Abstract
Objective
To determine whether 4000 IU vitamin D3/day (vs. 2000 IU/day) during pregnancy is safe and improves maternal/neonatal 25(OH)D in a dose-dependent manner.
Study Design
257 pregnant women 12–16 weeks’ gestation were enrolled. Randomization to 2000- vs. 4000 IU/day followed one-month run-in at 2000 IU/day. Participants were monitored for hypercalciuria, hypercalcemia and 25(OH)D status.
Results
Maternal 25(OH)D (n=161) increased from 22.7(SD 9.7) at baseline to 36.2(SD 15) and 37.9(SD 13.5) in the 2000- and 4000 IU groups, respectively. While maternal 25(OH)D change from baseline did not differ between groups, 25(OH)D monthly increase differed between groups (p<0.01). No supplementation-related adverse events occurred. Mean cord blood 25(OH)D (ng/mL) was 22.1±10.3 in 2000- and 27.0±13.3 in 4000 IU group (p=0.024). After controlling for race and study site, preterm birth and labor were inversely associated with pre-delivery- and mean 25(OH)D, but not baseline 25(OH)D,.
Conclusions
Maternal supplementation with 2000 and 4000 IU vitamin D/day during pregnancy improved maternal/neonatal vitamin D status. Evidence of risk reduction in infection, preterm labor and preterm birth was suggestive, requiring additional studies powered for these endpoints.
Keywords: vitamin D, cholecalciferol, pregnancy, health outcomes
Introduction
With avoidance of sunlight exposure due to lifestyle changes, concerns regarding skin cancer, and the resultant widespread use of sunscreen, fewer Americans are meeting their needs for vitamin D (1–3). A study published in 2002 by the Centers for Disease Control (CDC) and our laboratory at the Medical University of South Carolina (MUSC) revealed that 42% of African American women in their childbearing years exhibited vitamin D deficiency (hypovitaminosis D) (4). More recent publications suggest that the rate of deficiency is higher than previously reported (3, 5–7).
Until recently, there was no Recommended Dietary Allowance (RDA) for vitamin D; only an Adequate Intake (AI), which remained at 200 IU vitamin D/day for decades (8). A 2010 review of the vitamin D requirements by the Institute of Medicine (IOM) resulted in a revised RDA of 600 IU vitamin D/day (9), and suggested that fewer Americans are deficient than previously reported (9). Using the IOM definition of deficiency of <20 ng/mL (50 nmol/L), however, two recent large studies of vitamin D status in pregnant women living at latitude 32°N (South Carolina) showed that African American women were eight times as likely as Hispanic women, and twenty times as likely as Caucasian women, to have vitamin D deficiency (5, 7, 10). Yet, little information exists that addresses the vitamin D requirements of the pregnant woman and her fetus.
The recent NICHD-sponsored randomized controlled trial of vitamin D supplementation using 400, 2000 or 4000 IU vitamin D3/day starting at 12 weeks of gestation showed that 400 IU/day was woefully inadequate in achieving vitamin D sufficiency (7). Optimal 1,25(OH)2D production,(i.e., the point at which its concentration has reached steady-state) was found to occur when total circulating 25(OH)D concentration was at least 40 ng/mL (or 100 nmol/L) (7). In addition, only the 4000 IU group achieved optimal status in all women throughout pregnancy, including second trimester, irrespective of race. The translation of vitamin D supplementation at these doses to other women receiving care in a non-university health setting has become essential.
This study was undertaken to shed light on the vitamin D requirements of pregnant women receiving their prenatal care at community health centers. The study’s primary hypothesis was that vitamin D supplementation of 2000 or. 4000 IU/day during pregnancy was safe and effective in achieving vitamin D sufficiency and would result in improved maternal and neonatal health status. It was further hypothesized that both the 4000 IU dose would result in a greater increase in 25(OH)D than the 2000 IU dose. The co-primary outcome measures were the change in circulating 25(OH)D concentration from baseline to the completion of pregnancy in the mother, and her neonate’s 25(OH)D concentration at birth.
Methods and Experimental Design
Study Design
This study was a two-center, randomized, double-blinded study of vitamin D supplementation (FDA IND #66,346; ClinicalTrials.gov #NCT00412087). The study was approved by MUSC’s Institutional Review Board for Human Research (HR# 16476) and the Palmetto Baptist Hospital Institutional Review Board for Human Research (PH IRB# 2007-25). A written informed consent was obtained from each participant. Women less than 16 weeks’ gestation were eligible for participation in the study.
A precedent NICHD vitamin D supplementation trial during pregnancy had begun in 2004 with baseline 25(OH)D analysis revealing marked deficiency among darker pigmented women (3). Further, because 400 IU vitamin D/day had already been shown to be ineffective in maintaining adequate vitamin D status during pregnancy (11–14) and because the majority of women being recruited in this study were either African American or Hispanic, with darker pigmentation and a greater likelihood of vitamin D deficiency, a control group that would receive 400 IU/day vitamin D was considered unethical by the scientific review committee as well as the research team. Hence, a control arm was not included a priori in the study design.
Study Setting
This study was conducted from November 21, 2006 through June 30, 2010 at Eau Claire Cooperative Health Center (ECCHC) in Columbia, South Carolina, and Northwoods Community Health Center (NCHC) in North Charleston, South Carolina. ECCHC and NCHC are both Section 330 community health centers. At least 60% of ECCHC’s and NCHC’s patient populations are of racial/ethnic minority groups, including African American and Hispanic women. The populations being served by ECCHC and NCHC are among the poorest in South Carolina; the majority of women are in the 100%-below the federally designated poverty level and many women rank in the 200%-below federal poverty level.
Inclusion and Exclusion Criteria
The inclusion criteria were: maternal age of 16 years or greater; confirmed singleton pregnancy of less than 16 completed weeks of gestation at the time of enrollment; and intention to receive ongoing prenatal care at the community health center where consent was obtained.
Mothers with pre-existing calcium or parathyroid conditions or who required chronic diuretic or cardiac medication therapy, including calcium channel blockers, were not eligible for enrollment into the study. Mothers with active thyroid disease (e.g., Graves, Hashimoto’s or thyroiditis) also were not eligible to participate in the study; however, mothers on thyroid supplement with normal serological parameters could participate in the study if they were without any other endocrine dysfunction.
Randomization and Intervention
Upon enrollment into the study, expectant mothers’ vitamin D status was assessed by measuring total circulating 25(OH)D and PTH. Based on this initial 25(OH)D level, the randomization to 2000 or 4000 IU vitamin D3 per day was stratified using a cut-point of 32 ng/mL. Randomization lists were generated by computer prior to the start of the study. Randomization assignment was blinded to all participants and to the investigators except for the study biostatistician. Dose groups were identified for logistical purposes using six letters (three per dose group) as an additional measure against inadvertent unblinding.
Adherence to Medication Regimen
Adherence to the vitamin D supplementation regimen was measured by maternal self-report and pill counts at each follow-up visit (15). If a woman missed one prenatal visit, her next month’s supply of vitamins was mailed to her or delivered to her residence. If a woman had more than two missed visits or if she failed to take at least 50% of the prescribed vitamin D pills, she was exited from the study.
Study Protocol
Gestational Age at Enrollment
Gestational age was based on last menstrual period. If a woman was unsure of this date, the obstetrical estimate at the time of the visit was used. If, at the 20-week fetal ultrasound it was determined by the obstetrician that the gestational age was incorrect, the revised gestational age was used.
Initial Study Visit
Baseline blood and urine samples were obtained following each participant’s consent at the initial visit (10 to <16 weeks). Irrespective of enrollment gestational age, vitamin D supplementation did not begin before the twelfth week of gestation (12 and 0/7th weeks).
Subsequent Study Visits
Participants were followed with monthly study visits, which continued until delivery. These visits coincided with routine obstetrical visits. There was one additional visit with mother and infant two-weeks’ postpartum.
Completion of Questionnaires
Participants completed questionnaires used in the NICHD vitamin D pregnancy trial (7), which included information regarding sociodemographic information, baseline health status and medical history at the first visit. At the second visit, the Block Food Frequency Questionnaire was completed to ascertain generalized eating pattern, with specific calculation of calcium and vitamin D intake (Block, Berkeley, California) (16–21). An interim maternal health history questionnaire also was completed at each visit with the assistance of the study coordinator to ascertain adverse events, and type and frequency of acute illnesses such as respiratory, gastrointestinal, and other viral and/or bacterial illnesses. A review of medications and doctor’s visits was obtained at that time. After delivery, the newborn record of each infant was reviewed for mode of delivery, birth weight (grams), and gestational age.
Blood and Urine Samples
Maternal blood samples were collected at the first visit, then every other obstetrical visit and at the time of delivery. Maternal urine samples were collected at each visit. Cord blood was obtained at delivery. If the cord blood sample could not be obtained, a neonatal blood sample was drawn within two weeks of delivery.
Materials
Source of Vitamin D
Vitamin D tablets (1600 and 3600 IU) were manufactured by Tishcon Corporation, Westbury, NY, a Good-Manufacturing-Practice (GMP) facility. Hoffman-La Roche, Ltd., Basel, Switzerland, supplied the cholecalciferol content contained in the vitamin D tablet manufactured by Tishcon Corporation. The tablet vitamin concentration was verified by the company every six months and by an independent laboratory chosen by the investigators (Heartland Assays, Ames, IA) using HPLC-UV to ensure the tablets met label claim throughout the study; these results were reported to the Investigational Drug Department at MUSC. Tablets were maintained in MUSC’s Investigational Drug Division of Pharmacy until the time that they were dispensed to enrolled subjects at ECCHC or NCHC.
Source of Prenatal Vitamins
Prenatal vitamins (400 IU vitamin D3/tablet) prescribed at study entry were Myadec Multivitamin-Multimineral Supplement (distributed by Pfizer Consumer Healthcare, Morris Plains, NJ). Those mothers unable to swallow a prenatal vitamin were given a Flintstones™ Complete chewable vitamin (Bayer Healthcare, Morristown, NJ; 400 IU vitamin D3 per vitamin).
Study Measures
Maternal Sociodemographic Questionnaire
Upon enrollment in the study, each mother was asked to complete a sociodemographic questionnaire to ascertain maternal age, race, educational level, occupation, and insurance status.
Race/Ethnicity Definition
Each mother was asked to describe the racial/ethnic group to which she belonged, by selecting any applicable categories from African-American, Caucasian, Hispanic, American Indian, Asian, and other.
Pregnancy Intake and Surveillance Survey
Upon enrollment, each woman was asked to complete a health assessment questionnaire to ascertain her use of medications (checklist) and over-the-counter preparations that may have influenced vitamin D/calcium homeostasis. Additional questions concerned use of cigarettes and alcohol, and overall health status.
Pregnancy Health Status, and Labor and Delivery Characteristics and Complications
Characteristics of each mother’s health status and complications during pregnancy, labor, and delivery were recorded. Complications at the time of delivery were listed according to ACOG definitions. In addition, if the mother required hospitalization, a copy of the hospital record was obtained after she signed a release of medical information form. Any acute illnesses or development of pregnancy-related conditions that were not preexisting also were recorded. When appropriate, the Data Safety and Monitoring Committee (DSMC) and the IRB were notified of any adverse events.
Season
The season that each blood sample was drawn was defined as Spring (April – May), Summer (June–September), Fall (October–November) and Winter (December –March).
Maternal Body Mass Index (BMI) Measurement
Pre-pregnancy height and weight of each mother were recorded at the first outpatient visit to determine BMI (weight {kg}/height2 {m2}). During subsequent visits, only the mother’s weight was recorded, and the initial height and updated weight were used to calculate BMI at each outpatient visit.
Neonatal Growth Parameters
At the post-partum visit, the infant’s weight in grams, head circumference in centimeters, and length in centimeters were recorded. The growth parameters were then plotted using Fenton growth curves, which facilitate the calculation of z-scores and which permit the more precise assessment of growth of infants who are born preterm (22).
Laboratory Measurements
Maternal and Cord Blood/Neonatal Total Circulating 25(OH)D Assays
A rapid, direct RIA developed in the Hollis laboratory and manufactured by Diasorin Corporation (Stillwater, MN) was used to measure total circulating 25(OH)D concentration in serum samples as previously described (7, 23). Based on clinical laboratory classifications (24, 25), a priori, deficiency was defined as total circulating 25(OH)D <50 nmol/L (20 ng/mL), insufficiency as ≥50 to <80 nmol/L (≥20 to <32 ng/mL), and sufficiency as ≥80 nmol/L (≥32 ng/mL) (25–28). The inter- and intra-assay coefficient of variation is ≤10%.
Maternal and Infant Concentrations of Serum Calcium, Creatinine, and Phosphorus
Maternal serum total calcium, creatinine, and inorganic phosphorus were measured bimonthly (maternal) and at delivery (cord blood) by MUSC’s Clinical Chemistry Laboratory using standard methodology and laboratory normative data.
Monthly Maternal Urinary Calcium/Creatinine Ratio
Urinary calcium and creatinine were measured monthly for each mother by the Clinical Chemistry Laboratory at MUSC,1 then expressed as a ratio [urinary Ca (mg/dL): Cr (mg/dL) ratio (converted to mmol/L/mmol/L)].
Maternal and Cord Blood Concentrations of Circulating Intact PTH
Intact PTH (iPTH) was measured in serum samples by immunoradiometric assay (IRMA) as previously described (7). The adult normal range for iPTH in our laboratory is 1.3–5.4 pmol/L. Higher vitamin D levels are associated with lower iPTH; as vitamin D status improves, iPTH declines (29).
Safety Monitoring
All study participants were monitored monthly for hypervitaminosis D. The first sign of hypervitaminosis D is hypercalciuria, of which, urinary calcium: creatinine ratio is the most sensitive indicator. Operationally, we defined a priori caution limits for hypervitaminosis D as a urinary calcium (mg/dL): creatinine (mg/dL) ratio ≥0.8. The study’s Data and Safety Monitoring Committee reviewed the quarterly summary reports that were generated for all subjects enrolled in the study. Whenever any patient was to exceed the caution limit, a specific case study was to be initiated to examine the contribution of confounding factors (e.g., diet, sunlight exposure, etc). Operationally, we were to stop vitamin D3 supplementation if the urinary calcium: creatinine ratio (measured monthly) exceeded 1.0 or if the circulating 25(OH)D level (measured bimonthly) exceeded 100 ng/mL, and the DSMC and IRB notified immediately. The PI of the study reviewed all laboratory results on a weekly basis to identify potentially abnormal values.
Statistical Methods
Sample Size and Power Considerations
A total of 148 participants were to be enrolled in the study with 74 per supplementation arm. For one primary endpoint of change in 25(OH)D level between baseline and final measurements, this sample size would support the detection of a 10 ng/mL difference between dose groups with 80% power using a two-sided t-test at α=0.05. This calculation assumed that the standard deviation of 25(OH)D measurements at a single time point was approximately 10, that there would be a low correlation (ρ = 0.25) between the baseline and final measurements, and that a substantial proportion (up to 50%) of participants may be lost to follow-up. This calculation was robust to changes in the assumption regarding the magnitude of correlation between measurements made over time; if a higher correlation were to be present, the study’s power would be increased.
Statistical Analyses
Primary analysis
The a priori primary data analysis focused on comparisons of change in serum 25(OH)D between the two dose groups. While participants were randomized within each stratum, as described above, the strata were extremely imbalanced at the close of the study due to the infrequent occurrence of 25(OH)D levels above 32 ng/mL (Figure 1). Thus, the primary comparison for planning purposes was a two-sample test of dose group differences in the change in 25(OH)D levels, regardless of stratum. This comparison also was performed using the Wilcoxon rank-sum test as a sensitivity analysis to the normality assumption. The latter test is reported as the primary analysis. Statistical significance is claimed for p<0.05. Due to the multi-site implementation of the study, we also report results controlled for site using multivariable models.
Figure 1.
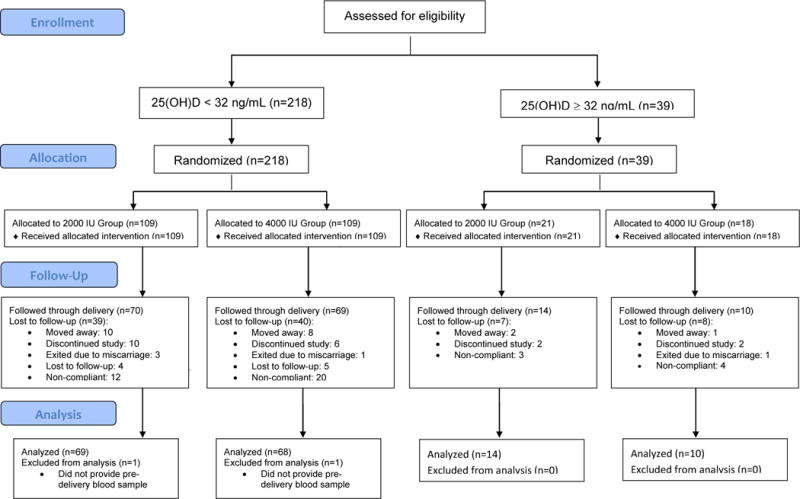
CONSORT Flow Diagram for this randomized clinical trial.
Secondary analysis
In secondary analyses, multi-level mixed-effects models were used to estimate the average monthly rate of change in 25(OH)D, compare this rate between dose groups, and explore the effects of covariates on the rate of change (30). These models included fixed effects for dose group, time, and the group-time interaction, and a random intercept effect, with additional covariate effects as required. Time was considered a continuous variable, measured in months rather than assuming structured visit occurrences. An unstructured covariance matrix was assumed. The same approach was used to evaluate the longitudinal association between 25(OH)D and calcium, iPTH (log-transformed), phosphorus, and urinary calcium, creatinine, and calcium: creatinine levels. The cumulative occurrence of pregnancy complications was compared between dose group levels using logistic regression. All analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary NC).
Participant attrition and missing data
Because the primary endpoint was change in 25(OH)D from baseline to delivery, the primary analysis was restricted to participants who remained in the study until delivery and provided a blood sample within 6 weeks prior to delivery, at delivery, or at the post-delivery visit (completers-only analysis). Typically, multiple imputation would be used to impute missing values in support of the favored intention-to-treat analytic approach. Because the multiple imputation model for this analysis would have required variables also measured in the final blood sample, however, it could not be used to impute cases with a missing final blood sample. Thus, to assess the primary findings’ robustness to assumptions about the missing data, we performed a sensitivity analysis under the following assumptions: cases with missing endpoints experienced no change in both groups; experienced the group-specific median change observed in completers; experienced no change in the 2000 IU group and minimal change in the 4000 IU group. In the secondary analyses using multi-level mixed-effects models for longitudinal modeling, all available data points were used, as it is not necessary to delete cases with missed timepoints when using this approach.
Results
Baseline characteristics
A total of 257 women consented to participate in this study. Of those, 161 (63%) provided complete data regarding the primary endpoint (see Figure 1, CONSORT Statement Flow Diagram). The sociodemographic characteristics of the active cohort are found in Table 1. After controlling for race and study site, no characteristics differed significantly between groups.
Table 1. Sociodemographic and Clinical Characteristics, by Self-Reported Race/Ethnicity.
Continuous and ordinal variables reported as median (range) unless otherwise noted, and compared between groups using the Wilcoxon rank-sum test. Categorical variables reported as frequency (%), and compared between groups using the chi-square test or Fisher’s exact test. P-values controlled for race and site were obtained using multivariable linear or logistic regression, when sample size permitted.
| Characteristic | Total Cohort N=257 | 2000 IU/day N=130 | 4000 IU/day N=127 | p-value | p-value controlled for race & site |
|---|---|---|---|---|---|
| Maternal age (mean ± SD) | 25.0 ± 5.1 | 24.5 ± 5.3 | 25.4 ± 5.0 | 0.076 | 0.116 |
| Gestational age at enrollment (mean ± SD) | 12.4 ± 1.8 | 12.4 ± 1.7 | 12.4 ± 2.0 | 0.48 | 0.60 |
| Planned pregnancy | 95 (37.0%) | 47 (36.2%) | 48 (37.8%) | 0.79 | 0.64 |
| Gravidity | 1 (0 – 7) | 1 (0 – 7) | 1 (0 – 7) | 0.37 | 0.29 |
| Primigravida | 97 (37.7%) | 51 (39.2%) | 46 (36.2%) | 0.62 | 0.55 |
| Parity | 1 (0 –4) | 0 (0 –4) | 1 (0 – 4) | 0.069 | 0.071 |
| BMI ≥ 30 | 70 (27.2%) | 32 (24.6%) | 38 (29.9%) | 0.34 | 0.34 |
| Race | |||||
| African-American | 124 (48.3%) | 61 (46.9%) | 63 (49.6%) | 0.56 | – |
| Caucasian | 25 (9.7%) | 12 (9.2%) | 13 (10.2%) | ||
| Hispanic | 101 (39.3%) | 55 (42.3%) | 46 (36.2%) | ||
| Other | 7 (2.7%) | 2 (1.5%) | 5 (3.9%) | ||
| Highest education achieved | |||||
| Less than high school | 72 (28.0%) | 35 (26.9%) | 37 (29.1%) | 0.85 | 0.74 |
| High school | 104 (40.5%) | 56 (43.1%) | 48 (37.8%) | ||
| Some college | 57 (22.2%) | 27 (20.8%) | 30 (23.6%) | ||
| Assoc. degree or higher | 24 (9.3%) | 12 (9.2%) | 12 (9.5%) | ||
| Employed | 116 (45.1%) | 60 (46.2%) | 56 (44.1%) | 0.74 | 0.61 |
| Season (April – September) | 163 (63.4%) | 83 (63.9%) | 80 (63.0%) | 0.89 | 0.83 |
| Insurance status | |||||
| None | 93 (36.2%) | 48 (36.9%) | 45 (35.4%) | 0.96 | 0.81 |
| Medicaid | 117 (45.5%) | 59 (45.4%) | 58 (45.7%) | ||
| Private | 47 (18.3%) | 23 (17.7%) | 24 (18.9%) | ||
| Prior obstetrical history | |||||
| Preterm birth | 12 (4.7%) | 5 (3.9%) | 7 (5.5%) | 0.57 | – |
| Preeclampsia | 11 (4.3%) | 5 (3.9%) | 6 (4.7%) | 0.77 | – |
| Gestational diabetes | 7 (2.7%) | 4 (3.1%) | 3 (2.4%) | 1.00 | – |
| Diabetes (Type I or II) | 5 (2.0%) | 2 (1.5%) | 3 (2.4%) | 0.68 | – |
| Chronic hypertension | 2 (0.8%) | 0 | 2 (1.6%) | 0.24 | – |
| Subjective health rating | 9 (3 – 10) | 9 (3 – 10) | 10 (5 – 10) | 0.29 | 0.18 |
Missing data as follows: gestational age, n=3.
The mean maternal baseline 25(OH)D was 22.7 ng/mL (SD 9.7); this did not differ significantly between dose groups (direct comparison p=0.43; controlled for study site p=0.43; controlled for race p=0.51). Estimated mean baseline serum intact PTH (iPTH) was 16.4 pg/mL (SD 6.9) and mean serum calcium was 9.3 mg/dL (SD 0.3). Intact PTH and calcium differed minimally between dose groups, with participants randomized to the 4000 IU group being 0.2 pg/mL lower in PTH (p=0.76) and 0.1 mg/dL lower in calcium (p=0.74) than those randomized to the 2000 IU group.
There were significant differences in baseline 25(OH)D according to race: African-American participants had the lowest values, with an estimated mean of 18.5 ng/mL (SD 8.4), while Hispanic and Caucasian participants had notably higher mean values of 26.1 ng/mL (SD 7.5) and 29.5 ng/mL (SD 14.4), respectively (p-value for overall comparison < 0.001). Baseline iPTH and calcium values did not differ significantly by race (iPTH, p=0.11; calcium, p=0.40).
Primary Analysis
Primary endpoint
The overall maternal 25(OH)D change from baseline was estimated to be +14.1 ng/mL (SD 12.7) (p<0.001). The mean change from baseline for the 2000 and 4000 IU groups was +12.9 ng/mL (SD 12.8) and +15.4 ng/mL (SD 12.6), respectively (group comparison of change p=0.23; p=0.40 adjusted for race and study site). At the last study visit prior to delivery, the average 25(OH)D levels were 36.2 ng/mL (SD 15.0) in the 2000 IU group and 37.9 ng/mL (SD 13.5) in the 4000 IU group. The difference between the two groups at this timepoint was not statistically significant in direct comparisons (p=0.29) or after controlling for race and study site (p=0.47). Overall, 31/83 (37.4%) of the 2000 IU group, and 36/78 (46.2%) of the 4000 IU group, achieved 40 ng/mL at this timepoint (p=0.27), the level at which conversion of 25(OH)D to 1,25(OH)2D is optimized during pregnancy (7).
Neonatal 25(OH)D as Measured in Cord Blood
The mean cord blood 25(OH)D level, as measured on 144 infants, was 0.7 (SD 0.3) times that of maternal predelivery 25(OH)D. Overall, the mean cord blood 25(OH)D level was 24.5 ng/mL (SD 12.0); 22.1 ng/mL (SD 10.3) in the 2000 IU group and 27.0 ng/mL (SD 13.3) in the 4000 IU group (p=0.024). The correlation between the infants’ and mothers’ 25(OH)D levels was estimated to be r=0.68 (p<0.001).
Parathyroid Hormone and Calcium
Predelivery calcium values did not differ significantly between dose groups: the estimated mean values were 8.9 mg/dL (SD 0.4) and 9.0 mg/dL (SD 0.4) in the 2000- and 4000 IU groups, respectively (p=0.17). The estimated change from baseline was −0.3 mg/dL in the 2000 IU group and −0.2 mg/dL in the 4000 IU group (p=0.22, ANCOVA). The significance of these results did not change after controlling for race and study site. Predelivery iPTH values differed significantly between dose groups, with estimated mean values of 17.5 pg/mL (SD 8.2) and 15.2 pg/mL (SD 9.3) in the 2000- and 4000 IU groups (p=0.023). This difference remained significant (p=0.044) after controlling for race. The dose groups also differed slightly in their PTH change from baseline. Specifically, participants randomized to the 2000 IU/day group had a mean increase in PTH of 0.9 pg/mL (SD 8.0), while those randomized to the 4000 IU/day group had a mean decline in PTH of 1.1 pg/mL (SD 9.0) (p=0.034, ANCOVA). This finding became marginally significant after controlling for race and site (p=0.054).
Complications of pregnancy
Table 2 describes the frequency of common complications of pregnancy and compares them between randomization groups. Among the 161 participants who provided a primary endpoint measure, the most common complication was infection, experienced by 74 women (46.0%). The other complications occurring in more than 10% of women were preterm labor (37/161, 23.0%), gestational diabetes (18/161, 11.2%) and preterm delivery (19/161, 11.8%). A total of 50 participants had either preterm labor or preterm delivery (31.1%). The remaining conditions occurred in less than 3% of the participants. The frequency of occurrence did not differ significantly between randomization groups for any of the conditions considered; however, the frequency of preterm labor was marginally significantly different between groups (p=0.091) and became more significant (p=0.060) after controlling for the effects of race. The total number of complications differed significantly between groups (p=0.044); this comparison remained marginally significant (p=0.061) when controlling for race.
Table 2. Clinical Conditions Reported during Pregnancy, Among Trial Completers (n=161).
Clinical Conditions of Pregnancy as defined by the following: (1) Preterm labor as documented in medical record that included hospitalization greater than 24 hours. (2) Preterm delivery less than 37 0/7 weeks of gestation as documented in medical record. (3) Hypertensive Disorders of Pregnancy as documented in the medical record, which included any one of the following—gestational hypertension, preeclampsia, HELLP Syndrome, worsening hypertension in women with existing hypertension. (4) Infection, Any—a new infection that was documented in the medical record that included urinary tract infections, bacterial vaginosis, flu or flu-like illness, or any illness that required antibiotic or antifungal therapy (did not include preexisting herpes simplex infections for which antiviral therapy was prescribed). (5) Gestational Diabetes as documented in the medical record following glucose tolerance test (did not include those with preexisting type 1 or type 2 diabetes). 6. Non-Repeat Cesarean Section—mode of delivery by cesarean section that was not repeat cesarean or vaginal delivery. 7. Any Comorbidity Listed Above—any of the comorbidities 1–6 listed above.
| Medical Condition | Total Cohort N=161 |
2000 IU/day N=83 |
4000 IU/day N=78 |
p-value |
|---|---|---|---|---|
| Preterm labor | 37 (23.0%) | 24 (28.9%) | 13 (16.7%) | 0.091 |
| Preterm delivery (<37 wks) | 19 (11.8%) | 10 (12.1%) | 9 (11.5%) | 1.00 |
| Preterm labor or delivery | 50 (31.1%) | 29 (34.9%) | 21 (26.9%) | 0.31 |
| Hypertension | 4 (2.5%) | 3 (3.6%) | 1 (1.3%) | 0.62 |
| Infection | 74 (46.0%) | 41 (49.4%) | 33 (42.3%) | 0.43 |
| Gestational diabetes | 18 (11.2%) | 11 (13.3%) | 7 (9.0%) | 0.46 |
| Any above comorbidity | 107 (66.5%) | 58 (54.2%) | 49 (45.8%) | 0.40 |
| Total comorbidity count | 0.044 | |||
| No comorbidities | 54 | 25 (30.1%) | 29 (37.2%) | |
| 1 comorbidity | 61 | 31 (37.4%) | 30 (38.5%) | |
| 2 comorbidities | 21 | 8 (9.6%) | 13 (16.7%) | |
| 3 comorbidities | 20 | 15 (18.1%) | 5 (6.4%) | |
| 4 comorbidities | 5 | 4 (4.8%) | 1 (1.3%) | |
| Non-repeat Caesarean | 58 (36.0%) | 31 (53.5%) | 27 (46.6%) | 0.61 |
Missing data as follows: nonrepeat Caesarean, n=13. P-values for the comparison of 2000 IU/day vs. 4000 IU/day were obtained using Fisher’s exact test, except for the analysis of total comorbidity count, which used Poisson regression.
Neonatal growth parameters
Figure 2 (A and B) illustrates the distribution of neonatal weight and head circumference across the major Fenton percentiles for each dose group. There was an association between percentile and dose group for neonatal weight (p=0.018, proportional odds model), but not head circumference (p=0.79, proportional odds model). Specifically, the 4000 IU group participants had 2.40 (95% CI 1.26, 4.61) times the odds of having an infant in the 50th percentile, compared to the 2000 IU group. This effect remained significant after controlling for the effects of race and study site (p=0.006).
Figure 2. A and B: Percent of Infants by Treatment Group Plotted by Fenton Growth Curve Percentiles for Weight (A) and Head Circumference (B).
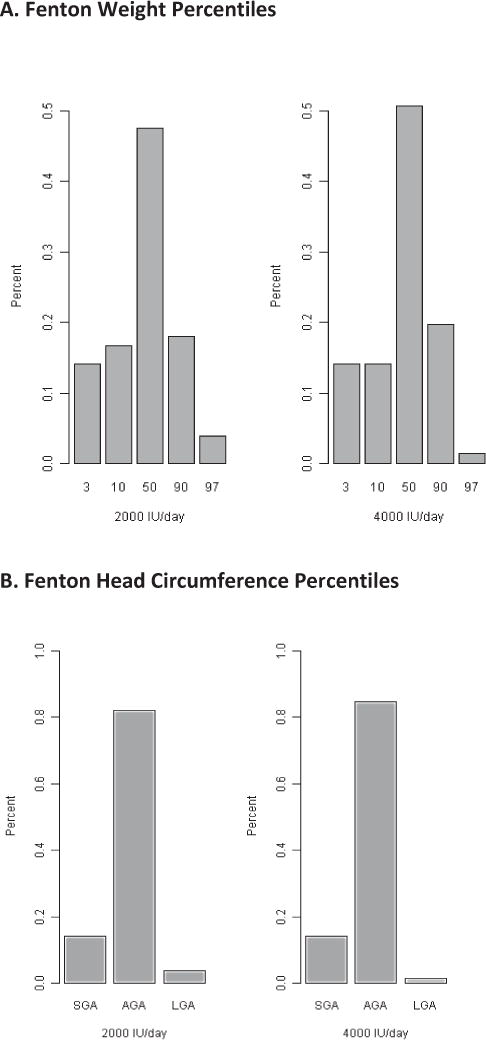
The percent of children within each treatment group who were at the 3rd, 10th, 50th, 90th, and 97th percentiles for weight (A) and head circumference (B). Less than the 10th percentile is defined as small-for-gestational age (SGA); 10–90th percentile as appropriate-for-gestational age (AGA); and >90th percentile as large-for-gestational age (LGA).
Safety parameters
Neither dose group experienced any instances of hypercalciuria or hypercalcemia. In longitudinal models assessing the relationship of 25(OH)D with serum calcium and phosphorus and urinary calcium, creatinine, and the calcium: creatinine ratio, statistically significant associations were observed with urinary calcium and urinary creatinine, after adjustment for race and study site. Specifically, we estimate that urinary calcium decreased 3.5 mg/dL per 10 ng/mL increase in 25(OH)D (p=0.02). Urinary creatinine decreased an estimated 16.5 mg/dL per 10 ng/mL increase in 25(OH)D (p=0.004). Figures 3 and 4 illustrate the time courses of serum calcium and the urinary calcium: creatinine ratios.
Figure 3.
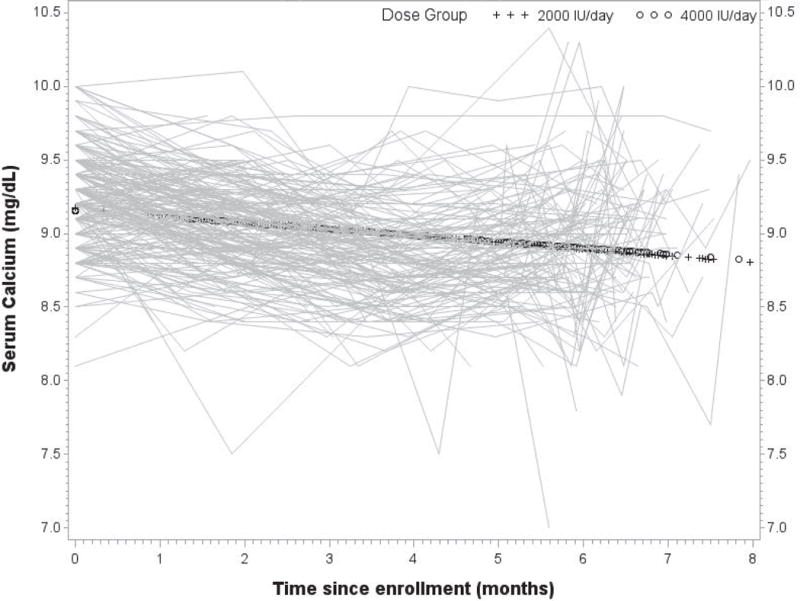
Longitudinal serum calcium (mg/dL) with group overlay by treatment group, with each participant’s serum calcium values plotted over time.
Figure 4.
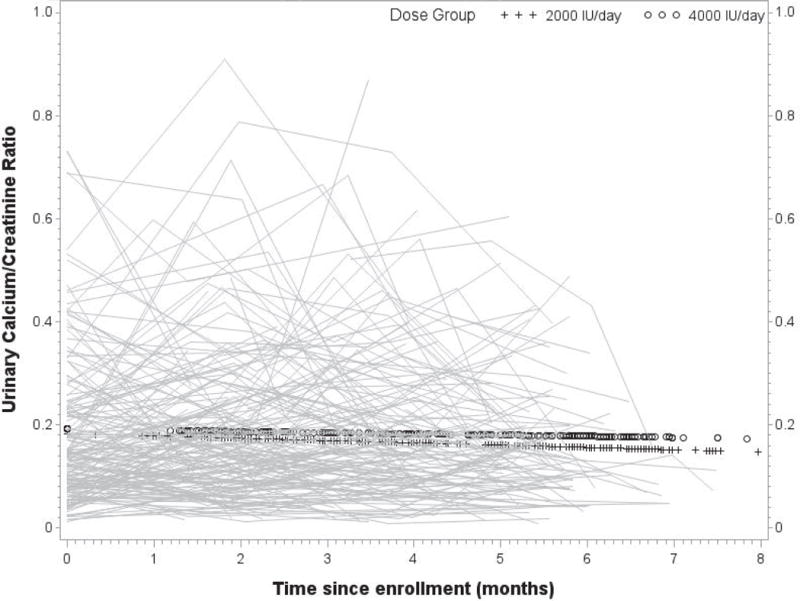
Longitudinal urinary calcium: creatinine ratios (mg/dL/mg/dL) with group overlay by treatment group, with each participant’s urinary calcium: creatinine ratios plotted over time.
Sensitivity Analysis
A sensitivity analysis was performed to determine the robustness of the primary findings to assumptions about the missing data. When all participants with missing final measurements were assumed to have experienced no change from baseline, the significance of our findings was unchanged in direction (p=0.69). However, when we assumed no change among those with missing endpoints in the 2000 IU group, but even a minimal 0.1 ng/mL change among those with missing endpoints in the 4000 IU group, the group comparison became statistically significant at a p-value of 0.025 or lower. Our most extreme imputation of the observed group medians, assuming that those with no final measurement were indistinguishable from the other members of their dose group, resulted in a highly significant group comparison at the p=0.0006 level.
Secondary Analyses
Serum 25(OH)D
Based on 1042 longitudinal measurements contributed by 257 participants, the monthly change in 25(OH)D level (Figure 5) was estimated to have a significant quadratic component (p<0.0001). This effect differed significantly between dose groups (p<0.01), such that estimated 25(OH)D levels of participants randomized to the 2000 IU group increased more slowly than the levels of those randomized to the 4000 IU group.
Figure 5.
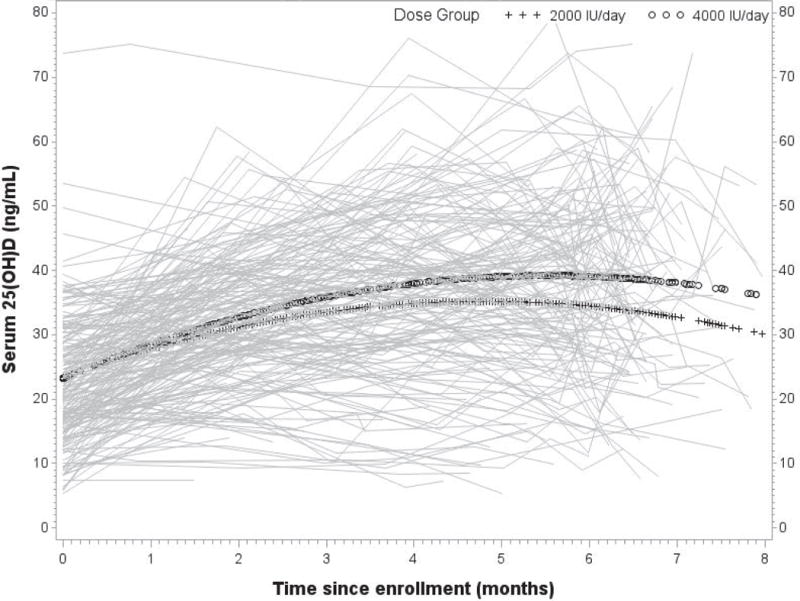
Longitudinal total circulating 25(OH)D (ng/mL) with group overlay by treatment
In an expanded model, race was a significant predictor of baseline status (p<0.0001) and 25(OH)D change over time (p<0.001), but not group differences in change over time. Baseline 25(OH)D level did not influence the change in 25(OH)D over time in a clinically significant manner. In the final longitudinal model of 25(OH)D, baseline values were estimated to be 19.2 ng/mL, 26.8 ng/mL, and 30.1 ng/mL among African-American, Hispanic, and Caucasian participants, respectively, assigned to the 2000 IU dose group. The 4000 IU dose group was estimated to have baseline values 0.11 ng/mL lower than the 2000 IU group (p=0.94). Model-based estimates of 25(OH)D levels over time are provided in Table 3 for each race-by-dose subgroup, assuming a six-month supplementation period.
Table 3. Estimated trajectory of serum 25(OH)D (ng/mL) according to dose group and race, based on quadratic model of serum 25(OH)D over time.
Model-based estimates of total circulating 25(OH)D concentrations over time for each race-by-dose subgroup.
| 2000 IU/day | 4000 IU/day | |||||
|---|---|---|---|---|---|---|
| Month | African-American | Hispanic | Caucasian | African-American | Hispanic | Caucasian |
| 0 | 19.2 | 26.8 | 30.1 | 19.0 | 26.7 | 30.0 |
| 1 | 24.6 | 30.1 | 33.4 | 25.5 | 31.0 | 34.3 |
| 2 | 28.8 | 32.7 | 36.1 | 30.5 | 34.4 | 37.9 |
| 3 | 31.6 | 34.4 | 38.1 | 34.2 | 37.0 | 40.7 |
| 4 | 33.2 | 35.4 | 39.6 | 36.4 | 38.6 | 42.8 |
| 5 | 33.5 | 35.6 | 40.4 | 37.2 | 39.4 | 44.1 |
| 6 | 32.5 | 35.1 | 40.5 | 36.6 | 39.2 | 44.7 |
Complications of pregnancy
Baseline 25(OH)D level was not predictive of complication occurrence except for preterm labor (OR=0.61 per 10 ng/mL, p=0.033) and combined preterm labor or delivery (OR=0.56 per 10 ng/mL, p=0.007), that became non-significant after controlling for race and study site (labor alone, OR=0.78 per 10 ng/mL, p=0.31; labor or delivery OR=0.64, p=0.052). Pre-delivery 25(OH)D was significantly predictive of preterm delivery (OR=0.56 per 10 ng/mL, p=0.004) and combined preterm labor or delivery (OR=0.73 per 10 ng/mL, p=0.015), which remained significant (delivery alone, OR=0.51 per 10 ng/mL, p=0.002; labor or delivery OR=0.73 per 10 ng/mL, p=0.018) after controlling for the effects of race and study site. Additional analyses by mean 25(OH)D (from 3rd visit onward) indicated negative associations with preterm delivery (OR=0.46 per 10 ng/mL, p=0.001) and infection (OR=0.74 per 10 ng/mL, p=0.026), after controlling for race and study site.
Comment
In this randomized trial of vitamin D supplementation in a diverse group of women receiving prenatal care at two community health center networks in South Carolina, women receiving daily doses of 2000 and 4000 IU did not differ in their pre-delivery vitamin D status; however, 4000 IU/day was superior to 2000 IU/day in raising maternal vitamin D status over time, achieving neonatal vitamin D sufficiency, and was associated with lower maternal intact PTH. While caution must be given when interpreting these findings, as the study was not designed as an equivalence study, but rather, to demonstrate superiority of one dose over the other, secondary analyses did show a dose-dependent effect with respect to preterm labor, preterm birth and risk of infection.
Although it was expected that both the 2000 and 4000 IU vitamin D/day groups would experience a rise in circulating 25(OH)D levels, with the greatest rise in those women in the 4000 IU group, there was concern that the 4000 IU group would have greater risk of hypervitaminosis D, which did not occur. In neither the 2000 nor the 4000 IU arms of the study were there any episodes of hypercalciuria or hypercalcemia. Urinary calcium-to-creatinine ratios and serum calcium, phosphorus, and creatinine levels were comparable in both groups and were well within normal limits for adults, including pregnant women. This is similar to our findings in the NICHD supplementation trial (7).
Based on national and regional data, it is clear that vitamin D deficiency is an emerging health issue that affects all ethnic and racial groups in the U.S., but more significantly those with darker pigmentation such as African Americans and Hispanics (4, 31). No group more vulnerable, the pregnant woman and her unborn fetus represents an important and often neglected segment of the U.S. population (26). It is during pregnancy that fetal cell signaling patterns are set into motion, affecting lifelong health status (28, 32–34). Most recently, the link between vitamin D deficiency and extraskeletal systems has been suggested: vitamin D deficiency is associated with acute and chronic infections (35–40), and later, lifelong sequelae, with notably increased risk of autoimmune diseases such as rheumatoid arthritis (41), systemic lupus erythematosus (42), multiple sclerosis (43, 44), type I diabetes (45, 46), and certain cancers (45, 47–52). Specific to the pregnant woman and her fetus, epidemiological studies report an association between vitamin D deficiency and an increased risk of hypertensive disorders of pregnancy, including preeclampsia (53–62), preterm birth (56), and mode of delivery (63). Additionally, chronic vitamin D deprivation in the mother and fetus appears to have lasting effects on skeletal integrity (26, 64–66) and fetal growth (67). These findings are supported by the earlier NICHD study (7, 68) and by this study, where secondary analyses found a suggestive pattern of associations between total circulating 25(OH)D and preterm labor, preterm birth and infection, even after controlling for race. In addition, neonates in the 4000 IU group were more likely to be average for gestational age by weight (AGA) than the 2000 IU group, which may reflect maternal glucose homeostasis, as larger babies are traditionally associated with gestational diabetes (69). We note, however, that the maternal associations were identified in secondary analyses, as the study was not powered to detect changes in the rate of complications as primary endpoints.
There were certain limitations in this study, the most notable being the nonadherence to protocol of some of the subjects. Women who were nonadherent would have a lower rise in their circulating 25(OH)D compared to women who were adherent. Thus, the treatment effect was reduced as a whole. The decline in 25(OH)D during the last trimester, possibly due to increased conversion to 1,25(OH)2D, may also reflect the cumulative drop-out of subjects during the course of pregnancy.
In evaluating our findings in the context of earlier studies, it is interesting to note that Mallet et al. (70) reported vitamin D supplementation of 1,000 IU/day during the last trimester of pregnancy resulted in only a 5–6 ng/ml increase in circulating 25(OH)D levels in maternal and cord serum. Similarly, Datta et al., in their study of 160 pregnant minority women in England who were provided with 800-1,600 IU vitamin D for the duration of their pregnancy found that circulating 25(OH)D levels (ng/mL) rose from 5.8 (SD 0.9) at the beginning of pregnancy to 11.2 (SD 6.3) at term (71). The Vieth et al (72) and Heaney et al (73, 74) data, and our own data with pregnant and lactating women (7, 75), have shown that doses exceeding 1,000 IU vitamin D/day (2,000–10,000 IU/day) are required to achieve a robust nutritional vitamin D status. Further, on a per kilogram basis, 400 IU has been shown to be adequate to achieve normal vitamin D status in neonates and infants; however, for a pregnant woman weighing on average 20 times that of a newborn infant receiving the same dose of vitamin D—namely 400 IU/day, that dose is irrelevant (7, 75–77).
The nutritional vitamin D status of the neonate is completely dependent on the vitamin D stores of the mother (7, 68, 78). This premise was reconfirmed by this study. Thus, if the mother is hypovitaminotic D, her infant will show depleted vitamin D stores. The effects of acute vitamin D deprivation are known to result in rickets in the rapidly growing child and osteopenia and osteoporosis in the mother. This result is especially prevalent in darker pigmented individuals, as reflected in a steady and significant rise in nutritional rickets in breastfed infants, mainly in the African American population (79–83). As was shown by this study and an earlier study, substantially improving the nutritional vitamin D status of the neonate at birth through higher dose maternal supplementation of vitamin D during pregnancy—above the suggested 400 or 600 IU/day—to achieve maternal sufficiency should alleviate this problem. Thus, we strongly believe that the current RDA for vitamin D in all age groups, but particularly pregnant women with darker pigmentation, is inadequate.
Detection of vitamin D deficiency early in pregnancy has implications for prevention and intervention, yet such studies have only recently been undertaken (26). Defining the prevalence of vitamin D deficiency in pregnant women and the optimal vitamin D supplementation strategies for these women and their developing infants will help to establish a prototype for recommendations applicable to other communities throughout the U.S. A reexamination of dietary vitamin D requirements of various vulnerable populations in the U.S., including pregnant women, was recommended by a Cochrane Review in 2002 (84), again in 2012 (85), and by the IOM in their 2010 statement (9).
Taken together with our findings from the NICHD vitamin D pregnancy study (7), 4000 IU vitamin D per day appears to be safe and effective in improving vitamin D levels over time. In addition, increased circulating 25(OH)D levels were associated with reduced occurrence of co-morbidities of pregnancy. As such, this study serves as the first step in establishing normative guidelines for vitamin D supplementation during pregnancy in a diverse group of women in a community health care setting. By evaluating the vitamin D status of pregnant women and their newborn infants who received care at community health centers, our findings serve as a model for other community health care centers in U.S. aimed at improving overall health status of its members, including alleviation of the growing problem of hypovitaminosis D throughout the U.S. Further, our findings are clinically relevant to community health centers and to those involved in the general health care of pregnant women and their neonates and infants throughout the U.S. Additional studies of vitamin D supplementation during pregnancy are warranted to determine optimal dosing and the physiological effects of vitamin D on both the mother and her developing fetus.
Acknowledgments
Sources of Financial Support: Funded in part by the Thrasher Research Fund; NIH RR01070 and UL1 RR029882; and MUSC Children’s Hospital Fund, and the Division of Neonatology, Medical University of South Carolina, Charleston, SC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the Center for Disease Control vitamin D meeting in Atlanta, GA in April 2010 and at the Pediatric Academic Societies Meeting in Vancouver, Canada in May 2010.
Disclosure: Dr. Bruce Hollis served as a scientific consultant for Diasorin, Inc., Stillwater, MN during the study period.
To convert mg/dL of calcium to mmol/L, multiply the value by 0.25. To convert mg/dL of creatinine to mmol/L, multiply by 0.088.
References
- 1.Vieth R, Bischoff-Ferrari H, Boucher B, Dawson-Hughes B, Garland C, Heaney R, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85:649–50. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton S, McNeil R, Hollis B, Davis D, Winkler J, Cook C, et al. Profound Vitamin D deficiency in a diverse group of women during pregnancy living in a sun-rich environment at latitude 32°N. International J Endocrinol. 2010 doi: 10.1155/2010/917428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson DD, Wagner CL, Hulsey TC, McNeil RB, Ebeling M, Hollis BW. Vitamin D Deficiency and Insufficiency is Common during Pregnancy. Am J Perinatol. 2011;28(1):7–12. doi: 10.1055/s-0030-1262505. Epub 2010/07/20. [DOI] [PubMed] [Google Scholar]
- 4.Nesby-O’Dell S, Scanlon K, Cogswell M, Gillespie C, Hollis B, Looker A, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: Third National Health and Nutrition Examination Survey: 1988–1994. Amer J Clin Nutr. 2002;76:187–92. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton SA, McNeil R, Hollis BW, Davis DJ, Winkler J, Cook C, et al. Profound Vitamin D Deficiency in a Diverse Group of Women during Pregnancy Living in a Sun-Rich Environment at Latitude 32 degrees N. Int J Endocrinol. 2010;2010:917428. doi: 10.1155/2010/917428. Epub 2011/01/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawodu A, Wagner CL. Mother-child vitamin D deficiency: an international perspective. Arch Dis Child. 2007;92(9):737–40. doi: 10.1136/adc.2007.122689. Epub 2007/08/24. doi: 92/9/737 [pii] 10.1136/adc.2007.122689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollis B, Johnson D, Hulsey T, Ebeling M, Wagner C. Vitamin D Supplementation during Pregnancy: Double Blind, Randomized Clinical Trial of Safety and Effectiveness. Journal of Bone and Mineral Research. 2011 doi: 10.1002/jbmr.463. http://onlinelibrary.wiley.com/doi/10.1002/jbmr.463/pdf. Epub June 2011. [DOI] [PMC free article] [PubMed]
- 8.Institute of Medicine. Subcommittee on Nutritional Status and Weight Gain during Pregnancy: Nutrition During Pregnancy. Washington, D.C.: National Academy Press; 1990. Calcium, Vitamin D, and Magnesium; pp. 318–35. [Google Scholar]
- 9.Food and Nutrition Board. Dietary Reference Intakes for Vitamin D and Calcium. Washington, D.C.: National Academy Press; 2010. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. [Google Scholar]
- 10.Johnson DD, Wagner CL, Hulsey TC, McNeil RB, Ebeling M, Hollis BW. Vitamin D Deficiency and Insufficiency is Common during Pregnancy. Am J Perinatol. 2010 Jul 16; doi: 10.1055/s-0030-1262505. Epub 2010/07/20. [DOI] [PubMed] [Google Scholar]
- 11.Delvin EE, Salle BL, Glorieux FH, Adeleine P, David LS. Vitamin D supplementation during pregnancy: effect on neonatal calcium homeostasis. J Pediatr. 1986;109(2):328–34. doi: 10.1016/s0022-3476(86)80396-1. [DOI] [PubMed] [Google Scholar]
- 12.Brooke OG, Brown IRF, Bone CDM, Carter ND, Cleeve HJW, Maxwell JD, et al. Vitamin D supplements in pregnant Asian women: Effects on calcium status and fetal growth. Br Med J. 1980;1:751–4. doi: 10.1136/bmj.280.6216.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maxwell JD, Ang L, Brooke OG, Brown IRF. Vitamin D supplements enhance weight gain and nutrional status in pregnant Asians. Brit J Obstet Gyneaecol. 1981;88:987–91. doi: 10.1111/j.1471-0528.1981.tb01686.x. [DOI] [PubMed] [Google Scholar]
- 14.Brooke OG, Butters F, Wood C. Intrauterine vitamin D nutrition and postnatal growth in Asian infants. Brit Med J. 1981;283:1024. doi: 10.1136/bmj.283.6298.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appelgren KE, Nietert PJ, Hulsey TC, Hollis BW, Wagner CL. Analyzing adherence to prenatal supplement: does pill count measure up? Int J Endocrinol. 2010;2010:631971. doi: 10.1155/2010/631971. Epub 2010/02/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Block G, DiSogra C. WIC dietary assessment validation study, final report. Alexandria, VA: Food and Nutrition Service, U.S. Department of Agriculture; 2001. Validation, self-administered, in low-income white, African-American and Hispanic women. [Google Scholar]
- 17.Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc. 1992;92:686–93. [PubMed] [Google Scholar]
- 18.Mares-Perlman JA, Klein BEK, Klein R, Ritter LL, Fisher MR, Freudenheim JL. A diet history questionnaire ranks nutrient intakes in middle-aged and older men and women similarly to multiple food records. J Nutr. 1993;123:489–501. doi: 10.1093/jn/123.3.489. [DOI] [PubMed] [Google Scholar]
- 19.Coates RJ, Eley JW, Block G, Gunter EW, Sowell AL, Grossman C. An evaluation of a food frequency questionnaire for assessing dietary intake of specific carotenoids and vitamin E among low-income black women. Am J Epidemiol. 1991;134:658–71. doi: 10.1093/oxfordjournals.aje.a116138. [DOI] [PubMed] [Google Scholar]
- 20.Sinha R, Patterson BH, Mangels AR, Levander OA, Gibson T, Taylor PR, et al. Determinants of plasma vitamin E in health males. Cancer Epidemiology Biomarkers and Prevention. 1993;2:473–9. [PubMed] [Google Scholar]
- 21.Sobell J, Block G, Koslowe P, Tobin J, Andres R. Validation of a retrospective questionnaire assessing diet 10–15 years ago. Am J Epidemiol. 1989;130:173–87. doi: 10.1093/oxfordjournals.aje.a115310. [DOI] [PubMed] [Google Scholar]
- 22.Fenton TR, Sauve RS. Using the LMS method to calculate z-scores for the Fenton preterm infant growth chart. Eur J Clin Nutr. 2007;61(12):1380–5. doi: 10.1038/sj.ejcn.1602667. [DOI] [PubMed] [Google Scholar]
- 23.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD. Determination of vitamin D status by radioimmunoassay with a 125I-labeled tracer. Clin Chem. 1993;39:529–33. [PubMed] [Google Scholar]
- 24.Laboratories MC. Laboratory Reference Data. Rochester, MN: Mayo Clinic; 2003. [Google Scholar]
- 25.Hollis BW, Wagner CL, Kratz A, Sluss PM, Lewandrowski KB. Normal serum vitamin D levels. Correspondence. N Engl J Med. 2005;352:515–6. doi: 10.1056/NEJM200502033520521. [DOI] [PubMed] [Google Scholar]
- 26.Hollis BW, Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. Am J Clin Nutr. 2004;79:717–26. doi: 10.1093/ajcn/79.5.717. [DOI] [PubMed] [Google Scholar]
- 27.Hollis B. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–22. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 28.Hollis BW, Wagner CL, Vitamin D. requirements during lactation: High-dose maternal supplementation as therapy to prevent hypovitaminosis D in both mother and nursing infant. Amer J Clin Nutr. 2004;80S:1752S–8S. doi: 10.1093/ajcn/80.6.1752S. [DOI] [PubMed] [Google Scholar]
- 29.Vieth R, Ladak Y, Walfish P. Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J Clin Endocrinal Metab. 2003;88:185–91. doi: 10.1210/jc.2002-021064. [DOI] [PubMed] [Google Scholar]
- 30.Singer J, Willett J. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 31.Reasner C, Dunn J, Fetchick D, Liel Y, Hollis B, Epstein S, et al. Alteration in vitamin D metabolism in Mexican-Americans. J Bone Mineral Res. 1990;5(1):13–7. doi: 10.1002/jbmr.5650050105. [DOI] [PubMed] [Google Scholar]
- 32.Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJP. Early growth and coronary heart disease in later life: longitudinal study. BMJ. 2001;322(7292):949–53. doi: 10.1136/bmj.322.7292.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dennison E, Arden N, Keen R, Syddall H, Day I, Spector T, et al. Birthweight, vitamin D receptor genotype and the programming of osteoporosis. Paediatr Perinat Epidemiol. 2001;15(3):211–9. doi: 10.1046/j.1365-3016.2001.00350.x. [DOI] [PubMed] [Google Scholar]
- 34.Ko P, Burkert R, McGrath J, Eyles D. Maternal vitamin D3 deprivation and the regulation of apoptosis and cell cyle during rat brain development. Dev Brain Res. 2004;153:61–8. doi: 10.1016/j.devbrainres.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Coussens AK, Wilkinson RJ, Hanifa Y, Nikolayevskyy V, Elkington PT, Islam K, et al. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proceedings of the National Academy of Sciences of the United States of America. 2012 doi: 10.1073/pnas.1200072109. Epub 2012/09/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 37.Laaksi I, Ruohola JP, Tuohimaa P, Auvinen A, Haataja R, Pihlajamaki H, et al. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86(3):714–7. doi: 10.1093/ajcn/86.3.714. Epub 2007/09/08. doi: 86/3/714 [pii] [DOI] [PubMed] [Google Scholar]
- 38.McNally JD, Leis K, Matheson LA, Karuananyake C, Sankaran K, Rosenberg AM. Vitamin D deficiency in young children with severe acute lower respiratory infection. Pediatr Pulmonol. 2009;44(10):981–8. doi: 10.1002/ppul.21089. [DOI] [PubMed] [Google Scholar]
- 39.Liu NQ, Hewison M. Vitamin D, the placenta and pregnancy. Archives of biochemistry and biophysics. 2012;523(1):37–47. doi: 10.1016/j.abb.2011.11.018. Epub 2011/12/14. [DOI] [PubMed] [Google Scholar]
- 40.Liu NQ, Kaplan AT, Lagishetty V, Ouyang YB, Ouyang Y, Simmons CF, et al. Vitamin D and the regulation of placental inflammation. J Immunol. 2011;186(10):5968–74. doi: 10.4049/jimmunol.1003332. Epub 2011/04/13. [DOI] [PubMed] [Google Scholar]
- 41.Merlino L, Curtis J, Mikuls T, Cerhan J, Criswell L, Saag K. Vitamin D intake is inversely associated with rheumatoid arthritis. Arthritis & Rheumatism. 2004;50(1):72–7. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- 42.Kamen DL, Cooper GS, Bouali H, Shaftman SR, Hollis BW, Gilkeson GS. Vitamin D deficiency in systemic lupus erythematosus. Autoimmun Rev. 2006;5(2):114–7. doi: 10.1016/j.autrev.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Hayes CE. Vitamin D: a natural inhibitor of multiple sclerosis. Proc Nutr Soc. 2000;59:531–5. doi: 10.1017/s0029665100000768. [DOI] [PubMed] [Google Scholar]
- 44.Munger K, Zhang S, O’Reilly E, Hernan M, Olek M, Willett W, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60–5. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 45.Holick MF. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–71. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 46.Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet. 2001;358:1500–03. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 47.Lefkowitz E, Garland C. Sunlight, vitamin D, and ovarian cancer mortality rates in US women. Int J Epidemiol. 1994;23:1133–6. doi: 10.1093/ije/23.6.1133. [DOI] [PubMed] [Google Scholar]
- 48.Garland F, Garland C, Gorham E, Young J. Geographic variation in breast cancer mortality in the United States: A hypothesis involving exposure to solar radiation. Prev Med. 1990;19:614–22. doi: 10.1016/0091-7435(90)90058-r. [DOI] [PubMed] [Google Scholar]
- 49.Garland C, Comstock G, Garland F, Helsing K, Shaw E, Gorham E. Serum 25(OH)D and colon cancer: Eight-year prospective study. Lancet. 1989;2:1176–8. doi: 10.1016/s0140-6736(89)91789-3. [DOI] [PubMed] [Google Scholar]
- 50.Grant WB. An estimate of premature cancer mortality in the US due to inadequate doses of solar ultraviolet-B radiation. Cancer. 2002;94:1867–75. doi: 10.1002/cncr.10427. [DOI] [PubMed] [Google Scholar]
- 51.Grant WB. An ecologic study of dietary and solar ultraviolet-B links to breast carcinoma mortality rates. Cancer. 2002;94:272–81. doi: 10.1002/cncr.10196. [DOI] [PubMed] [Google Scholar]
- 52.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Vitamin D Round Table Nutritional Aspects of Osteoporosis. 2. Elsevier Science; 2004. pp. 263–70. [Google Scholar]
- 53.Powe CE, Seely EW, Rana S, Bhan I, Ecker J, Karumanchi SA, et al. First Trimester Vitamin D, Vitamin D Binding Protein, and Subsequent Preeclampsia. Hypertension. 2010 doi: 10.1161/hypertensionaha.110.158238. HYPERTENSIONAHA.110.158238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robinson CJ, Alanis MC, Wagner CL, Hollis BW, Johnson DD. Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. Am J Obstet Gynecol. 2010;203(4):366 e1–6. doi: 10.1016/j.ajog.2010.06.036. Epub 2010/08/10. doi: S0002-9378(10)00811-2 [pii] 10.1016/j.ajog.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baker AM, Haeri S, Camargo CA, Jr, Espinola JA, Stuebe AM. A nested case-control study of midgestation vitamin D deficiency and risk of severe preeclampsia. J Clin Endocrinol Metab. 2010;95(11):5105–9. doi: 10.1210/jc.2010-0996. Epub 2010/08/20. doi: jc.2010-0996 [pii] 10.1210/jc.2010-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bodnar LM, Simhan HN. Vitamin D may be a link to black-white disparities in adverse birth outcomes. Obstet Gynecol Surv. 2010;65(4):273–84. doi: 10.1097/OGX.0b013e3181dbc55b. Epub 2010/04/21. doi: 0006254-201004000-00023 [pii] 10.1097/OGX.0b013e3181dbc55b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Halhali A, Diaz L, Avila E, Ariza AC, Garabedian M, Larrea F. Decreased fractional urinary calcium excretion and serum 1,25-dihydroxyvitamin D and IGF-I levels in preeclampsia. The Journal of Steroid Biochemistry and Molecular Biology. 2007;103(3–5):803–6. doi: 10.1016/j.jsbmb.2006.12.055. [DOI] [PubMed] [Google Scholar]
- 58.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal Vitamin D Deficiency Increases the Risk of Preeclampsia. 2007. pp. 3517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal Vitamin D Deficiency Increases the Risk of Preeclampsia. 2007. pp. 3517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hypponen E. Vitamin D for the prevention of preeclampsia? A hypothesis. Nutr Rev. 2005;63(7):225–32. doi: 10.1301/nr.2005.jul.225-232. [DOI] [PubMed] [Google Scholar]
- 61.Halhali A, Tovar AR, Torres N, Bourges H, Garabedian M, Larrea F. Preeclampsia is associated with low circulating levels of insulin-like growth factor 1 and 1,25-dihydroxyvitamin D in maternal and umbilical cord compartments. J Clin Endocrinol. 2000;85(5):1828–2833. doi: 10.1210/jcem.85.5.6528. [DOI] [PubMed] [Google Scholar]
- 62.Fischer D, Schroer A, Ludders D, Cordes T, Bucker B, Reichrath J, et al. Metabolism of vitamin D3 in the placental tissue of normal and preeclampsia complicated pregnancies and premature births. Clin Exp Obstet Gynecol. 2007;34(2):80–4. [PubMed] [Google Scholar]
- 63.Merewood A, Mehta SD, Chen TC, Bauchner H, Holick MF. Association between vitamin D deficiency and primary cesarean section. J Clin Endocrinol Metab. 2009;94(3):940–5. doi: 10.1210/jc.2008-1217. Epub 2008/12/25. doi: jc.2008-1217 [pii] 10.1210/jc.2008-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laboratories MC. Laboratory Reference Data. Rochester, MN: Mayo Clinic; 2004. [Google Scholar]
- 65.Delvin EE, Salle L, Glorieux FH, Adeleine P, David LS. Vitamin D suppementation during pregnancy : effect on neonatal calcium homeostasis. J Pediatr. 1986;109:328–34. doi: 10.1016/s0022-3476(86)80396-1. [DOI] [PubMed] [Google Scholar]
- 66.Marie P, Cancela L, LeBoulch N, Miravet L. Bone changes due to pregnancy and lactation: Influence of vitamin D status. Amer J Physiol. 1986;251:E400–E6. doi: 10.1152/ajpendo.1986.251.4.E400. [DOI] [PubMed] [Google Scholar]
- 67.Bodnar LM, Catov JM, Zmuda JM, Cooper ME, Parrott MS, Roberts JM, et al. Maternal Serum 25-Hydroxyvitamin D Concentrations Are Associated with Small-for-Gestational Age Births in White Women. J Nutr. 2010;140(5):999–1006. doi: 10.3945/jn.109.119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hollis BW, Wagner CL, Vitamin D. Pregnancy: Skeletal Effects, Nonskeletal Effects, and Birth Outcomes. Calcified Tissue International. 2012 doi: 10.1007/s00223-012-9607-4. Epub 2012/05/25. [DOI] [PubMed] [Google Scholar]
- 69.Burris HH, Rifas-Shiman SL, Kleinman K, Litonjua AA, Huh SY, Rich-Edwards JW, et al. Vitamin D deficiency in pregnancy and gestational diabetes mellitus. American Journal of Obstetrics and Gynecology. 2012 doi: 10.1016/j.ajog.2012.05.022. Epub 2012/06/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mallet E, Gugi B, Brunelle P, Henocq A, Basuyau JP, Lemeur H. Vitamin D supplementation in pregnancy: A controlled trial of two methods. Obstet Gynecol. 1986;68:300–4. doi: 10.1097/00006250-198609000-00002. [DOI] [PubMed] [Google Scholar]
- 71.Datta S, Alfaham M, Davies D, Winston J, Woodlead S, Evans J, et al. Vitamin D deficiency in pregnant women from a non-European ethnic minority population – an international study. Brit J Obstet Gyneaecol. 2002;109:905–8. doi: 10.1111/j.1471-0528.2002.01171.x. [DOI] [PubMed] [Google Scholar]
- 72.Vieth R, Chan PCR, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level (LOAEL) Amer J Clin Nutr. 2001;73(2):288–94. doi: 10.1093/ajcn/73.2.288. [DOI] [PubMed] [Google Scholar]
- 73.Heaney R, Davies K, Chen T, Holick M, Barger-Lux M. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–10. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 74.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–10. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 75.Wagner CL, Hulsey TC, Fanning D, Ebeling M, Hollis BW. High-dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: a 6-month follow-up pilot study. Breastfeed Med. 2006;1(2):59–70. doi: 10.1089/bfm.2006.1.59. Epub 2007/07/31. [DOI] [PubMed] [Google Scholar]
- 76.Wagner CL, Howard C, Hulsey TC, Lawrence RA, Taylor SN, Will H, et al. Circulating 25-hydroxyvitamin d levels in fully breastfed infants on oral vitamin d supplementation. Int J Endocrinol. 2010;2010:235035. doi: 10.1155/2010/235035. Epub 2010/01/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26(10):2341–57. doi: 10.1002/jbmr.463. Epub 2011/06/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hollis BW, Pittard WB. Evaluation of the total fetomaternal vitamin D relationships at term: Evidence for racial differences. J Clin Endorcrinol Metab. 1984;59:652–7. doi: 10.1210/jcem-59-4-652. [DOI] [PubMed] [Google Scholar]
- 79.Bachrach S, Fisher J, Parks JS. An outbreak of vitamin D deficiency rickets in a susceptible population. Pediatrics. 1979;64:871–7. [PubMed] [Google Scholar]
- 80.Sills I, Skuza K, Horlick M, Schwartz M, Rapaport R. Vitamin D deficiency rickets. Reports of its demise are exaggerated. Clin Pediatrics. 1994;33:491–3. doi: 10.1177/000992289403300808. [DOI] [PubMed] [Google Scholar]
- 81.Eugster EA, Sane KS, Brown DM. Need for a policy change to support vitamin D supplementation. Minnesota Med. 1996;79:29–32. [PubMed] [Google Scholar]
- 82.Kreiter S. AMB News and Views The Newsletter of the Academy of Breastfeeding Medicine. 2001. The reemergence of vitamin D deficiency rickets-the need for vitamin D supplementation. [Google Scholar]
- 83.Kreiter SR, Schwartz RP, Kirkman HN, Charlton PA, Calikoglu AS, Davenport ML. Nutritional rickets in African American breast-fed infants. J Pediatr. 2000;137:153–7. doi: 10.1067/mpd.2000.109009. [DOI] [PubMed] [Google Scholar]
- 84.Mahomed K, Gulmezoglu AM. Vitamin D supplementation in pregnancy (Cochrane Review) Cochrane Database of Systematic Reviews. Chicester, UK: John Wiley & Sons, Ltd; 1999. [Google Scholar]
- 85.De-Regil LM, Palacios C, Ansary A, Kulier R, Pena-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane database of systematic reviews. 2012;2:CD008873. doi: 10.1002/14651858.CD008873.pub2. Epub 2012/02/18. [DOI] [PMC free article] [PubMed] [Google Scholar]


