Abstract
Study Objectives:
This post hoc analysis evaluated the time to response that can be expected with sodium oxybate (SXB) for treatment of excessive daytime sleepiness (EDS) and cataplexy in patients with narcolepsy.
Methods:
Data were from a 4-week, double-blind, randomized, placebo-controlled trial (GHB-2; N = 136) of oral SXB 3 g, 6 g, and 9 g nightly, and its 12-month open-label extension (GHB-3). Two response definitions were utilized: ≥ 20% improvement in Epworth Sleepiness Scale (ESS) score (EDS responders), and ≥ 50% reduction in weekly cataplexy attacks (cataplexy responders). These thresholds were previously determined to be clinically relevant based on analysis of the relationship of Clinical Global Impression of Change with ESS and number of cataplexy attacks. Kaplan-Meier curves and median times to first response, based on above criteria, and to maximum response were estimated.
Results:
Among 86 patients randomized to SXB in GHB-2 and continued into GHB-3, 77.6% and 90.7% were EDS and cataplexy responders, respectively. The median (95% CI) times to first response were 37 (31–50) days for EDS and 25 (17–29) days for cataplexy, and median times to maximum response were 106 (85–164) days for EDS and 213 (94–279) days for cataplexy. GHB-3 results among 31 patients initially randomized to placebo were consistent with those treated with SXB throughout, but with longer times to maximum response.
Conclusions:
Response onset, assessed as clinically meaningful improvements in EDS and cataplexy, was observed in most patients within 2 months; a longer period is needed to achieve maximum response. Clinicians should recognize that time to initial and maximum response may take weeks to months.
Citation:
Bogan RK, Roth T, Schwartz J, Miloslavsky M. Time to response with sodium oxybate for the treatment of excessive daytime sleepiness and cataplexy in patients with narcolepsy. J Clin Sleep Med 2015;11(4):427–432.
Keywords: narcolepsy, excessive daytime sleepiness, cataplexy, sodium oxybate, time to response
Despite recent advances in the understanding of the chronic neurological disorder known as narcolepsy, it remains a condition that often has a long delay between symptom onset, symptom recognition and, most importantly, clinical diagnosis and treatment.1 This delay likely results, at least in part, from the reported low awareness among physicians and the general population of narcolepsy symptoms and their associated impact.1,2
Accumulating evidence supports an autoimmune response for the loss of hypocretin-producing neurons in the brain that is the underlying pathophysiology of narcolepsy.3 Symptomatically, the disease is characterized by the pentad of excessive daytime sleepiness (EDS), cataplexy, hypnagogic or hypnopompic hallucinations, sleep paralysis, and disturbed nocturnal sleep. Since narcolepsy has no known cure, patient management is driven by the symptomatic response.
Symptom presentation among narcolepsy patients is variable, since not all symptoms may be present, and when present, may differ in severity and may be described in different ways. Among the pentad, EDS and cataplexy are the two symptoms that are most frequently recognized.2 By definition, EDS is present in all narcolepsy patients, and although the presence of cataplexy provides confirmation of a narcolepsy diagnosis, it is only present in up to 70% of patients but is considered the most pathognomonic of all the symptoms.4 Thus, these two symptoms represent the main symptoms patients want treated and are the targets of currently approved narcolepsy therapies.5
BRIEF SUMMARY
Current Knowledge/Study Rationale: Sodium oxybate is approved for the treatment of excessive daytime sleepiness and cataplexy in patients with narcolepsy. Since it is important to allow sufficient time to observe a clinically relevant response before changing a treatment strategy, this analysis determined the time to response with sodium oxybate for improvement in excessive daytime sleepiness and cataplexy.
Study Impact: Clinically meaningful improvements in excessive daytime sleepiness and cataplexy were seen within 2 months in most patients, and maximum response required a longer period. Clinicians should recognize that the time course to initial and maximum response to sodium oxybate may take weeks to months.
Sodium oxybate (SXB) is the only medication approved for the treatment of both EDS and cataplexy in narcolepsy,6 and clinical trials have demonstrated that SXB results in significant improvement for both of these symptoms relative to placebo.7–10 Patient management with SXB relies on titration to efficacy and tolerability, and as with any of the recommended narcolepsy therapies in clinical practice, there may be variability in the observed response both in terms of the degree of clinical improvement as well as the time needed to achieve a clinical response. Knowing the time frame in which, on average, a response can be expected may be helpful to set expectations for patients as well as clinicians. Therefore, the objective of this analysis was to determine the time to response with SXB for the treatment of EDS and cataplexy in patients with narcolepsy. These analyses were based on data from previous SXB clinical studies.11,12
METHODS
This is a post hoc analysis using data from a previously published randomized, placebo-controlled, double-blind, parallel-group trial (GHB-2; N = 136)11 and its open-label extension (GHB-3; N = 118).12 In the open-label extension, patients who enrolled were treated for at least an additional 12 months with active drug regardless of their randomization group in the parent study. Both studies were performed in accordance with the Declaration of Helsinki, and all patients provided written informed consent prior to participation (ClinicalTrials.gov identifier NCT00049803).
In GHB-2, fixed doses of oral SXB 3 g, 6 g, and 9 g nightly were evaluated versus placebo over a 4-week treatment duration. For the open-label extension, patients were initiated on SXB 6 g nightly after a 3- to 5-day washout from GHB-2, with subsequent titration of SXB, based on efficacy and tolerability, up to 7.5 g and 9 g nightly or down to 4.5 g and 3 g nightly. In both the double-blind and open-label periods, concomitant treatment with stable doses of stimulants was permitted.
Clinical response information including the number of cataplexy attacks were recorded by patients in a daily diary. Daytime sleepiness was evaluated using the Epworth Sleepiness Scale (ESS), a patient-reported measure of EDS,13 at study visits. Results in the current analysis are based on evaluations of the patients and their diaries at discrete time points during the study. These time points were at the end of GHB-2, every 2 weeks during the first month of GHB-3 followed by every 2 months up to month 18, and then every 3 months until month 24.
The percent of patients who were responders and the time to therapeutic response was determined using two responder definitions, one for EDS based on observed ESS scores, and one for cataplexy based on cataplexy attacks derived from the patient diaries for the week prior to the evaluation time point. An EDS responder was defined as a patient who reported ≥ 20% reduction from baseline in score on the ESS, and a cataplexy responder was a patient with ≥ 50% reduction from baseline in weekly cataplexy attacks. These definitions were determined to be clinically relevant based on an analysis of data pooled from two randomized, placebo-controlled, double-blind, multicenter 4-week and 8-week trials of SXB for the treatment of narcolepsy with cataplexy.14 These data were used to examine the relationship of Clinical Global Impression of Change responses of “very much improved” or “much improved” with both the ESS and the number of cataplexy attacks. In that analysis, receiver operating characteristic curve analysis identified these responder cutoff values as providing the optimal balance between sensitivity (true responders) and specificity (false positives).14
Using the responder definitions, Kaplan-Meier survival curves were generated for first response (i.e., ≥ 20% improvement in ESS or ≥ 50% reduction in weekly cataplexy attacks) and the maximum response, which was the greatest improvement achieved by each patient for each outcome. Patients were censored if the event of interest did not occur, whether through discontinuation or lack of response. From the Kaplan-Meier analysis, median times to first response and maximum response were estimated along with their 95% confidence intervals (95% CI). These analyses encompass the GHB-2 and GHB-3 time periods for patients randomized to SXB in GHB-2, and the GHB-3 time period for patients randomized to placebo in GHB-2 and who subsequently initiated SXB in GHB-3.
RESULTS
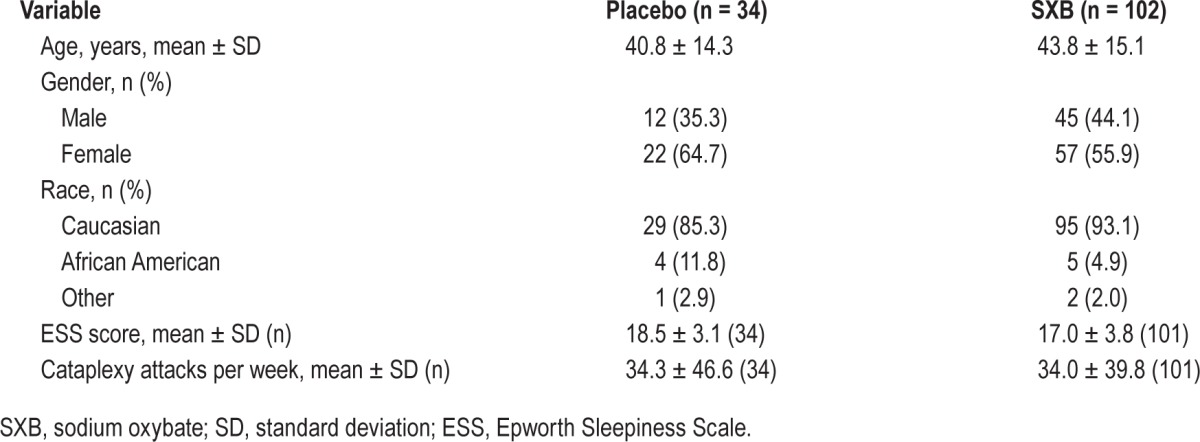
A total of 136 patients enrolled in GHB-2; patients were primarily female (60.3%), Caucasian (89.2%), with a mean ± standard deviation age of 43.1 ± 15.0 years, and their demographic and clinical characteristics were balanced between treatment groups (Table 1). Of these patients, 120 completed GHB-2 (10 discontinuations were due to adverse events), and 118 patients enrolled in the GHB-3 open-label extension and 81 completed the 12-month extension; 11 of the 37 discontinuations were due to adverse events as previously described.12 For the current analysis, data were available from 117 patients, of whom 31 were originally randomized to placebo in GHB-2 and 86 were randomized to SXB. Overall, 74.0% of patients met the ESS response criteria, and 91.5% met the response criteria for cataplexy.
Table 1.
Baseline demographic and clinical characteristics of the patients in GHB-2.
Based on the ESS response criterion, 77.6% of the patients who were randomized to treatment with SXB in GHB-2 and continued treatment with SXB in GHB-3 were considered ESS responders, and 90.7% were cataplexy responders (Figure 1). Among the patients initially randomized to placebo in GHB-2 and who were subsequently initiated on SXB in GHB-3, 64.5% met the criterion for ESS responders and 93.5% for cataplexy responders (Figure 1).
Figure 1. Proportion of patients achieving responder status.
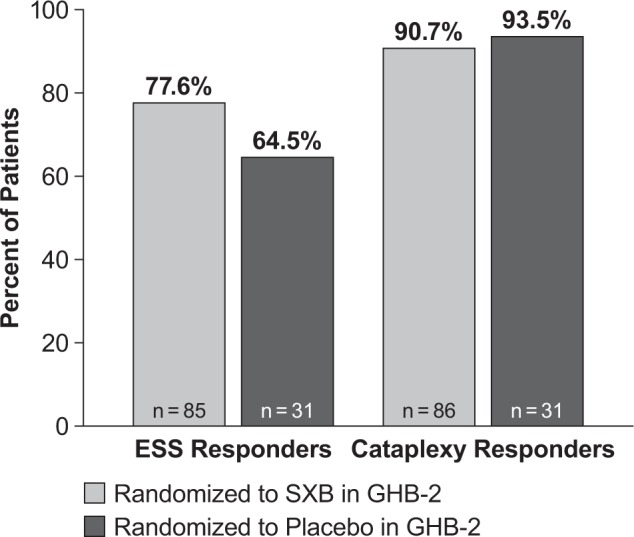
Data for patients treated with sodium oxybate in Study GHB-2 reflect both the GHB-2 and GHB-3 study periods; data for patients treated with placebo in GHB-2 reflect only open-label treatment with SXB in GHB-3. EDS responders were defined as ≥ 20% reduction in score on the ESS, and cataplexy responders were defined as a patient who achieved ≥ 50% reduction in weekly cataplexy attacks. EDS, excessive daytime sleepiness; ESS, Epworth Sleepiness Scale, SXB, sodium oxybate.
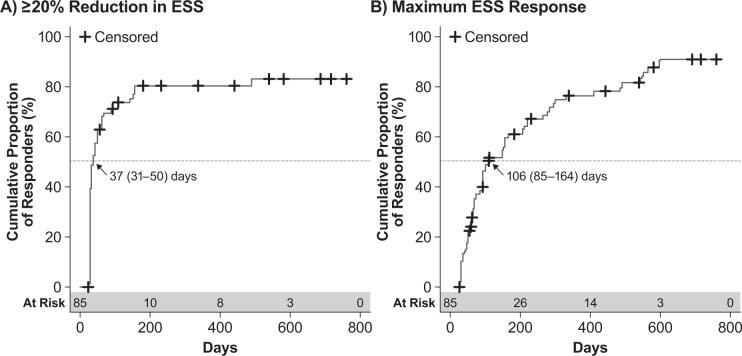
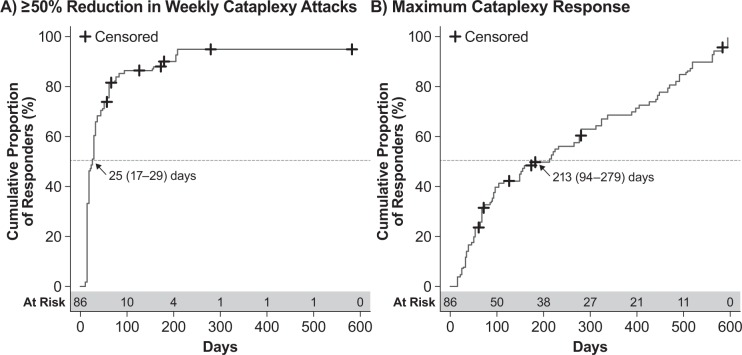
As per the Kaplan-Meier analysis, patients treated with SXB in both GHB-2 and GHB-3 achieved first ESS response with a median (95% CI) of 37 (31–50) days (Figure 2A) and achieved maximum ESS response with a median (95% CI) of 106 (85–164) days (Figure 2B). Among these same patients, the estimated median (95% CI) times were 25 (17–29) days (Figure 3A) to first cataplexy response and 213 (94–279) days to achieve maximum cataplexy response (Figure 3B).
Figure 2. Kaplan-Meier analysis of ESS response in patients treated with sodium oxybate across GHB-2 and GHB-3.
(A) Time to first response, defined as ≥ 20% reduction in ESS score. (B) Time to maximum response, defined as the greatest reduction in ESS score achieved by the patient. Values shown are the median time to response and the 95% confidence interval; censored patients are those for whom the event of interest did not occur through either discontinuation or lack of response. ESS, Epworth Sleepiness Scale.
Figure 3. Kaplan-Meier analysis of cataplexy response in patients treated with sodium oxybate across GHB-2 and GHB-3.
(A) Time to first response, defined as ≥ 50% reduction in weekly cataplexy attacks. (B) Time to maximum response, defined as the greatest reduction in weekly cataplexy attacks achieved by the patient. Values shown are the median time to response and the 95% confidence interval; censored patients are those for whom the event of interest did not occur through either discontinuation or lack of response.
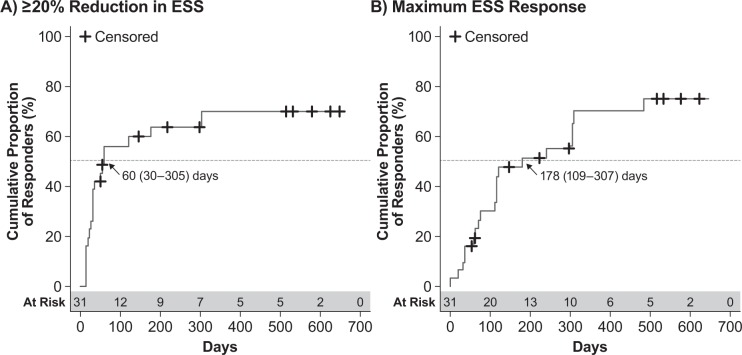
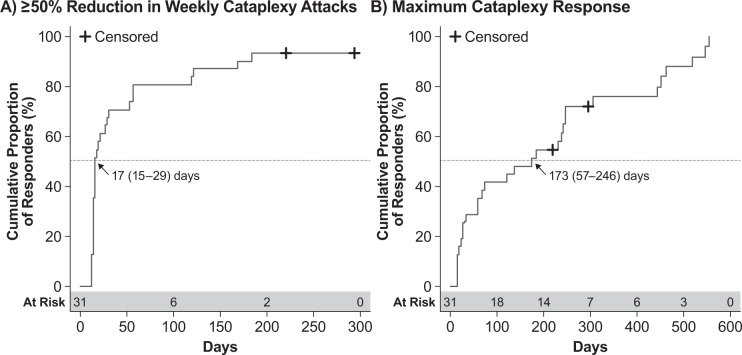
Patients who were randomized to placebo in GHB-2 and received SXB only in the open-label extension achieved first ESS response with a median (95% CI) time of 60 (30–305) days (Figure 4A). Maximum ESS response in these patients was achieved with a median (95% CI) of 178 (109–307) days (Figure 4B). Similarly, the initial 50% reduction in weekly cataplexy attacks was achieved with a median (95% CI) of 17 (15–29) days (Figure 5A), and maximum response was achieved with a median (95% CI) of 173 (57–246) days (Figure 5B). These times to response among the patients initially randomized to placebo in GHB-2 were generally consistent with those treated with SXB throughout GHB-2 and GHB-3.
Figure 4. Kaplan-Meier analysis of ESS response in GHB-3 among patients treated with placebo in GHB-2.
(A) Time to first response, defined as ≥ 20% reduction in ESS score. (B) Time to maximum response, defined as the greatest reduction in ESS score achieved by the patient. Values shown are the median time to response and the 95% confidence interval; censored patients are those for whom the event of interest did not occur through either discontinuation or lack of response. ESS, Epworth Sleepiness Scale.
Figure 5. Kaplan-Meier analysis of cataplexy response in GHB-3 among patients treated with placebo in GHB-2.
(A) Time to first response, defined as ≥ 50% reduction in weekly cataplexy attacks. (B) Time to maximum response, defined as the greatest reduction in weekly cataplexy attacks achieved by the patient. Values shown are the median time to response and the 95% confidence interval; censored patients are those for whom the event of interest did not occur through either discontinuation or lack of response.
DISCUSSION
This study extends the available information on treatment benefits of SXB by providing an analysis for extrapolating clinical trial results to a potential practical application in the clinical setting that may help enhance narcolepsy treatment decisions. It was previously reported in the double-blind phase of the 4-week clinical trial that reduction in the frequency of cataplexy attacks was observed within the first 2 weeks after initiating treatment with SXB.11 However, those results were for a numerical improvement in the degree of symptoms at specific time points rather than the time to a clinically meaningful benefit, since what constitutes such a benefit had not been previously defined. Data from the current analysis suggest the time frame for which patients treated with SXB may be expected to initially achieve clinically meaningful improvements, both for EDS and cataplexy, as well as the time frame in which patients are likely to achieve maximum treatment benefits for these two outcomes.
The results show that onset of a clinically meaningful therapeutic response, assessed as ≥ 20% reduction in ESS score or ≥ 50% reduction in weekly cataplexy attacks, was observed in high proportions of patients, with the majority of patients within 2 months. For the cataplexy outcome, the results appear consistent with the clinical trial data in which a converse analysis showed that at least an approximately 50% reduction from baseline in weekly cataplexy attacks was the median change after 2 weeks of treatment with SXB 6 g and 9 g.11 It is interesting to note that parallel to a greater response rate for cataplexy, relative to EDS, the time to a cataplexy response was shorter. The question arises as to the reason for these results, and it can be queried whether it is due to the fact that patients notice a change in cataplexy, which is a very specific event, more readily than for EDS, which is more of a general perception of overall state. It might therefore be interesting to look at EDS rated by a significant other of each patient to determine if they might observe more readily the difference in EDS. Alternatively, it is possible there may be differential sensitivity between the two scales used to measure these events, or that there is a differential mechanism mediating the pharmacologic activity of SXB on these two diverse clinical endpoints.
Of note, SXB is titrated to therapeutic effect in clinical practice, with a starting dose of 4.5 g per night administered in two equal, divided doses (2.25 g at bedtime and 2.25 g taken 2.5 to 4 h later), and increased by 1.5 g per night at weekly intervals to the effective dose range of 6 g to 9 g per night.6 The need to allow appropriate time to observe a therapeutic response with SXB has previously been recognized in practical treatment recommendations as being of a duration of 2 to 3 months.15,16 This time frame was primarily based on the observations in the long-term extension that maximum ESS response was obtained after 2 months of treatment.12 The initial response times reported here are generally consistent with both the recommendations and the data reported in the clinical trial, and further suggest that while a 1- to 2-month period is adequate for evaluating patients for a clinically relevant response, the time course to achieve maximum response may require a longer period of observation. This requirement for weeks to months to observe a full therapeutic effect has been thought to be consistent with the restorative effects of SXB on sleep in a manner similar to that whereby such a duration is necessary to recover from sleep debt.16 While the mechanism of action has not been fully delineated, the unique paradox, compared with stimulants, between pharmacokinetic and pharmacodynamic effects is important to consider. SXB has a time to maximum plasma concentration of 0.5 to 1.25 hours and an elimination half-life of 0.5 to 1 hour,6 indicating that drug exposure is typically 4 hours per dose; yet the daytime impact on EDS and cataplexy are apparent. In addition, upon withdrawal of SXB there is a substantial delay in return of cataplexy and presumably EDS rather than a rebound effect. It was also observed that responses in patients initially randomized to placebo were consistent with results of patients treated with SXB throughout the study.
This study has several limitations, including that it was a post hoc analysis of data from studies that included open-label treatment in a long-term extension; retention in long-term extensions is generally based on patient self-selection, with those patients remaining in the study generally those in whom the treatment is both tolerable and effective. Additionally, most of the patients in these clinical trials were on concomitant therapy with stable doses of stimulants. Another limitation that should be considered when extrapolating the results to clinical practice is that even though cataplexy attacks were calculated as a weekly rate based on patient diaries, the first evaluation for changes in EDS occurred at the 30-day study visit and at discrete time points corresponding to study visits thereafter. In this regard, it should also be noted that both the weekly cataplexy attacks and the EDS, based on ESS scores, are patient-reported measures rather than objective clinical assessments. Nevertheless, these results suggest that it is important to maintain an adequate period for patient observation that is at least as long as that suggested by the median response times reported in this study, it is possible that clinically meaningful effects may occur earlier, and that the median time for initial response would have been shorter if earlier and more frequent assessments were performed. Lastly, although the change in weekly cataplexy attacks was evaluated as a clinically meaningful outcome, changes in cataplexy severity were not evaluated.
In summary, treatment with SXB resulted in onset of clinically meaningful therapeutic responses for reductions in EDS and cataplexy in most patients within 2 months, with maximum response achieved in most patients after a longer period. Clinicians should recognize that although a clinically relevant response may be expected in the majority of patients, the time course to initial and maximum response may take weeks to months. Additional data from clinical practice may more clearly characterize the time to onset and magnitude of therapeutic response that may be expected in patients treated with SXB.
DISCLOSURE STATEMENT
This analysis was funded by Jazz Pharmaceuticals, Inc. Dr. Bogan is a shareholder and an employee of SleepMed Inc; has received consultant fees from Jazz Pharmaceuticals, Inc., UCB, and XenoPort; has received industry-funded research support from Jazz Pharmaceuticals, Inc., Phillips, ApniCure, and Fisher Paykel; and has been a Speakers Bureau member for Teva, Jazz Pharmaceuticals, Inc., UCB, and XenoPort. Dr. Roth has received grant/research support from Sunovion; consultant fees from Bayer, Eisai, Flamel, Intec, Intra-Cellular, Jazz Pharmaceuticals, Inc., McNeil, Merck, Novartis, Pfizer, Phillips, and Sunovion. Dr. Schwartz has received consultant fees from Jazz Pharmaceuticals, Inc., and Teva; and has been a Speakers Bureau member for Teva. Dr. Miloslavsky is an employee of Jazz Pharmaceuticals, Inc. E. Jay Bienen, PhD, of The Curry Rockefeller Group, LLC, provided editorial assistance in developing this manuscript, which was funded by Jazz Pharmaceuticals, Inc.
ABBREVIATIONS
- CI
confidence interval
- EDS
excessive daytime sleepiness
- ESS
Epworth Sleepiness Scale
- SD
standard deviation
- SXB
sodium oxybate
REFERENCES
- 1.Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med. 2014;15:502–7. doi: 10.1016/j.sleep.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg R, Kim AY. The AWAKEN Survey: knowledge of narcolepsy among physicians and the general population. Postgrad Med. 2014;126:78–86. doi: 10.3810/pgm.2014.01.2727. [DOI] [PubMed] [Google Scholar]
- 3.De la Herran-Arita AK, Garcia-Garcia F. Narcolepsy as an immune-mediated disease. Sleep Disord. 2014:792687. doi: 10.1155/2014/792687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institute of Neurological Disorders and Stroke. Narcolepsy Fact Sheet. Bethesda, MD: National Institutes of Health; 2013. [Google Scholar]
- 5.Morgenthaler TI, Kapur VK, Brown T, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30:1705–11. doi: 10.1093/sleep/30.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palo Alto, CA: Jazz Pharmaceuticals, Inc.; 2014. Apr, Xyrem [sodium oxybate] oral solution US prescribing information. [Google Scholar]
- 7.U.S. Xyrem Multicenter Study Group. Sodium oxybate demonstrates long-term efficacy for the treatment of cataplexy in patients with narcolepsy. Sleep Med. 2004;5:119–23. doi: 10.1016/j.sleep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Black J, Houghton WC. Sodium oxybate improves excessive daytime sleepiness in narcolepsy. Sleep. 2006;29:939–46. doi: 10.1093/sleep/29.7.939. [DOI] [PubMed] [Google Scholar]
- 9.Xyrem International Study Group. A double-blind, placebo-controlled study demonstrates sodium oxybate is effective for the treatment of excessive daytime sleepiness in narcolepsy. J Clin Sleep Med. 2005;1:391–7. [PubMed] [Google Scholar]
- 10.Xyrem International Study Group. Further evidence supporting the use of sodium oxybate for the treatment of cataplexy: a double-blind, placebo-controlled study in 228 patients. Sleep Med. 2005;6:415–21. doi: 10.1016/j.sleep.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 11.The US Xyrem Multi-Center Study Group. A randomized, double blind, placebo-controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsy. Sleep. 2002;25:42–9. [PubMed] [Google Scholar]
- 12.U.S. Xyrem Multicenter Study Group. A 12-month, open-label, multicenter extension trial of orally administered sodium oxybate for the treatment of narcolepsy. Sleep. 2003;26:31–5. [PubMed] [Google Scholar]
- 13.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 14.Steffen AD, Lai C, Weaver TE. Development of a definition of response to narcolepsy treatment. Sleep. 2014;37:A361. (Abstract Suppl) [Google Scholar]
- 15.Guilleminault C, Fromherz S. Narcolepsy: diagnosis and management. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5th ed. Philadelphia, PA: Elsevier Saunders; 2011. [Google Scholar]
- 16.Mignot EJ. A practical guide to the therapy of narcolepsy and hypersomnia syndromes. Neurotherapeutics. 2012;9:739–52. doi: 10.1007/s13311-012-0150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]