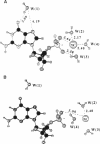
Abstract
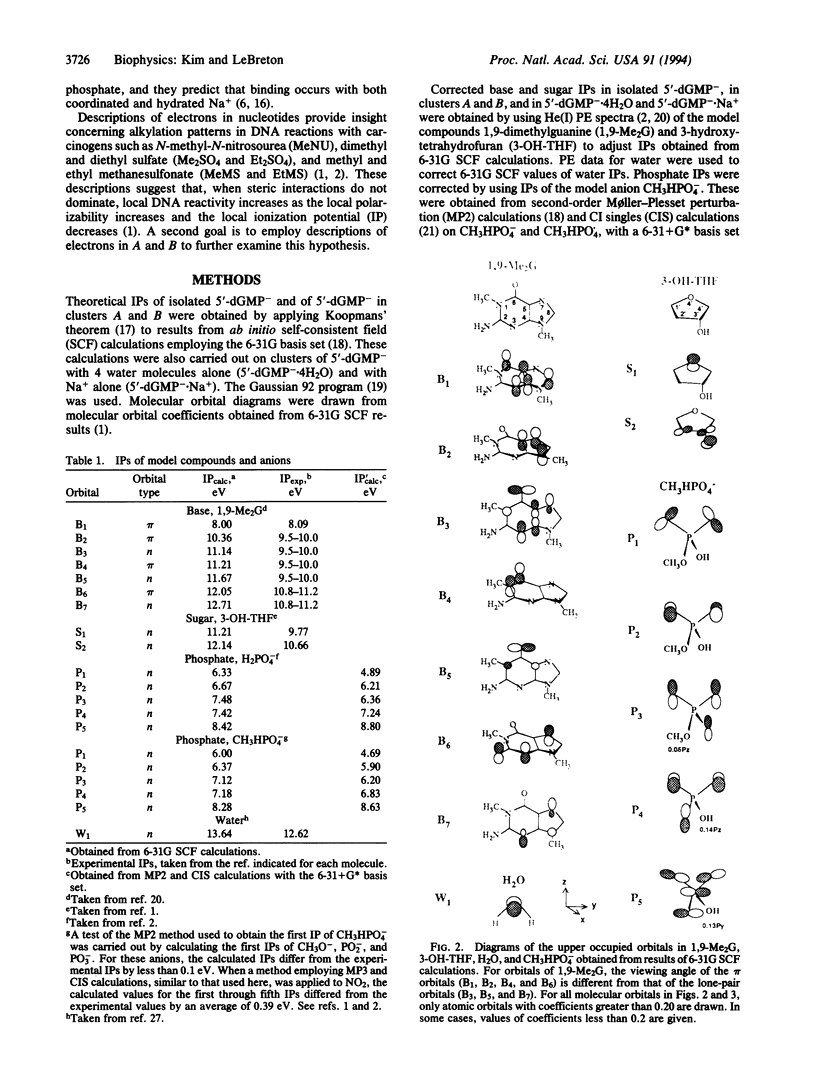
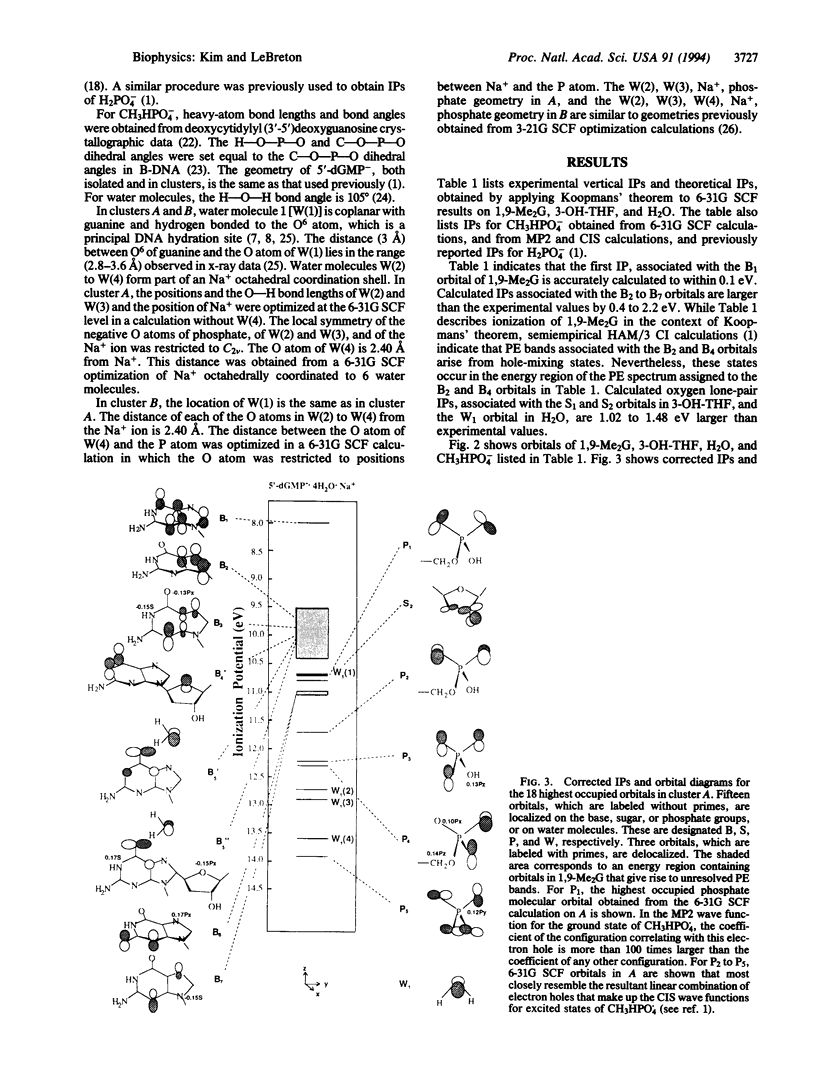
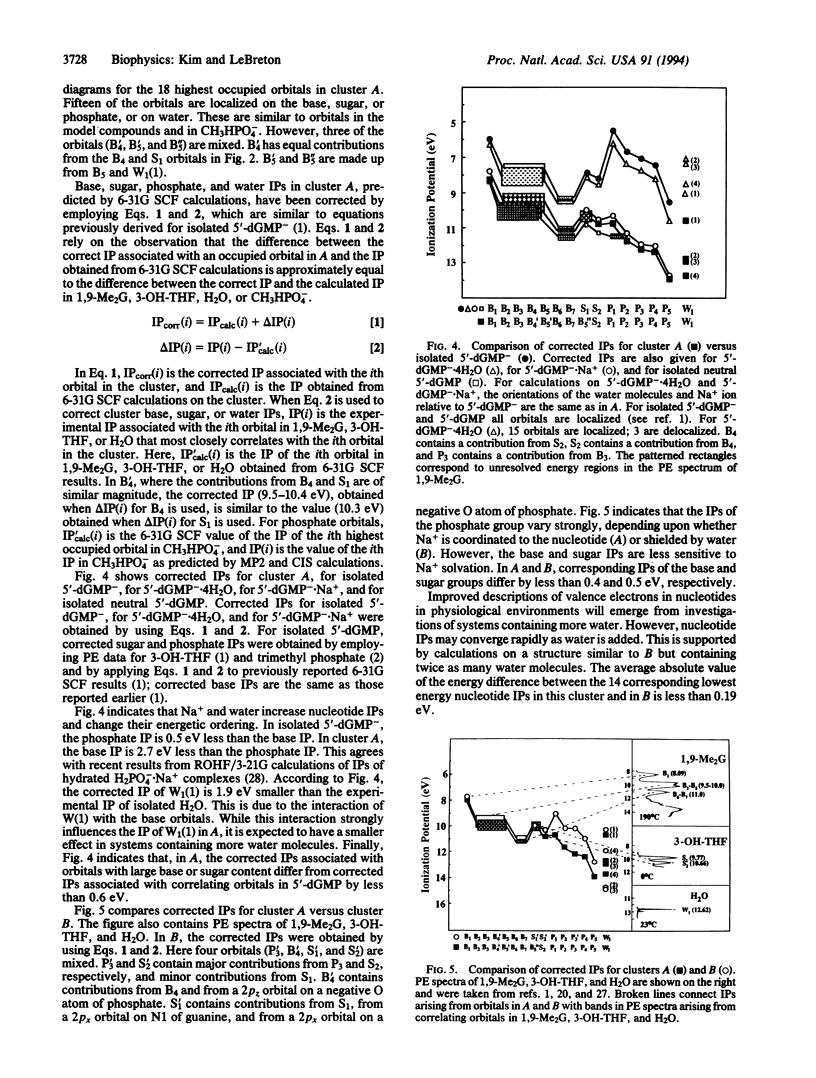
UV photoelectron data for 1,9-dimethylguanine, 3-hydroxytetrahydrofuran, and water, and results from ab initio self-consistent field (SCF) and post-SCF molecular orbital calculations were employed to describe valence electrons in clusters of 2'-deoxyguanosine 5'-phosphate (5'-dGMP-) with four water molecules and a phosphate-bound sodium ion. Two clusters (A and B) were examined. In A, Na+ is coordinated to 5'-dGMP-. In B, Na+ and 5'-dGMP- are separated by water. In 5'-dGMP- clusters, the nucleotide valence ionization potentials (IPs) are different from those previously reported for isolated 5'-dGMP-. The smallest IP in isolated 5'-dGMP- (4.6 eV) arises from the phosphate group; the smallest IPs in A (8.2 eV) and B (7.9 eV) arise from the base. In the clusters, the IPs associated with the seven upper occupied base orbitals differ from corresponding IPs in 1,9-dimethylguanine by less than 0.4 eV. In A and B, the smaller IP of the base, compared with the phosphate group, is consistent with reactivity data indicating that DNA and RNA are subject to electrophilic SN2 attack by carcinogens such as N-methyl-N-nitrosourea, dimethyl and diethyl sulfate, and methyl and ethyl methanesulfonate, in which more than 75% of nucleotide alkylation occurs at the bases.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Hukins D. W. Optimised parameters for A-DNA and B-DNA. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1504–1509. doi: 10.1016/0006-291X(72)90243-4. [DOI] [PubMed] [Google Scholar]
- Clementi E., Corongiu G. Simulations of the solvent structure for macromolecules. III. Determination of the Na+ counter ion structure. Biopolymers. 1982 Apr;21(4):763–777. doi: 10.1002/bip.360210404. [DOI] [PubMed] [Google Scholar]
- Cruse W. B., Egert E., Kennard O., Sala G. B., Salisbury S. A., Viswamitra M. A. Self base pairing in a complementary deoxydinucleoside monophosphate duplex: crystal and molecular structure of deoxycytidylyl-(3'-5')-deoxyguanosine. Biochemistry. 1983 Apr 12;22(8):1833–1839. doi: 10.1021/bi00277a014. [DOI] [PubMed] [Google Scholar]
- Ford G. P., Scribner J. D. Prediction of nucleoside-carcinogen reactivity. Alkylation of adenine, cytosine, guanine, and thymine and their deoxynucleosides by alkanediazonium ions. Chem Res Toxicol. 1990 May-Jun;3(3):219–230. doi: 10.1021/tx00015a006. [DOI] [PubMed] [Google Scholar]
- Jayaram B., Sharp K. A., Honig B. The electrostatic potential of B-DNA. Biopolymers. 1989 May;28(5):975–993. doi: 10.1002/bip.360280506. [DOI] [PubMed] [Google Scholar]
- Klein B. J., Pack G. R. Calculations of the spatial distribution of charge density in the environment of DNA. Biopolymers. 1983 Nov;22(11):2331–2352. doi: 10.1002/bip.360221103. [DOI] [PubMed] [Google Scholar]
- Kopka M. L., Fratini A. V., Drew H. R., Dickerson R. E. Ordered water structure around a B-DNA dodecamer. A quantitative study. J Mol Biol. 1983 Jan 5;163(1):129–146. doi: 10.1016/0022-2836(83)90033-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg J. M., Seeman N. C., Day R. O., Rich A. RNA double-helical fragments at atomic resolution. II. The crystal structure of sodium guanylyl-3',5'-cytidine nonahydrate. J Mol Biol. 1976 Jun 14;104(1):145–167. doi: 10.1016/0022-2836(76)90006-1. [DOI] [PubMed] [Google Scholar]
- Schneider B., Cohen D., Berman H. M. Hydration of DNA bases: analysis of crystallographic data. Biopolymers. 1992 Jul;32(7):725–750. doi: 10.1002/bip.360320703. [DOI] [PubMed] [Google Scholar]
- Seibel G. L., Singh U. C., Kollman P. A. A molecular dynamics simulation of double-helical B-DNA including counterions and water. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6537–6540. doi: 10.1073/pnas.82.19.6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhof E. Water: an integral part of nucleic acid structure. Annu Rev Biophys Biophys Chem. 1988;17:125–144. doi: 10.1146/annurev.bb.17.060188.001013. [DOI] [PubMed] [Google Scholar]