Abstract
Objectives
The goal of cartilage repair techniques such as microfracture (MFX) or matrix-associated autologous chondrocyte transplantation (MACT) is to produce repair tissue (RT) with sufficient glycosaminoglycan (GAG) content. Sodium magnetic resonance imaging (MRI) offers a direct and noninvasive evaluation of the GAG content in native cartilage and RT. In the femoral cartilage, this method was able to distinguish between RTs produced by MFX and MACT having different GAG contents. However, it needs to be clarified whether sodium MRI can be useful for evaluating RT in thin ankle cartilage. Thus, the aims of this 7-T study were (1) to validate our sodium MRI protocol in cadaver ankle samples, (2) to evaluate the sodium corrected signal intensities (cSI) in cartilage of volunteers, (3) and to compare sodium values in RT between patients after MFX and MACT treatment.
Materials and Methods
Five human cadaver ankle samples as well as ankles of 9 asymptomatic volunteers, 6 MFX patients and 6 MACT patients were measured in this 7-T study. Sodium values from the ankle samples were compared with histochemically evaluated GAG content. In the volunteers, sodium cSI values were calculated in the cartilages of ankle and subtalar joint. In the patients, sodium cSI in RT and reference cartilage were measured, morphological appearance of RT was evaluated using the magnetic resonance observation of cartilage repair tissue (MOCART) scoring system, and clinical outcome before and after surgery was assessed using the American Orthopaedic Foot and Ankle Society score and Modified Cincinnati Knee Scale. All regions of interest were defined on morphological images and subsequently transferred to the corresponding sodium images. Analysis of variance, t tests, and Pearson correlation coefficients were evaluated.
Results
In the patients, significantly lower sodium cSI values were found in RT than in reference cartilage for the MFX (P = 0.007) and MACT patients (P = 0.008). Sodium cSI and MOCART scores in RT did not differ between the MFX and MACT patients (P = 0.185). No significant difference in sodium cSI was found between reference cartilage of the volunteers and the patients (P = 0.355). The patients showed significantly higher American Orthopaedic Foot and Ankle Society and Modified Cincinnati scores after treatment than they did before treatment. In the volunteers, sodium cSI was significantly higher in the tibial cartilage than in the talar cartilage of ankle joint (P = 0.002) and in the talar cartilage than in the calcaneal cartilage of subtalar joint (P < 0.001). Data from the cadaver ankle samples showed a strong linear relationship between the sodium values and the histochemically determined GAG content (r = 0.800; P < 0.001; R2 = 0.639).
Conclusions
This study demonstrates the feasibility of in vivo quantification of sodium cSI, which can be used for GAG content evaluation in thin cartilages of ankle and subtalar joints at 7 T. A strong correlation observed between the histochemically evaluated GAG content and the sodium values proved the sufficient sensitivity of sodium MRI to changes in the GAG content of cartilages in the ankle. Both MFX and MACT produced RT with lower sodium cSI and, thus, of lower quality compared with reference cartilage in the patients or in the volunteers. Our results suggest that MFX and MACT produce RT with similar GAG content and similar morphological appearance in patients with similar surgery outcome. Sodium MRI at 7 T allows a quantitative evaluation of RT quality in the ankle and may thus be useful in the noninvasive assessment of new cartilage repair procedures.
Keywords: sodium MRI, ankle joint, 7 T, cartilage repair, glycosaminoglycan content
Defects of talar cartilage are common, and most of them are associated with ankle sprains.1 Patients with persistent pain in the ankle joint usually receive surgical treatment. Currently, microfracture (MFX) is the treatment of choice for smaller primary osteochondral talar lesions.2 Especially in larger cartilage defects, cell-based techniques such as autologous chondrocyte implantation (ACI) and matrix-associated autologous chondrocyte transplantation (MACT) are applied to the ankle.3,4 Several knee studies suggest that cell-based repair techniques, rather than MFX, lead more often in hyaline-like repair tissue (RT) and could provide a better clinical outcome.5,6 However, it should be noted that there are metabolic, biochemical, and biomechanical differences between knee and ankle cartilage and that ankle cartilage has a greater capacity for repair when compared with knee cartilage.7 Although MFX procedures are more costeffective, are less technically demanding, and have lower complication rates than cell-based techniques do, their role in the ankle repair is not strictly defined yet.
The negatively charged glycosaminoglycans (GAGs) provide strong electrostatic and osmotic forces responsible for the functional and structural properties of cartilage. Because the noninvasive monitoring of the GAG content in cartilage is of great interest, magnetic resonance imaging (MRI) techniques such as delayed gadolinium-enhanced MRI of cartilage (dGEMRIC),8,9 GAG chemical exchange saturation transfer,10 T1ρ mapping,11 or sodium imaging12 have been developed for the noninvasive evaluation of the native cartilage and cartilage RT.
Owing to low sodium concentration, very short transverse relaxation times, and the need for special multinuclear hardware, sodium MRI is a challenging method. However, recent hardware (7-T systems, phase-array coils) and software developments (measurement and image reconstruction techniques) resulted in many exciting in vivo cartilage studies.13 Sodium MRI is very sensitive to even small changes in cartilage GAG content and can be thus used as a reference technique for the evaluation of other GAG-sensitive techniques.10
The negatively charged GAGs in cartilage are counterbalanced by the positively charged sodium ions. Therefore, a change in GAG content is reflected by a change of signal in sodium image.14 The evaluation of GAG content in the knee cartilage using sodium imaging was successfully achieved in healthy humans15 and patients.16,17 Sodium MRI of the knee was able to distinguish not only between native hyaline cartilage and RT but also between RT after MACT and RT after bone marrow stimulation repair techniques.18 Although a sodium MRI study of the ankle cartilage has not been published yet, an article on sodium imaging of thewrist cartilage suggests that this method could be applied in joints smaller than the knee.19
The aims of this 7-T sodium MRI study were (1) to validate a sodium MRI protocol by comparing MRI results with histochemical analysis of GAG content in ankle samples, (2) to evaluate the sodium cSI indicating GAG content in tibial and talar cartilage of healthy volunteers, (3) and to compare sodium cSI values in RT between the patients after MFX and MACT treatment.
MATERIALS AND METHODS
Subjects
This cross-sectional feasibility study was approved by the institutional review board, and written informed consent was obtained from all patients and volunteers.
Nine asymptomatic volunteers without any pain, ankle malalignment (evaluated through the clinical examination), or history of trauma or surgery were recruited to obtain baseline sodium values in ankle cartilages. This group consisted of 6 women and 3 men with mean (SD) age of 28.7 (5.5) years (range, 23–39 years) and mean body mass index (BMI) (SD) of 23.9 (2.7) kg/m2 (range, 19.8–27.7 kg/m2).
From the cohort of more than 70 patients treated with MFX, Pridie drilling, ACI or MACT technique, 6 MFX patients and 6 MACT patients with similar age, BMI, and defect size agreed to undergo additional MRI examination at 7 T. Inclusion criteria were as follows: a singular osteochondral defect (Hepple 3 and 4) or a symptomatic deep chondral singular defect (Outerbridge grade 3 or 4) with stable adjacent cartilage. Exclusion criteria were progressed osteoarthritis or rheumatoid arthritis, kissing lesions, and instability or malalignment of the joint. Briefly, a marrow stimulation-based MFX technique was carried out as described by Steadman et al.20 A 3-dimensional scaffold seeded with autologous chondrocytes was used for MACT treatment: Hyalograft C (Fidia Advanced Biopolymer, Abano, Italy) in 4 patients, CaReS (Arthro Kinetics, Esslingen, Germany) in 1 patient, and Carticel (Genzyme Biosurgery, Cambridge, MA) in 1 patient.21
In the 6 MFX patients (2 women, 4 men), the mean (SD) age at MRI was 35.8 (10.0) years, the mean (SD) postoperative interval was 99.5 (33.1) months, the mean (SD) BMI was 27.0 (5.5 kg/m2), and the mean (SD) defect size was 1.26 (0.41) cm2. In the 6 MACT patients (4 women, 2 men), the mean (SD) age at MRI was 35.0 (6.0) years, the mean (SD) postoperative interval was 85.7 (23.9) months, the mean (SD) BMI was 26.1 (3.1) kg/m2, and the mean (SD) defect size was 1.53 (0.45) cm2.
Clinical Outcome
The American Orthopaedic Foot and Ankle Society (AOFAS) score22 and the Modified Cincinnati Knee Scale23 were evaluated before and after the surgery to monitor the clinical outcome. The AOFAS score reflects a patient’s perception of pain and function. The maximum is 100 points (50, function; 40, pain; 10 alignment). The Modified Cincinnati Knee Rating evaluates limitations in daily life and sports, ranging from severe limitations (zero points) to full functionality (10 points).
Ankle Specimens
Eight human cadaver fresh-frozen ankle joints (midtibia to foot base) were obtained from a local anatomy department with the approval of the university institutional review board. On the basis of morphological MRI, talar and tibial cartilage in the ankle samples was evaluated according to the guidelines of International Cartilage Repair Society. Specimens with very thin cartilage due to advanced osteoarthritis or with full-thickness defects (International Cartilage Repair Society grade of 3 or 4) were excluded from further evaluations. Thus, only 5 ankle samples, from 3 women and 2 men with median age of 46 years (range, 41–60 years), were used for further evaluations.
Magnetic Resonance Imaging
All MRI measurements were performed on a 7-T whole-body system (MAGNETOM; Siemens Healthcare, Erlangen, Germany). Proton images were recorded using a 28-channel knee array coil (Quality Electrodynamics LLC, Cleveland, OH). Sodium images were acquired with a 15-channel sodium-only knee array coil (Tx birdcage/Rx array) (QED, Quality Electrodynamics LLC, Cleveland, OH) and reconstructed online with adaptive combine algorithm using signal intensities from each coil element. Special care was taken to position the ankles of the subjects and the ankle samples in the same way for both proton and sodium measurements, which was made easier by identical housing of both coils.
Proton density-weighted 2-dimensional (2D) turbo spin echo (TSE) images with fat suppression in the sagittal and coronal planes were used for the morphological evaluation. In addition to product sequences, a work-in-progress TSE sequence optimized for ultrahigh-field systems (WIP 729C; Siemens Healthcare, Erlangen, Germany) providing higher resolution was used for subject measurements.
Sodium images were acquired with a standard 3-dimensional gradient echo (3D-GRE) sequence optimized for sodium imaging of cartilage. The small acquisition bandwidth of 80 hertz per pixel (readout length of 12.5 milliseconds), together with asymmetric readout, was used to maximize signal-to-noise ratio in the sodium images. Although sodium signal in cartilage exhibits a biexponential T2* decay with short and long components, a relatively long echo time used for sodium MRI minimized signal contribution from the short component. As a result, the broadening of the point-spread-function due to a very fast relaxation of the short T2* component was minimized. Sodium MRI protocols used for the subjects and the ankle samples were identical (Table 1).
TABLE 1.
Parameters of Proton and Sodium MR Sequences Used in the Current Study
| Sequence | TR/TE, millisecond |
Flip Angle, degrees |
Bandwidth, hertz per pixel |
Field of View, mm2 |
Matrix Size | Resolution, mm2 |
ST, mm | No. Slices | No. Averages | TA, min:s | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ex vivo | Sag. LR 2D-TSE | 4000/26 | 145 | 243 | 140 × 140 | 448 × 448 | 0.31 × 0.31 | 3.0 | 15 | 1 | 3:18 |
| Cor. 2D-TSE | 3000/25 | 179 | 243 | 140 × 140 | 448 × 448 | 0.31 × 0.31 | 3.0 | 15 | 1 | 2:29 | |
| Sodium 3D-GRE | 17/8.34 | 50 | 80 | 200 × 200 | 112 × 112 | 1.79 × 1.79 | 3.0 | 40 | 12 | 31:53 | |
| In vivo | Sag. LR 2D-TSE | 4000/26 | 170 | 243 | 140 × 140 | 448 × 448 | 0.31 × 0.31 | 3.0 | 15 | 1 | 3:18 |
| Cor. 2D-TSE | 3000/25 | 180 | 243 | 140 × 140 | 448 × 448 | 0.31 × 0.31 | 3.0 | 15 | 1 | 2:29 | |
| Sag. HR 2D-TSE | 4610/24 | 140 | 254 | 130 × 130 | 480 × 480 | 0.27 × 0.27 | 1.5 | 59 | 1 | 5:10 | |
| Sodium 3D-GRE | 17/8.34 | 50 | 80 | 200 × 200 | 112 × 112 | 1.79 × 1.79 | 3.0 | 40 | 12 | 31:53 |
2D indicates 2-dimensional; 3D, 3-dimensional; cor., coronal; GRE, gradient echo; LR/HR, lower/higher resolution; MR, magnetic resonance; sag., sagittal; ST, section thickness; TA, acquisition time. TR/TE, repetition/echo time; TSE, turbo spin echo.
Evaluation of Proton MRI
Proton images served for the morphological evaluation of cartilage repair site after surgery using the magnetic resonance observation of cartilage repair tissue (MOCART) scoring system.24 The MOCART is a reproducible grading system that has been applied to different cartilage repair techniques.16,18 Scores of zero and 100 points represent poor and excellent outcomes, respectively. Moreover, the thickness of reference cartilage and RT was measured in the proton images, in the region where sodium cSI values were evaluated in the MFX and MACT patients. Five measurements of tissue thickness were made in equidistant positions over each region. The mean thickness of reference cartilage and RT was calculated for each patient.
Postprocessing of Sodium Images
Cylindrical phantom filled with a homogenous saline solution was measured to correct sodium images for spatially inhomogeneous sodium knee array coil. First, phantom images were acquired using the same geometry as that for the images of the subjects and samples. Second, signal intensities of the phantom images were inverted (1/signal intensity) and rescaled to the values of 1 to 4095 to produce a correction matrix. Finally, the corrected images were obtained by multiplying the original image data with the correction matrix. All images were corrected using in-house written IDL (Interactive Data Language; Research Systems, Inc, Boulder, CO) and Matlab (MATLAB 7.7; The Mathworks, Natick, MA) scripts.
Sodium cSI maps of cartilage were calculated from the sodium images corrected for inhomogeneous B1 field. For the correction of sodium signal intensities in cartilage, the subjects were measured together with 10% wt/wt agarose gel phantoms having different concentrations of sodium (100, 150, 200, 250, and 300 mmol/L), as previously described by Shapiro et al.25 After correcting the signal intensity of the phantoms for their T1 and T2* relaxation times (T1, 30.1 milliseconds; T2*, 6.2 milliseconds) and for a 50-degree flip angle, a calibration curve was obtained. Sodium cSI maps were calculated using linear regression, and they were corrected for the mean sodium T1 and T2* of native cartilage taken from previously published measurements (T1, 20 milliseconds; T2*, 4.5 milliseconds)12,19,26 and for the 50-degree flip angle. Because the average water content from our histochemical evaluations was 70%, similar to 75% reported by Treppo et al,27 the values in the final sodium cSI maps were divided by a factor of 0.7 (Figs. 1, 2).
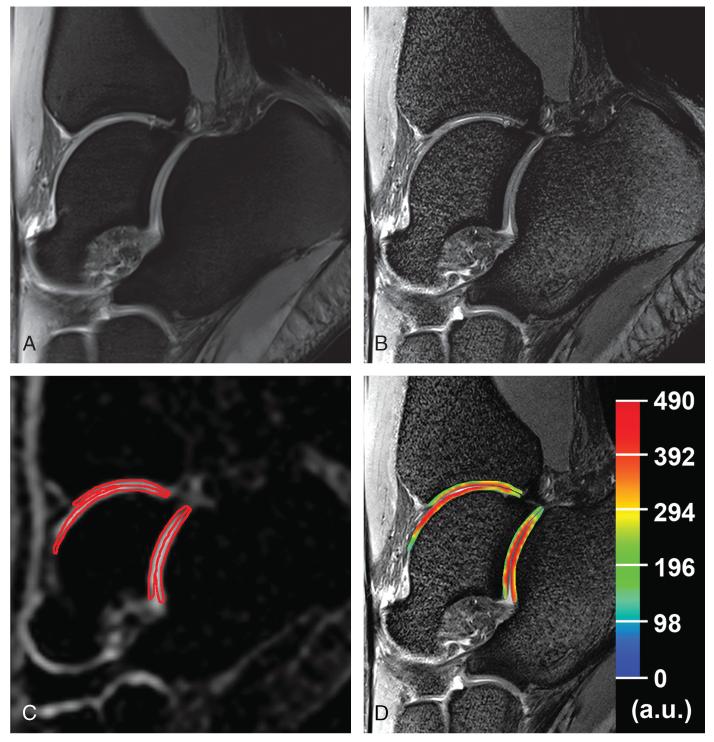
FIGURE 1.
Proton and corresponding sodium images of the ankle joint in a 23-year-old healthy woman. A, Sagittal proton density-weighted 2D-TSE image with fat suppression with lower resolution (0.31 × 0.31 × 3.0 mm3). B, Sagittal proton density-weighted 2D-TSE image with fat suppression (WIP 729C; Siemens, Erlangen, Germany) with higher resolution (0.27 × 0.27 × 1.5 mm3). C, Sagittal sodium 3D-GRE image with red contours representing the ROI evaluations of cartilages in the ankle and subtalar joints. D, Color-coded cSI map of sodium in cartilages overlaid on the corresponding morphological image.
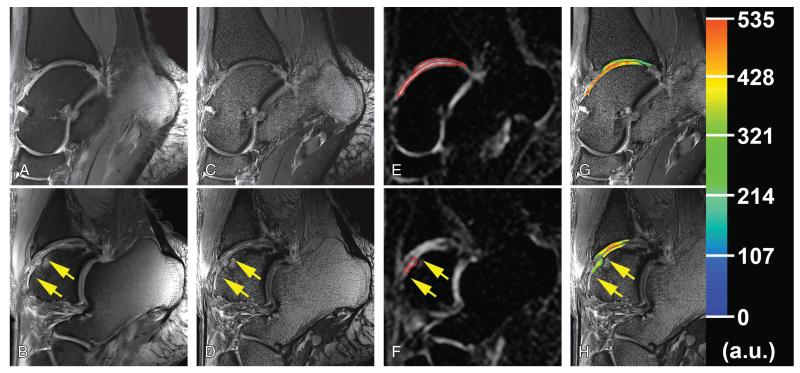
FIGURE 2.
Proton and corresponding sodium images from the medial side (upper row) and from the lateral side (lower row) of the ankle joint in a 35-year-old man obtained 54 months after the MFX treatment. Cartilage RT is situated between arrows. A and B, Sagittal proton density-weighted 2D-TSE image with fat suppression with lower resolution (0.31 × 0.31 × 3.0 mm3). C and D, Sagittal proton density-weighted 2D-TSE image with fat suppression (WIP 729C; Siemens) with higher resolution (0.27 × 0.27 × 1.5 mm3). E and F, Sagittal sodium 3D-GRE image with red contours representing the ROI evaluations of cartilage RT, tibial, and talar cartilage in ankle joint. G and H, Color-coded sodium cSI maps in cartilage and RT overlaid on the corresponding morphological images. Please note the lower sodium cSI values in RT than that in reference cartilage on the contralateral side of the ankle joint.
Evaluations of Sodium Images
For region of interest (ROI) analyses, sodium cSI maps of cartilage were rescaled to the resolution of proton 2D-TSE images and overlaid with the corresponding sagittal 2D-TSE image. The ROIs were defined by a senior radiologist (S.T., with 22 years of experience in musculoskeletal MRI) in both lower- and higher-resolution 2D-TSE images. The ROIs were afterward transferred to sodium cSI maps and evaluated using the JiveX DICOM Viewer (JiveX 4.3; VISUS Technology Transfer GmbH, Bochum, Germany).
In each patient, one 2D-TSE slice containing the largest amount of cartilage RT was selected and a repair ROI including the whole RT was selected. In addition, reference ROIs were defined by covering the whole morphologically normal-appearing tibial and talar cartilage on the side (medial or lateral) opposite to the cartilage repair site. If present, special attention was paid to exclude synovial fluid from ROIs (Fig. 2). In total, 3 mean sodium cSI values per patient were obtained for cartilage and RT.
In each volunteer, 2 contiguous 2D-TSE slices from the lateral side and 2 from the medial side were selected. In each selected slice, 4 ROIs covering the whole tibial and talar cartilage in the ankle joint as well as talar and calcaneal cartilage in the subtalar joint were defined (Fig. 1). These ROIs were transferred on the corresponding sodium cSI maps, and mean sodium cSI values were obtained. The cSI values from the same side and from the same cartilage type were averaged, resulting in 4 mean sodium cSI for each side of the ankle per volunteer. For comparison with the patients, sodium cSI from the lateral and medial sides were averaged into global values.
In each human ankle specimen, two 2D-TSE images from the lateral, central, and medial sides of the ankle joint were selected to enable comparison between sodium MRI and histochemical analysis. Two ROIs covering the whole tibial and talar cartilage in the ankle joint were selected in each morphological image. Sodium-normalized signal intensities (NSIs) were then calculated by multiplying each mean signal intensity with a factor derived from the signal intensity of the reference sample attached to knee coil. Sodium NSI values from the images on the same side (lateral, central, medial) were averaged for each cartilage type, resulting in 6 mean sodium signal intensity values per ankle specimen.
Histochemical Analyses
Tibial and talar cartilage from the human ankle samples was divided into lateral, central, and medial compartments (Fig. 3). From each cartilage compartment, 4 to 6 cartilage pieces of approximately 1-mm3 volume were harvested in the anterior-posterior direction for the biochemical evaluations. Each piece of cartilage was weighed, dried at 60°C for 24 hours, weighed again to obtain wet and dry weights, and followed by enzymatic digestion. Water content was calculated from the dry and wet weights. For quantitative biochemical analysis of GAG content in the cartilage samples, Blyscan Sulfated Glycosaminoglycan Assay kit (Biocolor Ltd, Belfast, Ireland) was used28 and optical absorbance of single-tissue digestion at 656 nm was performed in duplicates on a microplate spectrophotometer (Fluostar-Optima; BMG-Labtech, Offenburg, Germany). The sulfated GAG content was then estimated using a standard curve determined from calibration measurements of chondroitin-4 sulfate. Finally, the total GAG content in each cartilage compartment was calculated as the ratio between the sum of GAG weights from all cartilage pieces and the sum of wet weights from all cartilage pieces and expressed as a percentage.
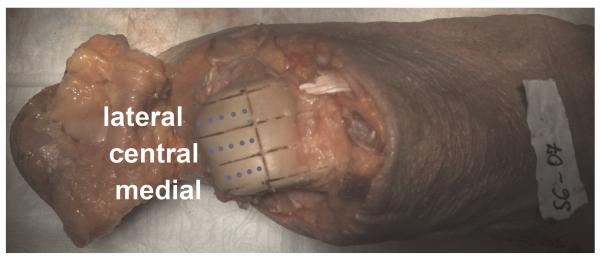
FIGURE 3.
Each talar cartilage of the human ankle samples was divided into lateral, central, and medial segments. Dots represent cartilage pieces (4–6) of approximately 1-mm3 volume, which were sampled in the anterior-posterior direction from each cartilage compartment for the biochemical evaluations. Tibial cartilage was divided and sampled similarly to talar cartilage. Figure 3 can be viewed online in color at www.investigativeradiology.com.
Statistical Analyses
To compare sodium cSI in tibial and talar reference cartilage between the volunteers as well as the MFX and MACT patients, 1-way analysis of variance (ANOVA) with the treatment type as a factor was calculated. For comparing sodium cSI from repair and reference cartilage between and within the MFX and MACT group, we used a mixed-model ANOVA with the treatment type and the tissue type (repair, reference) as a factor. Post hoc comparison was achieved with 2-tailed t tests corrected according to Bonferroni-Holm. The difference in sodium cSI between the lateral and medial sides of all 4 cartilage types and between global cSI of all 4 cartilages in the volunteers was evaluated by 2-way repeated measures ANOVA with side and cartilage type as a factor. Post hoc Bonferroni-corrected comparisons were then calculated to show differences in sodium cSI between cartilages of ankle and subtalar joint. Differences in the MOCART and clinical outcome scores between the MFX and MACT patients were evaluated with independent samples t tests.
A Pearson correlation coefficient (r) was determined to evaluate linear relation between 2 variables. The strength of association was classified as noncorrelation (r < 0.3), low (r = 0.3–0.5), moderate (r = 0.5–0.7), or strong (r > 0.7). All statistical evaluations were performed using the SPSS software (IBM SPSS Statistics 21.0), and a P value ≤ 0.05 was considered statistically significant.
RESULTS
Patients
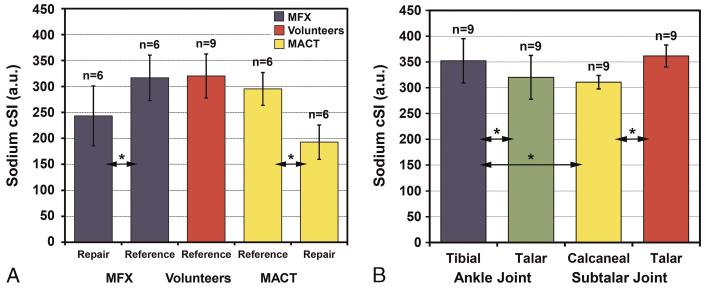
Independent-samples t test did not reveal any significant differences in age, postoperative interval, BMI, or the defect size between the MFX and MACT groups. One-way ANOVA did not show any significant differences in sodium cSI between the volunteers as well as the MFX and MACT patients for both tibial (P = 0.300) and talar reference cartilage (P = 0.391). The mixed-model ANOVA found a significant difference in cSI between talar reference cartilage and talar RT in the ankle joint (P < 0.001). Post hoc paired t tests revealed significantly lower sodium cSI in talar RT than that in talar reference cartilage in the MFX (P = 0.007) and MACT patients (P = 0.008) (Fig. 4). The difference between the MFX and MACT patients was not statistically significant in talar reference cartilage (P = 0.355) as well as in talar RT (P = 0.185) with independent-samples t tests (Fig. 5A). The mean sodium cSI values from RT and reference cartilage of the patients and the volunteers are in Table 2.
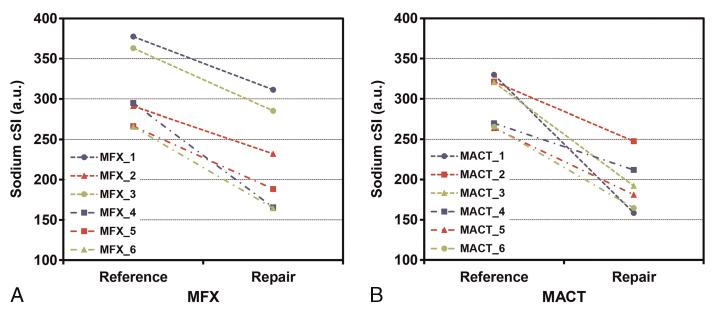
FIGURE 4.
A, Graphs indicating the sodium cSI values for native talar cartilage and talar RT for each MFX (A) and MACT (B) patient. Each line represents 1 patient. All mean sodium cSI values from the ROIs placed in native cartilage are higher than the values from the corresponding RT areas. Both cartilage repair surgeries resulted in significantly lower sodium cSI values compared with native cartilage (MFX, P = 0.007; MACT, P = 0.008). Figure 4 can be viewed online in color at www.investigativeradiology.com.
FIGURE 5.
A, Mean sodium cSI values from talar reference cartilage and talar RT of the MFX and MACT patients as well as those from talar reference cartilage of the volunteers. Note the significant decrease in mean sodium cSI of repair tissue after MFX and MACT compared with the corresponding values from reference cartilage. There was no significant difference in the sodium cSI of reference cartilage between the MFX and MACT patients and the volunteers. No significant difference in the sodium cSI was observed between MFX and MACT RT. B, Mean sodium cSI from tibial and talar cartilage of the ankle joint and that from calcaneal and talar cartilage of the subtalar joint from all 9 volunteers. Error bars stand for standard deviations, asterisk (*) represents the significant difference, and “n” stands for the number of independent observations. Figure 5 can be viewed online in color at www.investigativeradiology.com.
TABLE 2.
Sodium Concentration, Morphological Score, and Clinical Outcome in the Patients and the Volunteers
| Sodium cSI (a.u.) |
MOCART |
AOFAS Score |
Cincinnati Score |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Tibial RC | Talar RC | Talar RT | Score | Preoperative | Postoperative | Preoperative | Postoperative | ||
| MFX | Mean (SD) | 348 (54) | 317 (44) | 243 (58) | 75.8 (16.6) | 43.8 (10.3) | 88.0 (10.4) | 2.8 (1.5) | 7.2 (0.8) |
| Range | 299–438 | 266–377 | 166–311 | 45–95 | 35–55 | 75–100 | 1–4 | 6–8 | |
| MACT | Mean (SD) | 317 (35) | 295 (32) | 193 (33) | 65.0 (20.7) | 45.4 (21.6) | 90.2 (10.3) | 3.2 (1.5) | 7.4 (1.8) |
| Range | 280–365 | 264–330 | 158–248 | 40–85 | 15–75 | 76–100 | 1–5 | 5–10 | |
| Volun. | Mean (SD) | 352 (43) | 320 (42) | ||||||
| Range | 285–407 | 253–376 | |||||||
a.u. indicates arbitrary units; AOFAS, American Orthopaedic Foot and Ankle Society; cSI, corrected signal intensity; MACT, matrix-associated autologous chondrocyte transplantation; MFX, microfracture; MOCART, magnetic resonance observation of cartilage repair tissue; RC, reference cartilage; RT, repair tissue; volun., volunteers.
The mean MOCART evaluations showed that majority of the patients had complete filling of the defect (MFX, 5; MACT, 5) with visible demarcating border of RT (MFX, 4; MACT, 5), with surface damaged to less than 50% of depth (MFX, 3; MACT, 5), and with isointense signal intensity of RT (MFX: 5, MACT: 3). Independent samples t test did not show significant difference in the MOCART scores between the MFX and MACT patients (P = 0.341). All patients showed significantly higher AOFAS scores after the treatment than before the treatment (MFX, P < 0.001; MACT, P = 0.009). Similarly, the Modified Cincinnati Knee Rating scores were significantly higher after the treatment than before the treatment (MFX, P = 0.018; MACT, P = 0.006). No statistically significant difference was observed between the MFX and MACT patients when comparing the results of AOFAS (pretreatment, P = 0.950; posttreatment, P = 0.745) or the Modified Cincinnati Knee Rating (pretreatment, P = 0.666; posttreatment, P = 0.829) using independent samples t tests. Table 2 shows the mean scores of MOCART and clinical outcome evaluations.
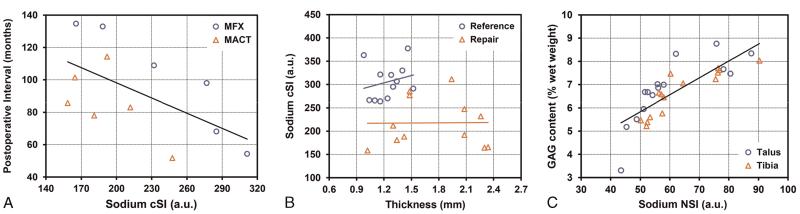
There was a medium negative correlation between postoperative interval and sodium cSI in RT (r = −0.590; P = 0.043; R2 = 0.349) (Fig. 6A). No linear association was found between MOCART scores and sodium cSI in RT (r = −0.255; P = 0.424; R2 = 0.065) as well as between clinical outcome scores after the surgery and sodium cSI in RT (AOFAS: r = −0.207, P = 0.519, R2 = 0.043; Cincinnati rating: r = 0.136, P = 0.672, R2 = 0.019).
FIGURE 6.
A, Plot represents the medium linear association between the sodium cSI values and postoperative interval of the MFX (circles) and MACT (triangles) patients (r = −0.590; P = 0.043; R2 = 0.349). Data are fitted by linear regression line, y = −1.2534x + 160.51. B, No linear association was found between the sodium cSI values and tissue thickness for reference cartilage (circles) (r = 0.232; P = 0.468; R2 = 0.054) and for RT (triangles) (r = 0.011; P = 0.973; R2 < 0.001). C, Plot demonstrates a strong linear correlation between the sodium NSI and the biochemically assessed GAG content from talar cartilage (circles) and from tibial (triangles) cartilage of the human ankle samples (r = 0.800; P < 0.001; R2 = 0.639). Data are fitted by linear regression line, y = 0.0727x + 2.1965. Figure 6 can be viewed online in color at www.investigativeradiology.com.
The mean (SD) thickness measured in the proton images was smaller in reference cartilage (MFX, 1.27 [0.22] mm; MACT, 1.22 [0.11] mm) than that in RT (MFX, 1.82 [0.42] mm; MACT, 1.69 [0.53] mm). No linear correlation was found between sodium cSI and tissue thickness in reference cartilage (r = 0.232, P = 0.468, R2 = 0.054) and in RT (r = 0.011; P = 0.973; R2 < 0.001) (Fig. 6B).
Volunteers
Sodium cSI values were calculated for the medial and lateral sides of all 4 cartilages of ankle and subtalar joint in the volunteers (Table 3). Although 2-way repeated-measures ANOVA did not show any significant differences in sodium cSI between the lateral and medial sides of tibial and talar cartilage of ankle joint as well as talar and calcaneal cartilage of the subtalar joint (P = 0.695), there was a significant difference between all 4 types of cartilages in ankle and subtalar joint (P = 0.007). Post hoc comparisons revealed significant difference in mean sodium cSI between tibial and talar cartilage of ankle joint (P = 0.002), between tibial cartilage of ankle joint and calcaneal cartilage of subtalar joint (P = 0.050), and between calcaneal cartilage and talar cartilage of subtalar joint (P < 0.001) (Fig. 5B). All other comparisons were not statistically significant.
TABLE 3.
Sodium Concentrations in the Cartilages of the Volunteers
| Sodium cSI (arbitrary units) |
|||||
|---|---|---|---|---|---|
| Ankle Joint |
Subtalar Joint |
||||
| Tibial | Talar | Talar | Calcaneal | ||
| Medial | Mean (SD) | 353 (42) | 320 (43) | 362 (21) | 311 (13) |
| Range | 278–408 | 251–374 | 336–409 | 294–335 | |
| Lateral | Mean (SD) | 352 (45) | 320 (43) | 361 (22) | 310 (13) |
| Range | 289–407 | 254–378 | 337–413 | 294–336 | |
| Global | Mean (SD) | 352 (43) | 320 (42) | 362 (21) | 311 (13) |
| Range | 285–407 | 253–376 | 336–411 | 294–335 | |
Ankle Samples
From the 5 ankle samples, 15 ROIs from talar cartilage and 15 ROIs from tibial cartilage were obtained. The mean (SD) sodium NSI value in the tibial cartilage was 63.8 (12.2) (range, 50.2–90.3) and in talar cartilage was 60.1 (13.8) (range, 43.5–87.6). The mean (SD) GAG content was 6.7% (1.0%) of wet weight (range, 5.2%–8.0%) in tibial and 6.8% (1.4%) of wet weight (range, 3.3%–8.8%). A strong linear relationship was observed between the sodium NSI and the histochemically assessed GAG content (r = 0.800; P < 0.001; R2 = 0.639) (Fig. 6C).
DISCUSSION
This study demonstrates, for the first time, the feasibility of in vivo sodium MRI of the native cartilage and cartilage RT in the ankle joint at 7 T. Sodium MRI offers quantitative biochemical evaluation of the status of native articular cartilage in volunteers and that of cartilage RT in patients after MFX and MACT treatment.
Because of ethical reasons, there is a lack of histological data concerning RT composition after MFX and MACT.4 It is therefore of substantial interest to develop and optimize biochemical MRI techniques for the noninvasive evaluation of native cartilage and quality of RT in the ankle. Morphological29 and biochemical MRI techniques such as T2 and T2* mapping,30-32 diffusion-weighted imaging,33 or dGEMRIC34 have provided interesting data about cartilage and RT in the ankle.15Although sodium MRI was successfully used for the evaluation of RT quality in the knee joint,16-18 to our knowledge, it has not yet been applied for the evaluation of ankle.
Although sodium MRI is challenging and requires multinuclear hardware, dedicated array coils, and preferably very high field strength (>3 T), recent in vivo studies demonstrated its clinical potential for the evaluation of cartilage repair and osteoarthritis.13 In addition, sodium imaging seems to be more sensitive to subtle changes in GAG content than do other GAG-sensitive techniques that have also specific limitations. The dGEMRIC is limited by the need for intravenous contrast agent and the time delay between its administration and the MRI.8,9,34 The T1ρ maps are affected not only by GAG concentration but also by dipolar relaxation mechanisms.11 The GAG chemical exchange saturation transfer is sensitive to patient motion and static magnetic field inhomogeneities, which require sophisticated postprocessing corrections.10
To validate the sensitivity of sodium MRI to the GAG content in thin ankle cartilages, measurements on the human cadaver ankle samples were first performed. A strong correlation observed between the histochemically evaluated GAG content and sodium NSI proved the sufficient sensitivity of this method to changes in the GAG content of tibial and talar cartilage and encouraged us to use sodium MRI for the in vivo evaluation of ankle cartilage. We found significantly lower sodium cSI in talar cartilage RT after both surgical procedures when compared with reference cartilage in the patients or in the volunteers. The sodium cSI values in tibial and talar reference cartilage of the patients were not significantly different from the values measured in the volunteers. Sodium cSI in RT did not differ significantly between the MFX and MACT patients, and the same results were observed for AOFAS and Modified Cincinnati Knee scores before and after the surgery. Both scores were higher after cartilage repair in each MFX and MACT patient. In addition, sodium cSI values in the cartilages of asymptomatic volunteers were determined in the ankle and subtalar joints.
At present, it is not clear whether clinical outcome after cartilage repair of the talar dome is different for MFX and MACT. Excellent outcomes at short term and midterm have been reported after MFX in the ankle.22,35,36 The first short-term and midterm results also report a good or excellent outcome after MACT.3,4,37 A few studies that directly compared the outcomes after MFX and MACT/ACI achieved a comparable outcome at midterm.31,33,38 This is in accordance with our observations of similar AOFAS and Modified Cincinnati Knee scores for the MFX and MACT patients.
The presented 7-T sodium MRI data indicate a significantly lower GAG content in RT after MFX and MACT compared with native cartilage, which can reflect less-favorable mechanical properties of RT. This is in agreement with previous sodium studies in the knee, which reported lower GAG concentration in RT of patients after MFX and MACT than in reference cartilage.16-18 In the ankle, dGEMRIC, which is sensitive to cartilage and RT GAG content, also showed a lower GAG content in RT after matrix-induced chondrogenesis than that in reference cartilage.34
In the previous knee studies, differences in the biochemical properties of RT from the 2 repair procedures were manifested by differences in sodium values18 and in T2 relaxation times.39,40 In contrast to these reports, we did not observe any significant difference in sodium cSI between the MFX and MACT RT in ankle joint. This discrepancy between knee and ankle studies could be explained by differences in the biochemical composition of the knee and ankle cartilage and by a greater capacity of ankle cartilage for repair compared with the knee joint.7 Furthermore, recent T2 mapping study at 7 T also did not find significant differences between MFX and MACT cartilage RT in talus.31
The fact that the sodium cSI did not correlate with the MOCART score or with the clinical outcome scores suggests that sodium MRI provides complementary information to morphological and clinical results. Although the cartilage GAG content and clinical outcome (pain, function) might not be directly related, only RT with sufficient concentration of GAGs can provide long-lasting functional and structural substitution for native cartilage. Because both types of cartilage repair techniques showed a decrease of sodium cSI in RT with time after surgery, sodium MRI might be useful for the evaluation of relationship between the GAG content and RT resilience over time. Although mid-term and long-term MFX RT seems to be more prone to degeneration, further long-term follow-up studies with sodium MRI will be needed for assessing optimal cartilage repair algorithms for ankle joint in clinical routine.
To achieve a sufficiently high signal-to-noise ratio, sodium images are, in general, acquired with low resolution. Because the cartilage layers in the ankle are thin (mean [SD] thicknesses of talar cartilage are approximately 1.35 [0.22] mm in males and 1.11 [0.28] mm in females41) and in-plane resolution in sodium images is 1.79 × 1.79 mm2, partial volume effects from the surrounding tissues could cause underestimation (in the presence of bone in a voxel) or overestimation (in the presence of synovial fluid) of sodium signal intensities and thus affect the accuracy of the presented results. To minimize this limitation, the phase encoding was set to the anterior-posterior direction and ROIs were selected carefully in the morphological images and transferred to the corresponding sodium images. Moreover, because ROIs containing many pixels covering the whole native cartilage or repair site were selected (in the volunteers, ROIs from 2 slices were averaged together), the overall partial volume effect on mean sodium cSI was averaged and similar in different types of cartilage and in different patients. As demonstrated in Figure 6B, the difference in sodium cSI values observed between reference cartilage and RT is not substantially affected by the tissue thickness and the corresponding partial volume effects. In addition, sodium cSI values are relative numbers; thus, the correction for possible systematic bias due to partial volume effect is not necessary.
Another limitation of the present data is that we used the same mean T1 and T2* relaxation times measured in the cartilage of healthy volunteers12,19,26 to correct the sodium maps in native cartilage and RT of patients. Although RT might have different relaxation times compared with native cartilage, these data are not available yet. Furthermore, relaxation times in RT could change even among patients with the same type of cartilage repair. An additional limitation of our preliminary study is the low number of patients. However, the number of subjects was sufficient to demonstrate significant difference in sodium cSI values between RT and native cartilage. Another source of bias in our measurements was a different follow-up interval of patients.
Because of the previously mentioned limitations (mainly partial volume artifacts), we did not evaluate the sodium concentrations in the native cartilage and RT. However, calculated sodium cSI values should be close to the expected sodium concentrations in the ankle cartilage.
In conclusion, sodium MRI at 7 Tallows quantitative assessment of native cartilage and cartilage RT. In contrast to the knee, both MFX and MACT lead to RT with similar sodium cSI values but different from that in the native cartilage. Because sodium concentration in cartilage is proportional to the cartilage GAG content, sodium MRI might be beneficial in the noninvasive evaluation of cartilage repair surgery.
ACKNOWLEDGMENTS
The authors thank Michael Weber for his helpful critical revision of the statistical analysis and Claudia Kronnerwetter for her help with MRI measurements.
Sources of funding: Supported by the Vienna Science and Technology Fund (Project WWTF-LS11-018), by the Austrian Science Fund (FWF) P 25246 B24, by the Vienna Spots of Excellence of the Vienna Science and Technology Fund (WWTF), Vienna Advanced Imaging Center (VIACLIC FA102A0017), and by DACH (SNF 320030E-134474).
Footnotes
Conflicts of interest
The authors report no conflicts of interest.
REFERENCES
- 1.Ronga M, Grassi FA, Montoli C, et al. Treatment of deep cartilage defects of the ankle with matrix-induced autologous chondrocyte implantation (MACI) Foot Ankle Surg. 2005;11:29–33. [Google Scholar]
- 2.Zengerink M, Struijs PA, Tol JL, et al. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2010;18:238–246. doi: 10.1007/s00167-009-0942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giannini S, Battaglia M, Buda R, et al. Surgical treatment of osteochondral lesions of the talus by open-field autologous chondrocyte implantation: a 10-year follow-up clinical and magnetic resonance imaging T2-mapping evaluation. Am J Sports Med. 2009;37:112S–118S. doi: 10.1177/0363546509349928. [DOI] [PubMed] [Google Scholar]
- 4.Giannini S, Buda R, Vannini F, et al. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: surgical technique and results. Am J Sports Med. 2008;36:873–880. doi: 10.1177/0363546507312644. [DOI] [PubMed] [Google Scholar]
- 5.Knutsen G, Drogset JO, Engebretsen L, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. J Bone Joint Surg Am. 2007;89A:2105–2112. doi: 10.2106/JBJS.G.00003. [DOI] [PubMed] [Google Scholar]
- 6.Saris DB, Vanlauwe J, Victor J, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36:235–246. doi: 10.1177/0363546507311095. [DOI] [PubMed] [Google Scholar]
- 7.Kuettner KE, Cole AA. Cartilage degeneration in different human joints. Osteoarthritis Cartilage. 2005;13:93–103. doi: 10.1016/j.joca.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Trattnig S, Mamisch TC, Pinker K, et al. Differentiating normal hyaline cartilage from post-surgical repair tissue using fast gradient echo imaging in delayed gadolinium-enhanced MRI (dGEMRIC) at 3 tesla. Eur Radiol. 2008;18:1251–1259. doi: 10.1007/s00330-008-0859-3. [DOI] [PubMed] [Google Scholar]
- 9.Trattnig S, Burstein D, Szomolanyi P, et al. T1(Gd) gives comparable information as Delta T1 relaxation rate in dGEMRIC evaluation of cartilage repair tissue. Invest Radiol. 2009;44:598–602. doi: 10.1097/RLI.0b013e3181b4c236. [DOI] [PubMed] [Google Scholar]
- 10.Schmitt B, Zbyn S, Stelzeneder D, et al. Cartilage quality assessment by using glycosaminoglycan chemical exchange saturation transfer and (23)Na MR imaging at 7 T. Radiology. 2011;260:257–264. doi: 10.1148/radiol.11101841. [DOI] [PubMed] [Google Scholar]
- 11.Keenan KE, Besier TF, Pauly JM, et al. Prediction of glycosaminoglycan content in human cartilage by age, T1rho and T2 MRI. Osteoarthritis Cartilage. 2011;19:171–179. doi: 10.1016/j.joca.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wheaton AJ, Borthakur A, Shapiro EM, et al. Proteoglycan loss in human knee cartilage: quantitation with sodium MR imaging—feasibility study. Radiology. 2004;231:900–905. doi: 10.1148/radiol.2313030521. [DOI] [PubMed] [Google Scholar]
- 13.Zbyn S, Mlynarik V, Juras V, et al. Sodium MR imaging of articular cartilage pathologies. Curr Radiol Rep. 2014;2:41. doi: 10.1007/s40134-014-0041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mankin HJ. Biochemical and metabolic aspects of osteoarthritis. Orthop Clin North Am. 1971;2:19–31. [PubMed] [Google Scholar]
- 15.Borthakur A, Mellon E, Niyogi S, et al. Sodium and T1rho MRI for molecular and diagnostic imaging of articular cartilage. NMR Biomed. 2006;19:781–821. doi: 10.1002/nbm.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trattnig S, Welsch GH, Juras V, et al. 23Na MR imaging at 7 T after knee matrixassociated autologous chondrocyte transplantation preliminary results. Radiology. 2010;257:175–184. doi: 10.1148/radiol.10100279. [DOI] [PubMed] [Google Scholar]
- 17.Chang G, Madelin G, Sherman OH, et al. Improved assessment of cartilage repair tissue using fluid-suppressed Na inversion recovery MRI at 7 Tesla: preliminary results. Eur Radiol. 2012;22:1341–1349. doi: 10.1007/s00330-012-2383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zbyn S, Stelzeneder D, Welsch GH, et al. Evaluation of native hyaline cartilage and repair tissue after two cartilage repair surgery techniques with 23Na MR imaging at 7 T: initial experience. Osteoarthritis Cartilage. 2012;20:837–845. doi: 10.1016/j.joca.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 19.Borthakur A, Shapiro EM, Akella SV, et al. Quantifying sodium in the human wrist in vivo by using MR imaging. Radiology. 2002;224:598–602. doi: 10.1148/radiol.2242011039. [DOI] [PubMed] [Google Scholar]
- 20.Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391:S362–S369. doi: 10.1097/00003086-200110001-00033. [DOI] [PubMed] [Google Scholar]
- 21.Grigolo B, Lisignoli G, Piacentini A, et al. Evidence for redifferentiation of human chondrocytes grown on a hyaluronan-based biomaterial (HYAff 11): molecular, immunohistochemical and ultrastructural analysis. Biomaterials. 2002;23:1187–1195. doi: 10.1016/s0142-9612(01)00236-8. [DOI] [PubMed] [Google Scholar]
- 22.Saxena A, Eakin C. Articular talar injuries in athletes: results of microfracture and autogenous bone graft. Am J Sports Med. 2007;35:1680–1687. doi: 10.1177/0363546507303561. [DOI] [PubMed] [Google Scholar]
- 23.Noyes FR, Barber SD, Mooar LA. A rationale for assessing sports activity levels and limitations in knee disorders. Clin Orthop Relat Res. 1989;246:238–249. [PubMed] [Google Scholar]
- 24.Marlovits S, Singer P, Zeller P, et al. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57:16–23. doi: 10.1016/j.ejrad.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro EM, Borthakur A, Dandora R, et al. Sodium visibility and quantitation in intact bovine articular cartilage using high field (23)Na MRI and MRS. J Magn Reson. 2000;142:24–31. doi: 10.1006/jmre.1999.1932. [DOI] [PubMed] [Google Scholar]
- 26.Madelin G, Jerschow A, Regatte RR. Sodium relaxation times in the knee joint in vivo at 7 T. NMR Biomed. 2012;25:530–537. doi: 10.1002/nbm.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Treppo S, Koepp H, Quan EC, et al. Comparison of biomechanical and biochemical properties of cartilage from human knee and ankle pairs. J Orthop Res. 2000;18:739–748. doi: 10.1002/jor.1100180510. [DOI] [PubMed] [Google Scholar]
- 28.Pietila K, Kantomaa T, Pirttiniemi P, et al. Comparison of amounts and properties of collagen and proteoglycans in condylar, costal and nasal cartilages. Cells Tissues Organs. 1999;164:30–36. doi: 10.1159/000016640. [DOI] [PubMed] [Google Scholar]
- 29.Notohamiprodjo M, Kuschel B, Horng A, et al. 3D-MRI of the ankle with optimized 3D-SPACE. Invest Radiol. 2012;47:231–239. doi: 10.1097/RLI.0b013e31823d7946. [DOI] [PubMed] [Google Scholar]
- 30.Battaglia M, Vannini F, Buda R, et al. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: mid-term T2-mapping MRI evaluation. Knee Surg Sports Traumatol Arthrosc. 2011;19:1376–1384. doi: 10.1007/s00167-011-1509-x. [DOI] [PubMed] [Google Scholar]
- 31.Domayer SE, Apprich S, Stelzeneder D, et al. Cartilage repair of the ankle: first results of T2 mapping at 7.0 T after microfracture and matrix associated autologous cartilage transplantation. Osteoarthritis Cartilage. 2012;20:829–836. doi: 10.1016/j.joca.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Andreisek G, Weiger M. T2* mapping of articular cartilage: current status of research and first clinical applications. Invest Radiol. 2014;49:57–62. doi: 10.1097/RLI.0b013e3182a574e1. [DOI] [PubMed] [Google Scholar]
- 33.Apprich S, Trattnig S, Welsch GH, et al. Assessment of articular cartilage repair tissue after matrix-associated autologous chondrocyte transplantation or the microfracture technique in the ankle joint using diffusion-weighted imaging at 3 tesla. Osteoarthritis Cartilage. 2012;20:703–711. doi: 10.1016/j.joca.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Wiewiorski M, Miska M, Kretzschmar M, et al. Delayed gadolinium-enhanced MRI of cartilage of the ankle joint: results after autologous matrix-induced chondrogenesis (AMIC)-aided reconstruction of osteochondral lesions of the talus. Clin Radiol. 2013;68:1031–1038. doi: 10.1016/j.crad.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 35.Becher C, Driessen A, Hess T, et al. Microfracture for chondral defects of the talus: maintenance of early results at midterm follow-up. Knee Surg Sports Traumatol Arthrosc. 2010;18:656–663. doi: 10.1007/s00167-009-1036-1. [DOI] [PubMed] [Google Scholar]
- 36.Kuni B, Schmitt H, Chloridis D, et al. Clinical and MRI results after microfracture of osteochondral lesions of the talus. Arch Orthop Trauma Surg. 2012;132:1765–1771. doi: 10.1007/s00402-012-1595-3. [DOI] [PubMed] [Google Scholar]
- 37.Aurich M, Bedi HS, Smith PJ, et al. Arthroscopic treatment of osteochondral lesions of the ankle with matrix-associated chondrocyte implantation: early clinical and magnetic resonance imaging results. Am J Sports Med. 2011;39:311–319. doi: 10.1177/0363546510381575. [DOI] [PubMed] [Google Scholar]
- 38.Brix M, Chiari C, Nehrer S, et al. Cartilage repair of the ankle with microfracturing or autologous chondrocyte implantation/matrix associated autologous chondrocyte implantation: follow up from 1 to 14 years. Osteoarthritis Cartilage. 2012;20:S130–S131. [Google Scholar]
- 39.Welsch GH, Trattnig S, Domayer S, et al. Multimodal approach in the use of clinical scoring, morphological MRI and biochemical T2-mapping and diffusionweighted imaging in their ability to assess differences between cartilage repair tissue after microfracture therapy and matrix-associated autologous chondrocyte transplantation: a pilot study. Osteoarthritis Cartilage. 2009;17:1219–1227. doi: 10.1016/j.joca.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 40.Welsch GH, Mamisch TC, Domayer SE, et al. Cartilage T2 assessment at 3-T MR imaging: in vivo differentiation of normal hyaline cartilage from reparative tissue after two cartilage repair procedures - Initial experience. Radiology. 2008;247:154–161. doi: 10.1148/radiol.2471070688. [DOI] [PubMed] [Google Scholar]
- 41.Sugimoto K, Takakura Y, Tohno Y, et al. Cartilage thickness of the talar dome. Arthroscopy. 2005;21:401–404. doi: 10.1016/j.arthro.2004.12.005. [DOI] [PubMed] [Google Scholar]