Abstract
Objective
To assess the safety and health effects of vitamin D supplementation during pregnancy.
Methods and Design
Datasets from two randomized clinical trials were first analyzed separately then combined for this analysis using a common data dictionary. In the NICHD trial, women were randomized to 400, 2000, or 4000 IU vitamin D3/day, stratified by race. In the Thrasher Research Fund trial, participants were randomized to 2000 or 4000 IU vitamin D3/day. Study drugs were from the same manufacturing lot for both trials. Identical questionnaires were given for comparable sociodemographics & clinical characteristics. Outcome measures were: (1) maternal and neonatal 25(OH)D achieved, and (2) maternal comorbidities of pregnancy (COP). SAS 9.3 was used for all analyses.
Results
In the combined cohort, there were 110 controls, 201 in the 2000 IU group, and 193 in the 4000 IU group. No differences between groups in baseline 25(OH)D were found; however, delivery and cord blood values were greater in the 4000 IU group (p<0.0001), an effect that persisted even after controlling for race and study. A greater percent were vitamin D replete in the 4000 IU group (p<0.0001). There was a trend where the 4000 IU group had decreased rates of comorbidities of pregnancy. There was a strong association between COP and final maternal 25(OH)D; an effect that persisted even after controlling for race and study (p=0.006).
Conclusions
Supplementation with 4000 IU/day was associated with lower risk of hypovitaminosis D than Control and 2000 IU groups. While not statistically significant, there was a trend toward lower rates of COP as supplementation dose increased. Maternal delivery 25(OH)D was inversely associated with any comorbidity of pregnancy, with fewer events as 25(OH)D increased. Future studies are needed to confirm these findings and determine the mechanisms of action of such effects.
Keywords: vitamin D, cholecalciferol, pregnancy, health outcomes
Introduction
It is clear that vitamin D status during pregnancy varies around the globe as a function of maternal sunlight exposure, degree of skin pigmentation, latitude, lifestyle, body mass index (BMI) and the intake of oral vitamin D supplements (1-8). It is common knowledge that if a woman is deficient during her pregnancy, then her fetus also will be deficient during gestation (1, 9). Whether such variation in vitamin D status can be associated with worse pregnancy outcomes still remains an open question.
While studies conducted in the 1980's and 1990's gave some evidence that maternal deficiency was associated with abnormal fetal growth, dentition and maternal health, there were issues with these studies as they were conducted with small sample sizes and the amount of vitamin D given was often low with few differences noted between women who had received placebo and those who had received treatment—typically 400 IU vitamin D/day (10-14). Prior studies did not establish the optimal vitamin D requirements and blood levels during pregnancy. More recently, larger doses up to 10,000 IU/day in nonpregnant adults have been found to be safe (15-18) and such higher dosing necessary to achieve sufficiency in certain individuals. Yet, whether these findings are applicable to the pregnant woman has not been fully addressed.
To begin to answer the question of what constitutes vitamin D sufficiency during pregnancy, two recent randomized clinical trials conducted by our group were presented and published (1, 19). The largest was an NICHD-sponsored randomized controlled trial of vitamin D supplementation beginning at 12-16 weeks of gestation where healthy women were randomized to one of three treatment groups—400, 2000, and 4000 IU vitamin D3/day (1). In the second clinical trial sponsored by the Thrasher Research Fund, women were randomized at 12-16 weeks of gestation to either 2000 or 4000 IU vitamin D3/day (19). Vitamin D status and health characteristics were recorded for both studies. Because both studies were conducted concurrently by the same study team using a common data dictionary, the datasets were combined to increase sample size and to collectively address the following questions: (1) what are the potential health effects of vitamin D3 supplementation during pregnancy, and (2) what are the implications of vitamin D deficiency on the mother and her fetus?
Methods and Experimental Design
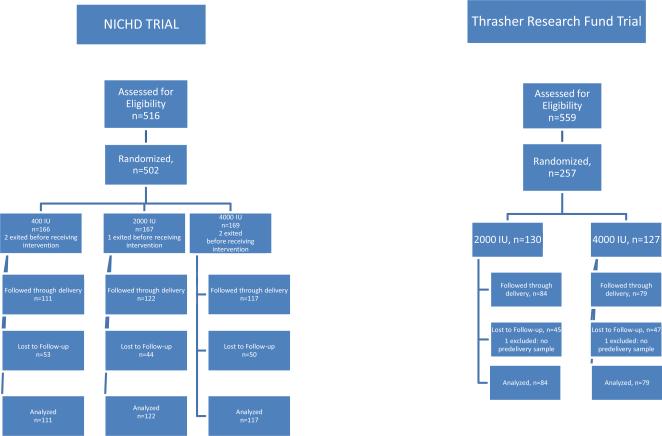
Datasets from the NICHD and Thrasher Research Fund vitamin D3 supplementation trials were combined for this analysis using a common data dictionary. In the NICHD trial, (as shown in Figure 1A), women were randomized to a total of 400, 2000 or 4000 IU vitamin D3/day, stratified by race with supplementation beginning between 12-16 weeks of gestation (1). In the Thrasher Research Fund (TRF) trial (see Figure 1B), after an initial run-in dose of 2000 IU/day for one-month, participants were randomized to total of either 2000 or 4000 vitamin D3/day, stratified by race (19). Details about both clinical trials have been published previously (1, 19) and are summarized below. All participants received study drugs from the same manufacturing lot. The studies administered identical questionnaires to produce comparable sociodemographic/clinical characteristics using the same criteria.
Figure 1.
Outcome measures for both clinical trials included the following: (1) maternal baseline and delivery 25(OH)D; (2) neonatal 25(OH)D concentration; (3) comorbidities of pregnancy (gestational diabetes, preeclampsia, hypertensive disorders of pregnancy; any infection; bacterial vaginosis (BV); preterm birth without preeclampsia as defined by an obstetrician).
Study Design of NICHD Trial
This study was a single-center, randomized controlled, double- blinded study of vitamin D supplementation (FDA IND #66,346; ClinicalTrials.gov #NCT00292591) approved by MUSC's Institutional Review Board for Human Research (HR#10727) (1). Women less with a singleton pregnancy than 12-16 weeks’ gestation were eligible to participate. The study was conducted from January 4, 2004 through August 31, 2010 at MUSC with women recruited from the surrounding community.
Study Design of Thrasher Research Fund Trial
This study was a two-center, randomized, double-blinded study of vitamin D supplementation (FDA IND #66,346; ClinicalTrials.gov #NCT00412087) approved by MUSC's Institutional Review Board for Human Research (HR# 16476) and the Palmetto Baptist Hospital Institutional Review Board for Human Research (IRB# 2007-25) (19). Women less than 16 weeks’ gestation were eligible for participation in the study. This study was conducted from November 21, 2006 through June 30, 2010 at Eau Claire Cooperative Health Center (ECCHC) in Columbia, South Carolina, and Northwoods Community Health Center (NCHC) in North Charleston, South Carolina. Women receiving their care at ECCHC delivered at Palmetto Baptist Hospital, Columbia, SC while women receiving their care at NCHC delivered at the Medical University of South Carolina, Charleston, SC.
Because 400 IU vitamin D/day had already been shown to be ineffective in maintaining adequate vitamin D status during pregnancy (11, 12, 20, 21) and because the majority of women being recruited in this study were either African American or Hispanic, with darker pigmentation and a greater likelihood of vitamin D deficiency, a control group that would receive 400 IU/day vitamin D was considered unethical by the scientific review committee as well as the research team. Hence, a control arm was not included a priori in the study design of the TRF trial.
Study Participants for Both Trials
The inclusion criteria for both trials were: maternal age of 16 years or greater; confirmed singleton pregnancy of less than 16 completed weeks of gestation at the time of enrollment; and plan to receive ongoing prenatal care until delivery where consent was obtained.
Exclusion Criteria for Both Trials
Mothers with pre-existing calcium or parathyroid conditions or who required chronic diuretic or cardiac medication therapy, including calcium channel blockers, were not eligible for enrollment into the study. Mothers with active thyroid disease (e.g., Graves, Hashimoto's or thyroiditis) also were not eligible to participate in the study; however, mothers on thyroid supplement with normal serological parameters could participate in the study if they were without any other endocrine dysfunction.
Randomization and Intervention
Overview
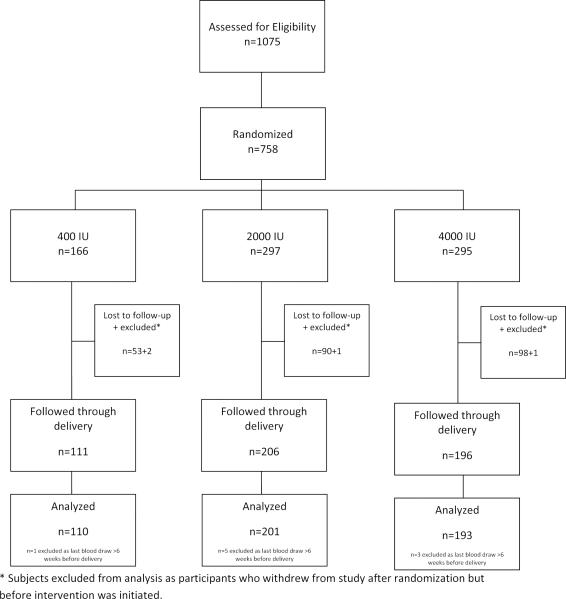
The schema for the combined cohort is summarized in Figure 2. Those women who received a total of 400 IU vitamin D3/day obtained 400 IU through their prenatal vitamin and 0 IU through the vitamin D placebo. Those women in the 2000 IU group for both trials received 400 IU/day from the prenatal vitamin and 1600 IU from a vitamin D tablet. Similarly, the women in the 4000 IU group received 400 IU/day from the prenatal vitamin and 3600 IU from the vitamin D tablet. The vitamin D tablets—placebo, 1600 IU and 3600 IU—were identical in appearance and were identified only by the manufacturer and the research pharmacy.
Figure 2.
Combined Pregnancy Vitamin D Cohort
a) NICHD Trial
Women were randomized to one of three treatment groups: control (400 IU vitamin D3/day), 2000 IU vitamin D3/day or 4000 vitamin D3/day following measurement of their baseline 25(OH)D concentration. Women with levels of 100 nmol/L (40 ng/mL) or less were eligible for randomization into one of the three treatment arms with further substratification by race within each treatment group. Women with baseline 25(OH)D levels greater than 100 to 150 nmol/L (>40 to 60 ng/mL, levels considered to be in the normal range at the time of study implementation) were randomized into one of two treatment groups (400 or 2000 IU/d of vitamin D3), whereas women with a baseline 25(OH)D level greater than 150 nmol/L (>60 ng/mL) were given 400 IU vitamin D3/d. The doses of vitamin D used in this study were selected based on current recommendations (400 IU/d), the upper safe intake level established in 1997 (2000 IU/d) (21) and the amount we calculated to be required to achieve nutritional vitamin D sufficiency (4000 IU/d) (9).
b) Thrasher Research Fund Trial
Following an initial month of 2000 IU vitamin D3/day, during which time baseline maternal 25(OH)D concentration was measured, each participant was randomized to receive either 2000 or 4000 IU of vitamin D3 daily for the remainder of her pregnancy. The randomization was stratified according to baseline 25(OH)D level, with two strata defined by a cut-point of 32 ng/mL. Following computer generated randomization, dose groups were identified for logistical purposes using six letters (three per dose group) as an additional measure against inadvertent unblinding.
Adherence to Medication Regimen
Adherence to the prescribed vitamin D supplementation regimen of 1 prenatal vitamin and the vitamin D supplement was measured by maternal self-report and pill counts at each follow-up visit for both studies (22). Because both trials were conducted on an intent-to-treat basis, women who missed more than two prenatal visits exited the study by their own accord; however, those women who came for their obstetrical appointments and who wanted to continue in the study irrespective of their compliance were allowed to do so
Study Protocol for Both Trials
Gestational Age at Enrollment
Participants could be consented and enrolled into the study before the initiation of vitamin D supplementation at 12-16 weeks of gestation. Gestational age was based on last menstrual period. If a woman was unsure of her gestational age, the obstetrical estimate at the time of the visit was used. If, at the 20-week fetal ultrasound it was determined by the obstetrician that the gestational age was incorrect, the revised gestational age was used and the discrepancy noted.
Initial Study Visit
Baseline blood and urine samples were obtained following each participant's consent at the initial visit; however, the earliest time of randomization following measurement of baseline total circulating 25(OH)D was 12 weeks’ gestation with the target upper limit of gestation of 16 weeks’ gestation. Irrespective of enrollment gestational age, vitamin D supplementation did not begin before the twelve week of gestation (12 and 0/7th weeks).
Subsequent Study Visits
Participants were followed with monthly study visits, which continued until delivery. These visits coincided with routine obstetrical visits. While the NICHD trial continued to follow the infants of the mothers through one year of age, both trials had an additional visit with mother and infant two-weeks’ postpartum.
Completion of Questionnaires
Following written, informed consent, mothers completed questionnaires used in the NICHD vitamin D pregnancy trial (1), which included information regarding sociodemographic information, baseline health status and medical history at the first visit. An interim maternal health history questionnaire also was completed at each visit with the assistance of the study coordinator to ascertain adverse events, discussing type and frequency of acute illnesses such as respiratory, gastrointestinal, and other viral and/or bacterial illnesses. A review of medications and doctor's visits was obtained at that time. After delivery, the newborn record of each infant was reviewed for mode of delivery. Birth weight (grams) and gestational age also were recorded.
Blood and Urine Samples
Whereas the NICHD trial collected maternal blood samples at each monthly visit, for the TRF trial, maternal blood samples were collected at the first visit then every other obstetrical visit and at the time of delivery. For both trials, maternal urine samples were collected at each visit. Cord blood was obtained at delivery. If the cord blood sample could not be obtained, a neonatal blood sample was drawn within two weeks of delivery. In fact, the vast majority of infant total circulating 25(OH)D levels (>95%) in both cohorts were obtained from cord blood. If a cord blood sample was missed, then every attempt was made to obtain the infant value within a few days of birth; thus, the values that were obtained are reflective of in utero vitamin D status.
Materials
Source of Vitamin D for Both Trials
Vitamin D tablets were manufactured by Tishcon Corporation, Westbury, NY, a Good-Manufacturing-Practice (GMP) facility. Hoffman-La Roche, Ltd., Basel, Switzerland, supplied the cholecalciferol content contained in the vitamin D tablet manufactured by Tishcon Corporation. The tablet vitamin concentration was verified by the company every six months and by an independent laboratory chosen by the investigators (Heartland Assays, Ames, IA) using HPLC-UV to ensure the tablets met label claim throughout the study; these results were reported to the Investigational Drug Department at MUSC. Tablets were maintained in MUSC's Investigational Drug Division of Pharmacy until the time that they were dispensed to enrolled subjects at ECCHC or NCHC.
Source of Prenatal Vitamins for Both Trials
Prenatal vitamins prescribed at the time of each subject's enrollment at ECCHC and NCHC were Myadec Multivitamin-Multimineral Supplement (distributed by Pfizer Consumer Healthcare, Morris Plains, NJ) with 400 IU vitamin D3/tablet. Those mothers who were unable to swallow a prenatal vitamin were given a Flintstones™ Complete chewable vitamin (Bayer Healthcare, Morristown, NJ), which provided 400 IU vitamin D3 per vitamin.
Study Measures
Maternal Sociodemographic Questionnaire
Upon enrollment in the study, each mother was asked to complete a sociodemographic questionnaire to ascertain maternal age, race, educational level, occupation, and insurance status.
Race/Ethnicity Definition
Each mother was asked to describe the racial/ethnic group to which she belonged, by selecting any applicable categories from African-American, Caucasian, Hispanic, American Indian, Asian, and other.
Pregnancy Intake and Surveillance Survey
Upon enrollment, each woman was asked to complete a health assessment questionnaire to ascertain her use of medications (checklist) and over-the-counter preparations that may have influenced vitamin D/calcium homeostasis. Additional questions concerned use of cigarettes and alcohol, and overall health status.
Pregnancy Health Status, and Labor and Delivery Characteristics and Complications
Characteristics of each mother's health status and complications during pregnancy, labor, and delivery were recorded. Complications at the time of delivery were listed according to American Congress of Obstetrician and Gynecologist (ACOG) definitions. In addition, if the mother required hospitalization, a copy of the hospital record was obtained after she signed a release of medical information form. Any acute illnesses or development of pregnancy-related conditions that were not preexisting also were recorded. When appropriate, the Data Safety and Monitoring Committee (DSMC) and the IRB were notified of any adverse events.
Season
The season that each blood sample was drawn was defined as Spring (April -May), Summer (June-September), Fall (October-November) and Winter (December -March).
Laboratory Measurements
Maternal and Cord Blood/Neonatal Total Circulating 25(OH)D Assays
A rapid, direct RIA developed in the Hollis laboratory and manufactured by Diasorin Corporation (Stillwater, MN) was used to measure total circulating 25(OH)D concentration in serum samples as previously described (1, 22). The laboratory participated in an independent quality assessment/assurance program (DEQAS) in place throughout both clinical trials.
Based on clinical laboratory classifications (23, 24), a priori, deficiency was defined as total circulating 25(OH)D <50 nmol/L (20 ng/mL), insufficiency as ≥50 to <80 nmol/L (≥20 to <32 ng/mL), and sufficiency as ≥80 nmol/L (≥32 ng/mL) (24, 25). The inter- and intra-assay coefficient of variation is ≤10%.
Maternal and Infant Concentrations of Serum Calcium, Creatinine, and Phosphorus
Maternal, cord blood, and infant serum total calcium, creatinine, and inorganic phosphorus were measured bimonthly (maternal) or at delivery (infant) by MUSC's Clinical Chemistry Laboratory using standard methodology and laboratory normative data. Results were reported to the PI and downloaded to the research database from the clinical chemistry registry in place for the medical center. The PI of the study reviewed all results on a weekly basis to identify potentially abnormal values.
Monthly Maternal Urinary Calcium/Creatinine Ratio for both Trials
A urine sample was obtained from the mother at each monthly obstetrical visit and was sent to the Clinical Chemistry Laboratory at MUSC for urinary calcium and creatinine measurement, and derivation of the urinary Ca (mg/dL): Cr (mg/dL) ratio.
Safety Monitoring
All study participants were monitored monthly for hypervitaminosis D. Operationally, for both clinical trials, we defined a priori caution limits for hypervitaminosis D as a urinary calcium (mg/dL): creatinine (mg/dL) ratio 0.8 and abnormal if the ratio exceeded 1.0. Whenever any patient was to exceed the caution limit, a specific case study was to be initiated to examine the contribution of confounding factors (e.g., diet, sunlight exposure, etc.). Operationally, we were to stop vitamin D3 supplementation if the urinary calcium: creatinine ratio (measured monthly) exceeded 1.0 or if the circulating 25(OH)D level (measured bimonthly) exceeded 100 ng/mL.
Data Safety and Monitoring Committee (DSMC)
As described previously (1, 19), during both trials, the DSMC reviewed the quarterly summary reports that were generated for all subjects enrolled in each study.
Statistical Analyses
Sample Size and Power Considerations
The sample size calculations for both studies have been described in detail in previous papers (1, 19). Briefly, the NICHD study had 90% power to detect a 10 ng/mL difference in final 25(OH)D between dose groups, assuming the use of a two-tailed ANOVA. The Thrasher study had 80% power to detect a 10 ng/mL difference between dose groups in the change between baseline and predelivery (25(OH)D. For the analysis of the combined data, in which final 25(OH)D is the preferred measure of circulating vitamin D status, at least 90% power will be available to detect a 10 ng/mL difference between dose groups.
Baseline demographic characteristics were summarized using frequencies and percentages for categorical characteristics, and median and range for continuous descriptors. Comparisons between dose groups were performed using chi-square tests for the categorical characteristics of race, insurance, education, and season; Poisson regression for count data (parity); and the Kruskal-Wallis test for the continuous quantity (age).
The primary analysis compared vitamin D status within six weeks of delivery between the three dose groups, using multivariable linear regression to adjust for study and participant race. In addition, the fraction of participants achieving the 25(OH)D thresholds of 32 ng/mL and 40 ng/mL (maternal), and 20 ng/mL and 32 ng/mL (neonatal), were compared between dose groups using multivariable logistic regression to adjust for study and participant race. In all multivariable models, participants with a race of “Other” were excluded due to small cell sizes.
Secondary endpoints for this analysis comprised comorbidities of pregnancy as follows: gestational diabetes, hypertensive disorders, maternal infection (any), bacterial vaginitis, and preterm birth without preeclampsia. The occurrence of these comorbidities was examined for association with dose group and with final maternal 25(OH)D using multivariable logistic regression. These models controlled solely for the effects of study and participant race, and included no additional independent variables.
In both the NICHD and Thrasher Research Fund clinical trials, more than one third of the women did not adhere to study protocol and took less than the prescribed number of vitamin D supplements per month. Because each trial was reported on an intent-to-treat basis, women who were nonadherent to protocol were included with women who were adherent. This resulted in a potential dilution of effect of each treatment, particularly in the higher treatment groups. As a result, the dataset was analyzed on the basis of final maternal 25(OH)D concentration attained and comorbidity rates.
Results
Baseline and final participant counts are found in Table 1. As shown in Figure 2, of the 758 women who participated in either the NICHD trial or the Thrasher Research Fund Trial from the point of randomization, 504 continued until their delivery. Of those women who completed the study, there were 110 controls, 201 in the 2000 IU group, and 193 in the 4000 IU group.
Table 1.
Baseline and Final Participant Counts
| Thrasher | NIH | Total | ||||
|---|---|---|---|---|---|---|
| 2000 IU | 4000 IU | Control | 2000 IU | 4000 IU | ||
| Baseline | 130 | 127 | 166 | 167 | 168 | 758 |
| Final | 84 | 79 | 110 | 117 | 114 | 504 |
Final totals: controls 110, 2000 IU 201, 4000 IU 193.
The combined cohort sociodemographic data are shown in Table 2. Differences were noted between the groups regarding maternal insurance status, parity and education with a greater number of women in the 2000 and 4000 IU groups having Medicaid or no insurance, higher parity and lower educational status.
Table 2.
Maternal Sociodemographic Characteristics by Treatment Group
| Characteristics | Control | 2000 IU | 4000 IU | p-value |
|---|---|---|---|---|
| Race/ethnicity (N, %) | ||||
| African-American | 28/110 (25.5) | 72/201 (35.8) | 68/193 (35.2) | 0.23 |
| Caucasian | 36/110 (32.7) | 42/201 (20.9) | 46/193 (23.8) | |
| Hispanic | 45/110 (40.9) | 85/201 (42.3) | 75/193 (38.9) | |
| Other | 1/110 (0.9) | 2/201 (1.0) | 4/193 (2.1) | |
| Age, years | 27 (18-41) | 26 (16-41) | 26 (17-44) | 0.40 |
| Parity | 2 (0-5) | 1 (0-7) | 1 (0-9) | 0.021 |
| Insurance | ||||
| None/Medicaid | 62/110 (56.4) | 149/201 (74.1) | 128/193 (66.3) | 0.006 |
| Education Some college or higher | 63/98 (64.3) | 87/191 (45.6) | 98/183 (53.6) | 0.010 |
| Season at enrollment | ||||
| Spring/Summer | 52/110 (47.3) | 106/201 (52.7) | 104/193 (53.9) | 0.52 |
Race, insurance, education, and season at enrollment compared between dose groups using chi-square tests. Age reported as median (min-max) and compared between dose groups using Kruskal-Wallis test. Parity compared between dose groups using Poisson regression.
As shown in Table 3, there were no differences in maternal baseline 25(OH)D, but there were consistent differences in maternal and cord blood 25(OH)D concentrations achieved with higher rates of sufficiency using various cutpoints in the 4000 IU group and 2000 IU group compared to the Control group (p-values generally <0.0001), an effect that persisted even after controlling for race and study.
Table 3.
Maternal and Neonatal 25(OH)D Status by Treatment Group
| Total Circulating 25(OH)D, ng/mL, Mean (SD) | Control | 2000 IU | 4000 IU | p-value (overall) | p-value (2000 vs. control) | p-value (4000 vs. control) |
|---|---|---|---|---|---|---|
| Baseline | 24.6 (10.9) | 23.2 (8.6) | 22.8 (9.7) | 0.21 | > 0.05 | > 0.05 |
| Within 6 weeks of delivery | 30.7 (14.1) | 37.1 (14.7) | 41.9 (16.2) | <0.0001 | < 0.001 | < 0.0001 |
| Neonatal/cord blood | 18.2 (10.1) | 21.2 (11.1) | 25.4 (12.9) | <0.0001 | > 0.05 | < 0.0001 |
| Maternal 25(OH)D ≥32 ng/mL, % | 57/110 (51.8) | 138/201 (68.7) | 147/193 (76.2) | <0.0001 | <0.0001 | <0.0001 |
| Maternal 25(OH)D ≥40 ng/mL, % | 39/110 (35.5) | 98/201 (48.8) | 121/193 (62.7) | <0.0001 | <0.0001 | <0.0001 |
| Neonatal 25(OH)D ≥20 ng/mL, % | 31/79 (39.2) | 96/165 (58.2) | 120/158 (76.0) | <0.0001 | 0.0007 | <0.0001 |
| Neonatal 25(OH)D ≥32 ng/mL, % | 10/79 (12.7) | 25/165 (15.2) | 41/158 (26.0) | 0.020 | 0.33 | 0.014 |
Continuous measures compared between dose groups using multivariable linear regression to adjust for study and participant race (excluding “Other”); pairwise comparisons against control performed using Dunnett's procedure. Dichotomous measures compared between dose groups using multivariable logistic regression to adjust for study and participant race (excluding “Other”).
Assessing maternal adverse events and comorbidities in the combined groups by treatment as shown in Table 4, there were no differences found. While each did not reach statistical significance, there was a trend of a lower rate of gestational diabetes and infection in the 4000 IU group compared to the Control and 2000 IU groups. Similarly, there was a trend of lower rates of hypertensive disorders of pregnancy and bacterial vaginosis with increasing dose, with the lowest rates noted in the 4000 IU group. Preterm birth without preeclampsia did not show a similar trend. When the comorbidities of pregnancy were combined together, there again was a dose effect trend with the lowest rate found in the 4000 IU group.
Table 4.
Association between Neonatal Growth and Comorbidities of Pregnancy by Supplementation Dose Group, Adjusted for Study and Race
| Control | 2000 IU | 4000 IU | p-value (overall) | p-value (2000 vs. control) | p-value (4000 vs. control) | NNT/H (2000 IU vs. control) | NNT/H (4000 IU vs. control) | |
|---|---|---|---|---|---|---|---|---|
| Neonatal birth weight, grams (SD) | 3233 (668) | 3382 (759) | 3231 (632) | 0.029 | > 0.05 | > 0.05 | -- | -- |
| Hypertensive Disorders of Pregnancy (N, %) | 9/110 (8.2) | 9/201 (4.5) | 4/193 (2.1) | 0.15 | 0.43 | 0.052 | NNT 27 | NNT 17 |
| Gestational Diabetes (N, %) | 8/110 (7.3) | 16/200 (8.0) | 10/191 (5.2) | 0.39 | 0.45 | 0.18 | NNH 138 | NNT 50 |
| Infection, any (N, %) | 45/110 (40.9) | 96/201 (47.8) | 75/193 (38.9) | 0.30 | 0.33 | 0.78 | NNH 15 | NNT 49 |
| Bacterial Vaginosis (N, %) | 12/110 (10.9) | 15/201 (7.5) | 9/193 (4.7) | 0.61 | 0.75 | 0.34 | NNT 23 | NNT 14 |
| Preterm Birth without Preeclampsia (N, %) | 14/107 (13.1) | 23/198 (11.6) | 26/192 (13.5) | 0.42 | 0.20 | 0.55 | NNT 69 | NNH 219 |
| Combined comorbidities (N, %) | 67/110 (60.9) | 125/201 (62.2) | 106/193 (54.9) | 0.43 | 0.97 | 0.30 | NNH 79 | NNT 17 |
NNT/H: Number Needed to Treat/Harm.
Excludes “Other” race due to small cell sizes. Continuous measures compared between dose groups using multivariable linear regression to adjust for study and participant race (excluding “Other”); pairwise comparisons against control performed using Dunnett's procedure. Dichotomous measures compared between dose groups using multivariable logistic regression to adjust for study and participant race (excluding “Other”).
Analyses of the combined datasets by final maternal 25(OH)D concentration attained, adjusted for study and race, are found in Table 5. Using the a priori cut-point of 32 ng/mL as the definition of sufficiency, there were clear differences in the rates of comorbidities for infection, hypertensive disorders of pregnancy, preterm birth without preeclampsia, and combined comorbidities. In more nuanced analyses, every 10 ng/mL increase in maternal 25(OH)D at delivery resulted in reduced odds of infection and preterm birth without preeclampsia, but these did not reach statistical significance. When the four main comorbidities of pregnancy were combined, for every 10 ng/mL increase in maternal 25(OH)D at delivery, the odds ratio was reduced to 0.84 (p=0.006).
Table 5.
Association between Comorbidities of Pregnancy and Final Maternal 25(OH)D level, Adjusted for Study and Race
| Comorbidity of Pregnancy | 25(OH)D < 32 ng/mL | 25(OH)D ≥ 32 ng/mL | OR per 10 ng/mL increase in 25(OH)D | 95% CI | p-value |
|---|---|---|---|---|---|
| Combined comorbidities (N, %) | 109/162 (67.3) | 189/342 (55.3) | 0.84 | 0.74 - 0.95 | 0.006 |
| Gestational DM (N, %) | 12/161 (7.5) | 22/340 (6.5) | 1.04 | 0.82 - 1.33 | 0.75 |
| Hypertensive disorders (N, %) | 10/162 (6.2) | 12/342 (3.5) | 0.77 | 0.57 - 1.06 | 0.11 |
| Infection, any (N, %) | 83/162 (51.2) | 133/342 (38.9) | 0.89 | 0.79 - 1.01 | 0.070 |
| Bacterial Vaginosis (N, %) | 14/162 (8.6) | 22/342 (6.4) | 0.93 | 0.74 - 1.18 | 0.54 |
| Preterm birth without preeclampsia (N, %) | 27/157 (17.2) | 36/340 (10.6) | 0.84 | 0.70 - 1.02 | 0.072 |
Excludes “Other” race due to small cell sizes. Continuous measures compared between dose groups using multivariable linear regression to adjust for study and participant race (excluding “Other”). Dichotomous measures compared between dose groups using multivariable logistic regression to adjust for study and participant race (excluding “Other”).
Discussion
While no differences were found between treatment groups at baseline in the combined datasets of two randomized clinical trials of vitamin D supplementation during pregnancy, delivery and cord blood values were greater in the 4000 IU group than in controls (p<0.0001), an effect that persisted even after controlling for race and study. After supplementation, a greater percent were vitamin D replete in the 4000 IU group (p<0.0001). When compared to the 400 IU group, there was a trend where the 4000 IU group had lower rates of comorbidities of pregnancy.
While there was sufficient power to detect health outcome differences in women during pregnancy as a function of treatment, the effect of nonadherence to vitamin D supplementation mitigated the potential effects of treatment in more than a third of the women in both the NICHD and the Thrasher Research Fund trials. As such, total circulating 25(OH)D then was more reflective of the true “group”—sufficient or insufficient—to which each mother belonged.
When using 25(OH)D concentration as the outcome, rather than treatment group, which has the inherent bias of compliance or adherence, there was a strong association between combined comorbidities of pregnancy and final maternal 25(OH)D; an effect that persisted even after controlling for race and study. Using an a priori cut-point of 20- and 32 ng/mL as the definitions of deficiency and sufficiency, respectively, there were statistically significant differences in the rates of comorbidities by both criteria for preeclampsia, infection, hypertensive disorders of pregnancy, and combined comorbidities. Maternal delivery 25(OH)D was inversely associated with any comorbidity of pregnancy, with fewer events as 25(OH)D increased.
The findings of this analysis support earlier observational and case control studies that have linked adverse pregnancy outcomes such as preeclampsia and maternal infection with vitamin D deficiency (26-31). The importance of adherence to a drug therapy and its impact, in this case, on total circulating 25(OH)D concentrations was readily apparent in this study. Identifying ways to improve adherence will result in improved vitamin D status and should be the focus of future studies. Further studies also are needed to confirm these findings in larger, prospective clinical trials with delineation of the putative mechanisms of action of vitamin D in immune regulation.
Highlights.
-
(1)
Two randomized clinical trial pregnancy vitD datasets were combined for analysis.
-
(2)
No differences in baseline 25(OH)D were noted between groups.
-
(3)
Maternal/cord blood 25(OH)D greatest in 4000 IU group.
-
(4)
Comorbidities of pregnancy were strongly associated with vitamin D status.
Acknowledgements
We would like to thank the women who participated in the NICHD and Thrasher Research Fund trials from 2004-2010, without whose help these research studies could not have happened. We also would like to thank the following for their tireless work throughout these clinical trials: at the Medical University of South Carolina study sites—Judy Shary, MS; Pamela G. Smith, RN; Betty Bivens, RA; and Martha L. Murphy, BS; and at the Eau Claire Cooperative Health Centers— Joyce Winkler, RN, MPH; Carolina Rodriguez Cook, MD; Gloria Warner, MBA; and Deborah Davis, MD. Lastly, we would like to thank Dr. Robert Woolson for his scholarly advice regarding analyses of this combined dataset.
Funded by: the Thrasher Research Fund; NIH RR01070 and UL1 RR029882; MUSC Children's Hospital Fund, and the Division of Neonatology, Medical University of South Carolina, Charleston, SC
Abbreviations
- NICHD
National Institute of Child Health and Human Development
- TRF
Thrasher Research Fund
- 25(OH)D
total circulating 25-hydroxy-vitamin D
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the Pediatric Societies Meeting in May 2012 held in Boston, MA and at the Vitamin D Workshop meeting held in June 2012 in Houston, TX.
References
- 1.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26(10):2341–57. doi: 10.1002/jbmr.463. PMCID: 3183324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamilton SA, McNeil R, Hollis BW, Davis DJ, Winkler J, Cook C, et al. Profound Vitamin D Deficiency in a Diverse Group of Women during Pregnancy Living in a Sun-Rich Environment at Latitude 32 degrees N. Int J Endocrinol. 2010;2010:917428. doi: 10.1155/2010/917428. PMCID: 3004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson DD, Wagner CL, Hulsey TC, McNeil RB, Ebeling M, Hollis BW. Vitamin D Deficiency and Insufficiency is Common during Pregnancy. Am J Perinatol. 2010 Jul 16; doi: 10.1055/s-0030-1262505. PMCID: PMID: 206409744. [DOI] [PubMed] [Google Scholar]
- 4.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3 synthesis in human skin. J Clin Endocrinal Metab. 1988;67:373–8. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 5.Moan J, Lagunova Z, Lindberg FA, Porojnicu AC. Seasonal variation of 1,25-dihydroxyvitamin D and its association with body mass index and age. J Steroid Biochem Mol Biol. 2009;113(3-5):217–21. doi: 10.1016/j.jsbmb.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Dawodu A, Wagner CL. Mother-child vitamin D deficiency: an international perspective. Arch Dis Child. 2007;92(9):737–40. doi: 10.1136/adc.2007.122689. PMCID: 2084036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawodu A, Wagner CL. Prevention of vitamin D deficiency in mothers and infants worldwide - a paradigm shift. Paediatr Int Child Health. 2012;32(1):3–13. doi: 10.1179/1465328111Y.0000000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawodu A. What's new in mother-infant vitamin D deficiency: a 21st century perspective. Medical principles and practice : international journal of the Kuwait University, Health Science Centre. 2012;21(1):2–3. doi: 10.1159/000331904. [DOI] [PubMed] [Google Scholar]
- 9.Hollis B, Wagner C. Assessment of dietary vitamin D requirements during pregnancy and lactation. Am J Clin Nutr. 2004;79:717–26. doi: 10.1093/ajcn/79.5.717. [DOI] [PubMed] [Google Scholar]
- 10.Marya R, Rathee S, Lata V, Mudgil S. Effects of vitamin D supplementation in pregnancy. Gynecol Obstet Invest. 1981;12:155–61. doi: 10.1159/000299597. [DOI] [PubMed] [Google Scholar]
- 11.Brooke OG, Brown IRF, Bone CDM, Carter ND, Cleeve HJW, Maxwell JD, et al. Vitamin D supplements in pregnant Asian women: Effects on calcium status and fetal growth. Br Med J. 1980;1:751–4. doi: 10.1136/bmj.280.6216.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooke OG, Butters F, Wood C. Intrauterine vitamin D nutrition and postnatal growth in Asian infants. Brit Med J. 1981;283:1024. doi: 10.1136/bmj.283.6298.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooke O, Brown I, Cleeve H, Sood A. Observations on the vitamin D state of pregnant Asian women in London. Br J Obstet Gynaecol. 1981;88:18–26. doi: 10.1111/j.1471-0528.1981.tb00931.x. [DOI] [PubMed] [Google Scholar]
- 14.Maxwell J, Ang L, Brooke O, Brown I. Vitamin D supplements enhance weight gain and nutritional status in pregnant Asians. Br J Obstet Gynaecol. 1981;88:987–91. doi: 10.1111/j.1471-0528.1981.tb01686.x. [DOI] [PubMed] [Google Scholar]
- 15.Vieth R. Vitamin D supplementation, 25-hydroxy-vitamin D concentrations, and safety. Am J Clin Nutr. 1999;69:842–56. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- 16.Heaney R, Davies K, Chen T, Holick M, Barger-Lux M. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–10. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 17.Hollis B, Wagner C. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D in both mother and nursing infant. Am J Clin Nutr. 2004;(Suppl 80):1752–8. doi: 10.1093/ajcn/80.6.1752S. [DOI] [PubMed] [Google Scholar]
- 18.Wagner CL, Hulsey TC, Fanning D, Ebeling M, Hollis BW. High-dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: a 6-month follow-up pilot study. Breastfeed Med. 2006;1(2):59–70. doi: 10.1089/bfm.2006.1.59. [DOI] [PubMed] [Google Scholar]
- 19.Wagner CL, McNeil R, Hamilton SA, Winkler J, Rodriguez Cook C, Warner G, et al. A randomized trial of vitamin D supplementation in 2 community health center networks in South Carolina. American Journal of Obstetrics and Gynecology. 2012 doi: 10.1016/j.ajog.2012.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delvin EE, Salle BL, Glorieux FH, Adeleine P, David LS. Vitamin D supplementation during pregnancy: effect on neonatal calcium homeostasis. J Pediatr. 1986;109(2):328–34. doi: 10.1016/s0022-3476(86)80396-1. [DOI] [PubMed] [Google Scholar]
- 21.Maxwell JD, Ang L, Brooke OG, Brown IRF. Vitamin D supplements enhance weight gain and nutrional status in pregnant Asians. Brit J Obstet Gyneaecol. 1981;88:987–91. doi: 10.1111/j.1471-0528.1981.tb01686.x. [DOI] [PubMed] [Google Scholar]
- 22.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD. Determination of vitamin D status by radioimmunoassay with a 125I-labeled tracer. Clin Chem. 1993;39:529–33. [PubMed] [Google Scholar]
- 23.Laboratories MC. Laboratory Reference Data. Mayo Clinic; Rochester, MN: 2003. Contract No.: Document Number. [Google Scholar]
- 24.Hollis BW, Wagner CL, Kratz A, Sluss PM, Lewandrowski KB. Normal serum vitamin D levels. Correspondence. N Engl J Med. 2005;352:515–6. doi: 10.1056/NEJM200502033520521. [DOI] [PubMed] [Google Scholar]
- 25.Hollis B. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–22. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 26.Bodnar LM, Simhan HN. Vitamin D may be a link to black-white disparities in adverse birth outcomes. Obstet Gynecol Surv. 2010;65(4):273–84. doi: 10.1097/OGX.0b013e3181dbc55b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal Vitamin D Deficiency Increases the Risk of Preeclampsia. 2007:3517–22. doi: 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson CJ, Wagner CL, Hollis BW, Baatz JE, Johnson DD. Maternal vitamin D and fetal growth in early-onset severe preeclampsia. American Journal of Obstetrics and Gynecology. 2011;204(6):556, e1–4. doi: 10.1016/j.ajog.2011.03.022. PMCID: 3136573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker V, Zhang X, Rastegar I, Liu P, Hollis B, Adams J, et al. Cord blood vitamin D status impacts innate immune responses. J Clin Endocrinal Metab. 2010 doi: 10.1210/jc.2010-1559. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bodnar LM, Krohn MA, Simhan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. The Journal of Nutrition. 2009;139(6):1157–61. doi: 10.3945/jn.108.103168. PMCID: 2682987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brannon PM. Vitamin D and adverse pregnancy outcomes: beyond bone health and growth. The Proceedings of the Nutrition Society. 2012:1–8. doi: 10.1017/S0029665111003399. [DOI] [PubMed] [Google Scholar]