Abstract
Study Objectives:
To determine whether occupational and neurophysiological decrements within shift work disorder (SWD) are differentially related to its two diagnostic symptoms, insomnia and excessive sleepiness.
Methods:
Thirty-four permanent night workers participated in an overnight lab protocol including a multiple sleep latency test (MSLT) and an event-related brain potential (ERP) task testing auditory target detection (P3a and P3b). At 16:00, each subject completed an Endicott Work Productivity Scale (EWPS), two Insomnia Severity Indices (ISI-Day, ISI-Night), and an Epworth Sleepiness Scale (ESS). Subjects were grouped by ISI and ESS scores into clinical phenotypes. This study compared EWPS and ERP results between alert insomniacs (“AI,” reporting insomnia without sleepiness), sleepy insomniacs (“SI,” reporting both insomnia and sleepiness), and controls.
Results:
The AI group was most impaired on the EWPS, significantly more impaired than controls (25.8 ± 14.8 vs. 12.3 ± 9.4, p < 0.05). SI were not statistically different from controls (19.5 ± 8.7 vs. 12.3 ± 9.4, p > 0.05). Compared to controls, AI showed significantly attenuated P3a response (Fcz, Czp, Cpz, mean difference [MD] 1.62–1.77, p < 0.05) and target-detection P3b response (Fcz, Czp, Cpz, MD 1.28–1.64, p < 0.05). P3b in SI was not different from controls (p > 0.10), and P3a was only different at one electrode site (Cpz, MD 1.43, p < 0.01). Neither the MSLT nor the ESS correlated with EWPS scores or ERP (P3a/P3b) amplitudes (p > 0.10). However, the mean of the ISI measurements correlated with the EWPS (r = 0.409, p < 0.01) and the attention-to-novelty P3a (r = −0.410, p < 0.01).
Conclusions:
Among shift work disorder patients, insomnia is linked to functional and cognitive impairments. Insomniacs with normal sleepiness showed more severe impairments than insomniacs who also reported excessive sleepiness.
Citation:
Belcher R, Gumenyuk V, Roth T. Insomnia in shift work disorder relates to occupational and neurophysiological impairment. J Clin Sleep Med 2015;11(4):457–465.
Keywords: insomnia, excessive sleepiness, shift work, event-related brain potentials, Insomnia Severity Index, multiple sleep latency test, Epworth Sleepiness Scale, circadian phase, work productivity
Shift work disorder (SWD) is a circadian rhythm disorder characterized by a chronic mismatch between a shift worker's sleep-wake schedule and his or her circadian clock.1–5 Clinically, this mismatch manifests in insomnia and/or excessive sleepiness. Diagnostic criteria for SWD require the presence of one or both of these symptoms, temporally related to a shift work schedule for at least three months, and not better explained by another medical, psychiatric, or sleep disorder.4
Many shift workers meeting diagnostic criteria for SWD report sleep difficulties consistent with those reported by patients with an insomnia disorder, while others report excessive sleepiness (either alone or in combination with insomnia symptoms).3,6 Studies comparing night shift workers with day workers estimate that 44.8% of night workers score greater than 10 on the Epworth Sleepiness Scale (compared to 32.7% of day workers), and 24.7% score greater than 13 (compared to 15.5% of day workers).6 With regard to insomnia, 18.5% of night workers met DSM-IV criteria for the disorder, while only 8.6% of day workers did.6 In conducting previous studies on SWD,1,2,7–9 we have observed that the population of shift workers reporting insomnia can be classified into two phenotypes: those reporting insomnia only, and those reporting insomnia alongside excessive sleepiness. We call the corresponding phenotypes “sleepy insomniacs” (SI) and “alert insomniacs” (AI). The phenotype presenting excessive sleepiness without insomnia (sleepy non-insomniacs [SN]) appears to be less common and is not considered in this study (see Methods).
BRIEF SUMMARY
Current Knowledge/Study Rationale: Workplace accidents, lost productivity, and neurophysiologic impairment are known to be associated with night shift work. This study aimed to determine whether these problems are differently related to insomnia and to excessive sleepiness, two hallmark symptoms that are diagnostic of shift work disorder (SWD) but are not reliably comorbid.
Study Impact: Our findings suggest that treatments focusing on excessive sleepiness in SWD may not sufficiently improve work-related outcomes, as overnight occupational and neurophysiologic impairment is more strongly correlated to insomnia than it is to sleepiness. Further research is needed to evaluate the impact of insomnia therapies on workplace safety and productivity in patients with SWD.
Functional impairments related to sleep deprivation and/ or circadian misalignment are common in shift work and represent a major productivity and safety risk.10–19 However, most research on performance and safety decrements among shift workers has focused on the deleterious effects of sleepiness,11,20–29 with less regard to shift-work related insomnia. Indeed, most of our knowledge on insomnia and performance is based upon findings in day workers.30–36 Given the different treatment paradigms for excessive sleepiness and insomnia (whether circadian-related or psychophysiologic), investigation into the potentially different occupational sequelae of these two symptoms remains an important priority for research on shift work and SWD. By considering two different clinical presentations of SWD in comparison with healthy night-working controls, this study aims to determine whether occupational and neurophysiological decrements within shift work disorder are differentially related to insomnia and excessive sleepiness.
In our previous research,9 we presented evidence of differences in neurophysiological markers, sleepiness, and sleep parameters between the AI and SI phenotypes of SWD. AI patients had increased amplitudes of the N1 ERP, an index of cortical hyperarousal54; normal MSLT during night-working hours; and sleep latency and maintenance problems during both diurnal and nocturnal sleep periods (with no statistically different differences in sleep parameters between the sleep periods: see Table 1). On the other hand, SI patients showed normal N1 amplitudes, suggesting normal cortical arousal and pathological levels of sleepiness during night shift hours.
Table 1.
Sleep diary, sleepiness, and circadian parameters by SWD phenotype.
These findings led us to evaluate and compare attention-related brain responses (P3a and P3b ERPs), as these provide additional insight into executive dysfunction potentially related to occupational impairment. These neurophysiological measures of brain activity associated with attention and memory are likely affected in people with higher sleep pressure due to either sleep restriction or circadian misalignment. In the current study, conducted within the same sample of night workers as our previous report, we measured the P300 ERP component. This component consists of the P3a and P3b markers, which are associated with involuntary attention switching between target task stimuli and irrelevant distracting stimuli. The P3a has latency around 200–300 ms from deviance onset and a frontocentral scalp distribution. ERP studies in humans with frontal lobe lesions have found that such patients produce a clear diminution of the P3a from a distracting stimulus. Frontal lobe engagement is therefore necessary for P3a generation and mechanisms of attention control.37 Unlike the P3a, the P3b marker is task relevant and associated with target detection. It is elicited when a subject's attention is focused on a stimulus.38 The P3b is associated with the process of memory comparison in the context of the previous stimuli, generating a brain potential with a parietal scalp distribution. The initial processing of a new stimulus engages the switching of attention that underlies P3a production, whereas the subsequent memory comparison engages the operations associated with P3b production. Based on previous research linking wakefulness, level of arousal, and sleep quality to the cognitive P3a and P3b ERP components,39–43 we expected that SWD patients predominantly suffering from insomnia may show different P3a and P3b markers compared to SWD patients who also experience excessive sleepiness.
We employed evoked response potentials and the Endicott Work Productivity Scale, a validated measure of work performance,44–46 to assess the functional abilities of night shift workers. The EWPS contains five subscales related to specific attributes of work performance: fatigue, executive function, interpersonal interactions, work efficiency, and counterproductive work behavior. Using groupwise comparisons of these measures between shift workers who present with insomnia, excessive sleepiness, both symptoms, or no symptoms, and using linear regression modeling to compare the symptoms head-to-head, this study aims to test the hypothesis that the two diagnostic symptoms of shift work disorder contribute differentially to occupational functioning and neurophysiology of attention related processes.
METHODS
This paper presents a secondary analysis from a larger study. Primary results from the MSLT, sleep diary, circadian phase assessment, and ISI and ESS questionnaires were presented in a previous report9 and are summarized in Table 1.
Subjects
Permanent night workers were recruited from healthcare and industrial settings to participate in an overnight laboratory sleep deprivation study. Subjects were required to have worked exclusively on a night shift for ≥ 6 months (≥ 3 night shifts per week, with each shift lasting 8–12 h and occurring between 19:00 and 08:00) and must not have worked a rotating shift or “picked up” daytime shifts during the 6 months preceding the laboratory study. They were required to have low probability for obstructive sleep apnea, restless leg syndrome, narcolepsy, and psychiatric disorders by providing responses in the normal range on the self-completed Berlin Questionnaire and staff-administered Hamilton Depression Scale, as well as in a clinical interview with a sleep medicine physician. Participants who had been diagnosed with insomnia or excessive sleepiness prior to beginning their nighttime work shift were excluded. They were required to be free from head injury, hearing problems, alcohol or substance abuse; must have been nonsmokers; and must not have consumed more than 300 mg of caffeine, on average, per day. Finally, they were required to be free from all CNS-acting medication as well as β-adrenergic blocking agents and other drugs which are known to affect the circadian rhythm, sleep-wake function, or melatonin production. Drugs encountered and disqualified included sertraline, tamoxifen, paroxetine, zolpidem, metoprolol, atenolol, bupropion, alprazolam, venlafaxine, methylphenidate, gabapentin, tramadol, diazepam, triazolam, levocetirizine, buspirone, citalopram, lisdexamfetamine, and trazodone. An exception was made for shift workers with insomnia symptoms who had taken melatonin or sleep-promoting agents while working nighttime shifts, but had subsequently discontinued medication and had been free of the drug and all potential metabolites for ≥ 2 weeks prior to the laboratory study.
Ninety-five night workers initially responded to flyers and online newsletter advertisements. Initial advertisements did not specify symptoms of SWD, while subsequent advertisements recruited night workers experiencing “sleepiness or trouble sleeping during the day,” based on recruitment needs. Of the 95 respondents, 35 (36.8%) were disqualified based on an online survey; 13 (13.7%) did not comply with pre-screening requests to keep a sleep diary or failed to keep a screening appointment; 5 (5.3%) were disqualified at the screening appointment; 3 (3.2%) were eligible but not enrolled; and 1 (1.1%) dropped out following enrollment. Thirty-eight subjects (40.0%) qualified based on inclusion criteria. Excluding one subject released for noncompliance, 37 subjects completed the study (mean age 35.41 ± 9.35 years, 62.2% female).
At the screening appointment, 26 of 37 qualifying subjects (70.2%) were diagnosed with SWD upon a clinical interview with a sleep medicine physician and review of the participant's screening sleep diary. The remaining 11 (29.7%) were asymptomatic (controls).
Self-Reported Sleep
For 2 weeks prior to the laboratory study, subjects kept a sleep diary reporting bedtime, wake time, latency to sleep, number of awakenings, use of alcohol and caffeine, and subjective sleep quality.
Laboratory Procedures
The laboratory study was scheduled to begin on an evening after the subject had worked ≥ 3 consecutive night shifts. They were instructed to sleep during the day before coming to the laboratory, as if sleeping before a subsequent night shift. On the study day, they arrived at 16:00 and were housed in a dimly lit (< 15 lux), sound-attenuated private bedroom and bathroom that they would occupy for 25 hours. Participants were aware of clock time. They were not permitted to sleep at any point during their entire experimental session, and were required to remain out of bed except for nap trials during the multiple sleep latency test (see below). Internet access, television, and cellular phone usage were permitted during unscheduled time, and 3 meals were provided at the subject's request in addition to snacks and water. Meals did not contain foods or drinks known to affect sleep or circadian rhythms such as caffeine, ethanol, milk, bananas, or tomatoes. Subjects were also prohibited from consuming crunchy foods that might injure the gums and permit blood contamination of the saliva samples (see below). Registered polysomnographic technologists and research associates continuously monitored all participants for wakefulness throughout the study.
Questionnaires
At 18:00, each subject completed an Epworth Sleepiness Scale (ESS), 2 separate Insomnia Severity Index (ISI) questionnaires, and the Endicott Work Productivity Scale (EWPS). Items on the separate ISIs were identical, but both verbal and written instructions prompted subjects to separately evaluate their diurnal sleep on one questionnaire (ISI-D) and nocturnal sleep on the other (ISI-N).
Phenotype Classification
Twenty-six subjects with SWD were classified into 3 phenotypes using scores from the ESS and ISI, as well as a diagnostic interview with a sleep medicine physician.9 Twelve subjects presented pathologic scores (≥ 10) on the ISI-D, ISI-N, or both, while scoring in the normal range (< 10) on the ESS. These subjects were classified “alert insomniacs” (AI). Within this subgroup, 11 subjects (91.7%) had pathologic scores on the ISI-D and 8 (66.7%) were flagged for pathologic scores on the ISI-N.
Eleven subjects with elevated scores on one or both ISIs as well as the ESS were classified into the sleepy insomniac (SI) group. Within this subgroup, 10 subjects (90.9%) had pathologic scores on the ISI-D, and 4 (36.4%) had pathologic scores on the ISI-N.
Eleven controls showed normal scores on the ESS and both ISIs, corroborating the diagnostic interview at the screening appointment.
Finally, 3 subjects showed pathologic sleepiness (ESS ≥ 10) but normal scores on both insomnia indices (ISI-D and ISIN < 10). These subjects were classified “sleepy non-insomniacs” (SN). Because of the small number of subjects who met criteria for this group, it was excluded from the analyses.
Circadian Phase Assessment
Beginning at 17:00, saliva samples were collected at 30-min intervals using a Salivette tube with a cotton insert (Sarstedt Group, Numbrecht, Germany). Prior to 17:00, subjects were instructed on the procedure of saliva collection and the amount of saliva required for each sample. Each sample was weighed to ensure that ≥ 1 mL of saliva was provided. Saliva was then extracted from the cotton insert in a frozen centrifuge, and samples were frozen at −20°C until being shipped over dry ice to SolidPhase, Inc. (Portland, Maine, USA), where they were radio-immunoassayed. The intra-assay precision was 2.6% to 20.1%, with functional sensitivity of 0.9 pg/mL and analytical sensitivity of 0.2 pg/mL.
Dim light melatonin onset (DLMO) is an individual's characteristic point in clock time marking the onset of elevated melatonin levels that normally coincide with the timing of the sleep period.47–49 DLMO was calculated as the time that the amplitude of the fitted LOWESS curve for melatonin concentration rose and remained above a subject's melatonin threshold for at least one hour. The threshold used was the average of the 5 lowest concentrations of melatonin during the 24-h phase assessment, plus 15% of the average of the 5 highest concentrations.
Objective Sleepiness Assessment
Objective sleepiness was assessed with a nocturnal multiple sleep latency test (MSLT), following standard research protocol. Electrode integrity was checked by physical inspection and electrical biocalibration before each nap. The first 5 naps (22:30 to 06:30) coincided with night shift hours, while the last 3 (08:30 to 12:30) evaluated the effects of acute sleep deprivation. In this report we are presenting the results of a 4-nap MSLT collected between 22:30 and 04:30, corresponding to night shift hours.
P3a and P3b Event Related Brain Potential
The typical oddball paradigm was used for an active auditory processing task. This paradigm consists of 4 types of regularly occurring sounds as well as a rare auditory target. Seventy percent of the regular sounds were simple tones (duration = 100 ms, frequency = 800 Hz) and served as a standard; 10% of regular sounds were complex tones (environmental sounds), which served as novel stimuli. Not included in the P3a/P3b analyses presented here are deviant frequency simple tones (10%, duration = 100 ms, frequency = 1,000 Hz) and deviant duration simple tones (10%, duration = 150 ms, frequency = 800 Hz). The irregular target was a high-pitch sound presented rarely (25 sounds across 14 min) and interspersed with all other sounds. Participants were instructed to press the button when they heard a target sound by using their dominant index finger. All sounds were presented through earplugs binaurally at a 75 dB SPL (sound pressure level) with 5 ms rise/fall time. All stimuli were presented at a constant inter-stimulus interval of 800 ms. Each session lasted 7.3 min. A total of 2 sessions with a short inter-session break (2 min) were presented to each subject. The clock time of the task was between 18:00 and 19:00 for each subject, a time in the 24-h cycle that occurs at neither the nadir for well-adjusted night workers nor the nadir for unadjusted night workers.1,9 It was thus a “neutral zone” in the circadian cycles of our subjects, and timing the ERP in this window permitted the examination of cognitive differences shortly after waking, without the confounding influence of acute sleep deprivation.
EEG Recording and ERP Data Analysis
EEG data was recorded via a 32-channel EEG cap (10-20 system, Easy Cap, Gilching, Germany) and an ASA system (ANT, the Netherlands). Electrooculogram (EOG) was recorded by 2 electrodes at the left and right canthus and 2 electrodes above and below the left eye of the subject. Impedances were kept < 10 kiloΩ, and a band-pass filter was set from 0.1 to 100 Hz. The sampling rate was 1,024 Hz.
Data were analyzed off-line using Brain Vision Analyzer software (Brain Products GmbH, Gilching, Germany). ERP data were separately analyzed for each sound type. Each segment began 100 ms prior to the stimulus onset and continued for 700 ms after the stimulus onset. A band-pass filter from 0.1 to 30 Hz was applied to segmented data. Segments in which the EEG or EOG exceeded ± 75 microvolts were excluded from the average. ERPs in response to standard and novel stimuli were averaged separately. Baseline correction (100 ms pre-stimulus interval) was applied to the averaged data. On average, ≥ 300 trials for the standard tone and ≥ 100 trials for the novel sounds were included for each subject.
To evaluate ERP data, the obligatory auditory N1 waveform was identified as the largest negative wave at latency 80 to 130 ms from sound onset in each individual grand average corresponding to each type of stimulus (standard and novel). The difference wave between ERPs to novel minus ERPs to standard was computed for each participant and then averaged per group. The peak amplitude and latency of the P3a and P3b of the difference wave were measured within an 200–300 ms and 400–600 ms time window from sound onset at the FCz and Cpz electrodes, as these are typically shown in studies to present the largest amplitude for P3a (FCz, frontal) and P3b (CPz, parietal) ERP components.
Statistical Analyses
Groupwise comparisons between strata of shift workers presenting different combinations of symptoms (AI and SI) and presenting no symptoms at all (controls) were performed. These phenotypic differences were analyzed using two-tailed Student's t-tests. Pearson bivariate correlations and linear regression modeling were also used, within the full sample, to clarify the differential contributions of SWD symptoms to variance in EWPS and ERP findings. All statistical comparisons of the P3a and P3b to novel sounds involved computing difference waves (amplitudes in response to novel sounds minus amplitudes to the standard tone). The time windows for mean amplitude comparisons were selected based on the peak amplitude of each brain response at FCz (frontal) and CPz (parietal) electrodes.
RESULTS
Endicott Work Productivity Scale
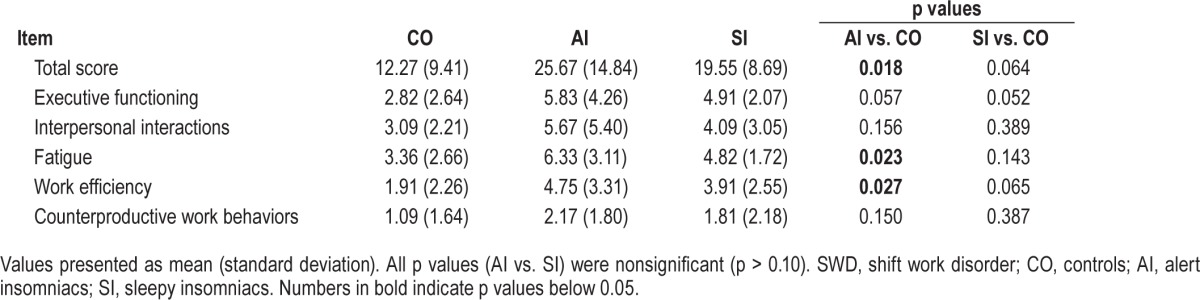
Night-working controls presented the lowest scores on the EWPS (12.27 ± 9.41), indicating the lowest level of occupational impairment. Subjects presenting insomnia alongside excessive sleepiness showed higher levels of impairment than controls (19.55 ± 8.69, p = 0.06), and SWD subjects only reporting insomnia had the greatest degree of impairment (25.67 ± 14.84, p < 0.05 vs. controls).
Summary data of the EWPS aggregate scores and subscales are presented in Table 2. An interesting and significant difference emerged on the fatigue subscale of the EWPS (items such as losing interest, becoming reckless, and falling asleep at work), with AI subjects reporting higher levels of fatigue than controls (6.3 ± 3.1 vs. 3.4 ± 2.62, p < 0.05 vs. controls). Paradoxically, SI subjects showed lower scores on the fatigue subscale than insomnia-only subjects, not significantly different from controls (4.82 ± 1.72, p > 0.10). This suggests that the EWPS may be sensitive to the distinction between fatigue and sleepiness. Compared to controls, AI subjects also reported higher levels of impairment in work efficiency (items such as working slowly, having to repeat tasks, and daydreaming): AI subjects scored 4.75 ± 3.31 compared to 1.91 ± 2.26 in controls (p < 0.05). SI subjects were not statistically different from controls on any of the EWPS subscales.
Table 2.
Endicott Work Productivity Scale scores and subscores by SWD phenotype.
Event-Related Potentials (P3a and P3b)
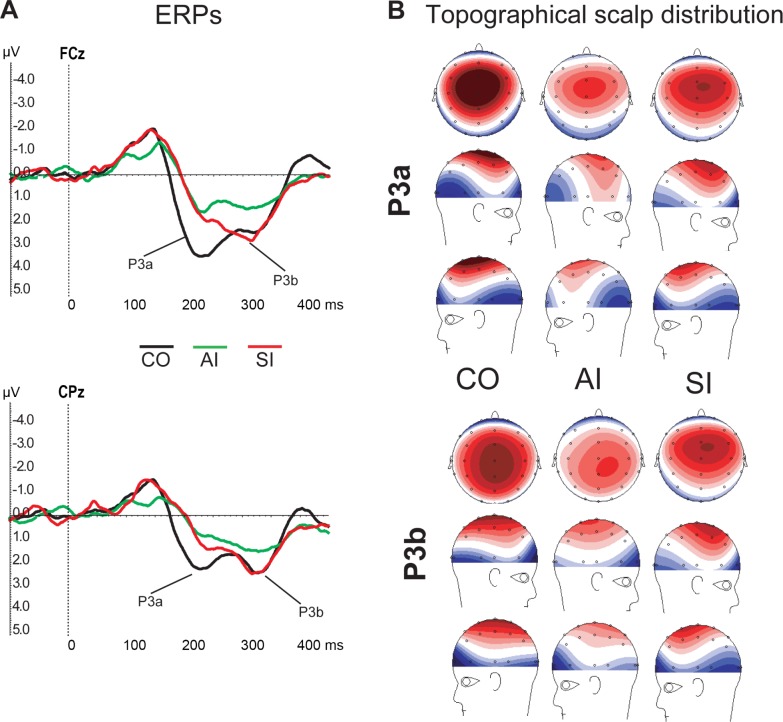
At the FCz, CZp, and CPz electrodes, alert insomniacs showed significantly attenuated P3a responses (mean difference 1.62–1.77, p < 0.05) and target-detection P3b responses (mean difference 1.28–1.64, p < 0.05) relative to controls (see Figure 1A). P3b in SI was not different from controls (p > 0.10), and P3a was only different at one electrode (CPz), mean difference 1.43, p < 0.01). Figure 1B illustrates the topographical scalp distribution of the P3a and P3b brain responses to the novel sounds.
Figure 1.
(A) Illustration of ERP difference waveforms corresponding to each group at FCz and CPz electrodes. (B) Topographical scalp distribution of P3a (200–240 ms) and P3b (340–380 ms) activities evaluated in each group. Positive voltage is colored red, negative voltage is colored blue, and white color corresponds to zero. CO, controls; AI, alert insomniacs; SI, sleepy insomniacs
Correlations to Insomnia and Sleepiness Metrics
Primary results from the MSLT, sleep diary, circadian phase assessment, and ISI and ESS questionnaires were presented in a previous report9 and are summarized in Table 1. Neither measure of sleepiness (MSLT or ESS) correlated with EWPS scores or ERP amplitudes (p > 0.10). However, the mean of the 2 ISI measures correlated with the EWPS (r = 0.409, p < 0.01) and the attention-to-novelty P3a (r = −0.410, p < 0.01). This suggests that insomnia in SWD is a strong predictor of occupational and neurophysiological impairment, compared to reports and objective measures of sleepiness.
Linear Modeling
Table 3 presents the results of 2 linear regression models using the ISI, ESS, and MSLT to predict EWPS scores and ERP amplitudes. The P3a marker at the CPz electrode was the only ERP amplitude impaired in both the AI and SI groups, and was thus selected as the ERP outcome variable for linear modeling. In Models 1a and 1b, the mean of 2 ISIs is the only significant predictor of EWPS score and P3a amplitude. We interpret this as further evidence that insomnia is a stronger predictor of occupational and neurophysiological impairment than sleepiness.
Table 3.
Linear regression models predicting Endicott Work Productivity Scale scores and P3a amplitude.
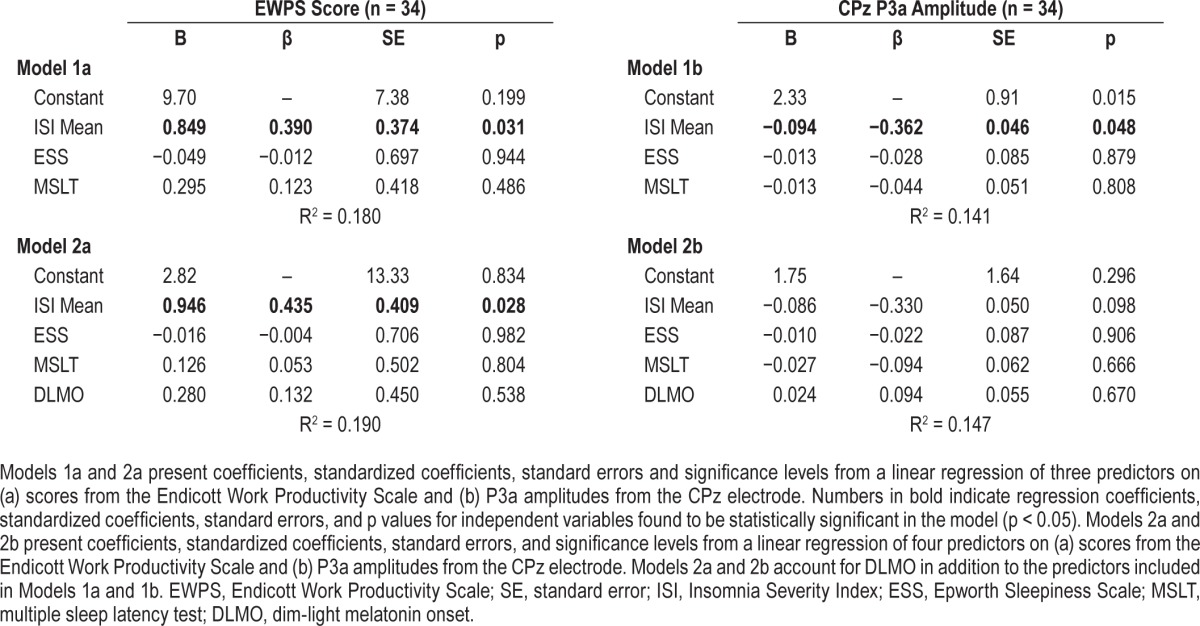
To determine if circadian phase is a factor in the ERP or EWPS assessments, dim-light melatonin onset (DLMO) was added to the other predictive variables in Model 2a and Model 2b. DLMO does not change the significance level or substantially affect the standardized effect size of the ISI on EWPS scores, although it does reduce the significance of the ISI in predicting P3a amplitudes. Results from this analysis confirm that a mean of the 2 ISI assessments is a robust predictor of occupational and neurophysiological functioning, such that higher levels of reported insomnia are linked to higher levels of occupational impairment as well as attenuated (impaired) ERP amplitudes.
Relationship between EWPS Scores and ERP Amplitudes
Total EWPS scores were correlated with the P3a, an attention-to-novelty response (Cpz, r = −0.344, p < 0.05). The fatigue subscale was also correlated to the P3a response (CPz, r = −0.523), p < 0.01), as was the executive function subscale (difficulty concentrating, organizing work, and forgetting information; CPz, r = −0.343, p < 0.05). Three subscales measuring interpersonal interactions, work efficiency, and counterproductive work behavior did not significantly relate to the P3a, and none of the EWPS scores related to P3b (target detection) amplitudes. These results suggest that lower EWPS scores in night workers with insomnia are more likely related to the attentional network (P3a) and less related to the memory-updating network (P3b).
DISCUSSION
The primary result of the present study is that insomnia is strongly linked to functional and cognitive impairments in shift workers, while sleepiness appears to make a smaller contribution. Insomniacs without excessive sleepiness showed more severe impairments than insomniacs who report excessive sleepiness. Finally, linear modeling and correlation analyses indicate that the ISI is a more robust predictor of functional sequelae than the MSLT or the ESS.
We have previously reported that there are etiological differences between the two insomnia phenotypes seen in SWD.9 It was concluded from this study that alert insomniacs (not suffering from excessive sleepiness) were likely to have a preexisting insomnia vulnerability, which evolved into an insomnia disorder in response to the circadian challenge of shift work. In contrast, the SI phenotype primarily had difficulty sleeping when required to sleep at an inappropriate circadian time (i.e., during the day). This was supported by diary data indicating that SWD patients with insomnia alone have disturbed sleep during both nocturnal and diurnal sleep periods, and ERP data showing an N1 response comparable to that seen in day-working individuals with an insomnia disorder. On the other hand, SWD patients reporting both insomnia and excessive sleepiness slept well on days off (i.e., reverting to nocturnal sleep) and showed normal N1 responses. Importantly, these sleep and ERP differences were not attributable to inter-individual differences in the circadian clock, as both groups showed comparable circadian misalignment (DLMO).
The results of the present study extend these findings into the occupational domain and emphasize the real-world stakes of clinical treatment for SWD. First, neurophysiologically, we report that the N1 marker is not the only difference between the two insomnia phenotypes of SWD: the attentional and target-detection markers P3a and P3b are also more impaired in the AI group. Second, we report that these neurophysiological impairments in alert insomniacs have practical and serious consequences for the workplace, as indicated by significantly elevated P3a and P3b responses and a strong, positive correlation between the EWPS and these ERP amplitudes. Comparing predictors of these impairments head-to-head, we found that the neurophysiological and occupational deficits within the context of SWD were not significantly related to circadian phase or degree of sleepiness, but instead to the severity of their insomnia.
This finding is novel in the context of shift work disorder, where many treatment protocols (e.g., stimulants) and occupational policies (e.g., duty hour restrictions) target the deleterious effects of excessive sleepiness rather than insomnia. On the other hand, it is unremarkable in the context of insomnia research, where multimodal evidence has examined functional and cognitive impairments in insomnia, with mixed findings, for decades (for a review, see Krystal55). One recent study of neurobehavioral impairments in day-working insomniacs determined that impairments stem from neurobiological differences between insomniacs and non-insomniacs, not from sleepiness related to reduced sleep time in insomnia56—a day worker finding consistent with our observations in shift workers. While our understanding of the exact neurological underpinnings of insomnia is still growing, it appears that neurophysiologic differences in insomniacs are linked to executive and occupational impairment. Our data suggest that these impairment-related neurological attributes are found in the AI phenotype of SWD, but not in the SI phenotype or in healthy night-working controls.
Another interesting result is that while the AI group had lower sleepiness on the ESS (by definition) as well as on the MSLT, they reported significantly greater fatigue. Many patients report sleepiness and fatigue interchangeably, but this paper provides clear evidence that while sleepiness is associated with MSLT latency, fatigue is not. To the clinician it is critical to differentiate between these two symptoms. These data suggest that, at least among shift workers, the treatment for fatigue is not to enhance alertness but rather to treat insomnia.
We had previously speculated that the treatment of insomnia in these two populations is different, with the SI phenotype needing a circadian readjustment and the AI group needing both circadian readjustment as well as treatment directly aimed at insomnia per se (e.g., CBT-I). The occupational productivity results described here are an additional call for the importance of aggressively treating this symptom, as work productivity and safety may improve in response to insomnia treatment.
This study has a few notable limitations. First, while the timing for our ERP task (18:00–19:00) was carefully selected to occur outside of the circadian nadir for our subjects, intergroup neurophysiologic differences might be different in this time window than during typical night-shift hours. While our study presents evidence of ERP impairment immediately after waking from a diurnal sleep period, future projects may consider collecting ERP data overnight. Second, our occupational impairment findings are limited to self-report. Research on occupational errors in natural environments is an important next direction for SWD, and intervention trials for SWD treatments should consider measuring work productivity and safety alongside the traditional clinical symptoms. Third, the small number of cases within each diagnostic subgroup required us to perform linear regression models within the entire sample instead of separately by group.
This study is part of a larger laboratory protocol, and general limitations to that project are described in a previous report.9 Summarized, the major limitations are the exclusion of shift workers who consumed large amounts of caffeine, smoked, had high pretest probability for sleep apnea or psychiatric conditions, or used hypnotics or other medications likely to affect the sleep-wake cycle. While these exclusions limit the generalizability of our findings to a true shift-working population, we feel that they reduced the influence of confounding factors in our investigation and were essential to the study's integrity. Finally, our study did not include an objective measure of sleep (e.g., PSG), and the laboratory protocol was only carried out once for each subject.
In conclusion, shift work disorder patients reporting insomnia without excessive sleepiness show greater work impairment and attentional impairment on ERP measures than patients reporting sleepiness and insomnia together. The degree of daytime impairment does not relate to degree of daytime sleepiness, but rather to the degree of insomnia and ERP impairment. These results reaffirm that insomnia within SWD demands clinical attention, as it is linked to productivity and neurophysiological impairments with serious consequences for safety and occupational health.
DISCLOSURE STATEMENT
This study is supported by grant K01OH009996-03 from CDC/NIOSH. Dr. Roth has served as a consultant for Abbott, Arcadia, AstraZeneca, Aventis, AVER, Bayer, BMS, Cypress, Ferrer, GlaxoSmithKline, Impax, Intec, Jazz, Johnson and Johnson, Merck, Neurocrine, Novartis, Proctor and Gamble, Pfizer, Purdue, Shire, Somaxon, and Transcept. He has received research support from Cephalon, Merck, Transcept, Speakers Bureau, Purdue, and Proctor and Gamble. The other authors have indicated no financial conflicts of interest. Research was performed at Henry Ford Hospital. This study does not involve off-label or investigational use of a drug.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the hard work of Ryan Howard, Laura Spear, Mike Gable, Jennifer Philport, Elaine Douglas, Brian King, and Drs. Niraj Parikh, Rami Abboud, Chirag Popat, Victor Gordon, Venkatesh Basappa-Krishnamurthy, and Narayan Neupane.
REFERENCES
- 1.Gumenyuk V, Howard R, Roth T, Korzyukov O, Drake CL. Sleep loss, circadian mismatch, and abnormalities in reorienting of attention in night workers with shift work disorder. Sleep. 2014;37:545–56. doi: 10.5665/sleep.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gumenyuk V, Roth T, Drake CL. Circadian phase, sleepiness, and light exposure assessment in night workers with and without shift work disorder. Chronobiol Int. 2012;29:928–36. doi: 10.3109/07420528.2012.699356. [DOI] [PubMed] [Google Scholar]
- 3.Wright KP, Jr., Bogan RK, Wyatt JK. Shift work and the assessment and management of shift work disorder (SWD) Sleep Med Rev. 2013;17:41–54. doi: 10.1016/j.smrv.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 4.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 5.Sack RL, Blood ML, Lewy AJ. Melatonin rhythms in night shift workers. Sleep. 1992;15:434–41. doi: 10.1093/sleep/15.5.434. [DOI] [PubMed] [Google Scholar]
- 6.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453–62. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 7.Gumenyuk V, Roth T, Korzyukov O, Jefferson C, Bowyer S, Drake CL. Habitual short sleep impacts frontal switch mechanism in attention to novelty. Sleep. 2011;34:1659–70. doi: 10.5665/sleep.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gumenyuk V, Roth T, Korzyukov O, et al. Shift work sleep disorder is associated with an attenuated brain response of sensory memory and an increased brain response to novelty: an ERP study. Sleep. 2010;33:703–13. doi: 10.1093/sleep/33.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gumenyuk V, Belcher R, Drake CL, Roth T. Differential sleep, sleepiness and neurophysiology in the insomnia phenotypes of shift work disorder. Sleep. 2015;38:119–26. doi: 10.5665/sleep.4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shift work and sleep: optimizing health, safety, and performance. J Occup Environ Med. 2011;53:S1–10. doi: 10.1097/JOM.0b013e31821aec20. quiz S11–2. [DOI] [PubMed] [Google Scholar]
- 11.Akerstedt T, Kecklund G. Shift work, severe sleepiness and safety. Ind Health. 2011;49:141–2. doi: 10.2486/indhealth.ms4902ed. [DOI] [PubMed] [Google Scholar]
- 12.Barger LK, Lockley SW, Rajaratnam SM, Landrigan CP. Neurobehavioral, health, and safety consequences associated with shift work in safety-sensitive professions. Cur Neurol Neurosci Rep. 2009;9:155–64. doi: 10.1007/s11910-009-0024-7. [DOI] [PubMed] [Google Scholar]
- 13.Berger AM, Hobbs BB. Impact of shift work on the health and safety of nurses and patients. Clin J Occ Nurs. 2006;10:465–71. doi: 10.1188/06.CJON.465-471. [DOI] [PubMed] [Google Scholar]
- 14.Clayton R. Safety: the social implications of shift work. Occup Health. 1982;34:319–21. [PubMed] [Google Scholar]
- 15.Folkard S. Shift work, safety, and aging. Chronobiol Int. 2008;25:183–98. doi: 10.1080/07420520802106694. [DOI] [PubMed] [Google Scholar]
- 16.Folkard S, Tucker P. Shift work, safety and productivity. Occup Med. 2003;53:95–101. doi: 10.1093/occmed/kqg047. [DOI] [PubMed] [Google Scholar]
- 17.Keller SM. Effects of extended work shifts and shift work on patient safety, productivity, and employee health. AAOHN J. 2009;57:497–502. doi: 10.3928/08910162-20091124-05. quiz 503–4. [DOI] [PubMed] [Google Scholar]
- 18.Vollmer ME. Health and safety consequences of shift work. Indiana Med. 1987;80:554–6. [PubMed] [Google Scholar]
- 19.Wagstaff AS, Sigstad Lie JA. Shift and night work and long working hours--a systematic review of safety implications. Scand J Work Environ Health. 2011;37:173–85. doi: 10.5271/sjweh.3146. [DOI] [PubMed] [Google Scholar]
- 20.Akerstedt T. Sleepiness as a consequence of shift work. Sleep. 1988;11:17–34. doi: 10.1093/sleep/11.1.17. [DOI] [PubMed] [Google Scholar]
- 21.Akerstedt T, Kecklund G, Knutsson A. Manifest sleepiness and the spectral content of the EEG during shift work. Sleep. 1991;14:221–5. doi: 10.1093/sleep/14.3.221. [DOI] [PubMed] [Google Scholar]
- 22.Akerstedt T, Torsvall L, Gillberg M. Sleepiness and shift work: field studies. Sleep. 1982;5(Suppl 2):S95–106. doi: 10.1093/sleep/5.s2.s95. [DOI] [PubMed] [Google Scholar]
- 23.Eldevik MF, Flo E, Moen BE, Pallesen S, Bjorvatn B. Insomnia, excessive sleepiness, excessive fatigue, anxiety, depression and shift work disorder in nurses having less than 11 hours in-between shifts. PloS One. 2013;8:e70882. doi: 10.1371/journal.pone.0070882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gold DR, Rogacz S, Bock N, et al. Rotating shift work, sleep, and accidents related to sleepiness in hospital nurses. Amer J Pub Heal. 1992;82:1011–4. doi: 10.2105/ajph.82.7.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halvani GH, Zare M, Mirmohammadi SJ. The relation between shift work, sleepiness, fatigue and accidents in Iranian Industrial Mining Group workers. Ind Health. 2009;47:134–8. doi: 10.2486/indhealth.47.134. [DOI] [PubMed] [Google Scholar]
- 26.Harma M, Sallinen M, Ranta R, Mutanen P, Muller K. The effect of an irregular shift system on sleepiness at work in train drivers and railway traffic controllers. J Sleep Res. 2002;11:141–51. doi: 10.1046/j.1365-2869.2002.00294.x. [DOI] [PubMed] [Google Scholar]
- 27.Kageyama T, Kobayashi T, Abe-Gotoh A. Correlates to sleepiness on night shift among male workers engaged in three-shift work in a chemical plant: its association with sleep practice and job stress. Ind Health. 2011;49:634–41. doi: 10.2486/indhealth.ms1239. [DOI] [PubMed] [Google Scholar]
- 28.Sallinen M, Kecklund G. Shift work, sleep, and sleepiness - differences between shift schedules and systems. Scand J Work Env Heal. 2010;36:121–33. doi: 10.5271/sjweh.2900. [DOI] [PubMed] [Google Scholar]
- 29.Van Dongen HP. Shift work and inter-individual differences in sleep and sleepiness. Chronobiol Int. 2006;23:1139–47. doi: 10.1080/07420520601100971. [DOI] [PubMed] [Google Scholar]
- 30.Drummond SP, Walker M, Almklov E, Campos M, Anderson DE, Straus LD. Neural correlates of working memory performance in primary insomnia. Sleep. 2013;36:1307–16. doi: 10.5665/sleep.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edinger JD, Means MK, Carney CE, Krystal AD. Psychomotor performance deficits and their relation to prior nights' sleep among individuals with primary insomnia. Sleep. 2008;31:599–607. doi: 10.1093/sleep/31.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez-Mendoza J, Calhoun S, Bixler EO, et al. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2010;33:459–65. doi: 10.1093/sleep/33.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, Morin CM. Insomnia and daytime cognitive performance: a meta-analysis. Sleep Med Rev. 2012;16:83–94. doi: 10.1016/j.smrv.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Kessler RC, Berglund PA, Coulouvrat C, et al. Insomnia and the performance of US workers: results from the America insomnia survey. Sleep. 2011;34:1161–71. doi: 10.5665/SLEEP.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shekleton JA, Flynn-Evans EE, Miller B, et al. Neurobehavioral performance impairment in insomnia: relationships with self-reported sleep and daytime functioning. Sleep. 2014;37:107–16. doi: 10.5665/sleep.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varkevisser M, Kerkhof GA. Chronic insomnia and performance in a 24-h constant routine study. J Sleep Res. 2005;14:49–59. doi: 10.1111/j.1365-2869.2004.00414.x. [DOI] [PubMed] [Google Scholar]
- 37.Knight RT, Nakada T. Cortico-limbic circuits and novelty: a review of EEG and blood flow data. Rev Neurosci. 1998;9:57–70. doi: 10.1515/revneuro.1998.9.1.57. [DOI] [PubMed] [Google Scholar]
- 38.Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalogr Clin Neurophysiol. 1975;38:387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- 39.Gosselin A, De Koninck J, Campbell KB. Total sleep deprivation and novelty processing: implications for frontal lobe functioning. Clin Neurophys. 2005;116:211–22. doi: 10.1016/j.clinph.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 40.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 41.Salmi J, Huotilainen M, Pakarinen S, Siren T, Alho K, Aronen ET. Does sleep quality affect involuntary attention switching system? Neurosci Lett. 2005;390:150–5. doi: 10.1016/j.neulet.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 42.Raz A, Deouell LY, Bentin S. Is pre-attentive processing compromised by prolonged wakefulness? Effects of total sleep deprivation on the mismatch negativity. Psychophysiology. 2001;38:787–95. [PubMed] [Google Scholar]
- 43.Balkin TJ, Bliese PD, Belenky G, et al. Comparative utility of instruments for monitoring sleepiness-related performance decrements in the operational environment. J Sleep Res. 2004;13:219–27. doi: 10.1111/j.1365-2869.2004.00407.x. [DOI] [PubMed] [Google Scholar]
- 44.Beaton DE, Tang K, Gignac MA, et al. Reliability, validity, and responsiveness of five at-work productivity measures in patients with rheumatoid arthritis or osteoarthritis. Arthritis Care Res. 2010;62:28–37. doi: 10.1002/acr.20011. [DOI] [PubMed] [Google Scholar]
- 45.Endicott J, Nee J. Endicott Work Productivity Scale (EWPS): a new measure to assess treatment effects. Psychopharmacol Bull. 1997;33:13–6. [PubMed] [Google Scholar]
- 46.Prasad M, Wahlqvist P, Shikiar R, Shih YC. A review of self-report instruments measuring health-related work productivity: a patient-reported outcomes perspective. Pharmacoeconomics. 2004;22:225–44. doi: 10.2165/00019053-200422040-00002. [DOI] [PubMed] [Google Scholar]
- 47.Lewy AJ, Sack RL. The dim light melatonin onset as a marker for circadian phase position. Chronobiol Int. 1989;6:93–102. doi: 10.3109/07420528909059144. [DOI] [PubMed] [Google Scholar]
- 48.Revell VL, Kim H, Tseng CY, Crowley SJ, Eastman CI. Circadian phase determined from melatonin profiles is reproducible after 1 wk in subjects who sleep later on weekends. J Pineal Res. 2005;39:195–200. doi: 10.1111/j.1600-079X.2005.00236.x. [DOI] [PubMed] [Google Scholar]
- 49.Revell VL, Eastman CI. How to trick mother nature into letting you fly around or stay up all night. J Biol Rhythms. 2005;20:353–65. doi: 10.1177/0748730405277233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee C, Smith MR, Eastman CI. A compromise phase position for permanent night shift workers: circadian phase after two night shifts with scheduled sleep and light/dark exposure. Chronobiol Int. 2006;23:859–75. doi: 10.1080/07420520600827160. [DOI] [PubMed] [Google Scholar]
- 51.Carskadon MA, Dement WC, Mitler MM, Guilleminault C, Zarcone VP, Spiegel R. Self-reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia. Am J Psychiatry. 1976;133:1382–8. doi: 10.1176/ajp.133.12.1382. [DOI] [PubMed] [Google Scholar]
- 52.Edinger JD, Fins AI. The distribution and clinical significance of sleep time misperceptions among insomniacs. Sleep. 1995;18:232–9. doi: 10.1093/sleep/18.4.232. [DOI] [PubMed] [Google Scholar]
- 53.Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6:179–88. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 54.Bastien CH. Insomnia: neurophysiological and neuropsychological approaches. Neuropsychol Rev. 2011;21:22–40. doi: 10.1007/s11065-011-9160-3. [DOI] [PubMed] [Google Scholar]
- 55.Krystal AD. Treating the health, quality of life, and functional impairments in insomnia. J Clin Sleep Med. 2006;3:63–72. [PubMed] [Google Scholar]
- 56.Shekleton JA, Flynn-Evans EE, et al. Neurobehavioral performance impairment in insomnia: relationships with self-reported sleep and daytime functioning. Sleep. 2014;37:107–16. doi: 10.5665/sleep.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]