Google Scholar]
- 21.Weigel P. H., DeAngelis P. L. Hyaluronan synthases: a decade-plus of novel glycosyltransferases. Journal of Biological Chemistry. 2007;282(51):36777–36781. doi: 10.1074/jbc.r700036200. [DOI] [PubMed] [Google Scholar]
- 22.Itano N., Sawai T., Yoshida M., et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. Journal of Biological Chemistry. 1999;274(35):25085–25092. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- 23.Rilla K., Oikari S., Jokela T. A., et al. Hyaluronan synthase 1 (HAS1) requires higher cellular udp-glcnac concentration than HAS2 and HAS3. The Journal of Biological Chemistry. 2013;288(8):5973–5983. doi: 10.1074/jbc.m112.443879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian X., Azpurua J., Hine C., et al. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature. 2013;499(7458):346–349. doi: 10.1038/nature12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tien J. Y. L., Spicer A. P. Three vertebrate hyaluronan synthases are expressed during mouse development in distinct spatial and temporal patterns. Developmental Dynamics. 2005;233(1):130–141. doi: 10.1002/dvdy.20328. [DOI] [PubMed] [Google Scholar]
- 26.Nardini M., Ori M., Vigetti D., Gornati R., Nardi I., Perris R. Regulated gene expression of hyaluronan synthases during Xenopus laevis development. Gene Expression Patterns. 2004;4(3):303–308. doi: 10.1016/j.modgep.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Siiskonen H., Kärnä R., Hyttinen J. M., Tammi R. H., Tammi M. I., Rilla K. Hyaluronan synthase 1 (HAS1) produces a cytokine-and glucose-inducible, CD44-dependent cell surface coat. Experimental Cell Research. 2014;320(1):153–163. doi: 10.1016/j.yexcr.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 28.Vigetti D., Viola M., Karousou E., de Luca G., Passi A. Metabolic control of hyaluronan synthases. Matrix Biology. 2014;35:8–13. doi: 10.1016/j.matbio.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Buse M. G. Hexosamines, insulin resistance, and the complications of diabetes: current status. The American Journal of Physiology: Endocrinology and Metabolism. 2006;290(1):E1–E8. doi: 10.1152/ajpendo.00329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeAngelis P. L. Monodisperse hyaluronan polymers: synthesis and potential applications. Current Pharmaceutical Biotechnology. 2008;9(4):246–248. doi: 10.2174/138920108785161550. [DOI] [PubMed] [Google Scholar]
- 31.Jing W., DeAngelis P. L. Synchronized chemoenzymatic synthesis of monodisperse hyaluronan polymers. The Journal of Biological Chemistry. 2004;279(40):42345–42349. doi: 10.1074/jbc.m402744200. [DOI] [PubMed] [Google Scholar]
- 32.Vigetti D., Ori M., Viola M., et al. Molecular cloning and characterization of UDP-glucose dehydrogenase from the amphibian Xenopus laevis and its involvement in hyaluronan synthesis. The Journal of Biological Chemistry. 2006;281(12):8254–8263. doi: 10.1074/jbc.m508516200. [DOI] [PubMed] [Google Scholar]
- 33.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 34.Libby P., Ridker P. M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 35.Viola M., Karousou E., D'Angelo M. L., et al. Regulated hyaluronan synthesis by vascular cells. doi: 10.1155/2015/208303. International Journal of Cell Biology. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kenagy R. D., Min S.-K., Clowes A. W., Sandy J. D. Cell death-associated ADAMTS4 and versican degradation in vascular tissue. Journal of Histochemistry and Cytochemistry. 2009;57(9):889–897. doi: 10.1369/jhc.2009.953901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kenagy R. D., Plaas A. H., Wight T. N. Versican degradation and vascular disease. Trends in Cardiovascular Medicine. 2006;16(6):209–215. doi: 10.1016/j.tcm.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kolodgie F. D., Burke A. P., Farb A., et al. Differential accumulation of proteoglycans and hyaluronan in culprit lesions: insights into plaque erosion. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22(10):1642–1648. doi: 10.1161/01.atv.0000034021.92658.4c. [DOI] [PubMed] [Google Scholar]
- 39.Kolodgie F. D., Burke A. P., Wight T. N., Virmani R. The accumulation of specific types of proteoglycans in eroded plaques: a role in coronary thrombosis in the absence of rupture. Current Opinion in Lipidology. 2004;15(5):575–582. doi: 10.1097/00041433-200410000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Moncayo-Arlandi J., López-García Al., Fernández M. C., Durán A. C., Fernández B. Osteoglycin deficiency does not affect atherosclerosis in mice. Atherosclerosis. 2014;237(2):418–425. doi: 10.1016/j.atherosclerosis.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 41.Heegaard A.-M., Corsi A., Danielsen C. C., et al. Biglycan deficiency causes spontaneous aortic dissection and rupture in mice. Circulation. 2007;115(21):2731–2738. doi: 10.1161/CIRCULATIONAHA.106.653980. [DOI] [PubMed] [Google Scholar]
- 42.Zen A. A. H., Caligiuri G., Sainz J., Lemitre M., Demerens C., Lafont A. Decorin overexpression reduces atherosclerosis development in apolipoprotein E-deficient mice. Atherosclerosis. 2006;187(1):31–39. doi: 10.1016/j.atherosclerosis.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 43.Riessen R., Wight T. N., Pastore C., Henley C., Isner J. M. Distribution of hyaluronan during extracellular matrix remodeling in human restenotic arteries and balloon-injured rat carotid arteries. Circulation. 1996;93(6):1141–1147. doi: 10.1161/01.CIR.93.6.1141. [DOI] [PubMed] [Google Scholar]
- 44.Vigetti D., Genasetti A., Karousou E., et al. Proinflammatory cytokines induce hyaluronan synthesis and monocyte adhesion in human endothelial cells through hyaluronan synthase 2 (HAS2) and the nuclear factor-kappaB (NF-kappaB) pathway. The Journal of Biological Chemistry. 2010;285(32):24639–24645. doi: 10.1074/jbc.m110.134536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Genasetti A., Vigetti D., Viola M., et al. Hyaluronan and human endothelial cell behavior. Connective Tissue Research. 2008;49(3-4):120–123. doi: 10.1080/03008200802148462. [DOI] [PubMed] [Google Scholar]
- 46.Chai S., Chai Q., Danielsen C. C., et al. Overexpression of hyaluronan in the tunica media promotes the development of atherosclerosis. Circulation Research. 2005;96(5):583–591. doi: 10.1161/01.res.0000158963.37132.8b. [DOI] [PubMed] [Google Scholar]
- 47.Kashima Y., Takahashi M., Shiba Y., et al. Crucial role of hyaluronan in neointimal formation after vascular injury. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0058760.e58760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vigetti D., Rizzi M., Viola M., et al. The effects of 4-methylumbelliferone on hyaluronan synthesis, MMP2 activity, proliferation, and motility of human aortic smooth muscle cells. Glycobiology. 2009;19(5):537–546. doi: 10.1093/glycob/cwp022. [DOI] [PubMed] [Google Scholar]
- 49.Vigetti D., Rizzi M., Moretto P., et al. Glycosaminoglycans and glucose prevent apoptosis in 4-methylumbelliferone-treated human aortic smooth muscle cells. Journal of Biological Chemistry. 2011;286(40):34497–34503. doi: 10.1074/jbc.m111.266312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cuff C. A., Kothapalli D., Azonobi I., et al. The adhesion receptor CD44 promotes atherosclerosis by mediating inflammatory cell recruitment and vascular cell activation. The Journal of Clinical Investigation. 2001;108(7):1031–1040. doi: 10.1172/jci200112455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spagnoli L. G., Orlandi A., Mauriello A., et al. Aging and atherosclerosis in the rabbit. 1. Distribution, prevalence and morphology of atherosclerotic lesions. Atherosclerosis. 1991;89(1):11–24. doi: 10.1016/0021-9150(91)90003-l. [DOI] [PubMed] [Google Scholar]
- 52.Stuhlsatz H. W., Loffler H., Mohanaradhakrishnan V., Cosma S., Greiling H. Topographic and age-dependent distribution of the glycosaminoglycans in human aorta. Journal of Clinical Chemistry and Clinical Biochemistry. 1982;20(10):713–721. doi: 10.1515/cclm.1982.20.10.713. [DOI] [PubMed] [Google Scholar]
- 53.Murata K., Yokoyama Y. Acidic glycosaminoglycans in human atherosclerotic cerebral arterial tissues. Atherosclerosis. 1989;78(1):69–79. doi: 10.1016/0021-9150(89)90160-3. [DOI] [PubMed] [Google Scholar]
- 54.Tovar A. M. F., Cesar D. C. F., Leta G. C., Mourao P. A. S. Age-related changes in populations of aortic glycosaminoglycans: species with low affinity for plasma low-density lipoproteins, and not species with high affinity, are preferentially affected. Arteriosclerosis, Thrombosis, and Vascular Biology. 1998;18(4):604–614. doi: 10.1161/01.atv.18.4.604. [DOI] [PubMed] [Google Scholar]
- 55.Chajara A., Delpech B., Courel M.-N., Basuyau J.-P., Lévesque H. Effect of aging on neointima formation and hyaluronan, hyaluronidase and hyaluronectin production in injured rat aorta. Atherosclerosis. 1998;138(1):53–64. doi: 10.1016/S0021-9150(98)00004-5. [DOI] [PubMed] [Google Scholar]
- 56.Varga R., Eriksson M., Erdos M. R., et al. Progressive vascular smooth muscle cell defects in a mouse model of Hutchinson-Gilford progeria syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(9):3250–3255. doi: 10.1073/pnas.0600012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vigetti D., Viola M., Karousou E., et al. Hyaluronan-CD44-ERK1/2 regulate human aortic smooth muscle cell motility during aging. The Journal of Biological Chemistry. 2008;283(7):4448–4458. doi: 10.1074/jbc.m709051200. [DOI] [PubMed] [Google Scholar]
- 58.Kita T., Kume N., Minami M., et al. Role of oxidized LDL in atherosclerosis. Annals of the New York Academy of Sciences. 2001;947:199–206. doi: 10.1111/j.1749-6632.2001.tb03941.x. [DOI] [PubMed] [Google Scholar]
- 59.Kita T., Kume N., Yokode M., et al. Oxidized-LDL and atherosclerosis. Role of LOX-1. Annals of the New York Academy of Sciences. 2000;902:95–102. doi: 10.1111/j.1749-6632.2000.tb06304.x. [DOI] [PubMed] [Google Scholar]
- 60.Viola M., Bartolini B., Vigetti D., et al. Oxidized low density lipoprotein (LDL) affects hyaluronan synthesis in human aortic smooth muscle cells. Journal of Biological Chemistry. 2013;288(41):29595–29603. doi: 10.1074/jbc.M113.508341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tammi R. H., Passi A. G., Rilla K., et al. Transcriptional and post-translational regulation of hyaluronan synthesis. FEBS Journal. 2011;278(9):1419–1428. doi: 10.1111/j.1742-4658.2011.08070.x. [DOI] [PubMed] [Google Scholar]
- 62.Vigetti D., Genasetti A., Karousou E., et al. Modulation of hyaluronan synthase activity in cellular membrane fractions. The Journal of Biological Chemistry. 2009;284(44):30684–30694. doi: 10.1074/jbc.m109.040386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hascall V. C., Wang A., Tammi M., et al. The dynamic metabolism of hyaluronan regulates the cytosolic concentration of UDP-GlcNAc. Matrix Biology. 2014;35:14–17. doi: 10.1016/j.matbio.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Evanko S. P., Wight T. N. Intracellular localization of hyaluronan in proliferating cells. Journal of Histochemistry and Cytochemistry. 1999;47(10):1331–1341. doi: 10.1177/002215549904701013. [DOI] [PubMed] [Google Scholar]
- 65.Petrey A. C., de la Motte C. A. Hyaluronan, a crucial regulator of inflammation. Frontiers in Immunology. 2014;5, article 101 doi: 10.3389/fimmu.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hascall V. C., Majors A. K., de la Motte C. A., et al. Intracellular hyaluronan: a new frontier for inflammation? Biochimica et Biophysica Acta–General Subjects. 2004;1673(1-2):3–12. doi: 10.1016/j.bbagen.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 67.Hanover J. A., Krause M. W., Love D. C. Bittersweet memories: linking metabolism to epigenetics through O-GlcNAcylation. Nature Reviews Molecular Cell Biology. 2012;13(5):312–321. doi: 10.1038/nrm3334. [DOI] [PubMed] [Google Scholar]
- 68.Vigetti D., Deleonibus S., Moretto P., et al. Role of UDP-N-acetylglucosamine (GlcNAc) and O-GlcNacylation of hyaluronan synthase 2 in the control of chondroitin sulfate and hyaluronan synthesis. The Journal of Biological Chemistry. 2012;287(42):35544–35555. doi: 10.1074/jbc.m112.402347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thoden J. B., Wohlers T. M., Fridovich-Keil J. L., Holden H. M. Human UDP-galactose 4-epimerase. Accommodation of UDP-N-acetylglucosamine within the active site. The Journal of Biological Chemistry. 2001;276(18):15131–15136. doi: 10.1074/jbc.m100220200. [DOI] [PubMed] [Google Scholar]
- 70.Hart G. W., Housley M. P., Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446(7139):1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 71.Xu Q., Yang C., Du Y., et al. AMPK regulates histone H2B O-GlcNAcylation. Nucleic Acids Research. 2014;42(9):5594–5604. doi: 10.1093/nar/gku236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kreppel L. K., Hart G. W. Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. Journal of Biological Chemistry. 1999;274(45):32015–32022. doi: 10.1074/jbc.274.45.32015. [DOI] [PubMed] [Google Scholar]
- 73.Vigetti D., Deleonibus S., Moretto P., et al. Natural antisense transcript for hyaluronan synthase 2 (HAS2-AS1) induces transcription of HAS2 via protein O-GlcN acylation. The Journal of Biological Chemistry. 2014;289(42):28816–28826. doi: 10.1074/jbc.m114.597401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Michael D. R., Phillips A. O., Krupa A., et al. The human hyaluronan synthase 2 (HAS2) gene and its natural antisense RNA exhibit coordinated expression in the renal proximal tubular epithelial cell. The Journal of Biological Chemistry. 2011;286(22):19523–19532. doi: 10.1074/jbc.m111.233916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Magistri M., Faghihi M. A., St Laurent G., Wahlestedt C. Regulation of chromatin structure by long noncoding RNAs: focus on natural antisense transcripts. Trends in Genetics. 2012;28(8):389–396. doi: 10.1016/j.tig.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee J. Y., Spicer A. P. Hyaluronan: a multifunctional, megaDalton, stealth molecule. Current Opinion in Cell Biology. 2000;12(5):581–586. doi: 10.1016/s0955-0674(00)00135-6. [DOI] [PubMed] [Google Scholar]
- 77.Hardie D. G. AMPK: a target for drugs and natural products with effects on both diabetes and cancer. Diabetes. 2013;62(7):2164–2172. doi: 10.2337/db13-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hardie D. G., Ross F. A., Hawley S. A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature Reviews Molecular Cell Biology. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vigetti D., Clerici M., Deleonibus S., et al. Hyaluronan synthesis is inhibited by adenosine monophosphate-activated protein kinase through the regulation of HAS2 activity in human aortic smooth muscle cells. Journal of Biological Chemistry. 2011;286(10):7917–7924. doi: 10.1074/jbc.m110.193656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stone J. D., Narine A., Shaver P. R., Fox J. C., Vuncannon J. R., Tulis D. A. AMP-activated protein kinase inhibits vascular smooth muscle cell proliferation and migration and vascular remodeling following injury. The American Journal of Physiology: Heart and Circulatory Physiology. 2013;304(3):H369–H381. doi: 10.1152/ajpheart.00446.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nagata D., Takeda R., Sata M., et al. AMP-activated protein kinase inhibits angiotensin II-stimulated vascular smooth muscle cell proliferation. Circulation. 2004;110(4):444–451. doi: 10.1161/01.CIR.0000136025.96811.76. [DOI] [PubMed] [Google Scholar]
- 82.Heickendorff L., Ledet T., Rasmussen L. M. Glycosaminoglycans in the human aorta in diabetes mellitus: a study of tunica media from areas with and without atherosclerotic plaque. Diabetologia. 1994;37(3):286–292. doi: 10.1007/bf00398056. [DOI] [PubMed] [Google Scholar]
- 83.Morita M., Yano S., Ishibashi Y., Nakata N., Kurioka S., Sugimoto T. Close relationship between serum hyaluronan levels and vascular function in patients with type 2 diabetes. Biomarkers. 2014;19(6):493–497. doi: 10.3109/1354750x.2014.940502. [DOI] [PubMed] [Google Scholar]
- 84.McDonald T. O., Gerrity R. G., Jen C., et al. Diabetes and arterial extracellular matrix changes in a porcine model of atherosclerosis. Journal of Histochemistry and Cytochemistry. 2007;55(11):1149–1157. doi: 10.1369/jhc.7A7221.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sainio A., Jokela T., Tammi M. I., Järveläinen H. Hyperglycemic conditions modulate connective tissue reorganization by human vascular smooth muscle cells through stimulation of hyaluronan synthesis. Glycobiology. 2010;20(9):1117–1126. doi: 10.1093/glycob/cwq076. [DOI] [PubMed] [Google Scholar]
- 86.Wang A., Hascall V. C. Hyaluronan structures synthesized by rat mesangial cells in response to hyperglycemia induce monocyte adhesion. The Journal of Biological Chemistry. 2004;279(11):10279–10285. doi: 10.1074/jbc.m312045200. [DOI] [PubMed] [Google Scholar]
- 87.Lewis A., Steadman R., Manley P., et al. Diabetic nephropathy, inflammation, hyaluronan and interstitial fibrosis. Histology and Histopathology. 2008;23(6):731–739. doi: 10.14670/HH-23.731. [DOI] [PubMed] [Google Scholar]
- 88.Wang A., de la Motte C., Lauer M., Hascall V. Hyaluronan matrices in pathobiological processes. FEBS Journal. 2011;278(9):1412–1418. doi: 10.1111/j.1742-4658.2011.08069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bogdani M., Johnson P. Y., Potter-Perigo S., et al. Hyaluronan and hyaluronan-binding proteins accumulate in both human type 1 diabetic islets and lymphoid tissues and associate with inflammatory cells in insulitis. Diabetes. 2014;63(8):2727–2743. doi: 10.2337/db13-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bogdani M., Korpos E., Simeonovic C. J., Parish C. R., Sorokin L., Wight T. N. Extracellular matrix components in the pathogenesis of type 1 diabetes. Current Diabetes Reports. 2014;14(12, article 552) doi: 10.1007/s11892-014-0552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen C. P., Hung W., Lin S. H. Effectiveness of hyaluronic acid for treating diabetic foot: a systematic review and meta-analysis. Dermatologic Therapy. 2014;27(6):331–336. doi: 10.1111/dth.12153. [DOI] [PubMed] [Google Scholar]
- 92.Edmonds M. E., Blundell M. P., Morris M. E., Thomas E. M., Cotton L. T., Watkins P. J. Improved survival of the diabetic foot: the role of a specialised foot clinic. The Quarterly Journal of Medicine. 1986;60(232):763–771. [PubMed] [Google Scholar]
- 93.Rathur H. M., Boulton A. J. M. Pathogenesis of foot ulcers and the need for offloading. Hormone and Metabolic Research. 2005;37(supplement 1):S61–S68. doi: 10.1055/s-2005-861398. [DOI] [PubMed] [Google Scholar]
- 94.Andrews K. L., Houdek M. T., Kiemele L. J. Wound management of chronic diabetic foot ulcers: from the basics to regenerative medicine. Prosthetics and Orthotics International. 2015;39(1):29–39. doi: 10.1177/0309364614534296. [DOI] [PubMed] [Google Scholar]
- 95.Katsumura C., Sugiyama T., Nakamuraa K., et al. Effects of advanced glycation end products on hyaluronan photolysis: a new mechanism of diabetic vitreopathy. Ophthalmic Research. 2004;36(6):327–331. doi: 10.1159/000081635. [DOI] [PubMed] [Google Scholar]
- 96.Neumann A., Schinzel R., Palm D., Riederer P., Münch G. High molecular weight hyaluronic acid inhibits advanced glycation endproduct-induced NF-kappaB activation and cytokine expression. FEBS Letters. 1999;453(3):283–287. doi: 10.1016/s0014-5793(99)00731-0. [DOI] [PubMed] [Google Scholar]
- 97.Wang H.-S., Tung W.-H., Tang K.-T., et al. TGF-β induced hyaluronan synthesis in orbital fibroblasts involves protein kinase C βII activation in vitro. Journal of Cellular Biochemistry. 2005;95(2):256–267. doi: 10.1002/jcb.20405. [DOI] [PubMed] [Google Scholar]
- 98.Slawson C., Copeland R. J., Hart G. W. O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends in Biochemical Sciences. 2010;35(10):547–555. doi: 10.1016/j.tibs.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Copeland R. J., Bullen J. W., Hart G. W. Cross-talk between GlcNAcylation and phosphorylation: roles in insulin resistance and glucose toxicity. American Journal of Physiology—Endocrinology and Metabolism. 2008;295(1):E17–E28. doi: 10.1152/ajpendo.90281.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Özcan S., Andrali S. S., Cantrell J. E. L. Modulation of transcription factor function by O-GlcNAc modification. Biochimica et Biophysica Acta. 2010;1799(5-6):353–364. doi: 10.1016/j.bbagrm.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gæde P. H., Jepsen P. V., Larsen J. N. B., Jensen G. V., Parving H.-H., Pedersen O. B. The Steno type 2 study: intensive multifactorial treatment reduces the incidence of cardiovascular disease in patients with type 2 diabetes. Ugeskrift for Laeger. 2003;165(26):2658–2661. [PubMed] [Google Scholar]
- 102.Marshall J. A., Bessesen D. H. Dietary fat and the development of type 2 diabetes. Diabetes Care. 2002;25(3):620–622. doi: 10.2337/diacare.25.3.620. [DOI] [PubMed] [Google Scholar]
- 103.Kang L., Lantier L., Kennedy A., et al. Hyaluronan accumulates with high-fat feeding and contributes to insulin resistance. Diabetes. 2013;62(6):1888–1896. doi: 10.2337/db12-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Choi S. W., Friso S. Epigenetics: a new bridge between nutrition and health. Advances in Nutrition. 2010;1(1):8–16. doi: 10.3945/an.110.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reddy M. A., Natarajan R. Epigenetic mechanisms in diabetic vascular complications. Cardiovascular Research. 2011;90(3):421–429. doi: 10.1093/cvr/cvr024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nathan D. M., Cleary P. A., Backlund J.-Y. C., et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. The New England Journal of Medicine. 2005;353(25):2643–2653. doi: 10.1056/nejmoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ihnat M. A., Thorpe J. E., Ceriello A. Hypothesis: the ‘metabolic memory‘, the new challenge of diabetes. Diabetic Medicine. 2007;24(6):582–586. doi: 10.1111/j.1464-5491.2007.02138.x. [DOI] [PubMed] [Google Scholar]
- 108.Ceriello A., Ihnat M. A., Thorpe J. E. The‘;Metabolic memory’: is more than just tight glucose control necessary to prevent diabetic complications? Journal of Clinical Endocrinology and Metabolism. 2009;94(2):410–415. doi: 10.1210/jc.2008-1824. [DOI] [PubMed] [Google Scholar]
- 109.Vigetti D., Viola M., Karousou E., et al. Epigenetics in extracellular matrix remodeling and hyaluronan metabolism. FEBS Journal. 2014;281(22):4980–4992. doi: 10.1111/febs.12938. [DOI] [PubMed] [Google Scholar]
- 110.Viollet B., Lantier L., Devin-Leclerc J., et al. Targeting the AMPK pathway for the treatment of Type 2 diabetes. Frontiers in Bioscience (Landmark Ed) 2009;14(9):3380–3400. doi: 10.2735/3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Patel A., MacMahon S., Chalmers J., et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. The New England Journal of Medicine. 2008;358(24):2560–2572. doi: 10.1056/nejmoa0802987. [DOI] [PubMed] [Google Scholar]
- 112.Turner R. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. The Lancet. 1998;352(9131):854–865. [PubMed] [Google Scholar]
- 113.Johnson J. A., Simpson S. H., Toth E. L., Majumdar S. R. Reduced cardiovascular morbidity and mortality associated with metformin use in subjects with type 2 diabetes. Diabetic Medicine. 2005;22(4):497–502. doi: 10.1111/j.1464-5491.2005.01448.x. [DOI] [PubMed] [Google Scholar]
- 114.Micic D., Cvijovic G., Trajkovic V., Duntas L. H., Polovina S. Metformin: its emerging role in oncology. Hormones. 2011;10(1):5–15. doi: 10.14310/horm.2002.1288. [DOI] [PubMed] [Google Scholar]
- 115.Bailey C. J. Metformin: effects on micro and macrovascular complications in type 2 diabetes. Cardiovascular Drugs and Therapy. 2008;22(3):215–224. doi: 10.1007/s10557-008-6092-0. [DOI] [PubMed] [Google Scholar]