SUMMARY
Evaluating anticoagulants in animal thrombosis models is a standard component of pre-clinical drug testing. Mice are frequently used for these initial evaluations because a variety of thrombosis models have been developed and are well characterized in this species, and the animals are relatively inexpensive to maintain. Because mice have a natural resistance to forming intravascular thrombi, vessel injury is required to induce intravascular clot formation. Several methods have been established for inducing arterial or venous thrombosis in mice. For the purpose of testing heparin-based drugs, we adapted a well-established model in which thrombus formation in the carotid artery is induced by exposing the vessel to ferric chloride. For studying anticoagulant effects on venous thrombosis, we use a model in which the inferior vena cava is ligated and the size of the resulting clots is measured. The most common adverse effect of anticoagulation therapy is bleeding. The effect of heparin-based anticoagulants can be tested in mice in a simple tail bleeding assay.
Keywords: Mouse, Heparin, Anticoagulant, Arterial thrombosis, Venous thrombosis, Tail bleeding time
GENERAL INTRODUCTION
Animal models of hemostasis and thrombosis have contributed substantially to our understanding of normal and pathologic blood coagulation in humans (1–5). Mice, because they are small in size, have a short gestation period, have high reproductive capacity, and are relatively inexpensive to maintain, are commonly used as test subjects for initial in vivo analyses of antithrombotic compounds intended for therapeutic use in humans. These animals have a number of features that make them suitable surrogates for testing anticoagulant drugs. Mice and humans have similar complements of plasma coagulation factors and regulatory proteins, and coagulation proteins from one species usually demonstrate reasonable activity (with a few exceptions) in the plasma of the other (6–8). Heparan sulfate, heparin and dermatan sulfate produce their anticoagulant effects through inhibition of the key coagulation protease thrombin, and through inhibition of proteases responsible for thrombin generation (9). These glycosaminoglycans primarily inhibit coagulation proteases indirectly by enhancing the activities of plasma serine protease inhibitors (serpins) such as antithrombin through allosteric- and template-based mechanisms (serpin-dependent effects). In some cases they directly inhibit proteases by binding to them (serpin-independent effect). The anion binding sites on coagulation proteases and serpins required for productive interactions with glycosaminoglycans are largely conserved between mice and humans, suggesting the two species have fundamentally similar mechanisms for regulating thrombin generation.
Similarities between human and murine plasma coagulation factors can be useful for evaluating a drug candidate, even when the compound interacts only with the human version of a protein target. The genomes of mice can be manipulated to produce constitutive or conditional deficiencies of a protein of interest (6–8). To generate a model for testing a drug that interacts exclusively with a human plasma protein, a mouse deficient in that protein can be “reconstituted” by intravenous infusion of its human counterpart. For coagulation proteases, this usually restores the wild type phenotype in hemostasis and thrombosis models (10–12).
There are important differences in normal (hemostasis) and pathologic (bleeding or thrombosis) coagulation between mice and humans that must be considered when interpreting results from mouse models. The size discrepancy alone between a large biped and a several thousand-fold smaller quadruped results in different forces on tissues, presenting different challenges for the respective hemostatic systems. Humans lacking coagulation factor IX have a condition (hemophilia B) characterized by a propensity to develop recurrent hemorrhage into large joints (hemarthrosis) such as knees, ankles, elbows that can be crippling (13). While factor IX deficient mice also have a hemorrhagic disorder, hemarthrosis is not a prominent feature, perhaps due to the relatively smaller mechanical forces on their joints (14). The atherosclerotic changes in large arteries that are a major contributor to arterial thrombosis in humans may develop over decades. Mice, with their shorter life-spans and high plasma levels of high density lipoprotein, are resistant to atherosclerosis, and require dietary or genetic manipulation to produce atherosclerotic plaque (15). Even mice genetically altered to develop atherosclerosis (for example, mice lacking apolipoprotein E) tend not to develop occlusive thrombi at sites of plaque rupture in the same manner as humans (16,17). In humans, venous thrombi form preferentially in the deep veins of the lower extremities and pelvis. Blood stasis aggravated by upright posture is a major contributor to formation of this type of clot (18). The long-term effects of hydrostatic forces on leg veins are unlikely to be important in mice, which rarely develop spontaneous venous thrombi in their extremities.
The natural resistance of mice to formation of occlusive thrombi requires that a vessel be injured in some manner to induce local thrombosis, and almost all mouse thrombosis models involve formation of a clot in a vessel that was healthy immediately prior to injury. This contrasts with thrombosis in humans, which typically occurs in a diseased vessel. A variety of techniques are employed to induce venous and arterial thrombosis in mice. In this chapter ferric chloride-induced injury of the carotid artery and ligature-induced venous stasis in the inferior vena cava are described. These techniques were chosen because they have been widely used, and because they are sensitive to heparin. The major adverse side effect of anticoagulants is bleeding. The last section of this chapter describes a simple heparin-sensitive tail bleeding time assay that can be used to evaluate the anti-hemostatic effects of a compound of interest.
FERRIC CHLORIDE-INDUCED ARTERIAL THROMBOSIS MODEL
1. INTRODUCTION
A variety of approaches are used to induce acute thrombus formation in large (e.g. carotid), medium (e.g. mesenteric) and small (e.g. cremaster) arteries in mice. Injury to the vascular endothelium to promote thrombus formation can be induced by chemical exposure (e.g. ferric chloride) (1–3,19–21), photochemical techniques (1–3), direct laser-based techniques (1–4), or mechanical methods (2). Thrombus formation is detected by monitoring changes in blood flow through the vessel with a Doppler flow-probe, by directly observing thrombus formation by intravital microscopy, or by observing histologic changed to the injured vessel. It has not been established which approach most closely reflects processes that occur during arterial thrombosis in humans. Many groups have found ferric chloride-induced injury to be a reproducible method for generating a clot that histologically resembles platelet-rich arterial thrombi in humans (19–23). Typically one or more small pieces of paper saturated with FeCl3 solution are applied to a vessels adventitial surface. FeCl3 defuses through the vessel wall to the luminal surface. Initially, it was thought that this treatment resulted in denudation of the vessel endothelium, exposing thrombogenic subendothelial matrix to flowing blood. While this may occur at high FeCl3 concentrations, recent work from Barr et al. suggests the endothelium remains largely intact after FeCl3 application (21). These investigators observed that erythrocytes initially bind to the altered endothelium, and ferric ions localize primarily to adherent erythrocytes and erythrocyte-derived structures and not to the endothelial cells themselves. Subsequently, platelets bind to adherent erythrocytes in a manner dependent on the platelet receptor glycoprotein 1b-α, with platelet aggregates eventually filling the lumen of the vessel.
We use ferric chloride-induced carotid artery occlusion to study the effects of heparin (23) and heparan-based anticoagulants on thrombus formation. Thrombus formation in mice induced by FeCl3 requires contributions from tissue factor-initiated coagulation (extrinsic pathway) (24) and from factors XII and XI (intrinsic pathway) (12,23,25). The method has been particularly useful for studying the effects of heparan-based compounds that are designed to inhibit specific protease components of the coagulation mechanism. Our approach involves establishing the lowest FeCl3 concentration that reproducibly induces vessel occlusion in untreated wild type mice, and then demonstrating that an anticoagulant compound prevents vessel occlusion at this FeCl3 concentration (22,23). If an antithrombotic effect is observed, the drug can then tested at progressively higher FeCl3 concentrations until the effect of the drug is overcome.
2. MATERIALS
A variety of inbred and mixed breeds of mice have been used to study hemostasis and thrombosis. C57Bl/6 mice have been used extensively in this regard and our work with the FeCl3 arterial thrombosis model is standardized with this readily available mouse line (22,23). Assay reproducibility can be enhanced by attention to a number of animal related factors (see Note 1).
Pentobarbital. Mice are placed under general anesthesia for the FeCl3 model with pentobarbital. This drug is a controlled substance and will require DEA licensure to obtain and use. Other general anesthetics may be used, but the sensitivity of the assay to vessel injury may be different than with pentobarbital (see Note 2).
FeCl3. A 20% stock solution is prepared by bringing 200 mg of FeCl3 up to 1 ml with de-ionized water. Subsequent dilutions of the stock are prepared with deionized water. We prepare fresh stock solution every one to two weeks.
Filter paper for application of FeCl3. Whatman 3MM Chromatography paper (catalog # 3030-917) is cut into small rectangles measuring ~1 × 1.5 millimeters.
Flow probe. We use a Transonic (Ithaca, New York) TS4020 transit time perivascular flow meter fitted with a 0.5VB504 Doppler flow probe (Catalog Mao-5VB). The flow meter is connected to an ML866 PowerLab 4/30 data acquisition system (AD Instruments, Dunedin, New Zealand) interfaced with a Macintosh computer.
Phosphate buffered saline (PBS). PBS is used to keep tissues moist during the procedure and as a vehicle for diluting heparan-based anticoagulants for intravenous administration. We use 1× Cellgro Dulbecco’s phosphate buffered saline without calcium or magnesium (Mediatech, Manassas, VA), but any source of sterile PBS should work.
3. METHODS
Mice are anesthetized by administration of pentobarbital (50 mg/kg) through an intraperitoneal injection. The injection is best given into the right side of the abdomen to avoid injuring the cecum or spleen.
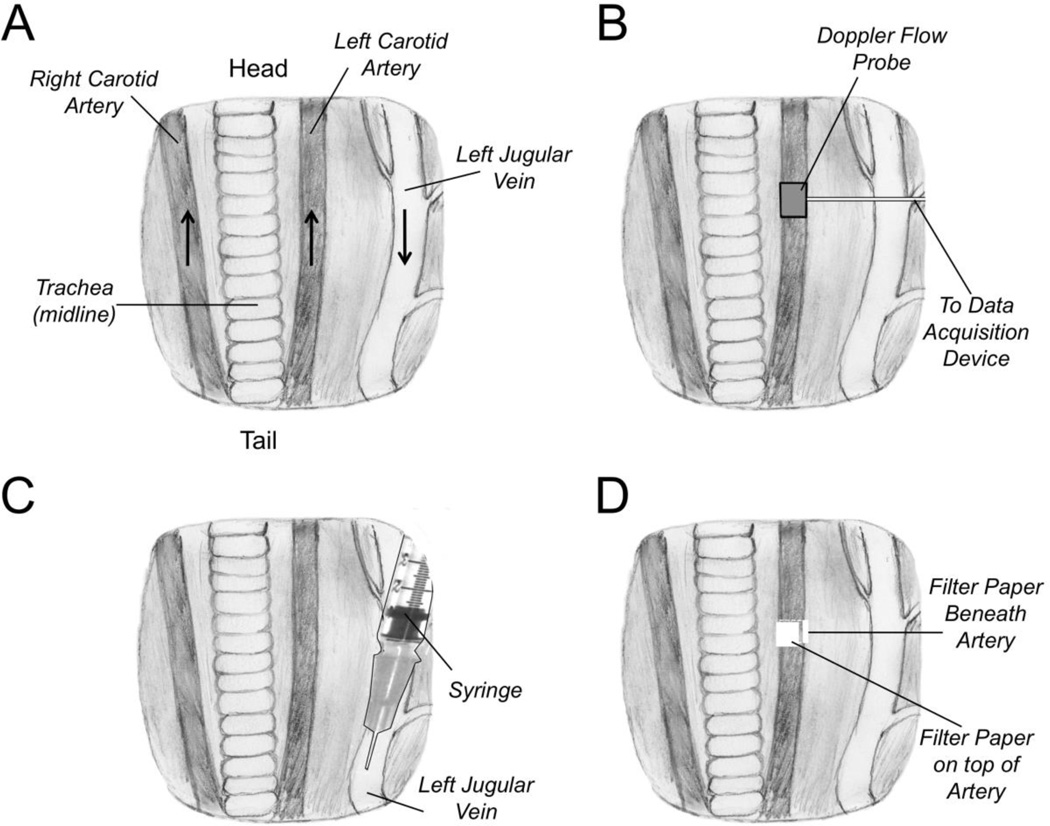
Once the animals are under anesthesia, they are placed on their backs on a 37 °C warming pad, and the extremities are immobilized with pieces of tape. The neck is opened along the midline and the carotid artery and jugular vein are exposed on one side of the neck. The carotid artery is separated from surrounding tissues by blunt dissection (Figure 1A) (see Note 3).
The flow probe is attached to the artery, and the area is bathed with PBS to insure proper signal transduction from the vessel to the probe (Figure 1B). A baseline flow rate is established (typically 0.5 to 0.8 ml/min in an adult mouse). The flow probe is then removed.
Anticoagulant compounds to be tested are diluted up to 100 µl with PBS. The drug (or vehicle control) is infused into the jugular vein using a 300 µl tuberculin syringe fitted with a 30-gauge needle, with the needle tip pointing toward the heart (the direction of blood flow) (Figure 1C) (Note 4).
Five minutes after drug infusion, the area around the carotid artery is dried with cotton Q-tips. Two Whatman chromatography paper pads are saturated with 50 µl each of FeCl3 solution (Figure 1D). Initial studies are typically performed at 3.5%. Fifty microliters is more solution than the pad can hold, and non-absorbed solution is discarded. The pads are applied to the surface of the carotid artery, on opposite sides of the artery from each other. After three minutes, the pads are removed, the area is washed with PBS, the Doppler flow probe is replaced (Figure 1B) and flow is monitored for up to 30 minutes. Changes in flow over time, and time to vessel occlusion are determined. Mice are sacrificed prior to recovering from anesthesia (Note 5).
Figure 1. Ferric Chloride Carotid Artery Thrombosis Model.
(A) Anatomy of ventral surface of the mouse neck showing the relative positions of the carotid arteries and jugular vein to the trachea, which runs along the midline. The positions of the animal’s head and tail relative to the drawing are indicated. The black arrows indicate the direction of blood flow in the major vessels. (B) A Doppler flow probe is placed on the carotid artery to monitor blood flow. (C) Anticoagulant compounds to be tested are administered through an intravenous injection into the jugular vein in the direction of blood flow (toward the heart). (D) Pieces of filter paper (two total) saturated with ferric chloride solution are applied beneath and on top of the carotid artery
INFERIOR VENA CAVA STASIS-INDUCED VENOUS THROMBOSIS MODEL
1. INTRODUCTION
Venous thrombi that cause symptoms in humans form primarily in the deep veins of the lower extremities and the pelvis (18). Embolization of clot to the pulmonary circulation is a major cause of mortality in patients with deep vein thrombosis. While platelets contribute to venous thrombus formation, the clots are predominantly comprised of fibrin and erythrocytes, and differ significantly in their histology from the platelet-rich thrombi that form in the arterial circulation. Blood has a tendency to pool in the deep leg veins in humans, due to hydrostatic forces created by our upright posture. The resulting stasis is a major contributor to venous thrombus formation in humans. Predisposition to venous thrombosis in humans also has a strong genetic component, and a number of inherited conditions have been identified that enhance basal thrombin generation and, consequently, risk for venous thromboembolism. The pathophysiologic processes that cause venous thrombosis in humans are, therefore, difficult to reproduce in mice. Injury to a vein with a chemical such as FeCl3 will cause formation of a venous thrombus, but the platelet-rich clots tend to look like those produced in the arterial circulation (5). Venous stasis models involving partial or complete ligation of the inferior vena cava in the abdomen have been developed to study the effects of anticoagulants on thrombus formation (1–5,26). Here we present a method that involves incomplete ligation of the inferior vena cava (26).
2. MATERIALS
C57Bl/6 mice. Same as in the FeCl3 thrombosis model (see Note 1).
Pentobarbital. Same as in the FeCl3 thrombosis model (see Note 2).
Phosphate buffered saline (PBS). Same as in the FeCl3 thrombosis model.
Surgical supplies. 4-0 Vicryl suture is used to ligate the inferior vena cava. 4-0 Steelex metal monofilament suture (Braun Catalog #F1614037).
3. METHODS
Mice are anesthetized by administration of pentobarbital (50 mg/kg) through an intraperitoneal injection as described in the section on the FeCl3 thrombosis model.
The mouse is placed on its back on a 37 °C warming pad, and the extremities are immobilized with pieces of tape. Heparan-based drug or vehicle in 100 µl of PBS is infused into a lateral tail vein using a 1 ml tuberculin syringe fitted with a 27-gauge needle. If the drug appears to compromise subsequent surgery, it can be administered shortly after the surgical procedure described below is complete (see Note 6).
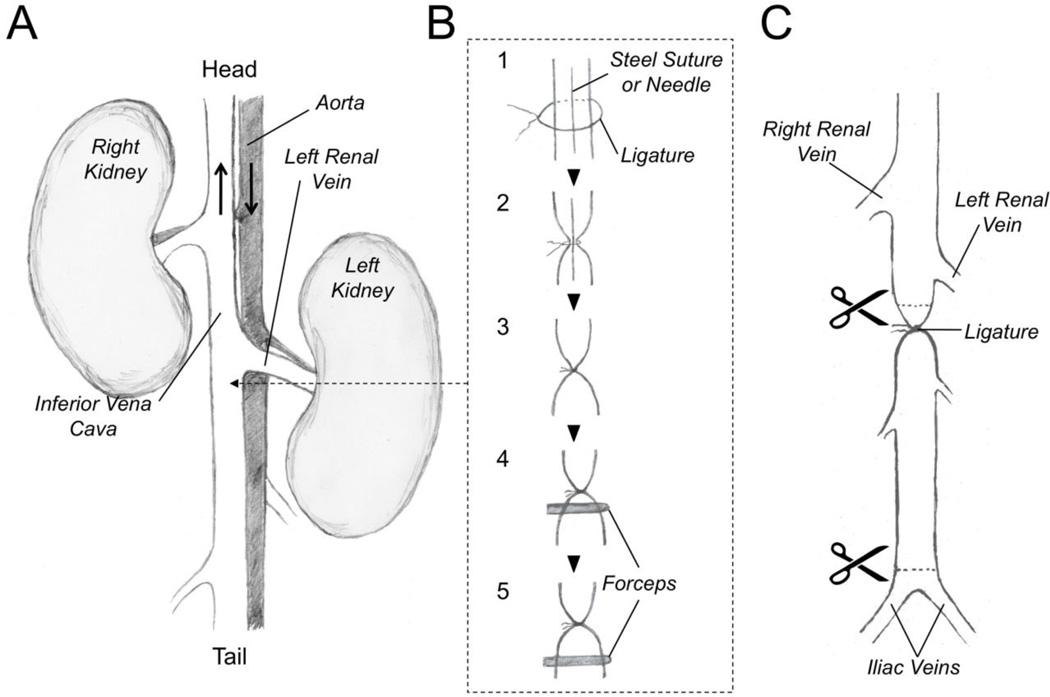
A midline vertical incision is made through the skin and abdominal wall with a scalpel. The inferior vena cava is exposed between the iliac bifurcation and the renal veins by pushing the abdominal contents (bowel) to the left side of the animal (Figure 2A). The bowel is kept moist by covering it with cotton gauze soaked in PBS. The vena cava is gently separated from the aorta by blunt dissection (see note 7).
A 4-0 coated Vicryl suture is placed underneath the vena cava immediately below the renal veins, and a 4-0 Steelex metal monofilament suture is placed longitudinally over the IVC (Figure 2B – Step 1) (see note 8). The Vicryl suture is tied over the IVC and the metal suture to stop blood flow through the vessel (Figure 2B – Step 2). Then the metal suture is gently removed by sliding it out from ligature (Figure 2B – Step 3). Removal of the steel suture restores a small amount of blood flow through the vena cava (see note 9).
A sterile forceps with serrated tip is used to compress (crimp) the vena cava immediately below the suture (toward the tail) for 20 seconds (Figure 2B – Step 4). The procedure is repeated at a location 5 mm below the first compression site (Figure 2B – Step 5). This “crush” injury will cause endothelial cell damage and serve as a nidus for thrombus formation within the vessel lumen (see note 9).
Bowel is returned to the abdominal cavity, and the abdominal wall is closed with sutures. The overlying skin is closed with surgical clips. The animal is observed until it recovers from anesthesia.
24 hours post surgery, the mouse is sacrificed with an intracardiac infusion of pentobarbital (100 mg/kg) and the abdomen is reopened. The vena cava is cut above the ligature and at the distal end above the iliac bifurcation (Figure 2C). Vessel contents are pushed out of the distal end of the vena cava by running forceps along the length of the vessel starting at the ligature. Expressed clot is placed in 10% formalin for 24 hours. After fixing, the clot is dried on a piece of filter paper and weighed (see note 10).
Figure 2. Inferior Vena Cava Venous Thrombosis Model.
(A) Anatomy of the retroperitoneum of the mouse as viewed from a ventral abdominal incision. The positions of the animal’s head and tail relative to the drawing are indicated. The black arrows indicate the direction of blood flow in the major vessels. (B) Thrombosis Model. Step 1 - A ligature is loosely placed around the inferior vena cava caudal to the left renal vein. A steel suture or other linear object such as a needle is also placed within the ligature. Step 2 – The ligature is tightened around the vessel and steel suture. Step 3 – The steel suture is gently removed from the ligature. This results in the ligature restricting flow through the vena cava without completely blocking it. Step 4 – Forceps are used to crimp the vena cava immediately below the suture, and (Step 5) 5 millimeters caudal to the suture to injure the vessel endothelium. (C) twenty-four hours after surgery, the abdomen is reopened, and the vena cava is excised for processing by cutting the vessel immediately above (cranial) to the ligature, and just above the iliac bifurcation.
TAIL BLEEDING MODEL
1. INTRODUCTION
Many antithrombotic drugs, including heparin, produce therapeutic effects by inhibiting processes required for normal hemostasic responses to injury. Therefor, the major trade-off for the beneficial drug effect is a significantly increased risk of bleeding (27). Newer oral agents, while exhibiting better safety profiles than older drugs such as heparin and warfarin, still target the key hemostatic proteases thrombin and factor Xa and, therefore, increase bleeding risk (27). Antithrombotic compounds are now under development that target plasma proteases such as factor XIa and factor XIIa that do not play major roles in hemostasis but that contribute to thrombus growth (28–31). The anticipated advantage of such drugs is not necessarily better efficacy than currently available agents, but an improved safety profile, permitting anticoagulation therapy to be applied to a wider range of patients. Mice deficient in factor XI or factor XII (the zymogen precursors of factor XIa and factor XIIa) exhibit resistance to injury-induced thrombosis in a number of models, including the FeCl3-induced carotid artery thrombosis and inferior vena cava ligation models described above, but do not have obvious hemostatic abnormalities (12,23,25). Drugs specifically targeting factor XIa and factor XIIa should, therefore, prevent thrombus formation but not increase bleeding. Drugs based on heparan-like structures have been reported that inhibit the activity of factor XIa (31). As heparans have a tendency to bind to multiple targets, it is important to test these compounds for off-target effects that could cause undesirable consequences, particularly bleeding.
The tail bleeding time has been used extensively to study hemostasis in mice (32–34). This assay is simple to perform, and is sensitive to the effects of heparin (23). Removal of the tip of the tail with a scalpel transects several blood vessels including two large lateral veins and the ventral artery. The tail tip is usually immersed in warm normal saline and time to cessation of bleeding and/or total blood loss can be determined. While wild type mice usually bleed for one to three minutes, mice with certain types of bleeding disorders or mice who have received anticoagulation therapy may have prolonged bleeding that is punctuated by periods of 1 to 2 minutes in which no bleeding occurs. For example mice lacking factor VIII or factor IX (hemophilia A or B, respectively) have similar initial tail bleeding times as wild type mice. However, shortly (30 to 60 seconds) after the initial cessation of bleeding, hemorrhage recurs (34). We observe mice for up to 30 minutes after tail transection to account for “rebleeding”, with the bleeding time recorded as the time it takes for all bleeding to stop. It must be recognized that any bleeding model only provides information about hemostasis in response to a specific-type of injury in a specific vascular bed, and will not necessarily reflect the propensity to bleed when injury involves other parts of the body. Nevertheless, the ease with which the tail bleeding time is performed has made it a mainstay of evaluating hemostasis in mice.
2. MATERIALS
3. METHODS
Mice are anesthetized by administration of pentobarbital (50 mg/kg) through an intraperitoneal injection as described in the section on the FeCl3 thrombosis model.
Once under anesthesia, the animal is placed on a 37 °C heating pad. Heparan based drug, or vehicle, in 100 °l of PBS is infused into a lateral tail vein using a 1 ml tuberculin syringe fitted with a 27-gauge needle (see Note 6).
The tail is transected with a scalpel 2 millimeters above the tip (see Note 11).
The bleeding tail is immediately immersed in a 1.7 ml microfuge tube filled with 1.2 ml of PBS kept at 37 °C with a heating block.
The animal is observed for up to 30 minutes. The time to cessation of bleeding (including rebleeding) is noted (see Note 12). Mice are sacrificed prior to recovering from anesthesia.
Volume of PBS plus blood is recorded to establish the amount of blood lost (see Note 13).
ACKNOWLEDGMENT
The work described in this manuscript was supported by awards HL81326, HL58837 and HL107152 from the National Heart, Lung and Blood Institute.
Footnotes
A number of animal-related factors can produce variability in this model. Different mouse strains vary in their propensity to form thrombi in response to FeCl3. Each laboratory should determine the sensitivity of the mouse line (inbred or mixed) to different concentrations of FeCl3 before testing anticoagulants. Older mice are larger than younger animals and have thicker vessel walls. This can alter the response to injury. We prefer to use mice that are in a relatively narrow age range (12–20 weeks) to limit this effect. Many investigators confine analysis to male mice, to avoid effects of variation in the levels of coagulation proteins during the estrus cycle in females. We have not noticed a significant inter-gender difference with the FeCl3 carotid artery injury model, but it is possible that certain anticoagulants may be sensitive to changes due to the estrus cycle.
The response of the animal to FeCl3 injury (i.e. the lowest concentration required to reproducibly induce thrombus formation) will vary with different anesthetics. For example, exposure of the carotid artery to 3.5% FeCl will reproducibly cause thrombus formation in C57Bl/6 mice anesthetized with pentobarbital (23), while the same mice anesthetized by isoflurane inhalation will occlude with 2.5% FeCl3. For each anesthetic, a range of FeCl3 concentrations should be tested to identify the lowest concentration that reproducibly causes vessel occlusion.
An obvious concern with using a surgery-based model to test anticoagulants is peri-operative bleeding. We find that if care is taken not to injure structures underlying the skin, the ferric chloride arterial injury model and the venous stasis model can be performed with minimal blood loss even on mice with severe hemophilia (factor VIII or IX deficiency) or in mice who have received heparin.
In our experience, FeCl3 arterial injury models are less sensitive to anti-platelet agents than to inhibitors of thrombin generation. However, when using relatively low FeCl3 concentrations, anti-platelet agents can influence results (produce an antithrombotic effect). Given this, we avoid using non-steroidal anti-inflammatory analgesics to treat pain because they can produce an anti-platelet effect that will alter results in the thrombosis model.
Changing the size or number of the FeCl3-soaked filter papers, or the duration of exposure of the vessel to FeCl3, will change the extent of vessel injury and influence the result. If these factors are standardized, the assay should have a high degree of reproducibility.
Administering drugs by tail vein injection is a skill that requires practice. The diameter of the target vessel is small and the skin and underlying tissue of the tail is tough and can be difficult to penetrate with a small-gauge needle. Warming the tail for a few minutes with a warm cloth to dilate the vessels can make injection easier.
While the anatomy of the carotid artery varies relatively little between mice, there can be considerable variation in the anatomy of the inferior vena cava, even among animals of the same strain. The number and size of collateral branches, and the positions of branch points of important vessels such as the renal veins can vary. Some investigators will ligate large collaterals so that flow to the vessel comes largely from the lower extremities, but we have not found that this affects results appreciably. We have observed that an occasional animal may develop paralysis of the hind limbs a few hours after the procedure. This might be caused by trauma to the aorta or to the small arteries that branch off of it at numerous points to supply the spinal chord. For beginners, it is often difficult to distinguish the vena cava from the aorta, leading to inadvertent ligation of both vessels. Practice is required to obtain proficiency in isolating and manipulate the inferior vena cava without injuring the aorta.
The 4-0 Steelex metal monofilament suture used in step 4 under Methods can be replaced with another object of comparable diameter, such as a 26-gauge needle. The extent of the partial obstruction of blood flow can be adjusted by using needles of different gauge.
This model requires a significant amount of practice to perform reproducibly compared to the FeCl3-induced arterial injury model, as the inferior vena cava is considerably more delicate than the carotid artery, the vessel is closely associated with the descending aorta in the abdominal cavity, and the required manipulations of the vessel are relatively complex. When crimping the vessel with forceps, excessive force can result in laceration.
A relatively large range of thrombus sizes should be expected, even in a control group of mice of the same strain. On occasion, a control mouse may even fail to develop a detectable thrombus. For this reason, it is usually necessary to test a larger number of animals than would be used in a more reproducible assay such as the FeCl3-induced arterial injury model.
While a common strategy is to transect the animal’s tail ~ 2 mm from the tip, some published models use injuries at different points on the tail. It is important to note that tail anatomy varies between animals, resulting in different amounts of tissue injury if a fixed distance from the tail tip is used as the point of injury. Some investigators chose to injure tails at a point where the cross-sectional areas are the same. This can be achieved by creating a template (typically a piece of plastic) with an aperture of desired cross-sectional area. The animal’s tail is drawn through the aperture and transected using the surface of the template to guide the scalpel.
Bleeding time should always be time to cessation of all bleeding, including rebleeding. Some investigators will determine when bleeding has stopped for at least 60 seconds as an indication that all bleeding has stopped. We prefer to observe the animal for a full 30 minutes to insure that bleeding does not recur.
We feel that the bleeding time alone is not sufficient to accurately reflect the extent of bleeding. A mouse may have a prolonged bleeding time, but lose relatively little blood during that time, compared to another animal that bleeds more briskly for a short period of time. We recommend measuring total blood loss as well as time to cessation of bleeding.
REFERENCES
- 1.Day SM, Reeve JL, Myers DD, Fay WP. Murine thrombosis models. Thromb Haemost. 2004;92:486–494. [PubMed] [Google Scholar]
- 2.Westrick RJ, Winn ME, Eitzman DT. Murine models of vascular thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:2079–2093. doi: 10.1161/ATVBAHA.107.142810. [DOI] [PubMed] [Google Scholar]
- 3.Sachs UJ, Nieswandt B. In vivo thrombus formation in murine models. Circ Res. 2007;100:979–991. doi: 10.1161/01.RES.0000261936.85776.5f. [DOI] [PubMed] [Google Scholar]
- 4.Furie B. Pathogenesis of thrombosis. Hematology 2009. 2009:255–258. doi: 10.1182/asheducation-2009.1.255. [DOI] [PubMed] [Google Scholar]
- 5.Diaz JA, Obi AT, Myers DD, Jr, Wrobleski SK, Henke PK, Mackman N, Wakefield TW. Critical review of mouse models of venous thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:556–562. doi: 10.1161/ATVBAHA.111.244608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogan KA, Weiler H, Lord ST. Mouse models of coagulation. Thromb Haemost. 2002;87:563–574. [PubMed] [Google Scholar]
- 7.Emeis JJ, Jirouskova M, Muchitsch EM, Shet AS, Smyth SS, Johnson GJ. A guide to murine coagulation factor structure, function, assays, and genetic alterations. J Thromb Haemost. 2007;5:670–679. doi: 10.1111/j.1538-7836.2007.02408.x. [DOI] [PubMed] [Google Scholar]
- 8.McManus MP, Gailani D. Animal Models of Diseases: Translational Medicine Perspective for Drug Discovery and Development. Bentham Scientific Publishers; 2012. Mouse models of coagulation factor deficiencies; pp. 67–121. [Google Scholar]
- 9.Tollefsen DM, Zhang L. Heparin and vascular proteoglycans. In: Marder VJ, Aird WC, Bennett JS, Schulman S, White GC, editors. Hemostasis and Thrombosis, Basic Principles and Clinical Practice. 6th edition. Philadelphia: Lippincott, Williams and Wilkins; 2013. pp. 585–597. [Google Scholar]
- 10.Kung SH, Hagstrom JN, Cass D, Tai SJ, Lin HF, Stafford DW, High KA. Human factor IX corrects the bleeding diathesis of mice with haemophilia B. Blood. 1998;91:784–790. [PubMed] [Google Scholar]
- 11.Geng Y, Verhamme IM, Smith SB, Sun MF, Matafonov A, Cheng Q, Smith SA, Morrissey JH, Gailani D. The dimeric structure of factor XI and zymogen activation. Blood. 2013;121:3962–3969. doi: 10.1182/blood-2012-12-473629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Renné T, Pozgajová M, Grüner S, Schuh K, Pauer HU, Burfeind P, Gailani D, Nieswandt B. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202:271–281. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carcao M, Moorehead P, Lillicrap D. Hemophilia A and B. In: Hoffman RH, Benz EJ, Silberstein LE, Heslop H, Weitz JI, Snastasi J, editors. Hematology, Basic Principles and Practice. 6th edition. Philadelphia: Saunders-Elsevier; 2013. pp. 1940–1960. [Google Scholar]
- 14.Lin HF, Maeda N, Smithies O, Straight DL, Stafford DW. A coagulation factor IX-deficient mouse model for human haemophilia B. Blood. 1997;90:3962–3966. [PubMed] [Google Scholar]
- 15.Zadelaar S, Kleemann R, Verschuren L, de Vries-Van der Weij J, van der Hoorn J, Princen HM, Kooistra T. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler Thromb Vasc Biol. 2007;27:1706–1721. doi: 10.1161/ATVBAHA.107.142570. [DOI] [PubMed] [Google Scholar]
- 16.Zhang SH, Reddick RL, Burkey B, Maeda N. Diet-induced atherosclerosis in mice heterozygous and homozygous for apolipoprotein E gene disruption. J Clin Invest. 1994;94:937–945. doi: 10.1172/JCI117460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb Vasc Biol. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 18.Lim W. Venous thromboembolism. In: Hoffman RH, Benz EJ, Silberstein LE, Heslop H, Weitz JI, Snastasi J, editors. Hematology, Basic Principles and Practice. 6th edition. Philadelphia: Saunders-Elsevier; 2013. pp. 2039–2047. [Google Scholar]
- 19.Eckly A, Hechler B, Freund M, Zerr M, Cazenave JP, Lanza F, Mangin PH, Gachet C. Mechanisms underlying FeCl3-induced arterial thrombosis. J Thromb Haemost. 2011;9:779–789. doi: 10.1111/j.1538-7836.2011.04218.x. [DOI] [PubMed] [Google Scholar]
- 20.Owens AP, 3rd, Lu Y, Whinna HC, Gachet C, Fay WP, Mackman N. Towards a standardization of the murine ferric chloride-induced carotid arterial thrombosis model. J Thromb Haemost. 2011;9:1862–1863. doi: 10.1111/j.1538-7836.2011.04287.x. [DOI] [PubMed] [Google Scholar]
- 21.Barr JD, Chauhan AK, Schaeffer GV, Hansen JK, Motto DG. Red blood cells mediate the onset of thrombosis in the ferric chloride murine model. Blood. 2013;121:3733–3741. doi: 10.1182/blood-2012-11-468983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Xu L. An optimized murine model of ferric chloride-induced arterial thrombosis for thrombosis research. Thromb Res. 2005;115:95–100. doi: 10.1016/j.thromres.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Cheng Q, Xu L, Feuerstein GZ, Hsu MY, Smith PL, Seiffert DA, Schumacher WA, Ogletree ML, Gailani D. Effects of factor IX or factor XI deficiency on ferric chloride-induced carotid artery occlusion in mice. J Thromb Haemost. 2005;3:695–702. doi: 10.1111/j.1538-7836.2005.01236.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Miller C, Swarthout RF, Rao M, Mackman N, Taubman MB. Vascular smooth muscle-derived tissue factor is critical for arterial thrombosis after ferric chloride-induced injury. Blood. 2009;113:705–713. doi: 10.1182/blood-2007-05-090944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng Q, Tucker EI, Pine MS, Sisler I, Matafonov A, Sun MF, White-Adams TC, Smith SA, Hanson SR, McCarty OJ, Renné T, Gruber A, Gailani D. A role for factor XIIa-mediated factor XI activation in thrombus formation in vivo. Blood. 2010;116:3981–3989. doi: 10.1182/blood-2010-02-270918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Revenko AS, Gao D, Crosby JR, Bhattacharjee G, Zhao C, May C, Gailani D, Monia BP, MacLeod AR. Selective depletion of plasma prekallikrein or coagulation factor XII inhibits thrombosis in mice without increased risk of bleeding. Blood. 2011;118:5302–5311. doi: 10.1182/blood-2011-05-355248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weitz JI. Antithrombotic drugs. In: Hoffman RH, Benz EJ, Silberstein LE, Heslop H, Weitz JI, Snastasi J, editors. Hematology, Basic Principles and Practice. 6th edition. Philadelphia: Saunders-Elsevier; 2013. pp. 2102–2119. [Google Scholar]
- 28.Schumacher WA, Luettgen JM, Quan ML, Seiffert DA. Inhibition of factor XIa as a new approach to anticoagulation. Arterioscler Thromb Vasc Biol. 2010;30:388–392. doi: 10.1161/ATVBAHA.109.197178. [DOI] [PubMed] [Google Scholar]
- 29.Löwenberg EC, Meijers JC, Monia BP, Levi M. Coagulation factor XI as a novel target for antithrombotic treatment. J Thromb Haemost. 2010;8:2349–2357. doi: 10.1111/j.1538-7836.2010.04031.x. [DOI] [PubMed] [Google Scholar]
- 30.Woodruff RS, Sullenger B, Becker RC. The many faces of the contact pathway and their role in thrombosis. J Thromb Thrombolysis. 2011;3:9–20. doi: 10.1007/s11239-011-0578-5. [DOI] [PubMed] [Google Scholar]
- 31.Al-Horani RA, Ponnusamy P, Mehta AY, Gailani D, Desai UR. Sulfated Pentagalloylglucoside is a potent, allosteric, and selective inhibitor of factor XIa. J Med Chem. 2013;56:867–878. doi: 10.1021/jm301338q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greene TK, Schiviz A, Hoellriegl W, Poncz M, Muchitsch EM. Animal Models Subcommittee of the Scientific And Standardization Committee Of The Isth. Towards a standardization of the murine tail bleeding model. J Thromb Haemost. 2010;8:2820–2822. doi: 10.1111/j.1538-7836.2010.04084.x. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Jennings NL, Dart AM, Du X-J. Standardizing a simpler, more sensitive and accurate tail bleeding assay in mice. World J Exp Med. 2012;2:30–36. doi: 10.5493/wjem.v2.i2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Broze GJ, Jr, Yin ZF, Lasky N. A tail vein bleeding time model and delayed bleeding in hemophiliac mice. Thromb Haemost. 2001;85:747–748. [PubMed] [Google Scholar]