Abstract
Introduction: Recent advances in microsurgery such as lymphaticovenous bypass (LVB) have been shown to decrease limb volumes and improve subjective symptoms in patients with lymphedema. However, to date, it remains unknown if these procedures can reverse the pathological tissue changes associated with lymphedema. Therefore, the purpose of this study was to analyze skin tissue changes in patients before and after LVB.
Methods and Results: Matched skin biopsy samples were collected from normal and lymphedematous limbs of 6 patients with unilateral breast cancer-related upper extremity lymphedema before and 6 months after LVB. Biopsy specimens were fixed and analyzed for inflammation, fibrosis, hyperkeratosis, and lymphangiogenesis. Six months following LVB, 83% of patients had symptomatic improvement in their lymphedema. Histological analysis at this time demonstrated a significant decrease in tissue CD4+ cell inflammation in lymphedematous limb (but not normal limb) biopsies (p<0.01). These changes were associated with significantly decreased tissue fibrosis as demonstrated by decreased collagen type I deposition and TGF-β1 expression (all p<0.01). In addition, we found a significant decrease in epidermal thickness, decreased numbers of proliferating basal keratinocytes, and decreased number of LYVE-1+ lymphatic vessels in lymphedematous limbs after LVB.
Conclusions: We have shown, for the first time, that microsurgical LVB not only improves symptomatology of lymphedema but also helps to improve pathologic changes in the skin. These findings suggest that the some of the pathologic changes of lymphedema are reversible and may be related to lymphatic fluid stasis.
Introduction
Lymphedema is a common complication of cancer treatment occurring in as many as 1 in 3 patients who undergo lymphadenectomy for breast cancer treatment.1 However, post-surgical lymphedema is not limited solely to breast cancer survivors, as recent studies have shown that nearly 1 in 8 patients treated for a variety of solid malignancies (including gynecological malignancies, melanoma, and sarcomas) also develop lymphedema.2 Post-surgical lymphedema in this setting is usually progressive and is a significant source of morbidity. While conventional treatments such as compression and manual lymphatic drainage are helpful in some patients, these treatments are time consuming, expensive, and palliative in nature, aiming to prevent progression of morbidity rather than cure the underlying disease.
Recent advances in microsurgical techniques have facilitated development of surgical methods to treat lymphedema.3,4 Examples of these techniques include lymphaticovenous bypass (LVB) and vascularized lymph node transfer.5–10 In these procedures, the primary aim is to bypass the obstructed lymphatics either by direct anastomoses of obstructed lymphatics to a regional vein or by transplantation of vascularized lymph nodes that promote lymphatic regeneration. Several studies have reported promising results with lymphaticovenous bypass procedures for the treatment of secondary lymphedema. For example, a number of retrospective studies have reported decreased limb volumes and subjective improvements in this patient population.11–14 More recently, using a prospective approach in 100 consecutive patients, Chang et al. from our group have reported both objective improvements in arm volumes and subjective improvements in symptoms in patients with upper extremity lymphedema treated with microsurgical lymphaticovenous bypass.15 However, while these studies are exciting, the cellular and molecular mechanisms that may contribute to these improvements remain unknown.
Our laboratory has studied the pathology of lymphedema using a mouse model. Using this approach, we have shown that inflammation and fibrosis play a critical role in the pathology of lymphedema.16–20 Additionally, we have shown (via a variety of interventions) that tissue responses to chronic lymphedema are fundamentally different than responses to resolving edema.19 Specifically, we have found that lymphedema results in activation and proliferation of CD4+ T helper 2 (Th2) cells and that these cells then promote production of profibrotic cytokines and growth factors including transforming growth factor-beta 1 (TGF-β1), interleukin-4 (IL-4), and interleukin-13 (IL-13).18–20 More importantly, we have found that inhibition of these pathways prevents development of fibrosis and is a useful means of treating lymphedema in the mouse model.19,20 These treatments lead to regression of the pathologic effects of lymphedema in this model including decreased inflammation, fibrosis, and hyperkeratosis.19
The purpose of the current study was to analyze the inflammatory and fibrotic pathways identified in our mouse models in patients treated with LVB. To accomplish this goal, we analyzed tissue changes in inflammation, fibrosis, hyperkeratosis, and lymphangiogenesis in patients before and 6 months after surgery. We report that treatment with LVB results in decreases in CD4+ cell inflammation, dermal fibrosis, hyperkeratosis, and lymphatic capillary number. These findings are important and provide a mechanistic rationale for the efficacy of LVB in this patient population.
Materials and Methods
Clinical evaluation, patient accrual, tissue biopsy, and serum collection
Six women with unilateral breast cancer-related upper extremity lymphedema were recruited into our Institutional Review Board (IRB) approved protocol designed to examine tissue and serum changes before and 6 months after lymphaticovenous bypass. The study was approved by the IRBs of both MD Anderson Cancer Center and Memorial Sloan Kettering Cancer Center (MSKCC). A lymphedema therapist performed qualitative assessment and quantitative volumetric analysis before and 6 months after LVB using our previously published methods.10
Briefly, volumetric analyses of patients' lymphedematous and unaffected limbs were performed using an optoelectronic limb volumeter (Perometer model and software; Pero-System, Wuppertal, Germany). Volume measurements were performed three times and averaged to ensure more consistent analysis. The volume differential (the excess volume of the lymphedematous limb compared to the unaffected contralateral limb) was defined as (volume of the lymphedematous limb - volume of the unaffected contralateral limb)/volume of the unaffected contralateral limb. The volume differential reduction (the reduction in the excess volume of the limb following the procedure) was defined as (preoperative volume differential - postoperative volume differential)/preoperative volume differential. In addition to volumetric analysis, subjective symptoms of lymphedema were assessed by our independent lymphedema therapist and recorded.
We used our previously described matched-control approach to harvest skin biopsies before and after LVB to minimize variability and increase our statistical power.18,19 Briefly, full thickness 5 mm skin biopsy specimens were harvested from the identical location of the distal forearm in both the lymphedematous and normal limbs preoperatively and 6 months after LVB. As a result, each patient had four biopsies performed: two preoperatively and two postoperatively. We have previously shown that this approach enables us to control for intra-patient variability since each patient served as their own preoperative normal skin biopsy control.18,19 Thus, by comparing tissue changes in both the normal and lymphedematous arm postoperatively with preoperative samples, we can determine if putative changes observed result from nonspecific changes that have occurred during the interval of time since surgery (i.e., both normal and lymphedematous limb would change) or if changes are limited to the arm treated with lymphaticovenous bypass.
Finally, serum samples were harvested preoperatively and 6 months after LVB using peripheral blood draws and centrifugation to isolate the serum component. Serum was then frozen at −80°C and used for analysis as outlined below.
Surgical approach
All patients were under general anesthesia during the procedures. Indocyanine green (ICG) lymphangiography was performed by intradermally injecting 0.01–0.02 mL of ICG (Akorn, Inc., Lake Forest, IL, USA) into each finger/toe web of the lymphedematous limb and imaged using a Hamamatsu Photodynamic Eye as previously reported.10 This imaging was used to classify lymphatic dysfunction using the MD Anderson ICG classification system (ICGN).10 Lymphaticovenous bypasses were then performed as previously reported and patency was assessed using isosulfan blue dye injections (Lymphazurin; United States Surgical Corp., Norwalk, CT).
Histology and immunohistochemistry
Biopsy specimens were fixed in 4% paraformaldehyde, paraffin-embedded, and sectioned at a thickness of 5 μm. Sections were stained with Hematoxylin (Dako, Carpinteria, CA) and Eosin (Thermo Scientific, Kalamazoo, MI) and slide images were captured (Mirax Scanner, Carl Zeiss, Munich, Germany). With both acanthosis and hyperkeratosis being hallmarks of lymphedema, we hypothesized that lymphaticovenular bypass may abrogate these pathologic processes. Therefore, epidermal thickness on the H&E sections was measured (top of stratum corneum to epidermal basement membrane) at four locations per sample (n=6 per group) in micrometers using Pannoramic Viewer (3DHISTECH, Budapest, Hungary).
For all immunohistochemical staining, tissue sections were blocked in appropriate secondary serum and stained with monoclonal or polyclonal antibodies (see below) using our previously published methods.18 In relation to our above hypothesis that LVB may decrease hyperkeratosis, we sought to determine the procedure's effect on keratinocyte proliferation. We performed immunohistochemistry for Ki67 (rabbit monoclonal #ab16667; Abcam, Cambridge, MA), a nuclear protein necessary for cell proliferation, and staining was visualized using 3,3-diaminobenzidine tetrahydrochloride (DAB; Dako, Carpinteria, CA). Following image capture, cell counts were performed by blinded reviewers at a magnification of 20X and repeated for a minimum of four fields per biopsy.
Based on our previous studies showing that human skin biopsies from lymphedematous limbs are heavily infiltrated with CD4+ cells, immunohistochemistry was performed on tissue sections to identify CD4 antigen (rabbit monoclonal #ab133616; Abcam).18 Following image capture, cell counts were recorded as above.
Because fibrosis is a hallmark of lymphedema, we analyzed the effects of LVB on fibrosis using immunohistochemical staining for collagen I (rabbit polyclonal #ab292; Abcam) and transforming growth factor-beta (TGF-β1; rabbit polyclonal #ab66043; Abcam). We chose to analyze TGF-β1 because we have previously shown that the expression of this profibrotic growth factor is significantly increased in clinical lymphedema samples and that inhibition of TGF-β1 signaling significantly decreases fibrosis and improves lymphatic function in a mouse model.16,18 We calculated the percentage of the image area with positive staining for collagen I as a function of total biopsy area using Metamorph image analysis software (Molecular Devices Corp., Sunnyvale, CA) and TGF-β1+ cell counts were performed by blinded reviewers as above.
Enzyme-linked immunosorbent assay (ELISA)
In order to study putative systemic changes that may occur after lymphaticovenous bypass, we collected serum from the peripheral blood of patients before and 6 months following surgery as described above. Based on our previous studies demonstrating changes in Th2-mediated immune responses in lymphedema, we analyzed the expression of interleukin-4 (IL-4) and immunoglobulin-E (IgE) using ELISA according to the manufacturer's directions (eBioscience, San Diego, CA) since these factors are known to correlate with Th2 cell differentiation.
Statistical analysis
We used the matched Student's t-test to compare preoperative and postoperative changes in tissue cell infiltration and cytokine expression and serum analyses. Data are presented as mean±standard deviation unless otherwise noted with p<0.05 considered significant.
Results
LVB decreases symptoms of lymphedema
The clinical characteristics of the patients accrued to our study are summarized in Table 1. Our group had previously shown that LVB results in significant decreases in arm volumes and improves subjective findings of lymphedema in the majority of patients, and, more importantly, that this response is maintained in long-term follow-up. In the current study, 3/6 patients experienced modest decreases in arm volumes at 6 months, however, when analyzed as a group the volumetric difference between pre and postop measures was not statistically significant. However, consistent with our previous reports,10,15 we found that 5/6 patients had decreased symptoms of lymphedema. The fact that we found no statistical differences in limb volumes is likely a type I statistical error since our study was not powered to evaluate limb volume changes but rather designed to analyze histologic changes which we have previously found to be much more sensitive than volumetric analysis.19
Table 1.
Patient Characteristics
| Age | BMI | Location (arm) | Duration (years) | ICGN Staging | Preop Radiation | Number of Bypasses | Symptom Relief |
|---|---|---|---|---|---|---|---|
| 63 | 29.6 | Right | 20 | 4 | No | 2 | Yes |
| 60 | 31.6 | Right | 2 | 3 | Yes | 4 | No |
| 43 | 29.2 | Left | 4 | 3 | Yes | 5 | Yes |
| 51 | 31.4 | Right | 4 | 3 | Yes | 2 | Yes |
| 48 | 26.4 | Right | 2 | 3 | Yes | 4 | Yes |
| 40 | 31.2 | Left | 4 | 4 | Yes | 6 | Yes |
BMI, Body mass index; ICGN, indocyanine green classification.
LVB is associated with decreased local tissue inflammation
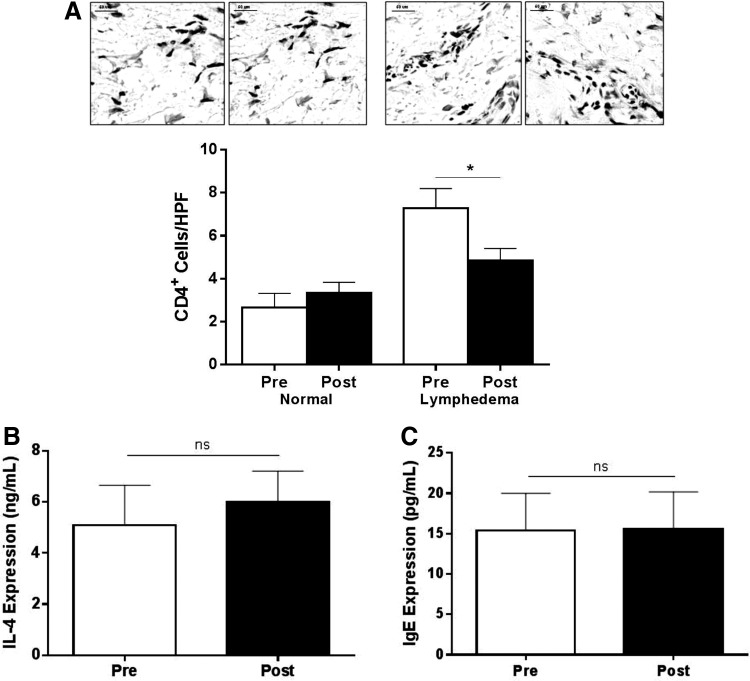
Using a mouse model as well as clinical biopsy specimens, we have previously shown that lymphedema is associated with a significant CD4+ cell inflammatory response and that inhibition of this response markedly decreased initiation and progression of lymphedema.20 In addition, we have previously shown that the severity of lymphedema is positively correlated with the degree of CD4+ inflammation.19 Consistent with these previous results, we found that biopsy specimens obtained from the lymphedematous arms had significantly increased CD4+ cell inflammation as compared with the normal limb before LVB (Fig. 1A; p<0.01). More importantly, we found that this response was significantly attenuated (p<0.01) 6 months after LVB, although the number of CD4+ cells present at this time point were still higher than normal (p=0.0492). Taken together, these findings suggest that although LVB markedly decreases inflammation locally, this process is not completely reversed.
FIG. 1.
LVB is associated with decreased local tissue inflammation. (A) Representative photomicrographs (120X) and quantification of CD4+ cells/HPF pre- and postoperatively in normal (left panels) and lymphedematous arms (right panels; *p<0.01). Systemic IL-4 (B) and IgE (C) protein expression following LVB.
We have previously shown that lymphedema is associated with a mixed T-helper-1 (Th1)/Th2 cell response. IL-4 is an inflammatory cytokine that promotes Th2 differentiation and can therefore be used as a means of analyzing systemic Th2 responses. However, analysis of IL-4 or IgE concentration (an antibody subtype that requires IL-4 expression for class switching) before and after surgery failed to show significant differences after LVB, suggesting that this treatment does not cause systemic changes (Fig. 1B, C). This hypothesis is consistent with our finding that the biopsy samples from the normal arm were little changed over time.
LVB decreases dermal fibrosis and TGF-β1 expression
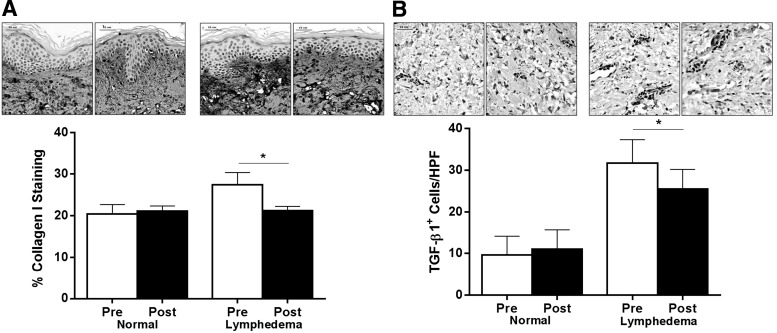
We have previously shown that fibrosis is a critical regulator of lymphatic regeneration and function.18 In addition, previous studies have shown that lymphatic vessels and the surrounding soft tissues become replaced by scar in chronic lymphedema.18,19 Comparison of normal and lymphedematous sections preoperatively demonstrated significant fibrosis and collagen deposition in the dermis in lymphedematous tissue samples (Fig. 2A; p<0.01). Collagen bundles could easily be seen interposed in the extracellular matrix of the dermis of lymphedematous samples. In contrast, normal skin had more scattered collagen type I deposition. Six months following LVB, collagen deposition was markedly decreased in the lymphedematous samples with levels approaching those noted in normal skin. In contrast, there were no interval changes in type I collagen deposition in normal skin biopsy samples. These findings provide a putative mechanism for the subjective improvements in lymphedema symptoms noted by the majority of our patients.
FIG. 2.
LVB decreases dermal fibrosis and TGF-β1 expression. (A) Representative photomicrographs (80X) and quantification of collagen I deposition pre- and postoperatively in normal (left panels) and lymphedematous arms (right panels; *p<0.01). (B) Representative photomicrographs (80X) and quantification of TGF-β1+ cells/HPF pre- and postoperatively in normal (left panels) and lymphedematous arms (right panels; *p<0.01).
Using a mouse model, we had previously shown that TGF-β1 plays a significant role in the regulation of tissue fibrosis in response to lymphedema.16–18 In addition, we previously showed that the expression of TGF-β1 is markedly increased in clinical biopsy samples harvested from lymphedematous tissues.18 Consistent with these reports, we found that the expression of TGF-β1 was markedly increased (3-fold) in the preoperative lymphedematous samples as compared to the matched control normal biopsy specimens (Fig. 2B: p<0.01). TGF-β1 expression was markedly decreased 6 months after LVB (28% decrease; Fig. 2B; p<0.01). However, consistent with our published report, we found that LVB improved but did not cure lymphedema.15 In the current study, we found that the expression of TGF-β1 was still significantly increased as compared with the normal limb.
LVB is associated with decreased hyperkeratosis and epidermal proliferation
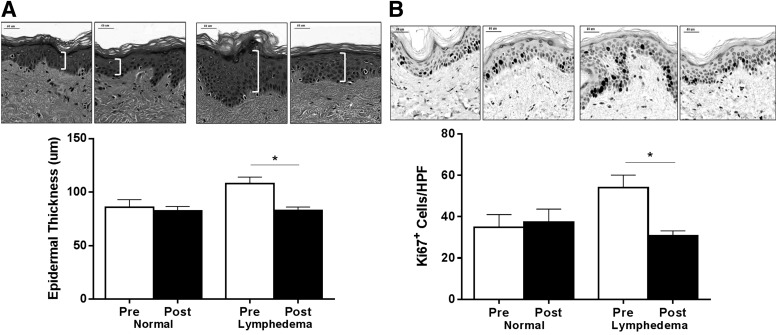
Hyperkeratosis is a clinical hallmark of lymphedema resulting in thickening of the skin.21 Consistent with this fact, we found skin samples harvested from the lymphedematous arms had significantly increased epidermal thickness as compared with the normal arm preoperatively (Fig. 3A). More importantly, we found that LVB was associated with a significant reduction in this feature and decreased the thickness of the epidermis to measurements within the normal range (Fig. 3A; p<0.01). In contrast, we found no differences in epidermal thickness in the normal arm as a function of time of biopsy, suggesting that the changes observed in hyperkeratosis were not due to global systemic changes.
FIG. 3.
LVB is associated with decreased hyperkeratosis and epidermal proliferation. (A) Quantification of hyperkeratosis as analyzed by epidermal thickness pre- and postoperatively in normal and lymphedematous arms (*p<0.01). Brackets demonstrate epidermal thickness in representative H&E sections (top) shown at 80X magnification. (B) Representative photomicrographs (80X) and quantification of Ki67+ proliferating keratinocytes/HPF pre- and postoperatively in normal and lymphedematous arms (*p<0.001).
Consistent with our finding that LVB is associated with decreased hyperkeratosis, we found that LVB resulted in significant decreases in the number of proliferating keratinocytes in the basal layer of the epidermis as assessed by Ki67 (a protein present during all active phases of the cell cycle) immunolocalization (Fig. 3B; p<0.001). In addition, similar to our observations in epidermal thickness, we found that the number of proliferating keratinocytes after LVB was indistinguishable from the normal limb.
LVB decreases the number of capillary lymphatic vessels
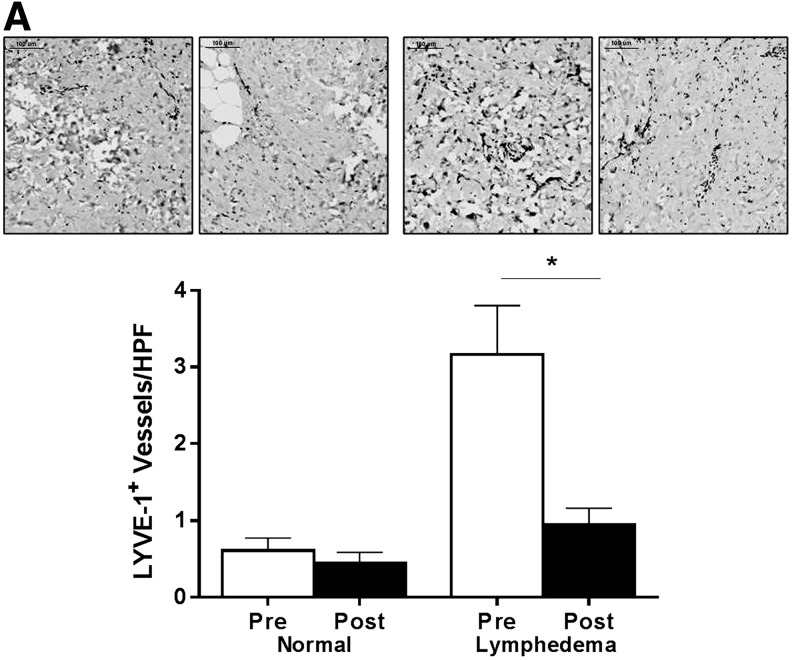
We have previously shown in our mouse model that chronic lymphedema is paradoxically associated with a significant increase in the number of capillary lymphatic vessels.16–18 Additionally, we have shown that this increase is attenuated if lymphedema progression is halted.18 Consistent with our preclinical studies, we found that tissue samples harvested from lymphedematous limbs had on average a 419% increase in the number of lymphatic capillaries as assessed using LYVE-1 (a lymphatic specific marker) immunolocalization (Fig. 4; p<0.01). More importantly, analysis of LYVE-1+ vessel counts postoperatively approximated the values obtained from the normal control limbs, suggesting that LVB can effectively reverse the physiologic changes that drive lymphangiogenesis in lymphedema (p=0.0029).
FIG. 4.
LVB decreases the number of capillary lymphatic vessels. (A) Representative photomicrographs (40X) and quantification of LYVE-1+ vessels/HPF pre- and postoperatively in normal and lymphedematous arms (*p<0.01).
Conclusions
In the current study, we have shown using human tissue samples that LVB decreased pathological tissue changes associated with lymphedema. These changes include decreased T cell inflammation, decreased fibrosis, diminished expression of pro-fibrotic cytokines, and decreased hyperkeratosis. These findings are interesting as they provide a cellular mechanism for our previously reported clinical results.
Inflammation has long been considered a pathological hallmark of lymphedema.22,23 Using a mouse model of lymphedema as well as clinical lymphedema specimens, our lab has recently shown that lymphedema results in a particular type of inflammation characterized by massive accumulation of CD4+ cells.19,20 In addition, using clinical biopsy specimens we have shown that the severity of lymphedema correlates with the degree of CD4+ cell inflammation.18 Using a variety of interventions with transgenic mice that lack T cells in general or CD4+ cells in particular, we have shown that CD4+ cells play an important role in the pathology of lymphedema and are necessary for fibrosis and lymphatic dysfunction after lymphatic injury.20 Consistent with these studies, in the current study we found that LVB markedly decreased tissue CD4+ cell counts only in the lymphedematous arm. The fact that CD4+ cell counts were unchanged in the normal arm during the postoperative period suggests that the changes in inflammation are localized to the lymphedematous arm (and likely related to our surgical intervention) rather than global systemic changes. These findings are important since, to our knowledge, they represent the first demonstration that the pathological tissue changes of lymphedema can be reversed using a physiological surgical intervention designed to decrease lymphatic stasis.
Tissue fibrosis is commonly observed in patients with lymphedema. Patients often complain of skin tightness, heaviness, and changes in skin turgor. In our study, we found that 5/6 patients had symptomatic improvements in these parameters. Consistent with this finding, we found that LVB was associated with a significant decrease in skin fibrosis as assessed by collagen type I immunohistochemistry. In fact, we found that the collagen content of lymphedematous limb skin biopsy samples was essentially indistinguishable from the normal limb. Consistent with this finding, we found that the expression of TGF-β1, a major regulator of fibrosis, was significantly decreased after LVB. This finding is important as we have previously shown that TGF-β1 expression plays a critical role in the regulation of tissue fibrosis in lymphedema.16–18 In fact, similar to our findings in the current study, we have previously shown that inhibition of TGF-β1 signaling using neutralizing antibodies or dominant negative adenoviral gene therapy markedly decreases tissue fibrosis in our mouse model of lymphedema.18 In addition, our lab and others have shown that TGF-β1 is an important anti-lymphangiogenic growth factor capable of inhibiting lymphatic function by directly decreasing lymphatic endothelial cell proliferation, migration, and function.16,24 Thus, it is possible that decreased TGF-β1 expression after LVB improves the symptoms of lymphedema via multiple mechanisms including decreased fibrosis and improved lymphatic function.
Analysis of the histological changes in the one patient in our study who did not experience symptomatic improvement provided some interesting insights. For example, although this patient had a statistically significant decrease in CD4+ cell inflammation (p=0.0313), in contrast to the other patients in our study there were no changes in the number of lymphatic vessels, the number of TGFβ+ cells, or the thickness of the epidermis after LVB. Therefore, it is possible that this patient had an incomplete response to LVB or that some of our bypasses thrombosed or became dysfunctional after surgery. Future studies with increased numbers of patients and more formal quality of life measurements will be needed to answer these questions.
Hyperkeratosis is a clinical feature of lymphedema and is noted most prominently in patients with advanced stage lymphedema. Consistent with this fact, we found that lymphedematous skin biopsy samples prior to LVB had increased epidermal thickness and increased numbers of proliferating basal keratinocytes as compared with normal skin biopsies. More importantly, we found that these pathological changes normalized after LVB. These findings support our studies with fibrosis and inflammation since these pathologic effects can be considered to be on a continuum and related to similar molecular mechanisms. For example, using a mouse model of lymphedema, we had previously shown that blockade of TGF-β1 using monoclonal antibodies markedly decreases dermal fibrosis and thickening.18 This hypothesis is also supported by a recent study demonstrating that blockade of TGF-β1/Smad signaling is associated with significantly decreased epithelial hyperplasia.25
In summary, we have shown for the first time that LVB can reverse some of the pathological changes associated with lymphedema including inflammation, fibrosis, and hyperkeratosis. These findings are important in that they not only represent effective translation of a mouse model of lymphedema to human subjects, but are also yet another step towards understanding the mechanisms behind this complex condition and its therapies. We hypothesize that the differences found in this study after LVB (i.e., decreased inflammation and fibrosis) would occur after any treatment for lymphedema that decreases lymphatic fluid stasis. This concept is supported by previous studies demonstrating decreased inflammation and expression of inflammatory cytokines after decongestive therapy.26 However, in our study, the patients did not have additional physical therapy or massage and simply continued their preoperative program. Therefore, we do not think that this fact contributed to our observations. Future studies designed to analyze histological changes after manual lymphatic drainage therapy or correlation of volumetric changes and inflammatory responses would be interesting and will provide additional insights.
Acknowledgments
We would like to thank the Molecular Cytology Core Facility (Core Grant P30 CA008748) at Memorial Sloan Kettering Cancer Center for assistance with tissue processing and imaging.
Funding: NIH R01 HL111130-01 (BJM); NIH/NCC 5T32 CA009501-25 (DAC).
Author Disclosure Statement
The authors have no conflicts of interest or disclosures. This work has not been previously presented and is not in consideration for publication at any other journals.
References
- 1.Petrek JA, Heelan MC. Incidence of breast carcinoma-related lymphedema. Cancer 1998;83:2776–2781 [DOI] [PubMed] [Google Scholar]
- 2.Cormier JN, Askew RL, Mungovan KS, Xing Y, Ross MI, Armer JM. Lymphedema beyond breast cancer: A systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer 2010;116:5138–5149 [DOI] [PubMed] [Google Scholar]
- 3.Mehrara BJ, Zampell JC, Suami H, Chang DW. Surgical management of lymphedema: Past, present, and future. Lymphat Res Biol 2011;9:159–167 [DOI] [PubMed] [Google Scholar]
- 4.Granzow JW, Soderberg JM, Kaji AH, Dauphine C. Review of current surgical treatments for lymphedema. Ann Surg Oncol 2014;21:1195–1201 [DOI] [PubMed] [Google Scholar]
- 5.Becker C, Assouad J, Riquet M, Hidden G. Postmastectomy lymphedema: Long-term results following microsurgical lymph node transplantation. Ann Surg 2006;243:313–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saaristo AM, Niemi TS, Viitanen TP, Tervala TV, Hartiala P, Suominen EA. Microvascular breast reconstruction and lymph node transfer for postmastectomy lymphedema patients. Ann Surg 2012;255:468–473 [DOI] [PubMed] [Google Scholar]
- 7.Baumeister RG, Siuda S, Bohmert H, Moser E. A microsurgical method for reconstruction of interrupted lymphatic pathways: Autologous lymph-vessel transplantation for treatment of lymphedemas. Scand J Plastic Reconst Surg 1986;20:141–146 [DOI] [PubMed] [Google Scholar]
- 8.Suami H, Chang DW. Overview of surgical treatments for breast cancer-related lymphedema. Plastic Reconst Surg 2010;126:1853–1863 [DOI] [PubMed] [Google Scholar]
- 9.O'Brien BM, Sykes P, Threlfall GN, Browning FS. Microlymphaticovenous anastomoses for obstructive lymphedema. Plastic Reconst Surg 1977;60:197–211 [DOI] [PubMed] [Google Scholar]
- 10.Chang DW. Lymphaticovenular bypass surgery for lymphedema management in breast cancer patients. Handchirurgie, Mikrochirurgie, plastische Chirurgie : Organ der Deutschsprachigen Arbeitsgemeinschaft fur Handchirurgie: Organ der Deutschsprachigen Arbeitsgemeinschaft fur Mikrochirurgie der Peripheren Nerven und Gefasse 2012;44:343–347 [DOI] [PubMed] [Google Scholar]
- 11.O'Brien BM, Mellow CG, Khazanchi RK, Dvir E, Kumar V, Pederson WC. Long-term results after microlymphaticovenous anastomoses for the treatment of obstructive lymphedema. Plastic Reconst Surg 1990;85:562–572 [DOI] [PubMed] [Google Scholar]
- 12.Huang GK, Hu RQ, Liu ZZ, Shen YL, Lan TD, Pan GP. Microlymphaticovenous anastomosis in the treatment of lower limb obstructive lymphedema: Analysis of 91 cases. Plastic Reconst Surg 1985;76:671–685 [PubMed] [Google Scholar]
- 13.Gloviczki P, Fisher J, Hollier LH, Pairolero PC, Schirger A, Wahner HW. Microsurgical lymphovenous anastomosis for treatment of lymphedema: A critical review. J Vasc Surg 1988;7:647–652 [DOI] [PubMed] [Google Scholar]
- 14.Campisi C, Eretta C, Pertile D, Da Rin E, Campisi C, Maccio A, Campisi M, Accogli S, Bellini C, Bonioli E, Boccardo F. Microsurgery for treatment of peripheral lymphedema: Long-term outcome and future perspectives. Microsurgery 2007;27:333–338 [DOI] [PubMed] [Google Scholar]
- 15.Chang DW, Suami H, Skoracki R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plastic Reconst Surg 2013;132:1305–1314 [DOI] [PubMed] [Google Scholar]
- 16.Clavin NW, Avraham T, Fernandez J, Daluvoy SV, Soares MA, Chaudry A, Mehrara BJ. TGF-beta1 is a negative regulator of lymphatic regeneration during wound repair. Am J Physiol Heart Circ Physiol 2008;295:H2113–2127 [DOI] [PubMed] [Google Scholar]
- 17.Avraham T, Clavin NW, Daluvoy SV, Fernandez J, Soares MA, Cordeiro AP, Mehrara BJ. Fibrosis is a key inhibitor of lymphatic regeneration. Plastic Reconst Surg 2009;124:438–450 [DOI] [PubMed] [Google Scholar]
- 18.Avraham T, Daluvoy S, Zampell J, Yan A, Haviv YS, Rockson SG, Mehrara BJ. Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair. Am J Pathol 2010;177:3202–3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avraham T, Zampell JC, Yan A, Elhadad S, Weitman ES, Rockson SG, Bromberg J, Mehrara BJ. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J 2013;27:1114–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zampell JC, Yan A, Elhadad S, Avraham T, Weitman E, Mehrara BJ. CD4(+) cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PloS One 2012;7:e49940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Safdar A. Principles and Practice of Cancer Infectious Diseases. Houston, TX, USA: Springer; 2011 [Google Scholar]
- 22.Olszewski WL. Pathophysiological aspects of lymphedema of human limbs: I. Lymph protein composition. Lymphat Res Biol 2003;1:235–243 [DOI] [PubMed] [Google Scholar]
- 23.Olszewski WL. The pathophysiology of lymphedema–2012. Handchirurgie, Mikrochirurgie, plastische Chirurgie: Organ der Deutschsprachigen Arbeitsgemeinschaft fur Handchirurgie: Organ der Deutschsprachigen Arbeitsgemeinschaft fur Mikrochirurgie der Peripheren Nerven und Gefasse 2012;44:322–328 [DOI] [PubMed] [Google Scholar]
- 24.Oka M, Iwata C, Suzuki HI, Kiyono K, Morishita Y, Watabe T, Komuro A, Kano MR, Miyazono K. Inhibition of endogenous TGF-beta signaling enhances lymphangiogenesis. Blood 2008;111:4571–4579 [DOI] [PubMed] [Google Scholar]
- 25.Buschke S, Stark HJ, Cerezo A, Pratzel-Wunder S, Boehnke K, Kollar J, Langbein L, Heldin CH, Boukamp P. A decisive function of transforming growth factor-beta/Smad signaling in tissue morphogenesis and differentiation of human HaCaT keratinocytes. Mol Biol Cell 2011;22:782–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foldi E, Sauerwald A, Hennig B. Effect of complex decongestive physiotherapy on gene expression for the inflammatory response in peripheral lymphedema. Lymphology 2000;33:19–23 [PubMed] [Google Scholar]