Abstract
Hypothesis
A chitosan-hydrogel-based nanoparticle (nanohydrogel) delivery system can be used to deliver therapeutic biomaterials across the round window membrane (RWM) into the inner ear in a mouse model.
Background
Delivering therapies to the inner ear has always been a challenge for the Otolaryngologist. Advances in biomedical nanotechnology, increased understanding of the RWM diffusion properties, and discovery of novel therapeutic targets and agents, have all sparked interest in the controlled local delivery of drugs and biomaterials to the inner ear using nanoparticles (NPs).
Methods
Fluorescently-labeled liposomal NPs were constructed and loaded into a chitosan-based hydrogel to form a nanohydrogel, and in vitro studies were performed to evaluate its properties and release kinetics. Furthermore, the nanohydrogel was applied to the RWM of mice, and perilymph and morphologic analysis were performed to assess the NP delivery and distribution within the inner ear.
Results
NPs with an average diameter of 160nm were obtained. In vitro experiments showed that liposomal NPs can persist under physiologic conditions for at least two weeks without significant degradation, and that the nanohydrogel can carry and release these NPs in a controlled and sustained manner. In vivo findings demonstrated that the nanohydrogel can deliver intact nanoparticles into the perilymphatic system and reach cellular structures in the scala media of the inner ear of our mouse model.
Conclusion
Our study suggests that the nanohydrogel system has great potential to deliver therapeutics in a controlled and sustained manner from the middle ear to the inner ear without altering inner ear structures.
Introduction
Hearing loss (HL) represents the most prevalent sensory disability worldwide, affecting over an estimated 275 million people (1). In the United States alone, it was estimated that at least 30 million adults suffered from some degree of HL in 2011 (2). Currently, sensorineural HL (SNHL), which accounts for the most severe-to-profound HL cases, has no effective, non-invasive options available for treatment. At this time, the options are limited to sound amplification devices (hearing aids) and cochlear implants, both of which have significant drawbacks, including highly variable effectiveness and the need for invasive procedures. While there are no accepted treatment options available to arrest or reverse the progression of SNHL, there has been intense interest in the development of new approaches, including the use of molecular therapies. Delivering therapies to the inner ear, however, has always been a challenge for the Otolaryngologist mainly due to the limited cochlear blood supply and the poor penetration of drugs through the blood-inner ear barrier following systemic administration. Therefore, the development of novel techniques for efficient delivery of emerging therapies is an essential component for the successful treatment of inner ear diseases.
Advances in biomedical nanotechnology, increased understanding of the round window membrane (RWM) diffusion properties, and discovery of novel therapeutic agents have all sparked interest in the controlled local delivery of drugs and biomaterials to the inner ear using nanoparticles (NPs). The intratympanic (IT) approach is currently the most effective and promising route for non-invasive delivery of therapy to the inner ear as it allows for the diffusion of various agents, including drugs and NPs through the RWM (3–10). After entering the perilymph, the therapeutic agent still has to cross additional structures, such as the vestibular membrane, in order to enter the endolymph and finally reach its target, the Organ of Corti. While various methods have been developed to deliver treatments through this pathway, they have faced several challenges, including poor RWM penetration of drugs, instability of the drug, and inconsistent inner ear pharmacokinetics. Nonetheless, the potential translational application of NP-based delivery was recently illustrated in a human ex vivo study were Roy et al (6) evaluated and demonstrated that the RWM of freshly frozen temporal bones is permeable to NP formulations of various sizes. Although these studies showed that the NPs can indeed cross the RWM and reach the inner ear, the results were highly variable and the authors suggested potential limitations including the use of limited in vitro and ex vivo conditions and inadequate contact time with the RWM.
In an effort to improve the efficiency and safety of the delivery of therapeutic agents across the RWM, investigators have recently developed numerous innovative therapy delivery systems using biodegradable synthetic and natural materials. More specifically, synthetic polylactic/glycolic acid nanoparticles (11,12) and thermo-reversible triblock copolymer poloxamer 407 hydrogels (13–15) have been used for the controlled release of encapsulated molecules including dexamethasone across the RWM in vivo with encouraging results. Similarly, natural hydrogels made of gelatin (16–20), hyaluronate (21–23), alginate (24), and chitosan (4,25–27) have also been used for local inner ear drug applications in vivo. Importantly, a recent clinical trial demonstrated that the delivery of Insulin-like growth factor-1 (IGF-1) using gelatin polymers is safe and may be efficacious for hearing recovery in the setting of steroid-refractive acute SNHL (28).
Our lab has developed a stable, safe, and controllable system that allows for reliable delivery of drugs with improved outcomes. We have successfully constructed a controlled and sustained local drug delivery system for inner ear disease including the delivery of dexamethasone (25) and gentamicin (27), using a Chitosan-Glycerophosphate (CGP) thermosensitive hydrogel, which is biocompatible and biodegradable, and when applied directly to the round window niche (RWN), becomes a semi-solid gel that attaches to and persists in the niche. The results clearly demonstrated that the drugs could be slowly and steadily released from the CGP-hydrogel into perilymph through the RWM. More recently, chitosanase was added as a regulator for this CGP-delivery system in order to enzymatically “turn off” the delivery of therapy to the inner ear fluids and vestibular structures when needed by rapidly degrading the CGP-hydrogel and efficiently removing the drug from the RWM (4). This addition makes it a very unique and promising system for local delivery of existing and emerging therapies.
While previously reported efforts, including our own, have demonstrated improvement in the controlled-delivery of drugs to the inner ear, the delivery of newer therapeutics, including biomaterials, remains a challenge mainly due to their instability in extracellular compartments such as the middle ear. Therefore, we believe that a biocompatible and modular NP system may enhance their stability, allowing for improved biological effects. Unfortunately, as mentioned above, the application of NPs without the use of controlled-delivery systems has resulted in inefficient delivery, suggesting that a companion system may be needed to aid in their delivery.
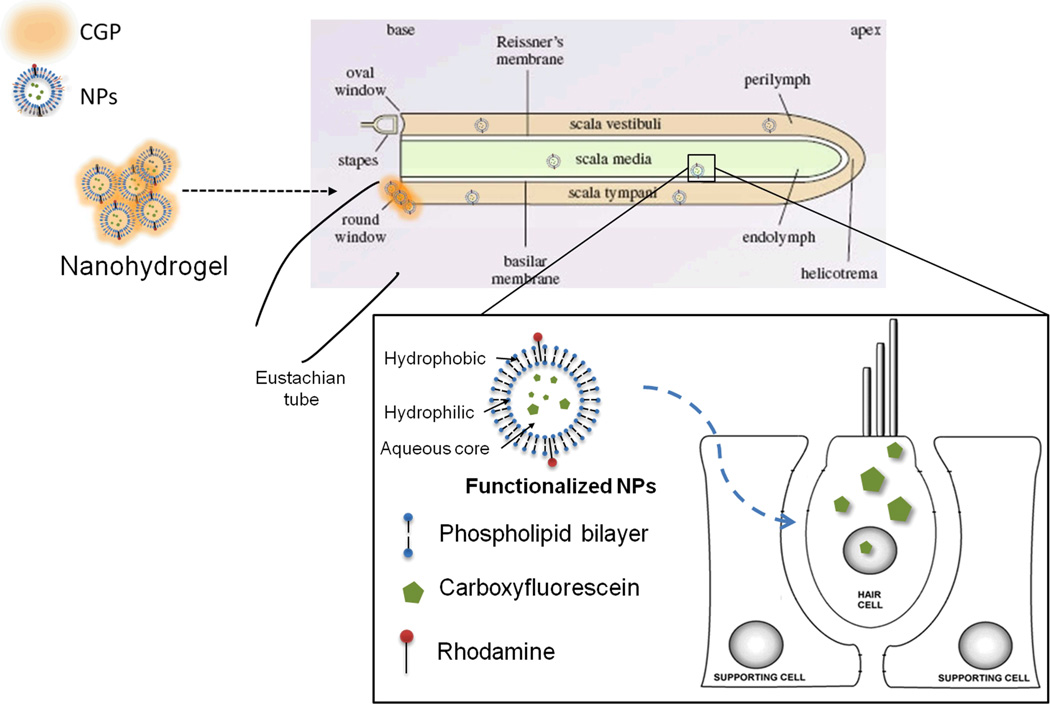
To this end, we hypothesized that local middle ear delivery of the NPs using a CGP-hydrogel-based nanoparticle (nanohydrogel) delivery system, which increases contact time with the RWM, may result in the successful and effective delivery of NPs to the inner ear (Figure 1).
Figure 1. Nanohydrogel delivery system.
A schematic representation of the nanohydrogel delivery system for inner ear application is shown. Rhodamine-labeled nanoparticles (NPs) are loaded onto the chitosan-glycerophosphate (CGP) - hydrogel to form the nanohydrogel, which is then placed directly on the round window membrane. NPs are slowly released across the RWM into the inner ear fluids ultimately reaching the cells of the inner ear to deliver their payload.
In the present study, we developed a novel nanohydrogel delivery system for inner ear application and evaluated its structure and release kinetics. In addition, we evaluated the inner ear distribution of NPs following local middle ear application of nanohydrogel in vivo using a mouse model.
Materials and methods
Fluorescent nanoparticle preparation, characterization and purification
The liposomes were prepared using the thin-film hydration technique from the aqueous assembly of 54umol% hydrogenated soy phosphatidylcholine (HSPC)/ 40umol% cholesterol (CHOL)/ 5umol% 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (mPEG2000-DSPE)/ 1umol% 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(Rhodamine B). All lipids and the mini-extruder were purchased from Avanti (Avanti Polar Lipids, Alabaster, Alabama). The lipids were hydrated with either 1X PBS or with 5(6)-Carboxyfluorescein (CF) (Sigma Aldrich, St. Louis, MO) for the self-quenching assay and the dual-labeled liposomes as described below. The hydrated lipids were incubated in a 60C water bath for 30min, followed by sonication for 1h. The samples were then extruded at least 15 times using a mini-extruder with a 0.1um membrane. The liposomes were purified using a PD-10 column (GE Healthcare, Piscataway, NJ) with 1X PBS, and their size was analyzed using a Zetasizer Nano range dynamic light scattering (DLS) machine (Malvern, Malvern, UK). The eluted samples were kept at 4C in the dark until use. (Z-Ave=160nm, PDI=0.16)
Hydrogel preparation
The CGP-hydrogel was freshly prepared essentially as previously described (4). Briefly, a 2% w/v solution of ultra pure chitosan (DDA 91.7%; Biosyntech, Quebec, Canada) in 0.1M HCl was prepared by magnetic stirring overnight at room temperature. This solution was kept at 4C until use. To obtain a thermosensitive hydrogel for the downstream experiments, a 55% w/v water soluble glycero-2-phosphate (GP) (EMD Millipore, Billerica, MA) solution was added dropwise while stirring by hand until the pH of the solution reached 7.2 +/− 0.1. The crosslinked hydrogel obtained was a highly viscous thermosensitive CGP-hydrogel, and it was kept on ice until use within 2 hours of preparation. The final concentration of chitosan and GP in the CGP-hydrogel were 1.8% (w/v) and 5% (w/v), respectively.
Nanohydrogel preparation for in vitro experiments
Immediately after crosslinking the CGP-hydrogel, the liposomes were added in a 1 to 5 ratio (v/v) while stirring by hand until a homogenous nanohydrogel formulation was obtained. The samples were kept on ice in the dark until use.
Ultra-structural analysis
The ultrastructure of each component of the nanohydrogel delivery system was evaluated using environmental scanning electron microscopy (SEM). Briefly, the samples were imaged on a FEI Quanta 600 field emission environmental scanning electron microscope (FE-ESEM) (FEI, Hillsboro, OR). The chamber was backfilled with 1.4 torr of water vapor and the images were collected with an accelerating voltage of 20 kV with a dwell time of 50 µs.
Nanoparticle stability and release kinetics in vitro
The stability of the liposomes was evaluated using the CF self-quenching technique as previously described (29). Briefly, a high concentration CF solution (0.2M) in PBS was prepared and loaded into the NPs and purified using a PD-10 Column (GE Healthcare, Piscataway, NJ). A Rhodamine label was introduced onto the NP surface to allow for NP tracking. The purified NPs were then loaded onto the CGP-hydrogel to form the nanohydrogel. Syringe samples were prepared as previously described (4). The collected PBS was analyzed for the CF and Rhodamine fluorescence intensity at the desired time points (over two weeks). Finally, TritonX-100 (TritonX) was added to the PBS samples to a final concentration of 1% v/v to disrupt the intact liposomes, followed by re-evaluation of the fluorescence intensity. The increase in CF fluorescence intensity (relative to Rhodamine) serves as a surrogate for the proportion of intact liposomes in the sample.
To assess the nanoparticle release from the nanohydrogel delivery system in vitro, samples were collected at days 1, 2, 5, 7. The liposome release was quantified by measuring the fluorescent intensity of the PBS samples using a FX Pro Molecular Imaging System (Bruker, Billerica, MA) using the appropriate filter sets. The exposure times were kept constant across samples.
In vivo application and assessment of the nanohydrogel delivery system
For the in vivo experiments, the nanohydrogel was prepared on the day in which it was to be used as previously described (4,27). The final solution contained approximately 3.8% (w/v) chitosan, 18.1% (w/v) GP. Purified dual-labeled liposomes loaded with a 120 µM CF solution in PBS were added to the CGP-hydrogel in a 1 to 5 ratio to form the final nanohydrogel solution. The samples were kept on ice and protected from light until use less than two hours after preparation.
Animal care and use was in accordance with the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania. C57BL/6J mice (Charles River, MA, USA), 6 to 8 weeks of age, of either gender, weighing 18 to 22g were used. Anesthesia was achieved by intraperitoneal injection of a Ketamine/Xylazine (100/10) cocktail. After anesthesia, as described in our previous studies (4,25,27), a post-auricular incision was made to approach the temporal bone. Briefly, using a microsurgical approach, a hole was drilled through the bulla with a 1-mm diamond burr to expose the RWN. A volume of approximately 0.3 µL of CGP-hydrogel, NPs, or nanohydrogel was carefully applied to the RWN using a custom-made flame-pulled glass syringe needle. The incision was then closed using 4-0 Nylon sutures. Animals successfully underwent the surgical procedure, recovered normally, and no complications were observed. In addition, the animals did not exhibit signs of distress, nor were there were any observable changes in behavior, such as log rolling or circling, suggesting that balance and auditory functions were preserved.
Perilymph collection
For the analysis of in vivo delivery, perilymph samples were harvested after 24 hours. The procedures were performed under anesthesia, as described in our previous studies (4,27), with the following modifications. While under deep anesthesia the mice were euthanized and the cochleae were quickly dissected off from the temporal bone to allow for the collection of approximately 0.3 µL of perilymph from the oval window (OW) with custom-made microcapillary tubes. The samples were diluted in 5 µL of deionized water, and were analyzed by fluorescence microscopy using the water-in-oil emulsion technique as previously described (30).
Tissue harvesting and preparation
At the designated time points the mice were euthanized and the cochleae on both sides were immediately dissected and fixed as previously described (4). The tissues were kept in the fixative at 4°C in the dark until processing. Samples were processed by the surface preparation technique (31,32).
Fluorescence microscopy
The membranous cochlea was decalcified in 0.5M EDTA, pH 8 for one hour at room temperature. The tissues were then washed with PBS and mounted on slides. Fluorescence microscopy images were captured using a Nikon Eclipse Ti Inverted Microscope System and NIS-Elements (Nikon, Melville, NY), and analyzed using ImageJ (National Institute of Health, Bethesda, MD).
Statistics
Statistical analysis was performed with Microsoft Excel 2007 (Microsoft, Redmond, WA). The t-student test was used to evaluate statistically significant differences between groups in all experiments. A p-value of less than 0.05 was considered statistically significant.
Results
Liposomal nanoparticles maintain stability for over two weeks in vitro
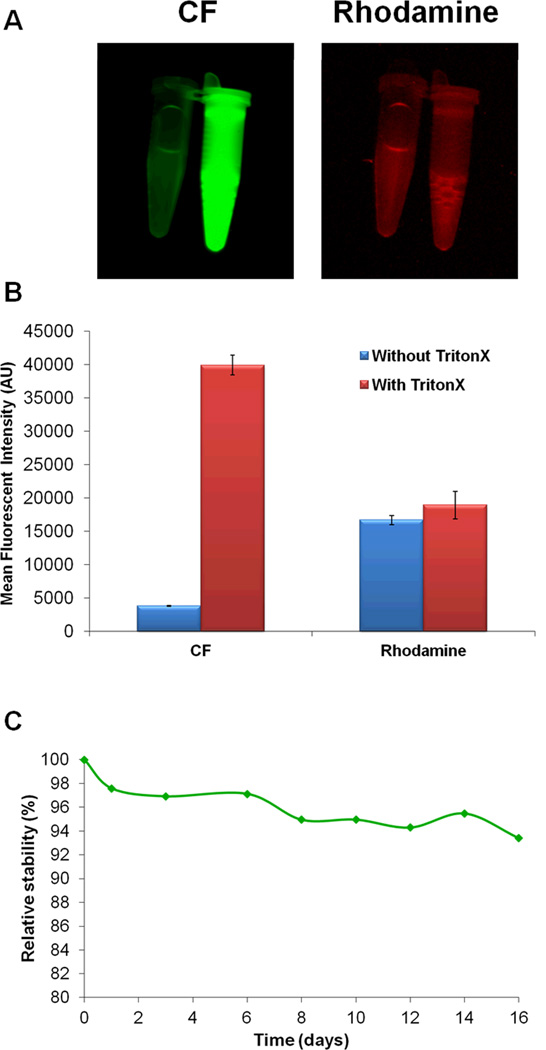
The stability of the liposomes was evaluated using a CF self-quenching assay as previously described (29), where the stability is inversely proportional to the rate of increase in CF fluorescence intensity owing to CF release from the liposome. Results were normalized to the fluorescence intensity of completely unquenched (released) CF by treating with TritonX, which is used to disrupt intact liposomes. This technique was validated with our synthesized liposomal nanoformulation as shown in Figures 2A and 2B. The results of the stability experiment demonstrated that the liposomes remain stable for extended periods of time (more than two weeks) under physiologic conditions as shown in Figure 2C, with a degradation/release rate of less than 1% per day.
Figure 2. Liposomal nanoparticle stability over time.
The stability of the liposomes was evaluated using the self-quenching CF technique. A) Representative images of the technique are shown to illustrate the significant increase in CF fluorescence intensity only after treatment with TritonX. Little release of CF was noted prior to treatment. No change in Rhodamine fluorescence was observed following treatment with TritonX. B) Quantified mean fluorescence intensity demonstrates approximately 10-fold increase in fluorescein fluorescence after treatment with TritonX to disrupt the liposomes and release the encapsulated dye. C) Relative stability (%) of the liposomes demonstrates that the proposed NPs are very stable.
Ultrastructural analysis demonstrates CGP-hydrogel incorporates nanoparticles
The results from the SEM ultrastructural analysis, summarized in Figure 3, confirmed the size and spherical shape of the liposomes, and further demonstrated their integrity (Left panel). In addition, images from CGP-hydrogel showed the characteristic matrix-like appearance, suggesting that this highly viscous and adherent hydrogel can carry biomolecules, such as liposomes (Right panel). Finally, the nanohydrogel surface images demonstrated a hydrogel background with a “patchy” pattern representing the embedded liposomes. These results suggested that CGP-hydrogel could serve as a platform for nanotherapy delivery to the inner ear.
Figure 3. Ultrastructural analysis.
The liposomes (left panel) were identified as spherical and relatively homogenous structures of 150–200nm in diameter in a background of salt solution. The CGP-hydrogel (center panel), as expected, had a matrix-like characteristic appearance, representing the cross-linked chitosan network. The nanohydrogel image (right panel) shows a hydrogel background with “patchy” surface representing the embedded liposomes.
Nanohydrogel releases intact nanoparticles in a sustained fashion over time in vitro
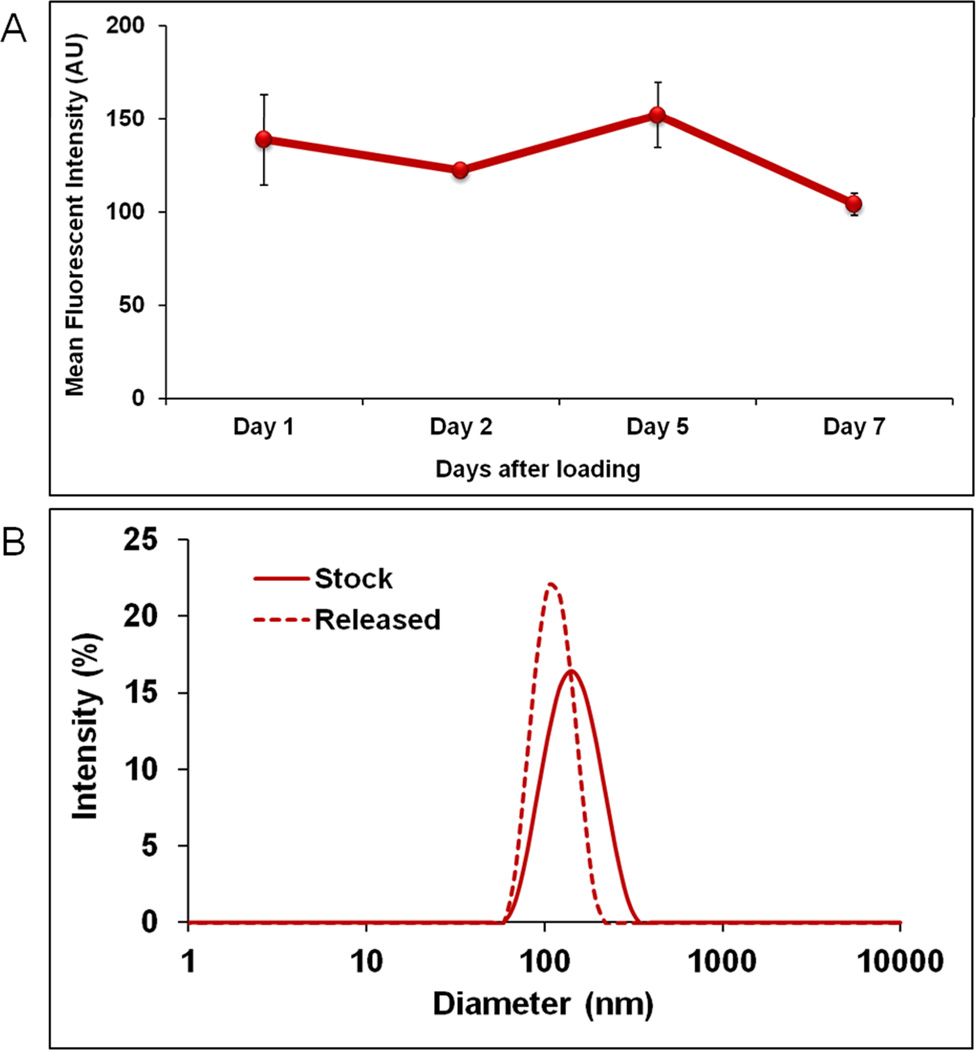
The release kinetics of NPs from the nanohydrogel was evaluated using the syringe model, as previously described (4). As illustrated in Figures 4A and 4B, a sustained and controlled release of NPs from the nanohydrogel was achieved throughout the experiment while maintaining their structural integrity as measured by the negligible change in size of the NPs using DLS. Together, these results suggest that the CGP-hydrogel can carry and release intact nanoparticles in a controlled manner, and support the use of nanohydrogel for inner ear application in vivo.
Figure 4. Nanohydrogel release kinetics and integrity.
A) Sustained and controlled release of NPs was detected up to 7 days by tracking the Rhodamine fluorescence in the collection. Mean Fluorescence Intensity ± SEM is shown. B) The integrity was confirmed by measuring the NP size in diameter (nm) before (stock) and after release using DLS. The results showed no significant size change. A solution of 1X PBS was used as control. At least three replicates were used for each group.
Nanohydrogel releases NPs across the RWM into the perilymph of the inner ear
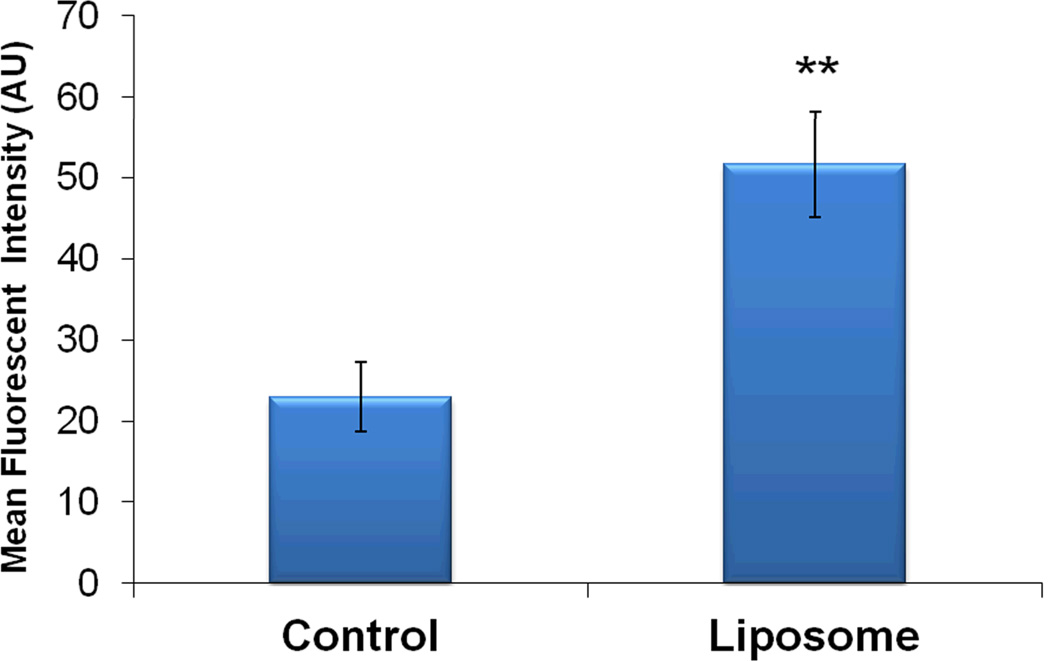
To assess the ability of the nanohydrogel to cross the RWM, an in vivo experiment was conducted using a mouse model as previously described (4,25,27). Following its application directly onto the RWN under direct microscopic visualization, the nanohydrogel successfully released liposomes across the RWM into the perilymph of the inner ear as demonstrated in Figure 5. This demonstrates that these liposomal NPs can be delivered into the inner ear pass the entry point using the nanohydrogel without violating the integrity of the RWM.
Figure 5. Perilymph analysis.
Following nanohydrogel application, NPs were detected in the perilymph of treated ear as measured by the Rhodamine fluorescence. Mean Fluorescence Intensity ± SEM is shown. (**P<0.01). At least two replicates were used per group.
Nanohydrogel delivers intact nanoparticles and their payload to the inner ear structures
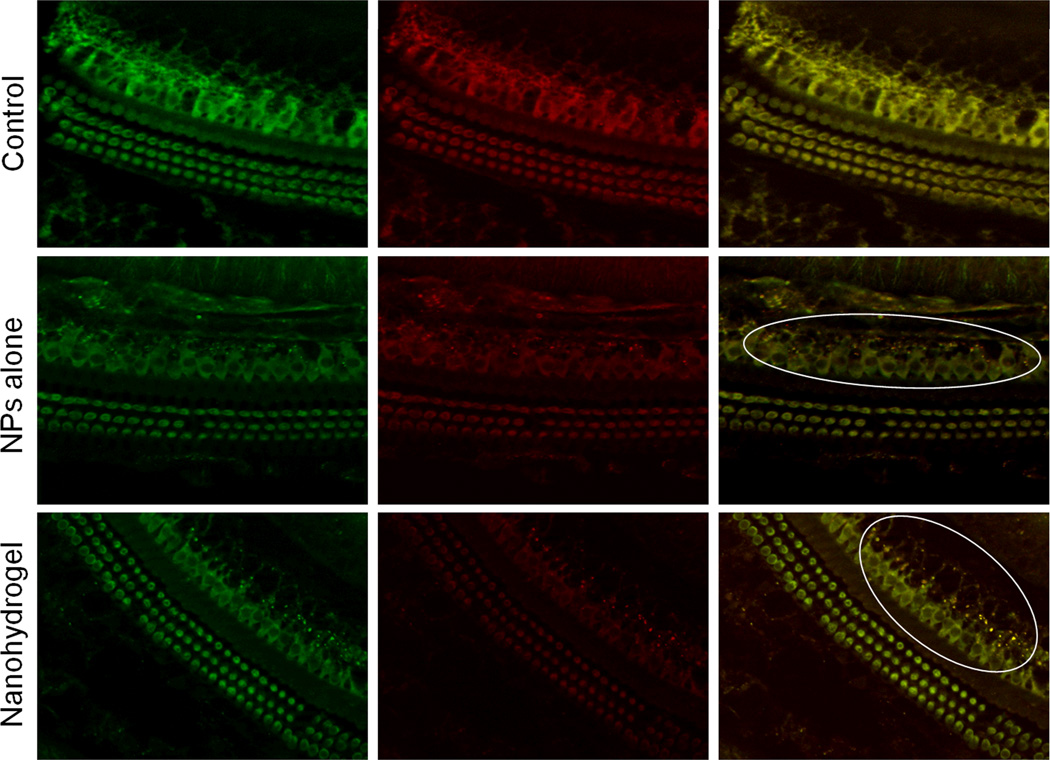
To evaluate whether the nanohydrogel delivery system can deliver structurally intact NPs and their payload to inner ear structures, an in vivo experiment was performed, where dual-labeled liposomes (Rhodamine on the surface, CF inside) were used to allow for their tracking and localization within the cochlea. Twenty four hours after the indicated treatments, the cochleae of mice were dissected, fixed and processed using the surface preparation technique. Fluorescent microscopy images demonstrated that these NPs are capable of crossing inner ear barriers and can reach inner ear cells in vivo as illustrated in Figure 6. As compared with NPs alone, the nanohydrogel-treated ears showed improved colocalization and therefore delivery of structurally intact NPs and their payload to cells of the inner ear. These findings support the use of nanohydrogel for effectively delivering NPs to the inner ear in a controlled manner.
Figure 6. Microscopic evaluation of the cochlea following nanohydrogel application.
Twenty four hours after nanohydrogel was applied onto the round window niche in vivo, NPs were detected in inner ear cells as evaluated by the CF (Left - Green) and Rhodamine (Center - Red) fluorescent signal colocalization (Right - Yellow dots) (Right). The right ears treated with mock surgery served as controls. White ovals were drawn to highlight the NPs. (Original magnification 40×)
Discussion
SNHL is one of the most common sensory deficits in humans, affecting millions of people worldwide. Although a lot of progress has been made in understanding the etiology and molecular mechanisms of this disease, there are currently no effective treatments that can target the pathological sites of the inner ear in order to reverse or improve recovery from SNHL. The most challenging issue for the treatment of inner ear diseases is to find ways to effectively deliver therapeutic agents to the inner ear without damaging the integrity or the function of delicate inner ear structures. The risks and limitations of systemic drug delivery for inner ear diseases have led otologists to explore the development of safe local delivery approaches that can not only carry drugs, but also bring therapeutic biomaterials into the inner ear in a sustained, controlled and safe manner. Unfortunately, such delivery systems are still in the early phase of development and have not been made available for clinical applications.
In an effort to improve the efficiency of drug delivery across the RWM, our lab has previously developed a novel CGP-hydrogel system that can deliver drugs to the inner ear in a stable, safe, and regulated manner, demonstrating significant improvement in outcomes (25,27). This hydrogel can be applied, as a liquid, directly to the RWN, and at body temperature becomes a semi-solid gel that attaches to and persists in the niche. More recently, chitosanase was added as a regulator for this CGP-delivery system, providing an enzymatic “turn off” switch for drug delivery to the inner ear and decreasing drug concentration when needed (4). Although the CGP-hydrogel delivery system can carry drugs into the inner ear in a sustained and controlled manner, it has not been used to deliver therapeutic biomaterials into desired cellular structures of the inner ear.
In this study, we demonstrated that liposomal nanoparticles maintain stability for over two weeks under physiologic conditions in vitro, and that the CGP-hydrogel-nanoparticle system (nanohydrogel) releases these stable nanoparticles across the RWM into the perilymph, ultimately delivering their payload to inner ear structures. This study demonstrated, for the first time, that nanohydrogel can be used as a controlled and effective delivery modality for bringing therapeutic agents in stable intact nanoparticles to cellular structures within the inner ear. While the surface preparation technique allows for verification NP delivery into the inner ear, as demonstrated in Figure 6, it is limited by its inability to determine the exact location of these NPs. Future studies will be conducted to demonstrate the ability of the NPs to penetrate specific areas and cells of the inner ear, including the Organ of Corti.
As with any new therapeutic approach, it is paramount to evaluate its safety profile for the potential translational application. In the context of local middle ear drug applications, the RWM acts as the main entry point, and thus, its integrity should be evaluated. While we have not specifically evaluated the integrity of the RWM in this particular study, unpublished data from our laboratory suggests that the membrane remains intact after CGP-hydrogel application. Nonetheless, the short- and long-term effects of our nanohydrogel delivery system to the RWM as well as to inner ear cellular structures will be addressed in subsequent studies.
Finally, this nanohydrogel delivery system, as opposed to current delivery systems, has the potential to incorporate cell-specific targeting ligands (i.e. scFv’s, affibodies, peptides, small proteins) through the use of a previously developed expression protein ligation (EPL)-“click” chemistry system (33), which is a highly efficient, site-specific system for the modular preparation of nanoformulations targeted to virtually any known biologically expressed ligand. This would represent a significant advancement in the ability to deliver therapy specifically to the cells of interest, in a safe, minimally-invasive manner.
Acknowledgments
This work was supported in part by the National Institute of Health Grants R01EB012065 (A.T) and R01CA157766 (A.T.).
Footnotes
The authors disclose no conflicts of interest.
References
- 1.WHO. Geneva: 2006. Primary Ear And Hearing Care Traning Resource: Advanced Level. [Google Scholar]
- 2.Lin FR, Niparko JK, Ferrucci L. Hearing loss prevalence in the United States. Arch Intern Med. 2011;171:1851–1852. doi: 10.1001/archinternmed.2011.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckiova D, Ranjan S, Newman TA, et al. Minimally invasive drug delivery to the cochlea through application of nanoparticles to the round window membrane. Nanomedicine (Lond) 2012;7:1339–1354. doi: 10.2217/nnm.12.5. [DOI] [PubMed] [Google Scholar]
- 4.Lajud SA, Han Z, Chi FL, et al. A regulated delivery system for inner ear drug application. J Control Release. 2013 doi: 10.1016/j.jconrel.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Steyger PS. Systemic aminoglycosides are trafficked via endolymph into cochlear hair cells. Sci Rep. 2011;1:159. doi: 10.1038/srep00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roy S, Glueckert R, Johnston AH, et al. Strategies for drug delivery to the human inner ear by multifunctional nanoparticles. Nanomedicine (Lond) 2012;7:55–63. doi: 10.2217/nnm.11.84. [DOI] [PubMed] [Google Scholar]
- 7.Salt AN, Plontke SK. Principles of local drug delivery to the inner ear. Audiol Neurootol. 2009;14:350–360. doi: 10.1159/000241892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheper V, Wolf M, Scholl M, et al. Potential novel drug carriers for inner ear treatment: hyperbranched polylysine and lipid nanocapsules. Nanomedicine (Lond) 2009;4:623–635. doi: 10.2217/nnm.09.41. [DOI] [PubMed] [Google Scholar]
- 9.Schuknecht HF. Ablation therapy for the relief of Meniere's disease. Laryngoscope. 1956;66:859–870. doi: 10.1288/00005537-195607000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, Zhang Y, Lobler M, et al. Nuclear entry of hyperbranched polylysine nanoparticles into cochlear cells. Int J Nanomedicine. 2011;6:535–546. doi: 10.2147/IJN.S16973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamura T, Kita T, Nakagawa T, et al. Drug delivery to the cochlea using PLGA nanoparticles. Laryngoscope. 2005;115:2000–2005. doi: 10.1097/01.mlg.0000180174.81036.5a. [DOI] [PubMed] [Google Scholar]
- 12.Ge X, Jackson RL, Liu J, et al. Distribution of PLGA nanoparticles in chinchilla cochleae. Otolaryngol Head Neck Surg. 2007;137:619–623. doi: 10.1016/j.otohns.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Piu F, Wang X, Fernandez R, et al. OTO-104: a sustained-release dexamethasone hydrogel for the treatment of otic disorders. Otol Neurotol. 2011;32:171–179. doi: 10.1097/MAO.0b013e3182009d29. [DOI] [PubMed] [Google Scholar]
- 14.Salt AN, Hartsock J, Plontke S, et al. Distribution of dexamethasone and preservation of inner ear function following intratympanic delivery of a gel-based formulation. Audiol Neurootol. 2011;16:323–335. doi: 10.1159/000322504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Dellamary L, Fernandez R, et al. Dose-dependent sustained release of dexamethasone in inner ear cochlear fluids using a novel local delivery approach. Audiol Neurootol. 2009;14:393–401. doi: 10.1159/000241896. [DOI] [PubMed] [Google Scholar]
- 16.Endo T, Nakagawa T, Kita T, et al. Novel strategy for treatment of inner ears using a biodegradable gel. Laryngoscope. 2005;115:2016–2020. doi: 10.1097/01.mlg.0000183020.32435.59. [DOI] [PubMed] [Google Scholar]
- 17.Fujiwara T, Hato N, Nakagawa T, et al. Insulin-like growth factor 1 treatment via hydrogels rescues cochlear hair cells from ischemic injury. Neuroreport. 2008;19:1585–1588. doi: 10.1097/WNR.0b013e328311ca4b. [DOI] [PubMed] [Google Scholar]
- 18.Inaoka T, Nakagawa T, Kikkawa YS, et al. Local application of hepatocyte growth factor using gelatin hydrogels attenuates noise-induced hearing loss in guinea pigs. Acta Otolaryngol. 2009;129:453–457. doi: 10.1080/00016480902725197. [DOI] [PubMed] [Google Scholar]
- 19.Iwai K, Nakagawa T, Endo T, et al. Cochlear protection by local insulin-like growth factor-1 application using biodegradable hydrogel. Laryngoscope. 2006;116:529–533. doi: 10.1097/01.mlg.0000200791.77819.eb. [DOI] [PubMed] [Google Scholar]
- 20.Lee KY, Nakagawa T, Okano T, et al. Novel therapy for hearing loss: delivery of insulin-like growth factor 1 to the cochlea using gelatin hydrogel. Otol Neurotol. 2007;28:976–981. doi: 10.1097/MAO.0b013e31811f40db. [DOI] [PubMed] [Google Scholar]
- 21.Borden RC, Saunders JE, Berryhill WE, et al. Hyaluronic acid hydrogel sustains the delivery of dexamethasone across the round window membrane. Audiol Neurootol. 2011;16:1–11. doi: 10.1159/000313506. [DOI] [PubMed] [Google Scholar]
- 22.Saber A, Laurell G, Bramer T, et al. Middle ear application of a sodium hyaluronate gel loaded with neomycin in a Guinea pig model. Ear Hear. 2009;30:81–89. doi: 10.1097/AUD.0b013e31818ff98e. [DOI] [PubMed] [Google Scholar]
- 23.Coleman JK, Littlesunday C, Jackson R, et al. AM-111 protects against permanent hearing loss from impulse noise trauma. Hear Res. 2007;226:70–78. doi: 10.1016/j.heares.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Noushi F, Richardson RT, Hardman J, et al. Delivery of neurotrophin-3 to the cochlea using alginate beads. Otol Neurotol. 2005;26:528–533. doi: 10.1097/01.mao.0000169780.84588.a5. [DOI] [PubMed] [Google Scholar]
- 25.Paulson DP, Abuzeid W, Jiang H, et al. A novel controlled local drug delivery system for inner ear disease. Laryngoscope. 2008;118:706–711. doi: 10.1097/MLG.0b013e31815f8e41. [DOI] [PubMed] [Google Scholar]
- 26.Saber A, Strand SP, Ulfendahl M. Use of the biodegradable polymer chitosan as a vehicle for applying drugs to the inner ear. Eur J Pharm Sci. 2010;39:110–115. doi: 10.1016/j.ejps.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Xu L, Heldrich J, Wang H, et al. A controlled and sustained local gentamicin delivery system for inner ear applications. Otol Neurotol. 2010;31:1115–1121. doi: 10.1097/MAO.0b013e3181eb32d1. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa T, Sakamoto T, Hiraumi H, et al. Topical insulin-like growth factor 1 treatment using gelatin hydrogels for glucocorticoid-resistant sudden sensorineural hearing loss: a prospective clinical trial. BMC Med. 2010;8:76. doi: 10.1186/1741-7015-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sila M, Au S, Weiner N. Effects of Triton X-100 concentration and incubation temperature on carboxyfluorescein release from multilamellar liposomes. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1986;859:165–170. [Google Scholar]
- 30.Chen AK, Cheng Z, Behlke MA, et al. Assessing the sensitivity of commercially available fluorophores to the intracellular environment. Anal Chem. 2008;80:7437–7444. doi: 10.1021/ac8011347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Malley BW, Jr, Li D, Turner DS. Hearing loss and cochlear abnormalities in the congenital hypothyroid (hyt/hyt) mouse. Hear Res. 1995;88:181–189. doi: 10.1016/0378-5955(95)00111-g. [DOI] [PubMed] [Google Scholar]
- 32.Xiang M, Gan L, Li D, et al. Essential role of POU-domain factor Brn-3c in auditory and vestibular hair cell development. Proc Natl Acad Sci U S A. 1997;94:9445–9450. doi: 10.1073/pnas.94.17.9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elias DR, Cheng Z, Tsourkas A. An intein-mediated site-specific click conjugation strategy for improved tumor targeting of nanoparticle systems. Small. 2010;6:2460–2468. doi: 10.1002/smll.201001095. [DOI] [PMC free article] [PubMed] [Google Scholar]