Abstract
Objectives
The anti-allergic ‘mast cell stabilising’ cromones release the anti-inflammatory protein annexin A1 (Anx-A1) from U937 cells. These drugs also inhibit polymorphonuclear leukocyte (PMN) trafficking. Since PMN can synthesise and release large amounts of Anx-A1, we hypothesised that a similar mechanism could operate upon application of cromones to these cells.
Methods and Results
Intravital microscopy was used to monitor the actions of cromones in the inflamed microcirculation. Reperfusion injury provoked a dramatic rise in adherent and emigrated leukocytes in the mesenteric vascular bed, associated with augmented tissue levels of myeloperoxidase (MPO). Nedocromil (2-20mg/kg) significantly (p<0.05) inhibited cell adhesion and emigration, as well as MPO release, in wild type but not Anx-A1−/− mice. Short pre-treatment of human PMN with nedocromil (10nM) inhibited cell adhesion (p<0.05) in the flow chamber assay, and this effect was reversed by a specific anti-AnxA1 or combination of anti-FPR1 and anti-FPR2 - but not an irrelevant control - antibodies. Western blotting experiments revealed that cromones stimulate PKC-dependent phosphorylation and release of Anx-A1 in human PMN.
Conclusions
We propose a novel mechanism to explain the anti-inflammatory actions of cromones on PMN trafficking, an effect that has long puzzled investigators.
Keywords: Nedocromil, cromoglycate, PKC, inflammation, FPR receptors
Cromones are a group of anti-allergic drugs of which sodium cromoglycate and sodium nedocromil are the exemplars. Early studies on the mechanism of action of these mast cell stabilisers suggested that they blocked mast cell degranulation, hence mediator release, when this triggered by various stimuli including IgE 1 . However these drugs can affect other facets of the inflammatory process including inhibition of eicosanoid 2 and cytokine 3 release.
Leukocyte emigration 4 is also inhibited by these drugs and, for instance, cromoglycate and ketotifen exhibit a protective role in neutrophil-dependent pathologies including intestinal 5 and pulmonary6 ischaemia-reperfusion (IR) model. Mast cells, located in close apposition to the vessels, are an important source of pro-inflammatory mediators released at the site of inflammation7. Mast-cell derived mediators activate adhesion molecules from the local environment leading to leukocyte recruitment (7) utilising a well-characterised multi step mechanism (reviewed in 8). Clearly inhibition of the release of mediators from these mast cells could explain the effect of the cromones on PMN trafficking. However, other work points to a separate, direct, effect of these compounds on the PMN 9.
Anx-A1 is a 37kDa glucocorticoid (GC)-regulated protein that mimics their effects in several in vivo and in vitro model systems 10-12. GCs not only induce the Anx-A1 gene in many cells but also increase secretion of the protein from existing intracellular pools by stimulating PKC activity13. Once secreted the protein acts in a paracrine/autocrine fashion, utilising formyl peptide receptors (FPR) 14, 15 to produce its biological effects. The N-terminal region of Anx-A1 bears the biological activity of the protein and phosphorylation on Ser27 residue is crucial for protein export and secretion 13. While studying the rapid mechanism of action of GC in a pro-monocytic U937 cell line, we noted that cromones promoted the rapid release of Anx-A116. This occurred through inhibition of phosphatase PP2A, that constitutively limits PKC activity thereby potentiating the phosphorylation and release of Anx-A1 16.
Anx-A1 is found in high amounts in circulating neutrophils and monocytes 17, from which the protein is externalized and cleaved after leukocyte adhesion to the endothelium in vitro 18. The protein strongly inhibits neutrophil recruitment in vivo 19 and in vitro 20, 21 . In this study, we sought to investigate the mechanism of action of cromones on PMN emigration.
Material & Methods
Two distinct, yet complementary models were used. In an in vivo reperfusion injury model using Anx-A1−/− and an in vitro flow chamber assay using human cells together with a specific neutralizing Anti-Anx-A1 antibody. We have also assessed the effect of the cromones on the internalisation of FPR1 and FPR2 by FACs analysis and in the flow chamber assay. Cromones were also tested in zymosan-induced peritonitis, an acute model of inflammation. A full description of the methods used is available in the Supplemental Materials.
Results
Alterations in the mouse mesenteric microcirculation following IR and the effect of nedocromil
In this model of IR in the mesenteric circulation, clamping and reopening of the superior mesenteric artery (SMA) induced a significant increase in the number of adherent and emigrated cells observed microscopically in WT mice, when compared to the sham operated animals (Figure 1A). This activation of the inflammatory process within the post-capillary venule endothelium is attenuated by the administration of (2mg/kg) nedocromil given 45 min pre-reperfusion.
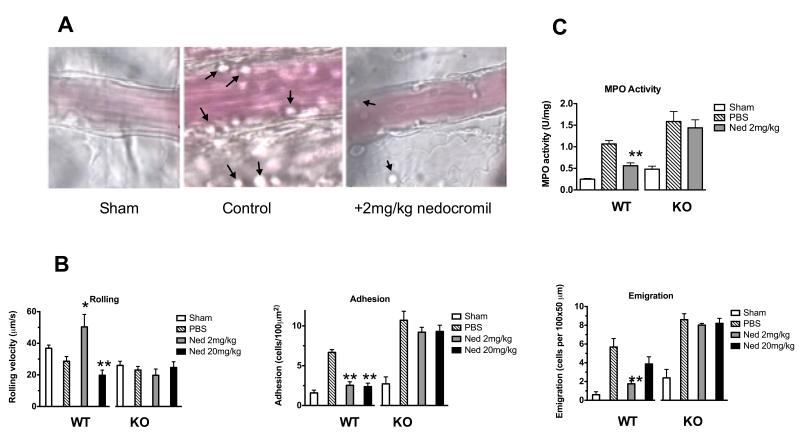
Figure 1. Nedocromil inhibits PMN migration and MPO tissue levels in a mouse model of ischaemia-reperfusion injury.
(A) Images are representative pictures of the mouse mesenteric microcirculation as observed in sham-operated mice (left hand frame) and following occlusion and reopening of the SMA (centre frame) and after administration of nedocromil (right hand frame). (B) Inhibitory effect of nedocromil on the leukocyte-endothelium interaction in IR inflamed mesenteric vessels of WT mice is abrogated in Anx-A1 KO mice. (C) Myeloperoxidase (MPO) releases in injured mesenteric tissues following IR injury. Data are mean ± SEM of n=6 mice per group. **p<0.01 vs sham group, *p<0.05 vs PBS group (see the Supplemental Materials file for details).
We then investigated the mechanism by which nedocromil exerts this protective effect. The IR protocol induced a reduction in VWBC (rolling velocity) in WT mice, which was associated with a significant increase in the degree of cell adhesion and emigration, as assessed 45 min after reperfusion (Figure 1B). Treatment of mice with a low (2mg/kg) dose of nedocromil strongly inhibited cell adhesion and emigration but had no effect on VWBC. At the higher dose of 20mg/kg, nedocromil inhibited all three parameters under observation- cell rolling velocity, adhesion and emigration - although the latter did not reach statistical significance (Figure 1B).
We next tested the potential involvement of Anx-A1 in the observed intravascular effects of nedocromil using Anx-A1−/− mice. In these animals, the IR procedure did not alter VWBC but produced the expected increase in cell adhesion and emigration. Within the time frame of these experiments, no difference between the genotypes observed with respect to the cellular response in the microcirculation (Figure 1B). However, nedocromil (2 mg/kg or 20 mg/kg) was without any discernable effect on the leukocyte-endothelium interactions promoted by the IR procedure in Anx-A1−/− mice. Taken together, these in vivo data strongly suggests that nedocromil exerts its protective effects in the inflamed microcirculation through the anti-inflammatory protein Anx-A1. Haemodynamic parameters were also measured. Administration of nedocromil (2 or 20mg/kg) significantly increased cell flux and wall shear rate in the WT mouse relative to PBS treatment (Table I; see supplementary data), but the compound did not alter the haemodynamic parameters in Anx-A1−/− mice (Table II; see supplementary data).
Measurement of myeloperoxidase (MPO) in the mesenteric tissue
PMN accumulation into the mesenteric tissue was also assessed by quantifying deposition of MPO by infiltrated cells. Mesenteric tissue samples from WT and Anx-A1−/− mice that had been subjected to IR exhibited a significant increase in MPO: 0.26 ± 0.04 U/mg to 1.06 ± 0.08 U/mg and 0.49 ± 0.02 U/mg to 1.58 ± 0.23 U/mg for WT and Anx-A1−/−, respectively (Figure 1C). Mesenteric tissue samples from WT mice that had been treated with nedocromil (2mg/kg) had a significantly less MPO activity (0.56 ± 0.06 U/mg) but, importantly the inhibitory effect of nedocromil was lost (1.64 ± 0.19 U/mg) in the Anx-A1−/− mice. Of interest, Anx-A1−/− mouse tissue displayed a trend to higher MPO values than WT tissue samples (Figure 1C).
Effects of nedocromil on human neutrophil-endothelium interaction
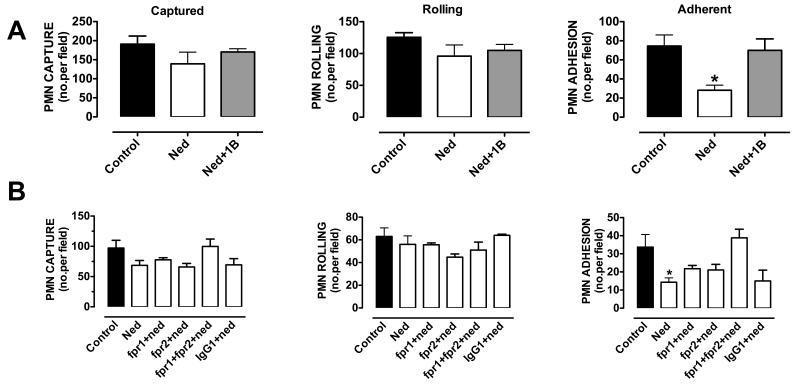
HUVECs, stimulated with TNF-α for 4 h (a time point that allows sufficient time for de novo synthesis of adhesion molecules 20) were used in this assay. Activation of HUVEC monolayers provoked a marked capture of PMNs and both rolling and adherent leukocytes could be visualized and counted off-line. Whilst nedocromil (10nM), pre-incubated for 5 min with the PMNs, had no effect on the number of leukocytes rolling or captured on the endothelium, the compound significantly decreased (>50%) the number of cells adherent on the endothelium (n=3, p<0.05; Figure 2A).
Figure 2. Cromones inhibit PMN adhesion to HUVEC.
Inhibitory effect of nedocromil on PMN interaction with HUVECs under flow is abrogated (A) by the use of a specific neutralizing anti-Anx-A1 antibody (20μg/ml) and (B) by the use of a combination of specific blocking anti-FPR1 and anti-FPR2 antibodies (5μg/ml). Data are presented as mean ± SEM of 3 independent experiments, *p<0.05 vs control group (see the Supplemental Materials file for details).
To ascertain the role of Anx-A1 in the mechanism of action of nedocromil, the effect of a specific neutralizing anti-Anx-A1 antibody was tested. PMNs were pre-incubated for 20 min with 10 μg/ml neutralizing Anx-A1, or irrelevant isotype-matched, monoclonal antibodies and then incubated for 5 min with nedocromil (10nM). The anti-adhesive effect of nedocromil was almost completely reversed (p<0.05) by the anti-Anx-A1 antibody (Figure 2A). The non-neutralizing control monoclonal antibody was without any effects in the system (data not shown).
Since Anx-A1 exerts its anti-inflammatory effects through the activation of formyl peptide receptors, we attested the effect of highly specific blocking anti-FPR122 and anti-FPR220 antibodies (5μg/ml, 1h prior treatment; Figure 2B). Interestingly, the anti-adhesive effect of nedocromil sodium was partially abrogated by either antibody but the combination of both could totally blocked nedocromil inhibitory adhesive effect of the PMNs on the monolayer. The non-neutralizing monoclonal antibody was without any effects in this system (data not shown).
Effect of nedocromil on FPR family receptors
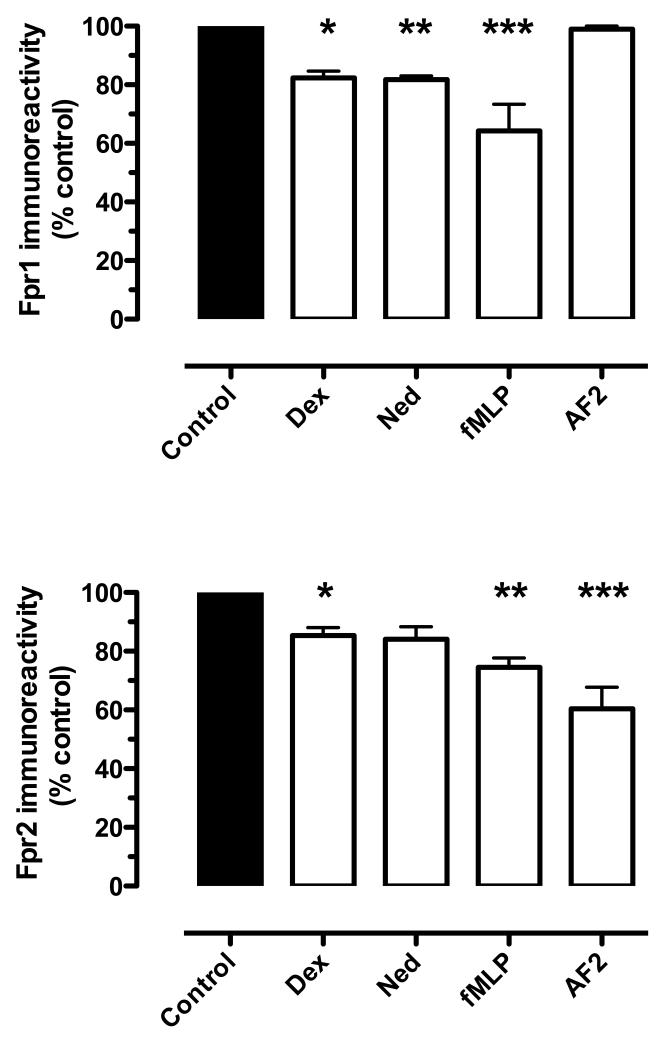
To provide additional support for our notion that Anx-A1 acts through FPR receptors in this flow chamber system, the expression of FPR-1 and FPR-2 receptors on PMN cell surface were analyzed by FACs after treatment with cromones. Figure 3 indicates that nedocromil rapidly (within 5 min) causes internalisation of both receptors (15-20%). Unrelated anti-inflammatory drugs were assessed as positive controls.
Figure 3. Assessment by FACS analysis of FPR-1 and FPR-2 receptor internalization in PMNs produced by cromones.
Human PMN were incubated with either nedocromil (10nM) or dexamethasone (2nM) for 5min at 37°C; after immediate transfer to 4°C, FPR1 and FPR2 cell surface expressions were determined with specific antibodies and flow cytometry analysis. Data are presented as mean ± SEM of 3 independent experiments, *p<0.05 vs control group (see the Supplemental Materials file for details).
Collectively, these in vivo and in vitro data support our hypothesis that cromones can exert their anti-PMN effects by releasing endogenous Anx-A1. Thus, the effect of these drugs on Anx-A1 disposition in human PMN was further investigated.
Effect of cromones on Anx-A1 phosphorylation and secretion by human PMNs
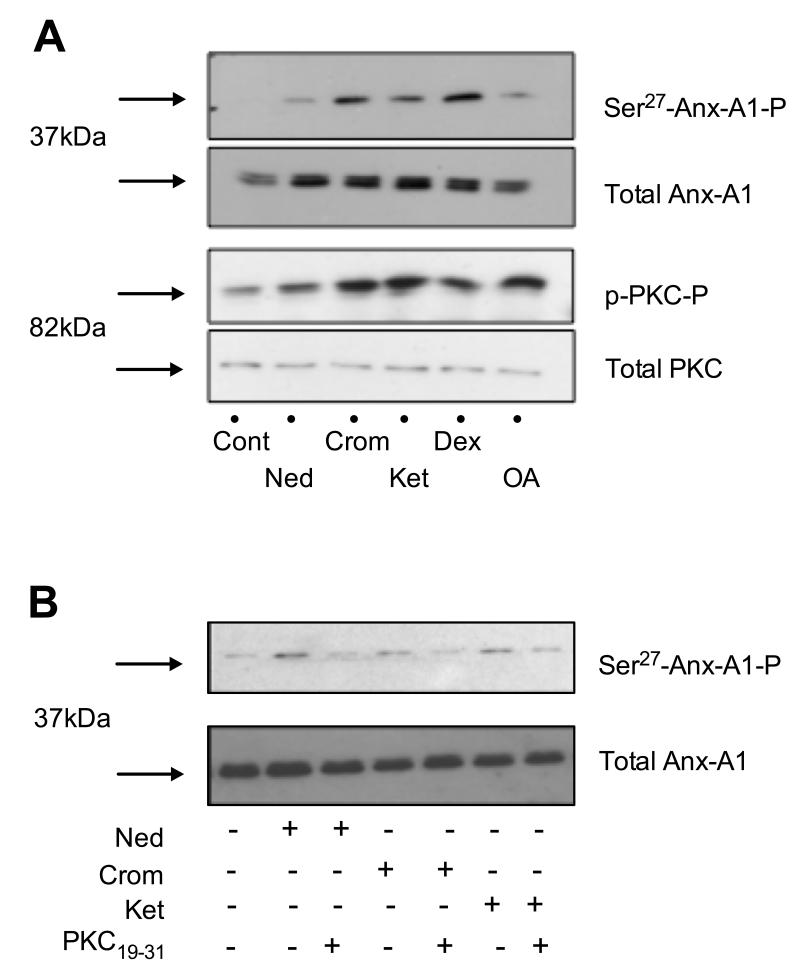
To address our next aim, the phosphorylation of PKC and Anx-A1 in cell lysates of PMNs pre-treated with different drugs was determined. Western blotting analysis reveals the rapid phosphorylation of protein kinase C (PKC) in human PMN cell lysates after 5 min treatment with either nedocromil (10nM), cromoglycate (10nM) or ketotifen (10nM) (Figure 4A). This is accompanied by an increase in phosphorylated Anx-A1 at Ser27, which we have shown to be essential for secretion of the protein 13, 16. No changes in total amount of Anx-A1 could be detected in the PMN extracts (Figure 4A). The same phosphorylation pattern of the protein was observed when PMNs were treated with either dexamethasone (2nM) or okadaic acid (10nM).
Figure 4. Crucial Anx-A1 phosphorylation by human PMN following treatment with cromones.
(A) Effect of the mast cell stabilisers on Anx-A1 and PKC phosphorylation. (B) Suppressive effect of PKC19-31 peptide on Anx-A1 phosphorylation (see the Supplemental Materials file for details).
The increments in phospho-Ser27 Anx-A1 would be indicative of protein mobilization and secretion, according to our model. We next therefore monitored cell-surface Anx-A1 in human PMN, using flow-cytometry analysis. Under basal conditions, very few PMN (<3%) displayed Anx-A1 on their plasma membrane (as detected with a highly specific monoclonal antibody; Figure 5A and B), while pre-treatment with ketotifen (10nM) or dexamethasone (2nM) significantly increased the proportion of positive cells (Figure 5B), with cromoglycate, nedocromil and okadaic acid being somewhat less active in this assay (Figure 5B).
Figure 5. Anx-A1 membrane expression and secretion by FACs and ELISA.
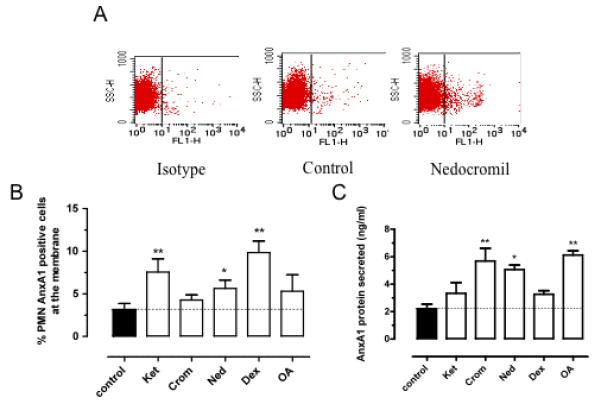
(A) Representative dot plot of membrane expression of the Anx-A1 positive cell population amongst resting (control) or treated isolated human PMN. (B) Represents the % of positive PMN expressing Anx-A1 on cell surface after cromone treatment. (C) ELISA assay measuring Anx-A1 protein in the supernatant of PMNs. Data are presented as mean ± SEM of 3 independent experiments, **p<0.01 or *p<0.05 vs control (see the Supplemental Materials file for details).
The final step of Anx-A1 mobilization in activated PMN is secretion in the medium. Thus, we next assessed, using a validated ELISA 23, whether we could detect release of Anx-A1 into the extracellular mileu following cromone treatment. Nedocromil, cromoglycate and okadaic acid were found to be more effective inducers of Anx-A1 release into the medium than either ketotifen or dexamethasone (Figure 5C). These observations could explain, in part, the apparent discrepancies observed between the drug effects in the static adhesion assay, presented in Figure 1S (see supplementary data).
Effect of cromones in an acute model of inflammation
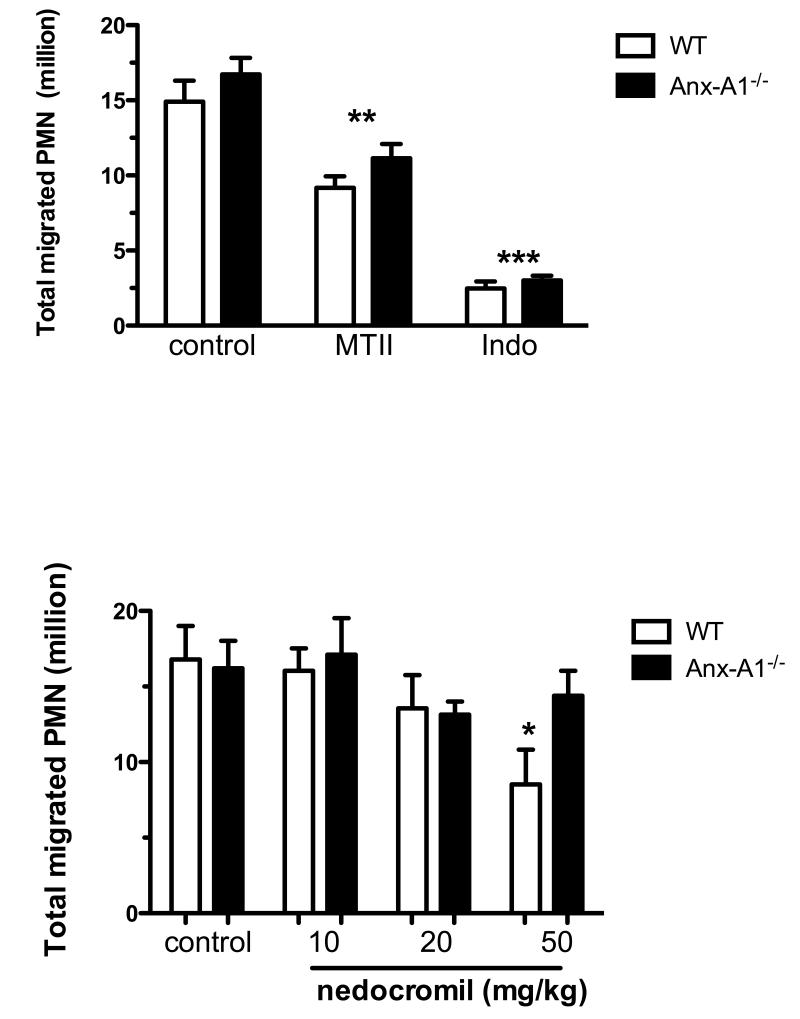
Finally, we tested whether the effects of cromones on PMN/endothelium interaction could be translated to attenuation of PMN infiltration in a model of acute peritonitis. The marked (>10 million cells) PMN accumulation promoted by zymosan was susceptible to inhibition by two unrelated anti-inflammatory drugs, the melanocortin analogue melanotan II and indomethacin (a cyclo-oxygenase inhibitor; Figure 6A). As neither of these drugs act through an Anx-A1-dependent pathway, they displayed similar inhibition of cell influx in both WT and Anx-A1−/− mice (MTII: 26% and 33%; indomethacin: 80% and 83%, respectively).
Figure 6. Cromones inhibit the intense PMN accumulation of the zymosan-induced peritonitis model.
(A) Effect of unrelated anti-inflammatory drugs on the number of PMN migrated. (B) Effect of increasing doses of nedocromil on PMN migration into the inflamed peritoneal cavity. Data are presented as mean ± SEM of n=6 mice per group,*p<0.05 vs. control group (see the Supplemental Materials file for details).
Nedocromil was tested next in this model; probably because of poor pharmacokinetics, the doses required to inhibit PMN migration in this model are higher than one would anticipate from the in vitro data, with doses below 10mg/kg being without effect (Figure 6B). Importantly though, as in the case of dexamethasone24, nedocromil acts in a genotype dependent manner with doses of ranging from 10 to 50 mg/kg, producing a graded inhibition between 5% and 49% in WT mice, whereas no consistent effect was observed in Anx-A1−/− mice. Cromoglycate (data not shown) produced a comparable inhibitory profile although does of 50-200 mg/kg were necessary to inhibit PMN migration in this model.
Discussion
There is now compelling evidence supporting the notion that mast cells regulate leukocyte influx in disease. Indeed efficacy of ‘mast cell stabilising drugs’ as well as the phenotype of mast cell-deficient mice support this concept in lung inflammation 25, gut inflammation 26, blood-brain barrier permeability 27, plasma extravasation 28 and intestinal inflammation 29. Despite this, others studies show how leukocyte influx can also occur in a mast cell-independent manner 9 and that mast cell stabilisers may act on inflammatory cells other than the mast cell, although a mechanism that could explain such anti-inflammatory effects has never been offered. Our study indicates Anx-A1 as a major molecular target for these effects of the cromones.
We have recently reported that Anx-A1, a GC-regulated anti-inflammatory protein, is secreted by anti-allergic drugs when cells have first been ‘primed’ with low GC doses 16. We proposed that this occurred secondary to the PKC activation (triggered by GCs) necessary to initiate Anx-A1 phosphorylation and that the cromones potentiated this effect by inhibiting a phosphatase (probably PP2A), which limits PKC activation and activity at the plasma membrane. For this reason, the effect on Anx-A1 phosphorylation and secretion by PMN, of a well-characterised PP2A inhibitor such as okadaic acid was also assessed. Indeed, in line with our previous study using the U937 promonocytic cell line, a correlation between the effects of cromones and okadaic acid on Anx-A1 distribution in the PMN was observed. Thus, the model whereby two processes are required for Anx-A1 secretion, phosphorylation as a pre-requisite for externalization is not limited to the mast cell and monocyte, but also valid for human PMN, hence likely to be a paradigm in the biology of this protein.
We have not addressed the actual mechanism of Anx-A1 secretion from the PMN in this study. These cells contain large amounts of the protein (up to 4% of total intracellular proteins 30); the intracellular distribution is complex 31 with a high proportion (~50-60%) of Anx-A1 being located in gelatinase granules 32, which are externalised along with their contents following adherence to endothelial cells, after which it is eventually cleaved by PR3 and other proteases 33. Depending upon the mechanism of PMN activation Anx-A1 may be also released attached to microparticles 34. It is not clear whether it is the gelatinase pool or the cytosolic (not in granules/vesicles) Anx-A1 pool that is the target for PKC in the human PMN, but in our experiments (Figure 2S, see supplementary data) using markers to identify the different granule subsets in PMN, it seemed the latter pool of Anx-A1 to be mobilised following PKC-mediated phosphorylation.
Since Anx-A1 brings about anti-inflammatory effects through potent inhibition of leukocyte migration process, we hypothesised that the functional release of the protein by cromones could not only explain their inhibition of eicosanoid release 16 but also underlie their inhibitory effects on cell influx in acute and chronic inflammation. Mast cell activation produces a sustained leukocyte influx through release of mediators such as histamine. This can occur in an IgE-dependent, as well as independent, manner. One example of the latter is IR injury in which mast cells have shown to be crucial regulators of leukocyte influx (for review see 7). Therefore, in this present study, we have used the mouse mesenteric microcirculation inflamed with an IR procedure and monitored by intravital microscopy the process of leukocyte recruitment onto the post-capillary venule endothelium. In line with previous studies conducted in the rat using cromoglycate or ketotifen 35, nedocromil induced a decrease in leukocyte adhesion and emigration. This was absent in our Anx-A1−/− mouse colony providing a strong evidence for an important role for this effector of endogenous anti-inflammatory regulation. Conscious that Anx-A1−/− mice exhibit a tendency to exacerbated inflammatory responses 36, 37, we have also tested a supramaximal dose of nedocromil to ensure that its effect was not masked by the more pronounced inflammatory response seen in these knockout mice. It should be noted that no difference between WT and Anx-A1−/− mice could be demonstrated in this experimental settings, indicating that the endogenous protein is not operative within the time frame used in these experiments. In fact, we know that Anx-A1−/− mice display augmented cellular responses in the mesenteries post-IR at later time-points (>6h; unpublished data).
Moreover, using nedocromil as a prototype, we could assess the anti-inflammatory properties of cromones, in a model where an intense PMN accumulation occurs. In this experiment, a significant inhibition and, importantly, a marked reliance on endogenous Anx-A1, was observed. The effect of nedocromil was lost in Anx-A1−/− mice, in line with that reported for dexamethasone: historical data from our laboratory shows that given at 1mg/kg this steroid provokes an acute Anx-A1-dependent inhibition reaching ~80% inhibition in WT, but only 9% inhibition in Anx-A1−/− mice. As expected, the assay can discriminate the contribution of endogenous Anx-A1 since a melanocortin receptor agonist and indomethacin provoked similar degrees of inhibition of PMN accumulation both in WT and Anx-A1−/− mice.
These actions of the cromones can be compared to those obtained with dexamethasone, used as a positive control since it also relies, in part, on Anx-A1 mobilization for its anti-leukocyte effects 37, 38. Additional evidence that this mechanism is relevant to the anti-inflammatory properties of cromones comes from our experiments using a specific neutralizing anti-Anx-A1 antibody in a flow chamber model. Human PMN-endothelial monolayer interactions were monitored here under defined flow conditions using a human system in vitro that physiologically resembles the events occurring in the microcirculation during inflammation. HUVECs were stimulated with TNF-α for 4 h to promote human PMN adherence 39. While nedocromil was without any effect on the capture and rolling of the PMN onto the HUVECs, a significant decrease in the number of PMN adherent to the monolayers was observed: this inhibition was abolished when the PMN were pre-treated with a specific anti-Anx-A1 neutralising antibody. This suggested that the anti-migratory effect of nedocromil on neutrophil recruitment is an Anx-A1 mediated event.
This study mainly addressed the mechanism by which the cromones exert their inhibitory effect on leukocyte recruitment (and used protocols to focus on the PMN as main target), and we have not investigated their effect on other molecules implicated in the trafficking cascade. It is described in the literature that cromoglycate can down-regulate vascular CD54 (or ICAM-1) expression in a rat lung ischemia-reperfusion model 6, on endothelial cells interacting with PBMC of asthmatic patients 40 or in biopsies of their bronchial mucosa 41. These effects could contribute to the cromone inhibitory effects seen on leukocyte recruitment in the IR model since increased levels of vascular ICAM-1 are to be. However, in the flow chamber assay, TNF-α-stimulation of HUVECs cells for 4 h increases E-selectin (CD62E) and little CD54 (which expression peaks at 16h of TNF-α stimulation; not shown). Moreover, it is unlikely to be working via any targets on the endothelium, since the drugs were added to the neutrophils prior to the flow assay.
In conclusion, we have established that cromones can act directly upon human and mouse PMN, to regulate their recruitment on activated endothelial monolayers or post-capillary venule endothelium, respectively. We also provide the first evidence, in vivo as well as in vitro, that these effects require the endogenous anti-inflammatory protein Anx-A1, showing – in human PMN – externalization and release of this protein. These findings could explain some observations originally made by Altounyan (reviewed in 42) who speculated that cromones might be endowed with clinical properties similar to corticosteroids. In addition to elucidating a mechanism through which cromones exert anti-inflammatory properties, our study also suggests further uses for these drugs in the context of pathologies driven by excessive PMN activation examples being vasculitis and gouty arthritis.
Supplementary Material
Acknowledgment
We acknowledge the support of the Wellcome Trust (085903/Z/08). We thank Guglielmo Rosignoli for his assistance. Human recombinant Anx-A1 protein was kindly provided by Dr Fulvio D’Acquisto.
Footnotes
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cox JS. Disodium cromoglycate (FPL 670) (‘Intal’): a specific inhibitor of reaginic antibody-antigen mechanisms. Nature. 1967;216:1328–1329. doi: 10.1038/2161328a0. [DOI] [PubMed] [Google Scholar]
- 2.Mattoli S, Mezzetti M, Fasoli A, Patalano F, Allegra L. Nedocromil sodium prevents the release of 15-hydroxyeicosatetraenoic acid from human bronchial epithelial cells exposed to toluene diisocyanate in vitro. Int Arch Allergy Appl Immunol. 1990;92:16–22. doi: 10.1159/000235218. [DOI] [PubMed] [Google Scholar]
- 3.Devalia JL, Rusznak C, Abdelaziz MM, Davies RJ. Nedocromil sodium and airway inflammation in vivo and in vitro. J Allergy Clin Immunol. 1996;98:S51–57. discussion S64-56. [PubMed] [Google Scholar]
- 4.Kay AB, Walsh GM, Moqbel R, MacDonald AJ, Nagakura T, Carroll MP, Richerson HB. Disodium cromoglycate inhibits activation of human inflammatory cells in vitro. J Allergy Clin Immunol. 1987;80:1–8. doi: 10.1016/s0091-6749(87)80183-5. [DOI] [PubMed] [Google Scholar]
- 5.Kanwar S, Kubes P. Ischemia/reperfusion-induced granulocyte influx is a multistep process mediated by mast cells. Microcirculation. 1994;1:175–182. doi: 10.3109/10739689409148272. [DOI] [PubMed] [Google Scholar]
- 6.Vural KM, Liao H, Oz MC, Pinsky DJ. Effects of mast cell membrane stabilizing agents in a rat lung ischemia-reperfusion model. Ann Thorac Surg. 2000;69:228–232. doi: 10.1016/s0003-4975(99)01052-8. [DOI] [PubMed] [Google Scholar]
- 7.Kubes P, Granger DN. Leukocyte-endothelial cell interactions evoked by mast cells. Cardiovasc Res. 1996;32:699–708. [PubMed] [Google Scholar]
- 8.Kelly M, Hwang JM, Kubes P. Modulating leukocyte recruitment in inflammation. J Allergy Clin Immunol. 2007;120:3–10. doi: 10.1016/j.jaci.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Furuta GT, Wang ZS, Wershil BK. Gastric inflammation during systemic anaphylaxis: neutrophil recruitment in stomach wall of mice does not require mast cell participation. Dig Dis Sci. 1998;43:2021–2027. doi: 10.1023/a:1018851012940. [DOI] [PubMed] [Google Scholar]
- 10.Croxtall JD, Flower RJ. Antisense oligonucleotides to human lipocortin-1 inhibit glucocorticoid-induced inhibition of A549 cell growth and eicosanoid release. Biochem Pharmacol. 1994;48:1729–1734. doi: 10.1016/0006-2952(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 11.Taylor AD, Loxley HD, Flower RJ, Buckingham JC. The role of lipocortin 1 (LC1) in the steroid feedback control of hypothalamo-pituitary-adrenocortical function. In vivo studies. Ann N Y Acad Sci. 1994;746:446–448. doi: 10.1111/j.1749-6632.1994.tb39281.x. [DOI] [PubMed] [Google Scholar]
- 12.Perretti M, Ahluwalia A, Harris JG, Harris HJ, Wheller SK, Flower RJ. Acute inflammatory response in the mouse: exacerbation by immunoneutralization of lipocortin 1. Br J Pharmacol. 1996;117:1145–1154. doi: 10.1111/j.1476-5381.1996.tb16709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solito E, Mulla A, Morris JF, Christian HC, Flower RJ, Buckingham JC. Dexamethasone induces rapid serine-phosphorylation and membrane translocation of annexin 1 in a human folliculostellate cell line via a novel nongenomic mechanism involving the glucocorticoid receptor, protein kinase C, phosphatidylinositol 3-kinase, and mitogen-activated protein kinase. Endocrinology. 2003;144:1164–1174. doi: 10.1210/en.2002-220592. [DOI] [PubMed] [Google Scholar]
- 14.Perretti M, D’Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- 15.Perretti M. The annexin 1 receptor(s): is the plot unravelling? Trends Pharmacol Sci. 2003;24:574–579. doi: 10.1016/j.tips.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Yazid S, Solito E, Christian H, McArthur S, Goulding N, Flower R. Cromoglycate drugs suppress eicosanoid generation in U937 cells by promoting the release of Anx-A1. Biochem Pharmacol. 2009;77:1814–1826. doi: 10.1016/j.bcp.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perretti M, Flower RJ. Measurement of lipocortin 1 levels in murine peripheral blood leukocytes by flow cytometry: modulation by glucocorticoids and inflammation. Br J Pharmacol. 1996;118:605–610. doi: 10.1111/j.1476-5381.1996.tb15444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perretti M, Croxtall JD, Wheller SK, Goulding NJ, Hannon R, Flower RJ. Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nat Med. 1996;2:1259–1262. doi: 10.1038/nm1196-1259. [DOI] [PubMed] [Google Scholar]
- 19.Getting SJ, Flower RJ, Perretti M. Inhibition of neutrophil and monocyte recruitment by endogenous and exogenous lipocortin 1. Br J Pharmacol. 1997;120:1075–1082. doi: 10.1038/sj.bjp.0701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayhoe RP, Kamal AM, Solito E, Flower RJ, Cooper D, Perretti M. Annexin 1 and its bioactive peptide inhibit neutrophil-endothelium interactions under flow: indication of distinct receptor involvement. Blood. 2006;107:2123–2130. doi: 10.1182/blood-2005-08-3099. [DOI] [PubMed] [Google Scholar]
- 21.Walther A, Riehemann K, Gerke V. A novel ligand of the formyl peptide receptor: annexin I regulates neutrophil extravasation by interacting with the FPR. Mol Cell. 2000;5:831–840. doi: 10.1016/s1097-2765(00)80323-8. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goulding NJ, Godolphin JL, Sharland PR, Peers SH, Sampson M, Maddison PJ, Flower RJ. Anti-inflammatory lipocortin 1 production by peripheral blood leucocytes in response to hydrocortisone. Lancet. 1990;335:1416–1418. doi: 10.1016/0140-6736(90)91445-g. [DOI] [PubMed] [Google Scholar]
- 24.Hannon R, Croxtall JD, Getting SJ, Roviezzo F, Yona S, Paul-Clark MJ, Gavins FN, Perretti M, Morris JF, Buckingham JC, Flower RJ. Aberrant inflammation and resistance to glucocorticoids in annexin 1−/− mouse. FASEB J. 2003;17:253–255. doi: 10.1096/fj.02-0239fje. [DOI] [PubMed] [Google Scholar]
- 25.Bruijnzeel PL, Warringa RA, Kok PT, Hamelink ML, Kreukniet J. Inhibitory effects of nedocromil sodium on the in vitro induced migration and leukotriene formation of human granulocytes. Drugs. 1989;37(Suppl 1):9–18. doi: 10.2165/00003495-198900371-00004. discussion 69-77. [DOI] [PubMed] [Google Scholar]
- 26.Kwasniewski FH, Landgraf RG, Jancar S. Small bowel injury associated to allergy is triggered by platelet-activating factor, mast cells, neutrophils and protected by nitric oxide. Int Immunopharmacol. 2008;8:371–378. doi: 10.1016/j.intimp.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Esposito P, Chandler N, Kandere K, Basu S, Jacobson S, Connolly R, Tutor D, Theoharides TC. Corticotropin-releasing hormone and brain mast cells regulate blood-brain-barrier permeability induced by acute stress. J Pharmacol Exp Ther. 2002;303:1061–1066. doi: 10.1124/jpet.102.038497. [DOI] [PubMed] [Google Scholar]
- 28.Javdan P, Figini M, Emanueli C, Geppetti P. Nedocromil sodium reduces allergen-induced plasma extravasation in the guinea pig nasal mucosa by inhibition of tachykinin release. Allergy. 1995;50:825–829. doi: 10.1111/j.1398-9995.1995.tb05056.x. [DOI] [PubMed] [Google Scholar]
- 29.Kaszaki J, Czobel M, Szalay L, Nagy S, Boros M. Endothelin-1 induces organ-specific histamine liberation and neutrophil granulocyte accumulation in the rat. Inflamm Res. 2008;57:396–402. doi: 10.1007/s00011-007-7224-x. [DOI] [PubMed] [Google Scholar]
- 30.Rosales JL, Ernst JD. Calcium-dependent neutrophil secretion: characterization and regulation by annexins. J Immunol. 1997;159:6195–6202. [PubMed] [Google Scholar]
- 31.Movitz C, Dahlgren C. Quantification of annexin I in subcellular fractions of human neutrophils reveals an exclusive cytosolic localisation. Cell Biol Int. 2001;25:963–969. doi: 10.1006/cbir.2001.0760. [DOI] [PubMed] [Google Scholar]
- 32.Perretti M, Christian H, Wheller SK, Aiello I, Mugridge KG, Morris JF, Flower RJ, Goulding NJ. Annexin I is stored within gelatinase granules of human neutrophil and mobilized on the cell surface upon adhesion but not phagocytosis. Cell Biol Int. 2000;24:163–174. doi: 10.1006/cbir.1999.0468. [DOI] [PubMed] [Google Scholar]
- 33.Vong L, D’Acquisto F, Pederzoli-Ribeil M, Lavagno L, Flower RJ, Witko-Sarsat V, Perretti M. Annexin 1 cleavage in activated neutrophils: a pivotal role for proteinase 3. J Biol Chem. 2007;282:29998–30004. doi: 10.1074/jbc.M702876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalli J, Norling LV, Renshaw D, Cooper D, Leung KY, Perretti M. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood. 2008;112:2512–2519. doi: 10.1182/blood-2008-02-140533. [DOI] [PubMed] [Google Scholar]
- 35.Gaboury JP, Johnston B, Niu XF, Kubes P. Mechanisms underlying acute mast cell-induced leukocyte rolling and adhesion in vivo. J Immunol. 1995;154:804–813. [PubMed] [Google Scholar]
- 36.Chatterjee BE, Yona S, Rosignoli G, Young RE, Nourshargh S, Flower RJ, Perretti M. Annexin 1-deficient neutrophils exhibit enhanced transmigration in vivo and increased responsiveness in vitro. J Leukoc Biol. 2005;78:639–646. doi: 10.1189/jlb.0405206. [DOI] [PubMed] [Google Scholar]
- 37.Damazo AS, Yona S, Flower RJ, Perretti M, Oliani SM. Spatial and temporal profiles for anti-inflammatory gene expression in leukocytes during a resolving model of peritonitis. J Immunol. 2006;176:4410–4418. doi: 10.4049/jimmunol.176.7.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allcock GH, Allegra M, Flower RJ, Perretti M. Neutrophil accumulation induced by bacterial lipopolysaccharide: effects of dexamethasone and annexin 1. Clin Exp Immunol. 2001;123:62–67. doi: 10.1046/j.1365-2249.2001.01370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hickey MJ, Reinhardt PH, Ostrovsky L, Jones WM, Jutila MA, Payne D, Elliott J, Kubes P. Tumor necrosis factor-alpha induces leukocyte recruitment by different mechanisms in vivo and in vitro. J Immunol. 1997;158:3391–3400. [PubMed] [Google Scholar]
- 40.Horvathova M, Podivinsky F, Gazdik F, Jahnova E. Effect of disodium cromoglycate treatment on peripheral blood mononuclear cell adhesion to cultured endothelium in allergic asthmatics. Physiol Res. 1998;47:445–451. [PubMed] [Google Scholar]
- 41.Hoshino M, Nakamura Y. The effect of inhaled sodium cromoglycate on cellular infiltration into the bronchial mucosa and the expression of adhesion molecules in asthmatics. Eur Respir J. 1997;10:858–865. [PubMed] [Google Scholar]
- 42.Edwards AM, Howell JB. The chromones: history, chemistry and clinical development. A tribute to the work of Dr R. E. C. Altounyan. Clin Exp Allergy. 2000;30:756–774. doi: 10.1046/j.1365-2222.2000.00879.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.