Abstract
Pregnancy is a critical time in the lifecycle of a woman where she is responsible not only for her own well-being, but also that of her developing fetus, a process that continues during lactation. Until recently, the impact of vitamin D status during this period had not been fully appreciated. Data regarding the importance of vitamin D in health have emerged to challenge traditional dogma, and suggest that vitamin D – through its effect on immune function and surveillance – plays a role beyond calcium and bone metabolism on the health status of both the mother and her fetus. Following birth, this process persists; the lactating mother continues to be the main source of vitamin D for her infant. Thus, during both pregnancy and lactation, maternal deficiency predicts fetal and infant deficiency; the significance of this is just beginning to be understood and will be highlighted in this review.
Keywords: breastfeeding, cholecalciferol, infant, innate immunity, lactation, neonate, pregnancy, vitamin D, women's health
Pregnancy represents a time of rapid change – changes in physical proportions, physiology and responsibility. During this time in a woman's lifecycle, she is responsible not only for her own well-being and health but also for that of her developing fetus. While the ‘right diet’ and the ‘right lifestyle’ cannot ensure a healthy baby at birth 100% of the time, the ‘wrong diet’ and the ‘wrong lifestyle’ such as diets lacking folate [1] or iron [2], and lifestyles involving alcohol and cigarettes are associ- ated with higher rates of congenital anomalies, adverse pregnancy outcomes, and direct sequelae in the offspring exposed to such ‘wrong’ con- ditions. Some aspects are more obvious to us because we see the direct effect or manifestation of the lack of a nutrient or the excess of an environmental toxin such as cigarette smoke (with impaired fetal growth), or the stigmata of fetal alcohol exposure (manifesting as fetal alcohol syndrome). Yet, in other cases, the effect of nutrient deprivation may be more subtle and take years to unfold (e.g., vitamin B12 deficiency). In addition, what was understood about a particular nutrient and its effect on maternal health during pregnancy and lactation has probably changed in the last decade with advances in molecular and cellular techniques that have helped to more fully define the effects of nutrient deprivation on gene expression and associated cell function. Such is the case with vitamin D, which has emerged from its obscure place in science as a forgotten vitamin that was only associated with bone and calcium metabo- lism to become one of the most celebrated and controversial vitamins/micronutrients in both medical and lay literature today [3].
To understand the role of vitamin D and its metabolites during pregnancy and lactation, one must first review the physiology of vitamin D – how it is made in the body through sunlight exposure, how and when it can be obtained by diet, and in what manner both forms – endogenous and dietary – are processed by the body. Following a brief overview of vitamin D physiology, we will review the cur- rently known effects of vitamin D deficiency on pregnancy outcomes and the later sequelae on both the mother and infant if deficiency con- tinues during lactation. Lastly, we will review the evidence from randomized controlled trials of improving pregnancy and lactation outcomes with vitamin D supplementation.
Overview of general vitamin D physiology & metabolism
There are two forms of vitamin D: D2 and D3. Vitamin D2, or ergocalciferol, is made by plants and vitamin D3, or cholecalciferol, is made by animals, including humans. Both forms require UV light, specifically UVB in the spectrum of 280–320 nm to catalyze the reaction. (For the remainder of this review, vitamin D will be referred to as vitamin D3 unless otherwise mentioned).
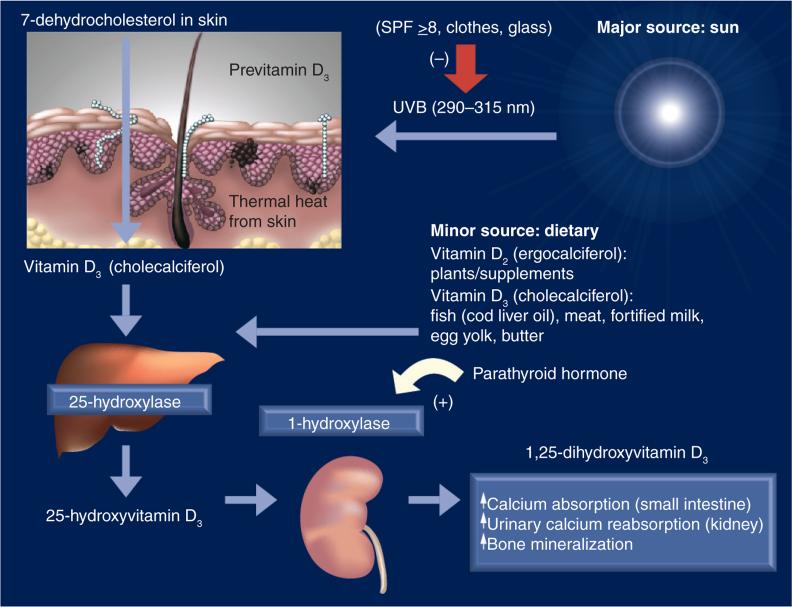
As shown in Figure 1, the first step in the formation of vitamin D is the conversion of epidermal 7-dehydrocholesterol to previtamin D following UVB exposure. Like other steroid hormones in the body whose main substrate is cholesterol, vitamin D requires a derivative of cholesterol, its 7-dehydrocholesterol precursor, but also sunlight at a specific wavelength and spectrum. This is a key point because without sunlight exposure we are dependent solely on dietary sources of vitamin D, which, except in rare cases, only account for up to 10% of the vitamin D in the body. This is supported by the findings of a recent vitamin D supplementation study involving over 494 women of diverse racial and ethnic backgrounds in South Carolina, USA who had completed the Block Food Frequency questionnaire, where the average daily diet provided only 200 IU/day vitamin D [4]. By comparison, following 10–15 min whole-body exposure of direct sunlight, the body generates 10,000–15,000 IU vitamin D within 24 h [5]. It is not surprising, then, that those living at or near the equator with 5–9 h of sunlight exposure have a mean total circulating 25-hydroxy vitamin D (25[OH]D) level almost twice that of most individuals living in the USA [6].
Figure 1. Vitamin D synthesis pathway.
SPF: Sun protection factor.
Reproduced with permission from [155].
Once formed, previtamin D undergoes a thermal reaction and is converted into vitamin D. It is then carried into the circulation mainly by the vitamin D-binding protein (VDBP), an α-globulin with a molecular weight of 58 kD, which has an avid affinity for vitamin D moieties. Most vitamin D is converted in the liver to 25(OH)D through the action of 25-hydroxylase. In order for this reaction to occur, a woman must have a healthy liver. Following its synthesis from either vitamin D2 or D3, 25(OH)D then enters the circulation where it is tightly bound to VDBP; a small fraction binds to albumin and other proteins. Only a small amount is unbound or free, but it is the unbound form that is taken up in the kidney and other extrarenal tissues for further processing within the cell [7].
The classic story of vitamin D is that circulating 25(OH)D is taken up by the kidney and converted to dihydroxyvitamin D (1,25[OH]2D or calcitriol), the active hormonal form of vitamin D, by the action of the enzyme 1-α-hydroxylase (also known as CYP27B1), a cytochrome P450 enzyme. 1,25(OH)2D's endocrine effects include the following:
Increased calcium and phosphorus absorption from the intestine
Increased urinary calcium reabsorption
Regulation of parathyroid hormone in a negative feedback loop
Through the actions of 1,25(OH)2D leading to enhanced intestinal absorption of calcium, reabsorption of urinary calcium and mobilization of calcium from bone, the body maintains calcium homeostasis. In this way the body will scavenge calcium at the expense of phosphorus to maintain calcium balance throughout the body because vital organs such as the heart, muscle and brain cannot function without adequate calcium. This process of calcium conservation can be maintained only when there is adequate vitamin D, which is necessary to provide enough substrate to form 25(OH)D, which in turn, is converted to 1,25(OH)2D. It is beyond the scope of this review to provide more detail on the skeletal effects of vitamin D. For a more complete discussion on vitamin D–calcium–bone metabolism during pregnancy and lactation, please refer to a classic review by Kovacs [8].
Defining vitamin D sufficiency
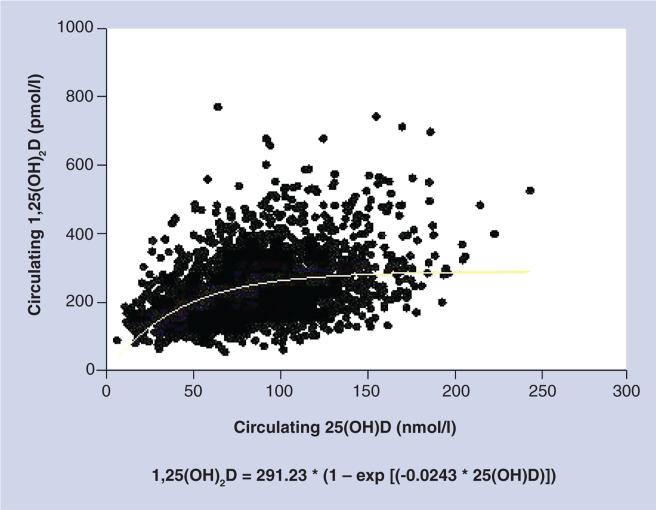
In order to discern the role that vitamin D plays in the health status of the pregnant woman and fetus and the lactating woman and her infant, one must have a common definition, and that includes the ‘definition’ of vitamin D sufficiency. There continues to be much debate regarding what constitutes frank deficiency, insufficiency and sufficiency. Depending on what biomarker one chooses, and if an outcome in question is bone metabolism versus immune function, there could be a different cut-off point for each category. Most, however, would agree – including the Institute of Medicine (IOM) in their most recent statement [9] – that levels below 50 nmol/l (or 20 ng/ml) represent deficiency; whether that label extends to 70 or even 80 nmol/l in the nonpregnant state is less clear. With regard to pregnancy, however, based on our recent randomized controlled trial with pregnant women, it is clear that optimization of 1,25(OH)2D does not occur until total circulating 25(OH)D levels have reached 40 ng/ml (100 nmol/l) (Figure 2) [4,10]. Does the definition of vitamin D sufficiency during pregnancy change to encompass this relationship between 25(OH)D and 1,25(OH)2D?
Figure 2. Relationship of circulating 25-hydroxyvitamin D on circulating dihydroxyvitamin D during pregnancy.
1,25(OH)2D: Dihydroxyvitamin D; 25(OH)D: 25-hydroxyvitamin D.
Reproduced with permission from [4].
Differences in vitamin D metabolism during pregnancy when compared with the nonpregnant state
As discussed above in the previous section, a striking difference exists in vitamin D metabolism during pregnancy and fetal development compared with nonpregnant and nonfetal states, a point that has been known for at least the past three decades but which has received little attention until recently [11–15]. The conversion of vitamin D to 25(OH)D appears unchanged during pregnancy, following first- and zero-order enzyme kinetics [4,16]; by contrast, the conversion of 25(OH)D to 1,25(OH)2D during pregnancy is unique and unparalleled during life. At no other time during life is 25(OH)D so closely linked with 1,25(OH)2D. By 12 weeks of gestation, 1,25(OH)2D levels are more than twice that of a nonpregnant adult and continue to rise two- to threefold from the nonpregnant baseline rising to over 700 pmol/l, attaining levels that would be toxic due to hypercalcemia to the nonpregnant individual, but which are essential during pregnancy [4,13,17].
It was first thought that this increase in circulating 1,25(OH)2D levels during pregnancy was due to an increase in the serum VDBP and while the total circulating levels would increase, the fracture of unbound or ‘free’ 1,25(OH)2D would remain the same [18]. This premise was challenged by Bikle et al., who demonstrated that free 1,25(OH)2D levels are increased during pregnancy despite the significant increase in VDBP levels [11]. Our recent randomized, controlled trial supports this premise [4]. In addition, data from our study demonstrate that a circulating 25(OH) D level of approximately 40 ng/ml is required to optimize the production of 1,25(OH)2D during human pregnancy through renal and/or placental production of the hormone (Figure 2) [4]. It is interesting to note that women with nonfunctional renal 1-α-hydroxylase and normal placental function fail to increase circulating 1,25(OH)2D3 during pregnancy [19], which supports enhanced renal production of calcitriol during pregnancy.
Some have thought that this rise is to ensure adequate delivery of calcium to the developing fetus, yet calcium homeostasis is not linked with 1,25(OH)2D concentration; they appear to be unlinked as 1,25(OH)2D rises above supraphysiologic nonpregnant levels. Contributing to this rise in 1,25(OH)2D may be calcitonin, also known to rise during pregnancy [20]: calcitonin stimulates renal 1-α-hydroxylase gene expression independent of calcium levels [21,22], and also protects by opposing hypercalcemia [22]. Prolactin has also been considered as a stimulator of the 1-α-hydroxylase; however, the increased concentration of 1,25(OH)2D sustained during pregnancy is not sustained during lactation [23]. This correlation between total circulating 25(OH)D and 1,25(OH)2D is, however, found in neonates, paralleling the association in the mother and reflective of the fetal state [4,15,17]. Thus, in both the mother and fetus, the rise in 1,25(OH)2D is dependent on substrate availability, in this case, 25(OH)D, and is independent of calcium homeostasis [4].
Why is calcium metabolism uncoupled from 1,25(OH)2D during this time? One of the leading theories is that 1,25(OH)2D is important for maternal tolerance to the foreign fetus whose DNA is only half that of the mother's, and in certain cases such as conception involving a donor egg, completely foreign. It is interesting that epidemiological studies involving pregnant women with preeclampsia – a clinical picture of inflammation and vasculitis – vitamin D deficiency has been implicated [24–26]. More work is warranted in this area to understand the possible role of vitamin D deficiency in preeclampsia.
Does fetal & neonatal vitamin D status play a role in later immune function?
The evolving emphasis on developmental origins of adult disease has led McGrath and others to investigate whether there are lasting effects following fetal and early infancy vitamin D deficiency, with the goal of understanding lasting effects of the early condition on later adult disease processes [27–42]. Because vitamin D status has not been a consistent concern during pregnancy, long-term data are sparse. The few studies that have been conducted have focused more on the neonatal effects of vitamin D during pregnancy, rather than the long latency and later potential health effects. With severe maternal vitamin D deficiency, the fetus may rarely develop neonatal seizures due to hypocalcemia or rickets in utero that is manifested at birth [43,44].
Supplementation with the current standard amount of vitamin D in prenatal vitamins – 400 IU vitamin D/day – during pregnancy has a minimal effect on circulating 25(OH)D concentrations in the mother and her infant at term [4,45]. Infants of women who were deficient throughout pregnancy will maintain or reach a state of deficiency more quickly than an infant whose mother was replete during pregnancy [46].
It is evident that vitamin D metabolism during pregnancy is unique in human physiology, but why would such a change occur? To begin to answer this question, a look at vitamin D's role beyond calcium metabolism is essential.
Emerging understanding of the immunological effects of vitamin D
While the endocrine effects of vitamin D are best understood as important for maintaining the health status of all of us, including the pregnant or lactating woman, the story does not end with that paradigm. At the turn of the 20th century, Mellanby in his study of rachitic children and dogs, noted an increased risk of respiratory infections in those afflicted [47,48]. Later, Weick [49] in 1967 and Rehman [50] in 1994 independently observed that children with rickets appear ill, with decreased energy and activity, and were more susceptible to respiratory illnesses. It would take the advances in molecular and cellular biology before the mechanisms of that association were better understood.
One of the mechanisms through which vitamin D acts is via an endogenous antimicrobial peptide cathelicidin (LL-37), generated by the innate immune system in response to microbial invasion. Rook and colleagues first noted that 1,25(OH)2D had noncalciotropic or noncalcium metabolism properties [51,52]. Both 1,25(OH)2D and 25(OH)D have the ability to induce the expression of cathelicidin in monocyte/macrophage and epidermal lineage in cells [53]. It was known that cathelicidin is activated through surface Toll-like receptor (TLR) 2 activation on monocytes and macrophages and that the vitamin D receptor element is contained in the regulatory region of these cell types [54–56].
Expansion of our understanding of vitamin D's role in immune function came in 2006; in their landmark study, Liu et al. demonstrated how vitamin D is intricately involved in the innate immune system [54]. Serum samples taken from African–American subjects with low 25(OH)D were inefficient in supporting cathelicidin mRNA induction; however, this stalemate could be reversed through the addition of 25(OH)D to those samples with low 25(OH)D levels. Thus, in this series of experiments, the addition of 25(OH)D3 restored the ability of sera from African–American individuals to support TLR2/1L-mediated induction of cathelicidin mRNA. A related study by Fabri et al. showed that vitamin D is required for IFN-γ-mediated antimicrobial activity of human macrophages, and is especially important in HIV and tuberculosis patients [57]. Both study findings have implications for the pregnant woman and her developing fetus and extends to the lactating woman and her breastfeeding infant.
The effects of vitamin D on the immune system are not limited to the innate immune system but also extend to the adaptive immune system. 1,25(OH)2D not only has the ability to act intracellularly in macrophages and monocytes, but also in T and B lymphocytes. The vitamin D receptor is found on activated (but not resting) human T cells [58] and B cells [59,60]. Whereas 1,25(OH)2D appears to activate the bactericidal process within macrophages and monocytes, it has the reverse effect in lymphocytes; in these cells it appears to act in an anti-inflammatory manner, with an overall dampening effect on lymphocyte function [61,62]. Evidence suggests that 1,25(OH)2D suppresses certain B-cell functions such as proliferation and immuno globulin production and retards the differentiation of B-lymphocyte precursors to mature plasma cells. In addition, 1,25(OH)2D appears to also act through the vitamin D receptor of T cells by inhibiting the proliferation of uncommitted T helper cells and promoting the proliferation of immunosuppressive regulatory T cells, with accumulation of these cells at inflammatory ‘hot spots’ [53]. These in vitro findings explain the significant association between vitamin D deficiency and autoimmune diseases [63], such as systemic lupus erythematosus [64], multiple sclerosis [65–74], rheumatoid arthritis [70,75,76], diabetes – both Types 1 [70,77–81] and 2 [82–84] – and certain cancers, such as colon [85–88], breast [89–94] and prostate cancer [65–98].
Vitamin D status during pregnancy around the globe
In the 1980s, after the laboratory techniques for measuring circulating 25(OH)D had been perfected [99], investigators began to measure the vitamin D status of pregnant women. The women of darker pigment, who had migrated from sunnier climates to the UK or France, for example, and who wore clothing that left little skin exposed were found to be the most deficient [100–105]. The first report of widespread vitamin D deficiency in women of childbearing age in the USA came from a report from the CDC in their Third National Health and Nutrition Examination Survey, from 1988 to 1994, which revealed that 42% of African–American women had 25(OH)D levels below serum concentrations of 15 ng/ml or 37.5 nmol/l [106]. Applying the IOM's more recent definition of deficiency – 25(OH)D level <20 ng/ml – to the dataset increases the prevalence to approximately 75% [9].
More recent studies in the USA suggest that the degree of deficiency is greatest in the African–American population but substantial in Hispanic and Caucasian women who have limited access to sunlight, either through limited activity outdoors, type of clothing, cultural practices or thorough use of sunscreen when outdoors [107,108]. In two studies involving over 1000 pregnant woman in South Carolina, USA, at latitude 32°N, 75% African–American, 33% Hispanic and 12% Caucasian women had evidence of deficiency [107,108], confirming the extent and severity of this health problem. In other areas of the world, deficiency is also commonplace, again reflecting a woman's lifestyle, degree of skin pigmentation, where she lives (i.e., the latitude and whether she is an urban dweller or lives in more rural areas) and the most important factor, whether she has sunlight exposure [109]. A long-standing unawareness of how vitamin D is made and of the short- and long-term health consequences of vitamin D insufficiency has led to widespread insufficiencies in most populations.
Vitamin D during pregnancy: why is it important?
From the prior section it is clear that vitamin D deficiency during pregnancy is widespread, yet what effect does deficiency have on the mother and her developing fetus, and, following delivery, on her breastfeeding infant? Some have suggested that such ‘deficiency’ is really how we were meant to live. If we look at individuals who live in sub-Saharan Africa or at the equator and who have sunlight exposure on a significant surface area of their bodies, we find that such individuals ‘live’ with a total circulating 25(OH)D of at least 40 ng/ml or 100 nmol/l. While such individuals have lower risk of diabetes, hypertension and autoimmune disease, other differences besides vitamin D status could well account for such differences such as the lack of highly processed foods and higher intakes of fiber instead of fat. It is unclear if individuals living in more developed countries had comparable vitamin D status to those living in a sun-rich environment, if such individuals would have improved health status on the basis of vitamin D's beneficial effect on health. Several large-scale studies are underway to assess this question in a prospective manner. Until then, we are left with retrospective analyses and case–control studies to assess the impact of vitamin D on health and its role in immune function in general and specific to the pregnancy and lactation states.
There are many epidemiological studies that report associations between vitamin D deficiency and altered health. Conversely, higher circulating 25(OH)D levels have been linked with improved glucose handling and β-cell function [110], and a reduction in risk for a growing list of long latency diseases that include cardiovascular disease [96,111–115], multiple sclerosis [66,71,73], rheumatoid arthritis [75], systemic lupus erythematosus [64], Type 1 and 2 diabetes [66,71,73,75,96,111–115] and various cancers [85,91,94,116–121]. Critics of this perspective counter that while such findings are intriguing, they do not provide definitive evidence of causality or a mechanism of action that come from randomized controlled trials and may lead to what is referred to as ‘circular epidemiology’ [122]. However, there is mounting evidence from laboratory studies that vitamin D – as a pre-prohormone – is essential in maintaining the immune system, with profound implications [54,123]. There are two related reviews on the topic that highlight the importance of optimization of circulating 25(OH)D levels to reduce the risk of long latency disease states [124,125].
It is quite plausible that in an ideal world, total circulating 25(OH)D should mirror what is attained by those who live and work in a sunrich environment because that is how we evolved thousands of years ago, with such systems still operational in modern-day humans. However, in many parts of the world, lifestyles have undergone dramatic changes in the last 50–60 years, which on an evolutionary scale is an insignificant time for humans, as a species, to have adapted to such a change, in this case, to have already adapted to substrate vitamin D deprivation. It makes sense then that our body stores of vitamin D should recapitulate that of individuals living in a sun-rich environment who have circulating 25(OH)D levels of 54–90 ng/ml [126–128], not shared by those who are sunlight deprived or covered from sunlight [129].
What are the effects of vitamin D deficiency during pregnancy?
Maternal considerations
Studies conducted in the 1980s showed that profound vitamin D deficiency was associated with impaired fetal growth; however, those studies were conducted during a time when the immune effects of vitamin D were not appreciated and thus, it is unclear from those studies what the mechanism of action was for this impaired growth. More recent epidemiologic and case– control studies show a correlation between vitamin D deficiency and adverse pregnancy outcomes, not limited to fetal growth. For example, Bodnar et al. found an association between vitamin D deficiency and maternal preeclampsia [130] that has been supported by Robinson et al. in their two nested case–control studies [25,131]; and most recently, by Wei et al., who found a longitudinal effect of maternal vitamin D status during mid-gestation and later development of preeclampsia in those meeting the IOM definition of deficiency (25[OH]D level <50 nmol/l) [132]. Others have found an association between the mode of delivery (higher risk of Cesarean delivery with vitamin D deficiency) [133] and bacterial vaginosis [134].
Fetal growth & neonatal anthropomorphic measures
Adequate nutritional vitamin D status during pregnancy is important for fetal skeletal development, tooth enamel formation and perhaps general fetal growth and development [102,103]. Mannion et al., comparing growth parameters in newborn infants with the maternal intakes of milk and vitamin D during pregnancy, found an association between vitamin D intake during pregnancy and birth weight, such that with every additional 40 IU of maternal vitamin D intake, there was an associated 11-g increase in birth weight [135]. Pawley and Bishop, in their study of 108 pregnant women and their offspring, found a significant association between umbilical cord 25(OH)D concentrations and head circumference at 3 and 6 months’ postnatal age that persisted after adjusting for confounding factors [136]. Maghbooli et al. found significantly wider posterior fontanelle diameter in neonates of mothers with vitamin D deficiency (as defined by a 25(OH)D level <34.9 nmol/l or ~14 ng/ml) compared with neonates whose mothers were not deficient [137].
Effect on neonatal & infant immune function
Extending such findings beyond anthropomorphic growth parameters and bone to the realm of immune function, a recent prospective study of neonates showed an increased risk of respiratory syncytial virus (RSV) bronchiolitis in those neonates with deficiency [10]. Specifically, Belderbos et al., in their analysis of 156 neonates followed them prospectively for 1 year with the primary outcome measure being the rate of RSV during the first year as a function of vitamin D status at birth [10]. Total circulating 25(OH)D was significantly lower in those with RNA PCR-confirmed RSV bronchiolitis/lower respiratory tract infection: 65 versus 84 nmol/l in those who did not have an RSV infection (p = 0.009). In addition, there was a sixfold higher risk of RSV in those with cord blood 25(OH)D <50 nmol/l (20 ng/ml) versus those with a concentration >75 nmol/l (30 ng/ml). The findings from this study are further supported by the work of Walker et al., who measured 25(OH)D induction of TLR antimicrobial production and its effect on in vitro monocyte responses [17]. Cord blood samples deficient in vitamin D had less effect on adult monocyte cathelicidin gene expression compared with vitamin D replete cord blood (>75 nmol/l). When cord blood was repleted with 25(OH)D, cathelicidin gene expression in vitro increased significantly. These studies strongly support the role of vitamin D on immune function and surveillance during the perinatal period.
Sunlight versus supplementation: is there a right answer?
There is no doubt that sunlight exposure is superior to oral supplementation in terms of vitamin D safety data. No one has ever died of too much vitamin D generated from sunlight exposure, but people have become toxic from ingesting too much oral vitamin D. It takes, of course, daily consumption of thousands of IUs of vitamin D as an adult – above 10,000 IU/day – to become toxic from oral vitamin D [138], yet there does not appear to be an upper limit of sunlight exposure and vitamin D synthesis from this route as sunlightderived vitamin D triggers down regulation of certain enzyme systems and upregulation of others in the body to dispose of any vitamin D and its metabolites not needed by the body. Judicious sunlight exposure is not a clear-cut entity, however, as to what amount of sunlight is sufficient to achieve optimal vitamin D status varies depending on the time of year, the time of day, where you live (i.e., latitude), degree of skin pigmentation, clothing and what surface area of skin is actually exposed. There is less of a ‘one size fits all’ prescription for prescribing sunlight exposure than, perhaps, a vitamin D supplement. In addition, there is the significant issue of the association of UV light's damaging effect on the epidermis and the underlying dermis leading to photoaging and certain skin cancers such as basal and squamous cell carcinomas [139,140]. If the source of vitamin D cannot be judicious sunlight exposure, then the only alternative for the pregnant and lactating woman is vitamin D supplementation.
Effectiveness of vitamin D supplementation during pregnancy
A Cochrane review on vitamin D supplementation during pregnancy in 2000 concluded that there was not enough evidence to evaluate the requirements and effects of vitamin D supplementation during pregnancy [141]. An updated Cochrane review in 2012 came to a similar conclusion. Unfortunately, the report failed to include any randomized controlled trial study where 400 IU/day was given as the control; this reflected the premise that 0 IU/day vitamin D is the norm and that 400 IU/day supplementation is the treatment and should not be viewed as a control group [142]. Yet, in many parts of the world, including the USA, Canada, Japan, Australia, and many countries in Europe, it would be unethical to not give 400 IU/day as part of the prenatal vitamin preparation; therefore, those women receiving 400 IU vitamin D/day would be considered as the control group for these areas of the world.
As shown in Table 1, the adequate intake for vitamin D during pregnancy of 400 IU/ day is grossly inadequate, especially with ethnic minorities. As predicted by vitamin D pharmaco kinetics, supplements of 1000 IU/day of vitamin D in pregnant women result in a 12.5–15.0 nmol/l increase in circulating 25(OH)D concentrations in both maternal and cord serum compared with nonsupplemented controls [101–103]. These findings were recently corroborated by our two randomized clinical trials involving vitamin D supplementation in pregnant women [4,143]. The significance of these findings and others for those who care for the pediatric population is that when a woman who has vitamin D deficiency gives birth, her neonate will also be deficient.
Table 1.
Historical vitamin D pregnancy supplementation trials: health characteristics of mothers and their infants.
| Study (year) | Country and population | Intervention | Therapy duration (months) | Baseline 25(OH)D (nmol/l) | End point 25(OH)D (nmol/l) | Findings | Ref. |
|---|---|---|---|---|---|---|---|
| Brooke et al. (1980) | UK/Asian | 0 IU, n = 59 | 3 | 16.3 | – | More SGA and larger fontanelle area with higher incidence of profound hypocalcemia in placebo group | [102] |
| 1000 IU D2/day, n = 67 | 3 | 20.0 | 168† | ||||
| Cockburn et al. (1980) | Edinburgh, Scotland | 0 IU, n = 633; 25(OH)D available in n = 82 | 7 | 32.5 at 24 weeks | 32.5 | 0 IU group infants gained less wt and showed decreased linear growth Higher incidence of abnormal dental defects at 3 years. 400 IU D2/day group infants had improved calcium and phosphorous levels and a lower incidence of hypocalcemia | [45] |
| 400 IU D2/day, n = 506; 25(OH)D available in n = 82 | 7 | 39.0 at 24 weeks | 42.8 | ||||
| Brooke et al. (1981) | UK/Asian (follow-up of neonates from Brooke et al. study [102]) | 0 IU, n = 59 | 3 | 16.3 | – | Follow-up at 1 year: placebo group infants gained less wt and had a lower rate of linear growth compared with 1000 IU D2/day infant group | [104] |
| 1000 IU D2/day, n = 67 | 3 | 20.0 | 168† | ||||
| Maxwell et al. (1981) | Asian women living in the UK (follow-up of neonates from Brooke et al. study [102]) | 0 IU D2/day, n = 67 | 3 | 16.3 | – | Supplemented mothers with better wt gain, improved nutritional status and less SGA infants | [156] |
| 1000 IU D2/day, n = 59 | 3 | 20.0 | 168† | ||||
| Marya et al. (1981) | India | 0 IU, n = 75 | 3 | Not reported | Not reported | 1200 IU D2/day group showed significantly lower alkaline phosphatase levels and increased fetal birth wt. 600,000 IU D2/day proved more efficacious than placebo or 1200 IU D2/day in terms of significantly greater fetal birth wt. Dependence of fetal calcium on maternal levels: fetal calcium was significantly lower (p = 0.001) in mothers with calcium below 8.5 mg percent than those with higher levels (≥9.7) | [105] |
| 1200 IU D2/day, n = 25 | 3 | ||||||
| 600,000 IU D2 ×1 at 6 and again at 7 months, n = 20 | Months 6 and 7 | ||||||
| Delvin et al. (1986) | Lyon, France | 0 IU, n = 20 (25[OH]D available in n = 13) | 3 | Day 230: 11 | 13 (cord: 7) | Better calcium, alkaline phosphatase and vitamin D status in mothers and neonates of 1000 IU D3/day group. 25(OH)D and 1,25(OH)2D strongly correlated in control group only (r = 0.859, p < 0.005) | [157] |
| 1000 IU D3/day, n = 20 (25[OH]D available in n = 14) | 3 | 22 | 26 (cord: 18) | ||||
| Mallet et al. (1986) | Northwest France | 0 IU, n = 21 | 3 | – | 9.4 (cord 5.3) | 1000 IU/day during the last trimester of pregnancy resulted in only a 5-6 ng /ml increase in circulating 25(OH)D levels in maternal and cord serum | [158] |
| 1000 IU D2/day, n = 27 | 3 | – | 25.3 (cord 15.7) | ||||
| 200,000 IU D2 at month 7, n = 20 | ×1 at month 7 | – | 26.0 (cord 18.2) | ||||
| Datta et al. (2002) | UK/minority | 160 women screened. 80 had 25(OH)D <8 ng/ml and were treated with 800 IU D3/day starting at their initial booking. Women still deficient at 36 weeks gestation (number not given) had dose increased to 1600 IU D3/day | 3–? | 5.8 | 11.2 | 50% women were profoundly deficient at time of enrollment as defined by 25(OH)D <8 ng/ml | [159] |
| Sahu et al. (2009) | Uttar Pradesh, northern India | Group A: sunlight only; exposure limited to face, hands and feet, n = 14 | Sunlight only | 25.8 | 23.8 | A significant increase in 25(OH)D at delivery only in group C: 34.2% group C achieved 25(OH)D >80 nmol/l vs 7% group A and 5.75% group B at delivery | [160] |
| Group B: 60,000 IU D3 + sunlight, n = 35 | Month 5 | 33.4 | 30.9 | ||||
| Group C: 120,000 IU D3 + sunlight, n = 35 | Month 5 and 7 | 40.1 | 53.4 | ||||
| Hollis et al. (2011) | Charleston, SC, USA | 400 IU D3/day, n = 111 | 7 | 61.6 | 78.9 | No adverse events due to vitamin D supplementation; 2000 and 4000 IU D3/day groups with higher mean by second trimester than control group with higher percentage, meeting IOM definition of sufficiency; 4000 IU D3/day group achieved greater percentage sufficiency by second trimester than other two groups Infants of mothers in 4000 IU D3/day group with greater percentage sufficiency as per IOM definition | [4] |
| 2000 IU D3/day, n = 122 | 7 | 58.3 | 98.3 | ||||
| 4000 IU D3/day, n = 117 | 7 | 58.2 | 111.0 | ||||
Based on known pharmacokinetic data during the pregnant and nonpregnant states, it is most likely that the wrong dose of supplementation was given or the assay for 25(OH)D was invalid. The response observed is one that would be expected after supplementation with 10,000 IU/day vitamin D3 for 3 months.
–: Not given; 1,25(OH)2D: Dihydroxyvitamin D; 25(OH)D: 25-hydroxyvitamin D; D2: Ergocalciferol; D3 Cholecalciferol; IOM: Institute of Medicine; r: Correlation coefficient; SGA: Small for gestational age, defined as third percentile; wt: Weight.
Results of two recent randomized trials during pregnancy
Two vitamin D supplementation studies involving a diverse group of pregnant women less than 16 weeks of gestation showed that 4000 IU vitamin D3/day was superior to 400 or 2000 IU/day by the second trimester in achieving circulating 25(OH)D of at least 40 ng/ml, the point at which 1,25(OH)2D begins to be optimized (Figure 2) [4,143]. If one's goal is to achieve the minimal 25(OH) D concentration of 20 ng/ml set forth by the IOM, then while 4000 IU/day was superior to 2000 IU/day in achieving this minimal goal, it was not statistically significant; both 2000 and 4000 IU/day will achieve this level in pregnant women. If one's goal, however, is to reach the point of 1,25(OH)2D optimization, then there is a clear advantage of taking 4000 IU/day.
It is important to note that in these two studies that involved 510 women's participation until delivery, according to each study's Data Safety and Monitoring Committee, there were no adverse events related to vitamin D supplementation [4]. All women were followed closely for any adverse events that may be related to vitamin D supplementation. In each category of adverse events, there was a trend where the higher dose groups had fewer events than those women randomized into the control group. Taken together, looking at the most common and severe comorbidities of pregnancy (hypertensive disorders of pregnancy, gestational diabetes, infection and preterm labor/preterm birth), preliminary analyses showed statistically significant differences between the groups with higher risk in the control group (400 IU group) when compared with both the 2000 and 4000 IU groups (p = 0.03) and higher risk in the 2000 IU group when compared with the 4000 IU group [144]. Confirmatory findings came from the second trial where lower risk for comorbidities of pregnancy was seen with higher 25(OH)D [143]. The study was powered to discern safety risks and as such, comorbidities of pregnancy were an outcome safety measure. Additional trials around the world will be necessary to confirm these findings. There are at least ten vitamin D supplementation trials that will be completed within the next 2 years.
Summation of the pregnancy studies on vitamin D supplementation show that 400 IU/day is woefully inadequate at raising the level of 25(OH)D in women to attain optimal production of 1,25(OH)2D. Without adequate sunlight exposure, vitamin D supplementation becomes necessary. Up to 4000 IU/day is not only safe but most effective in achieving the minimal level of 25(OH)D for optimal 1,25(OH)2D production by the second trimester. Because women of darker pigmentation are at the greatest risk for vitamin D deficiency, care must be taken to provide adequate vitamin D to achieve sufficiency in these at-risk women. Not only important during pregnancy for fetal development, the vitamin D status of the mother is essential during lactation for the transfer of vitamin D to the breastfeeding infant. In the next section the vitamin D requirements of the breastfeeding dyad are reviewed.
The needs of the breastfeeding dyad
It is again worth mentioning that the fetus of the woman who is vitamin D deficient during pregnancy will be deficient during gestation as the mother is her fetus’ sole source of vitamin D. Such deficiency states extend beyond pregnancy into lactation. If a breastfeeding woman is deficient, her breast milk will be deficient, and her recipient infant will also be deficient. It was thought for decades that breast milk was ‘naturally’ low in vitamin D because the women who were sampled in such studies had marginal levels of vitamin D. When a mother is replete in vitamin D, the transfer of vitamin D in her milk is sufficient to provide an adequate amount of substrate for her recipient breastfeeding infant. This was first documented by Cancela et al. in 1986, who showed the relationship between the vitamin D content of maternal milk and the vitamin D status of nursing women and breastfed infants, which was later validated by two pilot studies performed by our group almost two decades later [145]. It is also important to emphasize here that it is vitamin D and not 25(OH)D that preferentially gets into breast milk, which has important implications for dosing. The half-life of vitamin D is 12–24 h compared with the half-life of 25(OH)D, which is 2–3 weeks. Since it is vitamin D that is transferred into the milk, the mother needs a daily source of vitamin D in order to provide her infant with enough substrate to avoid deficiency [146].
In the first of three studies, fully lactating women starting at 1 month postpartum were randomized to receive either 1600 or 3600 IU vitamin D2 plus 400 IU vitamin D3 through their prenatal vitamin [146]. All were blinded to treatment. Vitamin D2 was chosen for this study to serve as a tracer of vitamin D from the mother into her milk and then into her recipient infant because most individuals have little vitamin D2 in their diet (which was shown to be the case in this study). The remarkable part of this study was that it supported the premise that if a mother becomes vitamin D replete then her milk vitamin D levels will increase such that her infant will have vitamin D-‘enriched’ milk. It was found that 4000 IU/day was not enough, however, to raise maternal serum levels to consistently yield at least 400 IU vitamin D/l breast milk. Approximately 20% of maternal vitamin D is transferred to the infant through maternal milk; so compared with the same pregnant woman, a lactating woman would need a higher vitamin D supplementation dosage to attain the same vitamin D status during lactation that she had achieved during pregnancy. Based on pharmacokinetic data and the rate of transfer to the infant through milk, it was hypothesized that a maternal vitamin D supplementation dose of approximately 6000 IU/day would be necessary to raise maternal serum vitamin D and, thus, milk vitamin D levels that would ultimately give the recipient breastfed infant at least 400 IU vitamin D/day.
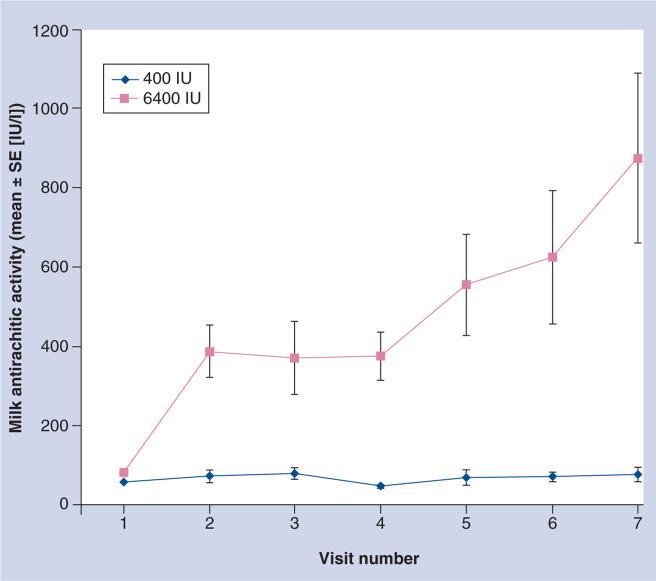
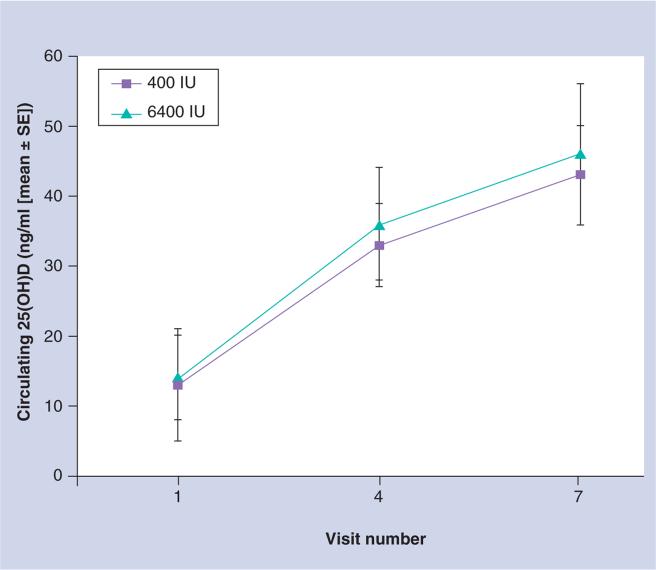
In the second pilot study, women were randomized at 1 month postpartum to a prenatal vitamin containing 400 IU/tablet plus either placebo or 6000 IU vitamin D/day [147]. The infants of the mothers randomized to 400 IU/day received 300 IU vitamin D/day while the infants of the mothers randomized to 6000 IU/day received placebo. In the latter arm of the trial, mothers were essentially the sole source of vitamin D for their young infants who had minimal sunlight exposure. Again, all were blinded to treatment. The results showed that the milk antirachitic concentration (which is the vitamin D content of the breast milk) was significantly higher in the 6000 IU group of women compared with the 400 IU group without evidence of toxicity (Figure 3). In addition, the infants of mothers in the 6000 IU arm had circulating 25(OH)D levels comparable to those infants who themselves were receiving 300 IU vitamin D/day (Figure 4). It was a small sample size but the results were significant and served as a proof of concept for a larger trial involving two study sites – Charleston, SC, USA and Rochester, NY, USA – to begin. The study began in 2006 with completion scheduled for 2012. While investigators and subjects remain blinded, it is important to note that there have been no cases of vitamin D toxicity during the trial.
Figure 3. Milk antirachitic activity as a function of maternal vitamin D3 dose: 400 versus 6400 IU/day.
SE: Standard error.
Adapted with permission from [147].
Figure 4. Infant circulating 25-hydroxyvitamin D as a function of maternal supplementation (400 vs 6400 IU vitamin D3/day) and infant supplementation (300 vs 0 IU vitamin D3/day).
25(OH)D: 25-hydroxyvitamin D; SE: Standard error.
Adapted with permission from [147].
The results of this trial will be helpful to women who are requesting best practice recommendations for achieving optimal vitamin D status for the mother and infant during lactation. Until then, the recommendations set forth by the American Academy of Pediatrics [148] and the IOM [9] are for the mother to take a prenatal vitamin and for her recipient infant to be given 400 IU vitamin D/day unless her infant receives at least a liter of infant formula/day. Such a recommendation – while it addresses the daily vitamin D needs of the infant – does not address the needs of the mother in the context of her own specific needs or what daily intake is necessary to achieve adequate milk transfer to her recipient infant.
The health effects of vitamin D deficiency during infancy have been well described in terms of calcium and skeletal metabolism; infants with prolonged vitamin D deficiency are at significant risk of developing rickets, a problem worsened in breastfed infants whose mothers were deficient during pregnancy and during lactation [147]. Without supplementation of that infant, the problem manifests as the classic signs and symptoms of rickets. For decades it was thought that the story ended there but as discussed earlier in this review, Mellanby and others more than a century ago had observed higher rates of respiratory infections in those children with vitamin D deficiency [48]. This is supported by more recent studies that showed a significant correlation between vitamin D deficiency and higher rates of RSV bronchiolitis and lower respiratory infections in the first year of life [10,149,150] and pneumonia in children under 5 years of age [150]; cord blood differences in innate immune responses [17] and higher risk of tuberculosis [151–154] in children with vitamin D deficiency and/or rickets. Additional studies are warranted to understand vitamin D's role during lactation and early infancy and to ascertain the optimal total circulating 25(OH)D concentration that is associated with improved health and growth.
Conclusion
There is widespread deficiency of vitamin D throughout the world in pregnant women due to changes in lifestyle, access to sunlight, use of sunscreen, and minimal vitamin D content in a variety of diets with few exceptions. Such deficiency using conservative parameter estimates is greatest in women of darker pigmentation and those with limited or without access to sunlight or vitamin D supplements.
Recent evidence suggests that unlike any other time during a woman's life, there is an uncoupling of the active hormone 1,25(OH)2D with calcium metabolism and a direct coupling with 25(OH) D such that optimal 1,25(OH)2D occurs when 25(OH)D concentration is at least 40 ng/ml (100 nmol/l). There is some evidence to suggest that vitamin D deficiency during pregnancy is linked with preeclampsia and other adverse health outcomes of pregnancy, but such associations warrant further study for confirmation and understanding the mechanism.
The implication of maternal deficiency during pregnancy is that the fetus is also affected, with known consequences on fetal growth, dentition, bone density and immune function and risk of infections such as RSV. As more data concerning vitamin D status during pregnancy are amassed, such sequelae will be better understood.
The impact of maternal deficiency extends beyond pregnancy into lactation as the mother continues to be the main source of vitamin D for the young infant. The traditional view that human milk is marginally sufficient and often deficient in vitamin D has prompted the American Academy of Pediatrics and the IOM to make recommendations regarding infant vitamin supplementation with 400 IU/day unless that infant consumes infant formula of 1 l or more per day. Such a view does not address why the milk is deficient and offers no alternatives to mothers who recognize the advantages of breast milk over formulas for optimal development. Studies that supplement the mother alone with higher doses of vitamin D are currently underway as a potential therapy that treats both the mother and infant through the mother.
Future research is needed to delineate the mechanisms of action of vitamin D on the health status of the mother and developing embryo and fetus beyond calcium and bone metabolism that extends into the realm of immune function and developmental origins of adult disease. Such research should include early infancy and the effect of vitamin D sufficiency during lactation.
Future perspective
We envision that over the next decade, the concept that vitamin D is actually a pre-prohormone and a potent mediator of the immune system will move from the perspective of heresy to one that is well-established. Such a paradigm shift will occur because of the mounting evidence that is being amassed to prove this point. What we know now is but a fraction of what we will learn in the decades to come. With improved understanding of vitamin D's mechanisms of action come effective interventions. When any precursor to a hormone is restored and health characteristics improve, the overall disease burden diminishes. As such, there are several groups who will directly benefit from this paradigm shift: women, most notably African–American and Hispanic women, and their developing fetuses, and lactating women and their recipient infants. This is what we expect will happen. Only the test of time may prove our hypothesis correct.
Executive summary.
Background
Vitamin D has emerged from being a forgotten vitamin that was only associated with bone and calcium metabolism to become one of the most celebrated and controversial vitamins/micronutrients in both medical and lay literature today.
General vitamin D physiology & metabolism
Without sunlight exposure we are dependent solely on dietary sources of vitamin D, which, except in rare cases, only account for up to 10% of the vitamin D in the body.
Defining vitamin D sufficiency
With regard to pregnancy, however, based on a recent randomized controlled trial with pregnant women, it is clear that optimization of dihydroxyvitamin D (1,25[OH]2D) does not occur until total circulating 25-hydroxyvitamin D (25[OH]D) levels have reached 40 ng/ml (100 nmol/l).
Differences in vitamin D metabolism during pregnancy when compared with the nonpregnant state
The conversion of vitamin D to 25(OH)D appears unchanged during pregnancy, following firstand zero-order enzyme kinetics; by contrast, the conversion of 25(OH)D to 1,25(OH)2D during pregnancy is unique and unparalleled during the lifespan. At no other time during the lifecycle is 25(OH)D so closely linked with 1,25(OH)2D. By 12 weeks of gestation, 1,25(OH)2D levels are more than twice that of a nonpregnant adult and continue to rise two- to threefold from the nonpregnant baseline rising to over 700 pmol/l, attaining levels that would be toxic owing to hypercalcemia in the nonpregnant individual, but are essential during pregnancy.
Does fetal & neonatal vitamin D status play a role in later immune function?
Supplementation with the current standard amount of vitamin D in prenatal vitamins – 400 IU vitamin D/day – during pregnancy has a minimal effect on circulating 25(OH)D concentrations in the mother and her infant at term. Infants of women who were deficient throughout pregnancy will maintain or reach a state of deficiency more quickly than an infant whose mother was replete during pregnancy.
Emerging understanding of the immunological effects of vitamin D
The effects of vitamin D on the immune system are not limited to the innate immune system but also extend to the adaptive immune system.
Vitamin D status during pregnancy around the globe
Degree of deficiency is greatest in women with darker pigmentation but a substantial number of women, irrespective of pigmentation, who have limited access to sunlight, either through limited activity outdoors, type of clothing, cultural practices or thorough use of sunscreen when outdoors, are vitamin D deficient.
Vitamin D during pregnancy: why is it important?
There are many epidemiological studies that report associations between vitamin D deficiency and altered health.
Conversely, higher circulating 25(OH)D levels have been linked with improved health.
The significance of these findings have with regard to pregnancy is just beginning to be understood.
What are the effects of vitamin D deficiency during pregnancy?
More recent epidemiologic and case–control studies show a correlation between vitamin D deficiency and adverse pregnancy outcomes – not limited to fetal growth, and include preeclampsia and bacterial vaginosis. In addition, adequate nutritional vitamin D status during pregnancy is important for fetal skeletal development, tooth enamel formation, and perhaps general fetal growth and development. There also is mounting evidence to suggest that vitamin D deficiency impacts on the immune function, not only of the mother, but also of the neonate and infant through the first year of life.
Sunlight versus supplementation: is there a right answer?
While sunlight is superior to vitamin D supplementation in its efficacy and safety, there are issues with what constitutes ‘judicious’ sunlight exposure. In addition, lifestyle changes limit the amount of sunlight available to many individuals making vitamin D supplementation to mimic the sunlight production of vitamin D the only viable option.
Effectiveness of vitamin D supplementation during pregnancy
400 IU vitamin D/day is inadequate in achieving sufficiency for the majority of pregnant women. To attain optimal production of 1,25(OH)2D, a 25(OH)D level of 40 ng/ml (100 nmol/l) or higher is required. Suffice it to say, when a woman who has vitamin D deficiency gives birth, her neonate also will be deficient.
Results of two recent randomized trials during pregnancy
Two vitamin D supplementation studies involving a diverse group of pregnant women less than 16 weeks of gestation showed that 4000 IU vitamin D3/day was superior to 400 or 2000 IU/day by the second trimester in achieving circulating 25(OH)D of at least 100 nmol/l (40 ng/ml), the point at which 1,25(OH)2D begins to be optimized.
The needs of the breastfeeding dyad
It is again worth mentioning that the fetus of the woman who is vitamin D deficient during pregnancy will be deficient during gestation as the mother is the fetus’ sole source of vitamin D. Such deficiency states extend beyond pregnancy into lactation. If a breastfeeding woman is deficient, her breast milk will be deficient, and her recipient infant also will be deficient. Conversely, when a mother is replete in vitamin D, the transfer of vitamin D in her milk is sufficient to provide adequate amount of substrate for her recipient breastfeeding infant.
Future perspective
We envision that over the next decade, the concept that vitamin D is actually a pre-prohormone and a potent mediator of the immune system will move from the perspective of heresy to one that is well-established. Such a paradigm shift will occur because of the mounting evidence that is being amassed to prove this point.
Acknowledgments
BW Hollis serves as a consultant for Diasorin Corporation, Stillwater, MN, USA. CL Wagner and BW Hollis are supported by NIH grants NIH R01 HD043921 and NIH R01 HD47511 and the Thrasher Research Fund.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Daly S, Mills JL, Molloy AM, et al. Minimum effective dose of folic acid for food fortification to prevent neural-tube defects. Lancet. 1997;350(9092):1666–1669. doi: 10.1016/S0140-6736(97)07247-4. [DOI] [PubMed] [Google Scholar]
- 2.Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am. J. Clin. Nutr. 2000;71(Suppl. 5):1280S–1284S. doi: 10.1093/ajcn/71.5.1280s. [DOI] [PubMed] [Google Scholar]
- 3.Maxmen A. Vitamin D on trial. The Scientist. 2012;26(3):44–50. [Google Scholar]
- 4••.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J. Bone Miner. Res. 2011;26(10):2341–2357. doi: 10.1002/jbmr.463. [This randomized controlled trial is the largest to date that assessed the safety and effectiveness of higher-dose vitamin D supplementation up to 4000 IU/day to attain physiologic levels of 25-hydroxyvitamin D that would occur if living in a sun-rich environment. The findings strongly suggest that 4000 IU/day is necessary to attain optimal dihydroxyvitamin D (1,25[OH]2D) production with no adverse events associated with supplementation at that dose.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuoka LY, Wortsman J, Haddad JG, Hollis BW. Skin types and epidermal photosynthesis of vitamin D3. J. Am. Acad. Dermatol. 1990;23(3):525–526. doi: 10.1016/s0190-9622(08)81116-4. [DOI] [PubMed] [Google Scholar]
- 6•.Luxwolda MF, Kuipers RS, Kema IP, Janneke Dijck-Brouwer DA, Muskiet FA. Traditionally living populations in east Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/l. Br. J. Nutr. 2012:1–5. doi: 10.1017/S0007114511007161. [Highlights the vitamin D status of individuals who dwell in east Africa with several hours of sunlight exposure daily. The levels attained by those living in such a climate are far above those who are city dwellers or wear protective clothing.] [DOI] [PubMed] [Google Scholar]
- 7•.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J. Clin. Endocrinol. Metab. 1986;63(4):954–959. doi: 10.1210/jcem-63-4-954. [First study to show that while vitamin D-binding protein (VDBP) increases during pregnancy along with 1,25(OH)2D, the increase in 1,25(OH)2D exceeds that of VDBP such that free 1,25(OH)2D levels are increased during pregnancy despite the increase in VDBP levels.] [DOI] [PubMed] [Google Scholar]
- 8•.Kovacs CS. Calcium and bone metabolism in pregnancy and lactation. J. Clin. Endocrinol. Metab. 2001;86(6):2344–2348. doi: 10.1210/jcem.86.6.7575. [Reviews calcium and bone metabolism during pregnancy and lactation.] [DOI] [PubMed] [Google Scholar]
- 9.Dietary Reference Intakes for Vitamin D and Calcium. National Academy Press; Washington, DC, USA: 2010. Food and Nutrition Board. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. [Google Scholar]
- 10.Belderbos ME, Houben ML, Wilbrink B, et al. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics. 2011;127(6):e1513–e1520. doi: 10.1542/peds.2010-3054. [DOI] [PubMed] [Google Scholar]
- 11.Bikle DD, Gee E, Halloran B, Haddad JG. Free 1,25-dihydroxyvitamin D levels in serum from normal subjects, pregnant subjects, and subjects with liver disease. J. Clin. Invest. 1984;74(6):1966–1971. doi: 10.1172/JCI111617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar R, Cohen WR, Silva P, Epstein FH. Elevated 1,25-dihydroxyvitamin D plasma levels in normal human pregnancy and lactation. J. Clin. Invest. 1979;63(2):342–344. doi: 10.1172/JCI109308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lund B, Selnes A. Plasma 1,25-dihydroxy- vitamin D levels in pregnancy and lactation. Acta Endocrinol. 1979;92(2):330–335. doi: 10.1530/acta.0.0920330. [DOI] [PubMed] [Google Scholar]
- 14.Steichen JJ, Tsang RC, Gratton TL, Hamstra A, DeLuca HF. Vitamin D homeostasis in the perinatal period: 1,25-dihydroxyvitamin D in maternal, cord, and neonatal blood. N. Engl. J. Med. 1980;302(6):315–319. doi: 10.1056/NEJM198002073020603. [DOI] [PubMed] [Google Scholar]
- 15.Seino Y, Ishida M, Yamaoka K, et al. Serum calcium regulating hormones in the perinatal period. Calcif. Tissue Int. 1982;34(2):131–135. doi: 10.1007/BF02411223. [DOI] [PubMed] [Google Scholar]
- 16.Heaney RP, Armas LA, Shary JR, Bell NH, Binkley N, Hollis BW. 25-hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am. J. Clin. Nutr. 2008;87(6):1738–1742. doi: 10.1093/ajcn/87.6.1738. [DOI] [PubMed] [Google Scholar]
- 17.Walker VP, Zhang X, Rastegar I, et al. Cord blood vitamin D status impacts innate immune responses. J. Clin. Endocrinol. Metab. 2011;96(6):1835–1843. doi: 10.1210/jc.2010-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouillon R, Van Assche FA, Van Baelen H, Heyns W, DeMoor P. Influence of the vitamin D-binding protein on serum concentrations of 1,25(OH)2D. J. Clin. Invest. 1981;67:589–596. doi: 10.1172/JCI110072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greer FR, Hollis BW, Napoli JL. High concentrations of vitamin D2 in human milk associated with pharmacologic doses of vitamin D2. J. Pediatr. 1984;105:61–64. doi: 10.1016/s0022-3476(84)80361-3. [DOI] [PubMed] [Google Scholar]
- 20.Stevenson JC, Hillyard CJ, MacIntyre I, Cooper H, Whitehead MI. A physiological role for calcitonin: protection of the maternal skeleton. Lancet. 1979;2(8146):769–770. doi: 10.1016/s0140-6736(79)92117-2. [DOI] [PubMed] [Google Scholar]
- 21.Shinki T, Ueno Y, DeLuca HF, Suda T. Calcitonin is a major regulator for the expression of renal 25-hydroxyvitamin D3-1alpha-hydroxylase gene in normocalcemic rats. Proc. Natl Acad. Sci. USA. 1999;96(14):8253–8258. doi: 10.1073/pnas.96.14.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong Y, Armbrecht HJ, Christakos S. Calcitonin, a regulator of the 25-hydroxyvitamin D3 1alpha-hydroxylase gene. J. Biol. Chem. 2009;284(17):11059–11069. doi: 10.1074/jbc.M806561200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carneiro RM, Prebehalla L, Tedesco MB, et al. Lactation and bone turnover: a conundrum of marked bone loss in the setting of coupled bone turnover. J. Clin. Endocrinol. Metab. 2010;95(4):1767–1776. doi: 10.1210/jc.2009-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bodnar LM, Simhan HN. Vitamin D may be a link to black–white disparities in adverse birth outcomes. Obstet. Gynecol. Surv. 2010;65(4):273–284. doi: 10.1097/OGX.0b013e3181dbc55b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson CJ, Alanis MC, Wagner CL, Hollis BW, Johnson DD. Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. Am. J. Obstet. Gynecol. 2010;203(4):366, e1–6. doi: 10.1016/j.ajog.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson CJ, Wagner CL, Hollis BW, Baatz JE, Johnson DD. Maternal vitamin D and fetal growth in early-onset severe preeclampsia. Am. J. Obstet. Gynecol. 2011;204(6):556, e1–4. doi: 10.1016/j.ajog.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.McGrath J, Feton F, Eyles D. Does ‘imprinting’ with low prenatal vitamin D contribute to the risk of various adult disorders? Med. Hypotheses. 2001;56:367–371. doi: 10.1054/mehy.2000.1226. [Provocative paper on the theory of vitamin D as an imprinting or epigenetic phenomenon during pregnancy and early infancy.] [DOI] [PubMed] [Google Scholar]
- 28.Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am. J. Clin. Nutr. 2007;85(1):6–18. doi: 10.1093/ajcn/85.1.6. [DOI] [PubMed] [Google Scholar]
- 29.Hollick M. Vitamin D deficiency. N. Engl. J. Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 30.Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am. J. Clin. Nutr. 2007;85(3):649–650. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 31.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J. Nutr. 2005;135(2):317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 32.Hollis B, Wagner CL, Kratz A, Sluss PM, Lewandrowski KB. Normal serum vitamin D levels. N. Engl. J. Med. 2005;352(5):515–516. doi: 10.1056/NEJM200502033520521. [DOI] [PubMed] [Google Scholar]
- 33.McGrath J. Hypothesis: is low prenatal vitamin D a risk-modifying factor for schizophrenia? Schizophr. Res. 1999;49:173–177. doi: 10.1016/s0920-9964(99)00052-3. [DOI] [PubMed] [Google Scholar]
- 34.McGrath J, Selten JP, Chant D. Long-term trends in sunshine duration and its association with schizophrenia birth rates and age at first registration-data from Australia and The Netherlands. Schizophr. Res. 2002;54:199–212. doi: 10.1016/s0920-9964(01)00259-6. [DOI] [PubMed] [Google Scholar]
- 35.Eyles D, Brown J, MacKay-Sim A, McGrath J, Feron F. Vitamin D3 and brain development. Neuroscience. 2003;118(3):641–653. doi: 10.1016/s0306-4522(03)00040-x. [DOI] [PubMed] [Google Scholar]
- 36.Brown J, Bianco J, McGrath J, Eyles D. 1,25-dihydroxyvitamin D-3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci. Lett. 2003;343:139–143. doi: 10.1016/s0304-3940(03)00303-3. [DOI] [PubMed] [Google Scholar]
- 37.Ko P, Burkert R, McGrath J, Eyles D. Maternal vitamin D3 deprivation and the regulation of apoptosis and cell cyle during rat brain development. Dev. Brain Res. 2004;153:61–68. doi: 10.1016/j.devbrainres.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Burne T, Becker A, Brown J, Eyles D, MacKay-Sim A, McGrath J. Transient prenatal vitamin D deficiency is associated with hyperlocomotion in adult rats. Behav. Brain Res. 2004;154:549–555. doi: 10.1016/j.bbr.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 39.Burne T, Feron F, Brown J, Eyles D, McGrath J, Mackay-Sim A. Combined prenatal and chronic postnatal vitamin D deficiency in rats impairs prepulse inhibition of acoustic startle. Physiol. Behav. 2004;81:651–655. doi: 10.1016/j.physbeh.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Cui X, McGrath JJ, Burne THJ, Mackay-Sim A, Eyles DW. Maternal vitamin D depletion alters neurogenesis in the developing rat brain. Int. J. Dev. Neurosci. 2007;25(4):227–232. doi: 10.1016/j.ijdevneu.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 41.O'Loan J, Eyles DW, Kesby J, Ko P, McGrath JJ, Burne THJ. Vitamin D deficiency during various stages of pregnancy in the rat; its impact on development and behaviour in adult offspring. Psychoneuroendocrinology. 2007;32(3):227–234. doi: 10.1016/j.psyneuen.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Kesby J, Burne T, McGrath J, Eyles D. Developmental vitamin D deficiency alters MK 801-induced hyperlocomotion in the adult rat: an animal model of schizophrenia. Biol. Psychiat. 2006;60:591–596. doi: 10.1016/j.biopsych.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 43.Hatun S, Ozkan B, Orbak Z, et al. Vitamin D deficiency in early infancy. J. Nutr. 2005;135(2):279–282. doi: 10.1093/jn/135.2.279. [DOI] [PubMed] [Google Scholar]
- 44.Schnadower D, Agarwal C, Oberfield S, Fennoy I, Pusic M. Hypocalcemic seizures and secondary bilateral femoral fractures in an adolescent with primary vitamin D deficiency. Pediatrics. 2006;118(5):22226–22230. doi: 10.1542/peds.2006-1170. [DOI] [PubMed] [Google Scholar]
- 45.Cockburn F, Belton NR, Purvis RJ, et al. Maternal vitamin D intake and mineral metabolism in mothers and their newborn infants. Brit. Med. J. 1980;5:11–14. doi: 10.1136/bmj.281.6232.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hollis BW, Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. Am. J. Clin. Nutr. 2004;79:717–726. doi: 10.1093/ajcn/79.5.717. [DOI] [PubMed] [Google Scholar]
- 47.Mellanby E. Experimental rickets. Medical Research (Great Britain). Special Report Series. 1921;61:1–78. [Google Scholar]
- 48.Mellanby E. An experimental investigation on rickets. Lancet. 1919;1:407–412. [Google Scholar]
- 49.Weick MT. A history of rickets in the United States. Am. J. Clin. Nutr. 1967;20(11):1234–1241. doi: 10.1093/ajcn/20.11.1234. [DOI] [PubMed] [Google Scholar]
- 50.Rehman P. Sub-clinical rickets and recurrent infection. J. Trop. Pediatr. 1994;40:58. doi: 10.1093/tropej/40.1.58. [DOI] [PubMed] [Google Scholar]
- 51.Rook G. Vitamin D and tuberculosis. Tubercle. 1986;67(2):155–156. doi: 10.1016/0041-3879(86)90011-5. [DOI] [PubMed] [Google Scholar]
- 52.Rook GA, Steele J, Fraher L, et al. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986;57(1):159–163. [PMC free article] [PubMed] [Google Scholar]
- 53.Bikle D, Adams J, Christakos S. Vitamin D: production, metabolism, mechanism of action, and clinical requirements. In: Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. In: Rosen C, editor. American Society for Bone and Mineral Research. Washington, DC, USA: 2008. pp. 141–149. [Google Scholar]
- 54.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 55.Liu P, Stenger S, Tang D, Modlin R. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 2007;179:2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 56.Zheng Y, Niyonsaba F, Ushio H, et al. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br. J. Dermatol. 2007;157(6):1124–1131. doi: 10.1111/j.1365-2133.2007.08196.x. [DOI] [PubMed] [Google Scholar]
- 57.Fabri M, Stenger S, Shin DM, et al. Vitamin D is required for IFN-g-mediated antimicrobial activity of human macrophages. Sci. Transl. Med. 2011;3(104):104ra102. doi: 10.1126/scitranslmed.3003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhalla A, Amento E, Clemens T, Holick M, Krane S. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J. Clin. Endocrinol. Metab. 1983;57(6):1308–1310. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 59.Tsoukas C, Provvedini D, Manolagas S. 1,25-dihydroxyvitamin D3: a novel immunoregulatory hormone. Science. 1984;224(4656):1438–1440. doi: 10.1126/science.6427926. [DOI] [PubMed] [Google Scholar]
- 60.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1 alpha,25-dihydroxyvitamin D3-binding macromolecules in human B lymphocytes: effects on immunoglobulin production. J. Immunol. 1986;136(8):2734–2740. [PubMed] [Google Scholar]
- 61.Bikle D. Nonclassic actions of vitamin D. J. Clin. Endocrinol. Metab. 2009;94(1):26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manolagas SC, Werntz DA, Tsoukas CD, Provvedini DM, Vaughan JH. 1,25-dihydroxy-vitamin D3 receptors in lymphocytes from patients with rheumatoid arthritis. J. Lab. Clin. Med. 1986;108(6):596–600. [PubMed] [Google Scholar]
- 63.Palmer MT, Lee YK, Maynard CL, et al. Lineage-specific effects of 1,25-dihydroxy-vitamin D3 on the development of effector CD4 T cells. J. Biol. Chem. 2011;286(2):997–100. doi: 10.1074/jbc.M110.163790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kamen DL, Cooper GS, Bouali H, Shaftman SR, Hollis BW, Gilkeson GS. Vitamin D deficiency in systemic lupus erythematosus. Autoimmune Rev. 2006;5(2):114–117. doi: 10.1016/j.autrev.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 65.Kieseier B, Giovannoni G, Hartung H. Immunological surrogate markers of disease activity in multiple sclerosis. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999;50:570–583. [PubMed] [Google Scholar]
- 66.Hayes CE. Vitamin D: a natural inhibitor of multiple sclerosis. Proc. Nutr. Soc. 2000;59:531–535. doi: 10.1017/s0029665100000768. [DOI] [PubMed] [Google Scholar]
- 67.Munger K, Zhang S, O'Reilly E, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60–65. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 68.Willer CJ, Dyment DA, Sadovnick AD, Rothwell PM, Murray TJ, Ebers GC. Timing of birth and risk of multiple sclerosis: population based study. BMJ. 2005;330(7483):120. doi: 10.1136/bmj.38301.686030.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chaudhuri A. Why we should offer routine vitamin D supplementation in pregnancy and childhood to prevent multiple sclerosis. Med. Hypotheses. 2005;64:608–618. doi: 10.1016/j.mehy.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 70.Ponsonby A, Lucas R, van der Mei IA. UVR, vitamin D and three autoimmune diseases – multiple sclerosis, Type 1 diabetes, rheumatoid arthritis. Photochem. Photobiol. 2005;81:1267–1275. doi: 10.1562/2005-02-15-IR-441. [DOI] [PubMed] [Google Scholar]
- 71.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 72.Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann. Rheum. Dis. 2007;66(9):1137–1142. doi: 10.1136/ard.2007.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kimball SM, Ursell MR, O'Connor P, Vieth R. Safety of vitamin D3 in adults with multiple sclerosis. Am. J. Clin. Nutr. 2007;86(3):645–651. doi: 10.1093/ajcn/86.3.645. [DOI] [PubMed] [Google Scholar]
- 74.Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann. Rheum. Dis. 2007;66(9):1137–1142. doi: 10.1136/ard.2007.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis Rheum. 2004;50(1):72–77. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- 76.Cutolo M, Otsa K, Uprus M, Paolino S, Seriolo B. Vitamin D in rheumatoid arthritis. Autoimmune Rev. 2007;7:59–64. doi: 10.1016/j.autrev.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 77.Boucher BJ. Strategies for reduction in the prevalence of NIDDM; the case for a population-based approach to the development of policies to deal with environmental factors in its aetiology. Diabetologia. 1995;38(9):1125–1129. doi: 10.1007/BF00402186. [DOI] [PubMed] [Google Scholar]
- 78.The EURODIAB Substudy 2 Study Group Vitamin D supplement in early childhood and risk for Type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1999;42:51–54. doi: 10.1007/s001250051112. [DOI] [PubMed] [Google Scholar]
- 79.Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of Type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 80.Hypponen E. Micronutrients and the risk of Type 1 diabetes: vitamin D, vitamin E, and nicotinamide. Nutr. Rev. 2004;62(9):340–347. doi: 10.1301/nr.2004.sept.340-347. [DOI] [PubMed] [Google Scholar]
- 81.Harris SS. Vitamin D in Type 1 diabetes prevention. J. Nutr. 2005;135(2):323–325. doi: 10.1093/jn/135.2.323. [DOI] [PubMed] [Google Scholar]
- 82.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in Type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2007;92(6) doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu S, Song Y, Ford ES, Manson JE, Buring JE, Ridker PM. Dietary calcium, vitamin D, and the prevalence of metabolic syndrome in middle-aged and older US women. Diabetes Care. 2005;28(12):2926–2932. doi: 10.2337/diacare.28.12.2926. [DOI] [PubMed] [Google Scholar]
- 84.Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among US adults. Diabetes Care. 2005;28(5):1228–1230. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- 85.Garland C, Comstock G, Garland F, Helsing K, Shaw E, Gorham E. Serum 25(OH)D and colon cancer: eight-year prospective study. Lancet. 1989;2:1176–1178. doi: 10.1016/s0140-6736(89)91789-3. [DOI] [PubMed] [Google Scholar]
- 86.Tseng M, Breslow RA, Graubard BI, Ziegler RG. Dairy, calcium, and vitamin D intakes and prostate cancer risk in the National Health and Nutrition Examination Epidemiologic Follow-up Study cohort. Am. J. Clin. Nutr. 2005;81(5):1147–1154. doi: 10.1093/ajcn/81.5.1147. [DOI] [PubMed] [Google Scholar]
- 87.Wu K, Feskanich D, Fuchs CS, Willett WC, Hollis BW, Giovannucci EL. A nested case control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. J. Natl Cancer Inst. 2007;99(14):1120–1129. doi: 10.1093/jnci/djm038. [DOI] [PubMed] [Google Scholar]
- 88.Hu J, Morrison H, Mery L, DesMeules M, Macleod M. Canadian Cancer Registries Epidemiology Research Group. Diet and vitamin or mineral supplementation and risk of colon cancer by subsite in Canada. Eur. J. Cancer Prev. 2007;16(4):275–291. doi: 10.1097/01.cej.0000228411.21719.25. [DOI] [PubMed] [Google Scholar]
- 89.Eisman JA, Suva LJ, Sher E, Pearce PJ, Funder JW, Martin TJ. Frequency of 1,25-dihydroxyvitamin D3 receptor in human breast cancer. Cancer Res. 1981;41(12 Pt 1):5121–5124. [PubMed] [Google Scholar]
- 90.Sher E, Eisman JA, Moseley JM, Martin TJ. Whole-cell uptake and nuclear localization of 1,25-dihydroxycholecalciferol by breast cancer cells (T47 D) in culture. Biochem. J. 1981;200(2):315–320. doi: 10.1042/bj2000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garland F, Garland C, Gorham E, Young J. Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev. Med. 1990;19:614–622. doi: 10.1016/0091-7435(90)90058-r. [DOI] [PubMed] [Google Scholar]
- 92.Mordan-McCombs S, Valrance M, Zinser G, Tenniswood M, Welsh J. Calcium, vitamin D and the vitamin D receptor: impact on prostate and breast cancer in preclinical models. Nutr. Rev. 2007;65(8 Pt 2):S131–S133. doi: 10.1111/j.1753-4887.2007.tb00341.x. [DOI] [PubMed] [Google Scholar]
- 93.Garland C, Gorham E, Mohr S, Grant W, Giovannucci E, Lipkin M. Vitamin D and prevention of breast cancer: pooled analysis. J. Steroids Biochem. 2007;103:708–711. doi: 10.1016/j.jsbmb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 94.Freedman DM, Chang SC, Falk RT, et al. Serum levels of vitamin D metabolites and breast cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol. Biomarkers Prev. 2008;17(4):889–894. doi: 10.1158/1055-9965.EPI-07-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Platz EA, Leitzmann MF, Hollis BW, Willett WC, Giovannucci E. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control. 2004;15:255–265. doi: 10.1023/B:CACO.0000024245.24880.8a. [DOI] [PubMed] [Google Scholar]
- 96.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J. Natl Cancer Inst. 2006;98(7):451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 97.Giovannucci E. Strengths and limitations of current epidemiologic studies: vitamin D as a modifier of colon and prostate cancer risk. Nutr. Rev. 2007;65(8 Pt 2):S77–S79. doi: 10.1111/j.1753-4887.2007.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 98.Mikhak B, Hunter DJ, Spiegelman D, Platz EA, Hollis BW, Giovannucci E. Vitamin D receptor (VDR) gene polymorphisms and haplotypes, interactions with plasma 25-hydroxyvitamin D and 1,25-dihydroxy- vitamin D, and prostate cancer risk. Prostate. 2007;67(9):911–923. doi: 10.1002/pros.20570. [DOI] [PubMed] [Google Scholar]
- 99.Hollis B, Roos B, Lambert P. Vitamin D in plasma: quantitation by a nonequilbrium ligand binding assay. Steroids. 1981;37:609–619. doi: 10.1016/s0039-128x(81)90137-9. [DOI] [PubMed] [Google Scholar]
- 100.Cockburn F, Belton N, Purvis R, et al. Maternal vitamin D intake and mineral metabolism in mothers and their newborn infants. Br. Med. J. 1980;231:1–10. doi: 10.1136/bmj.281.6232.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maxwell JD, Ang L, Brooke OG, Brown IRF. Vitamin D supplements enhance weight gain and nutrional status in pregnant Asians. Brit. J. Obstet. Gyneaecol. 1981;88:987–991. doi: 10.1111/j.1471-0528.1981.tb01686.x. [DOI] [PubMed] [Google Scholar]
- 102.Brooke OG, Brown IRF, Bone CDM, et al. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. Br. Med. J. 1980;1:751–754. doi: 10.1136/bmj.280.6216.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brooke OG, Butters F, Wood C. Intrauterine vitamin D nutrition and postnatal growth in Asian infants. Brit. Med. J. 1981;283:1024. doi: 10.1136/bmj.283.6298.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brooke O, Brown I, Cleeve H, Sood A. Observations on the vitamin D state of pregnant Asian women in London. Br. J. Obstet. Gynaecol. 1981;88:18–26. doi: 10.1111/j.1471-0528.1981.tb00931.x. [DOI] [PubMed] [Google Scholar]
- 105.Marya R, Rathee S, Lata V, Mudgil S. Effects of vitamin D supplementation in pregnancy. Gynecol. Obstet. Invest. 1981;12:155–161. doi: 10.1159/000299597. [DOI] [PubMed] [Google Scholar]
- 106.Nesby-O'Dell S, Scanlon K, Cogswell M, et al. Hypovitaminosis D prevalence and determinants among African–American and white women of reproductive age: Third National Health and Nutrition Examination Survey: 1988–1994. Am. J. Clin. Nutr. 2002;76:187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 107.Johnson DD, Wagner CL, Hulsey TC, McNeil RB, Ebeling M, Hollis BW. Vitamin D deficiency and insufficiency is common during pregnancy. Am. J. Perinatol. 2011;28(1):7–12. doi: 10.1055/s-0030-1262505. [DOI] [PubMed] [Google Scholar]
- 108.Hamilton SA, McNeil R, Hollis BW, et al. Profound vitamin D deficiency in a diverse group of women during pregnancy living in a sun-rich environment at latitude 32 degrees. N. Int. J. Endocrinol. 2010;2010:917428. doi: 10.1155/2010/917428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dawodu A, Wagner CL. Mother–child vitamin D deficiency: an international perspective. Arch. Dis. Child. 2007;92(9):737–740. doi: 10.1136/adc.2007.122689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chiu K, Chu A, Go V, Soad M. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am. J. Clin. Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 111.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49(5):1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 112.Zittermann A, Schleithoff SS, Koerfer R. Putting cardiovascular disease and vitamin D insufficiency into perspective. Br. J. Nutr. 2005;94(4):483–492. doi: 10.1079/bjn20051544. [DOI] [PubMed] [Google Scholar]
- 113.Zittermann A, Schleithoff SS, Koerfer R. Vitamin D insufficiency in congestive heart failure: why and what to do about it? Heart Fail. Rev. 2006;11(1):25–33. doi: 10.1007/s10741-006-9190-8. [DOI] [PubMed] [Google Scholar]
- 114.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Ann. Rheum. Dis. 2007;66(9):1137–1142. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 115.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch. Intern. Med. 2008;168(11):1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rao RK. Prospective study of colorectal cancer in the west of Scotland: 10-year follow-up. Br. J. Surg. 1990;77(12):1434. doi: 10.1002/bjs.1800771231. [DOI] [PubMed] [Google Scholar]
- 117.Lefkowitz E, Garland C. Sunlight, vitamin D, and ovarian cancer mortality rates in US women. Int. J. Epidemiol. 1994;23:1133–1136. doi: 10.1093/ije/23.6.1133. [DOI] [PubMed] [Google Scholar]
- 118.Grant WB. An estimate of premature cancer mortality in the US due to inadequate doses of solar ultraviolet-B radiation. Cancer. 2002;94:1867–1875. doi: 10.1002/cncr.10427. [DOI] [PubMed] [Google Scholar]
- 119.Rao RK. Prostate cancer. Trop. Doct. 2002;32(3):155–157. doi: 10.1177/004947550203200312. [DOI] [PubMed] [Google Scholar]
- 120.Holick MF. Vitamin D: importance in the prevention of cancers, Type 1 diabetes, heart disease, and osteoporosis. Am. J. Clin. Nutr. 2004;79:362–371. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 121.Egan KM, Signorello LB, Munro HM, Hargreaves MK, Hollis BW, Blot WJ. Vitamin D insufficiency among African–Americans in the southeastern United States: implications for cancer disparities (United States). Cancer Causes Control. 2008;19(5):527–535. doi: 10.1007/s10552-008-9115-z. [DOI] [PubMed] [Google Scholar]
- 122.Morgan V, McGrath J, Hultman C, et al. The offspring of women with severe mental disorder. In: Early Life Origins of Human Health and Disease. In: Newnham JP, Ross MG, editors. S Karger AG; Basel, Switzerland: 2009. pp. 202–204. [Google Scholar]
- 123.Martineau AR, Wilkinson RJ, Wilkinson KA, et al. A single dose of vitamin D enhances immunity to Mycobacteria. Am. J. Respir. Crit. Care. Med. 2007;176(2):208–213. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 124.Hollis BW. Circulating 25-hydroxy- vitamin D levels indicative of vitamin sufficiency: implications for establishing a new effective DRI for vitamin D. J. Nutr. 2005;135:317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 125.Holick MF. Vitamin D deficiency. N. Engl. J. Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 126.Haddock L, Corcino J, Vazquez MD. 25(OH)D serum levels in the normal Puerto Rican population and in subjects with tropical sprue and parathyroid disease. Puerto Rico Health Sci. 1982;1:85–91. [Google Scholar]
- 127.Haddad JG, Chyu K. Competitive protein-binding radioassay for 25-hydroxycholecalciferol. J. Clin. Endocrinal. Metab. 1971;33:992–995. doi: 10.1210/jcem-33-6-992. [DOI] [PubMed] [Google Scholar]
- 128.Matsuoka LY, Wortsman J, Hanifan N, Holick MF. Chronic sunscreen use decreases circulating concentrations of 25-hydroxyvitamin D: a preliminary study. Arch. Dermatol. 1988;124:1802–1804. [PubMed] [Google Scholar]
- 129.Hollis BW, Wagner CL, Drezner MK, Binkley NC. Circulating vitamin D3 and 25-hydroxyvitamin D in humans: an important tool to define adequate nutritional vitamin D status. J. Steroid Biochem. Mol. Biol. 2007;103(3–5):631–634. doi: 10.1016/j.jsbmb.2006.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J. Clin. Endocrinol. Metab. 2007;92(9):3517–3522. doi: 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Robinson CJ, Wagner CL, Hollis BW, Baatz JE, Johnson DD. Maternal vitamin D and fetal growth in early-onset severe preeclampsia. Am. J. Obstet. Gynecol. 2011:556, e1–4. doi: 10.1016/j.ajog.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wei SQ, Audibert F, Hidiroglou N, et al. Longitudinal vitamin D status in pregnancy and the risk of pre-eclampsia. BJOG. 2012 doi: 10.1111/j.1471-0528.2012.03307.x. doi:10.111 1/j.1471-0528.2012.03307 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 133.Merewood A, Mehta SD, Chen TC, Bauchner H, Holick MF. Association between vitamin D deficiency and primary Cesarean section. J. Clin. Endocrinol. Metab. 2009;94(3):940–945. doi: 10.1210/jc.2008-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bodnar LM, Krohn MA, Simhan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J. Nutr. 2009;139(6):1157–1161. doi: 10.3945/jn.108.103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mannion C, Gray-Donald K, Koski K. Milk restriction and low maternal vitamin D intake during pregnancy are associated with decreased birth weight. CMAJ. 2006;174(9):1273–1277. doi: 10.1503/cmaj.1041388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pawley N, Bishop NJ. Prenatal and infant predictors of bone health: the influence of vitamin D. Am. J. Clin. Nutr. 2004;80(6):1748S–1751S. doi: 10.1093/ajcn/80.6.1748S. [DOI] [PubMed] [Google Scholar]
- 137.Maghbooli Z, Hossein-Nezhad A, Shafaei A, Karimi F, Madani F, Larijani B. Vitamin D status in mothers and their newborns in Iran. BMC. 2007;7(1):1. doi: 10.1186/1471-2393-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vieth R, Chan PC, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am. J. Clin. Nutr. 2001;73(2):288–294. doi: 10.1093/ajcn/73.2.288. [DOI] [PubMed] [Google Scholar]
- 139.National Coalition for Skin Cancer Prevention . American Association for Health Education. Reston, VA, USA: 1998. The National Forum for Skin Cancer Prevention in Health, Physical Education, Recreation and Youth Sports. [Google Scholar]
- 140.Reichrath J. The challenge resulting from positive and negative effects of sunlight: how much solar UV exposure is appropriate to balance between risks of vitamin D deficiency and skin cancer? Prog. Biophys. Mol. Biol. 2006;92:9–16. doi: 10.1016/j.pbiomolbio.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 141.Mahomed K, Gulmezoglu AM. Vitamin D supplementation in pregnancy. Cochrane Database Syst. Rev. 2000;2:CD000228. doi: 10.1002/14651858.CD000228. [DOI] [PubMed] [Google Scholar]
- 142.De-Regil LM, Palacios C, Ansary A, Kulier R, Pena-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Sys. Rev. 2012;2:CD008873. doi: 10.1002/14651858.CD008873.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wagner C, McNeil R, Hamilton S, et al. Vitamin D (vitD) supplementation during pregnancy: Thrasher Research Fund RCT in SC Community Health Center Networks.. Presented at: Pediatric Academic Societies Annual Meeting; Vancover, BC, Canada. 1–4 May 2010; (Abstract 3737.375) [Google Scholar]
- 144.Wagner C, Johnson D, Hulsey T, et al. Vitamin D supplementation during pregnancy part 2 NICHD/CTSA Randomized Clinical Trial (RCT): outcomes.. Presented at: Pediatric Academic Societies Annual Meeting; Vancover, BC, Canada. 1–4 May 2010; (Abstract 1665.6) [Google Scholar]
- 145.Cancela L, LeBoulch N, Miravet L. Relationship between the vitamin D content of maternal milk and the vitamin D status of nursing women and breastfed infants. J. Endocrinol. 1986;110:43–50. doi: 10.1677/joe.0.1100043. [DOI] [PubMed] [Google Scholar]
- 146.Hollis BW, Wagner CL. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am. J. Clin. Nutr. 2004;80(Suppl. 6):1752S–1758S. doi: 10.1093/ajcn/80.6.1752S. [DOI] [PubMed] [Google Scholar]
- 147••.Wagner CL, Hulsey TC, Fanning D, Ebeling M, Hollis BW. High-dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: a 6-month follow-up pilot study. Breastfeed. Med. 2006;1(2):59–70. doi: 10.1089/bfm.2006.1.59. [The results of this pilot study suggest that if a mother is vitamin D replete, her breast milk will be vitamin D replete, and therefore, her recipient breastfeeding infant will be vitamin D replete. It refutes the dogma that human milk is inherently deficient or borderline sufficient in vitamin D, but rather that its content solely reflects maternal vitamin D status.] [DOI] [PubMed] [Google Scholar]
- 148.Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 149.Houben ML, Bont L, Wilbrink B, et al. Clinical prediction rule for RSV bronchiolitis in healthy newborns: prognostic birth cohort study. Pediatrics. 2011;127(1):35–41. doi: 10.1542/peds.2010-0581. [DOI] [PubMed] [Google Scholar]
- 150.Beser E, Cakmakci T. Factors affecting the morbidity of vitamin D deficiency rickets and primary protection. East Afr. Med. J. 1994;71(6):358–362. [PubMed] [Google Scholar]
- 151.Bikle DD. Vitamin D and the immune system: role in protection against bacterial infection. Curr. Opin. Nephrol. Hypertens. 2008;17(4):348–352. doi: 10.1097/MNH.0b013e3282ff64a3. [DOI] [PubMed] [Google Scholar]
- 152.Williams B, Williams AJ, Anderson ST. Vitamin D deficiency and insufficiency in children with tuberculosis. Pediatric Infect. Dis. J. 2008;27(10):941–942. doi: 10.1097/INF.0b013e31817525df. [DOI] [PubMed] [Google Scholar]
- 153.Morcos MM, Gabr AA, Samuel S, et al. Vitamin D administration to tuberculous children and its value. Boll. Chim. Farm. 1998;137(5):157–164. [PubMed] [Google Scholar]
- 154.Muhe L, Lulseged S, Mason KE, Simoes EA. Case–control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet. 1997;349(9068):1801–1804. doi: 10.1016/S0140-6736(96)12098-5. [DOI] [PubMed] [Google Scholar]
- 155.Wagner CL, Taylor SN, Hollis BW. Does vitamin D make the world go ‘round’? Breastfeed Med. 2008;3(4):239–250. doi: 10.1089/bfm.2008.9984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Maxwell J, Ang L, Brooke O, Brown I. Vitamin D supplements enhance weight gain and nutritional status in pregnant Asians. Br. J. Obstet. Gynaecol. 1981;88:987–991. doi: 10.1111/j.1471-0528.1981.tb01686.x. [DOI] [PubMed] [Google Scholar]
- 157.Delvin EE, Salle BL, Glorieux FH, Adeleine P, David LS. Vitamin D supplementation during pregnancy: effect on neonatal calcium homeostasis. J. Pediatr. 1986;109:328–334. doi: 10.1016/s0022-3476(86)80396-1. [DOI] [PubMed] [Google Scholar]
- 158.Mallet E, Gugi B, Brunelle P, Henocq A, Basuyau J, Lemeur H. Vitamin D supplementation in pregnancy: a controlled trial of two methods. Obstet. Gynecol. 1986;68:300–304. doi: 10.1097/00006250-198609000-00002. [DOI] [PubMed] [Google Scholar]
- 159.Datta S, Alfaham M, Davies D, et al. Vitamin D deficiency in pregnant women from a non-European ethnic minority population: an interventional study. Br. J. Obstetr. Gynaecol. 2002;109:905–908. doi: 10.1111/j.1471-0528.2002.01171.x. [DOI] [PubMed] [Google Scholar]
- 160.Sahu M, Das V, Aggarwal A, Rawat V, Saxena P, Bhatia V. Vitamin D replacement in pregnant women in rural north India: a pilot study. Eur. J. Clin. Nutr. 2009;63:1157–1159. doi: 10.1038/ejcn.2009.27. [DOI] [PubMed] [Google Scholar]