Abstract
Study Objectives:
To describe characteristics and surgical and clinical outcomes of obese children with obstructive sleep apnea (OSA).
Methods:
At our institution from 2000 to 2010, 143 obese children with an overnight polysomnography (OPSG) diagnosis of OSA, excluding children with comorbidities, were identified. Relationships between demographics, clinical findings, and the severity of OSA were assessed. Presurgery and postsurgery OPSG indices were compared. We defined cure as an apneahypopnea index (AHI) < 1.5/h on the postsurgery OPSG, and we compared the cure rates of different surgeries.
Results:
A total of 143 children, median age 12.4 y (interquartile range [IQR] 9.6–14.9) and BMI z-scores 2.8 (IQR 2.6–2.9), were included. Seventy-eight (55%) (Median age 12 y [IQR 9–15]) underwent surgery: 1 had tonsillectomy; 1 tonsillectomy + uvulopharyngopalatoplasty (UPPP); 23 adenotonsillectomy (AT); 27 AT + UPPP; 11 adenoidectomy + UPPP; 8 UPPP; and 7 AT + turbinate trim ± tongue base suspension. Overall, surgery cured 19 children (26%), but AHI improved in the majority of children (p = 0.001). Similarly, the arousal index, PETCO2, and SpO2 nadir improved significantly (p < 0.002, p = 0.019, p < 0.001, respectively). AHI improved significantly in children with mild-to-moderate OSA in comparison to severe OSA (p < 0.001). Children with enlarged tonsils and no history of prior surgery benefitted more often from surgery (p < 0.004 and p = 0.002, respectively). AT was the only surgery reducing the AHI significantly (p = 0.008). Children did not lose weight despite intervention. Adherence with PAP was poor.
Conclusions:
Surgery improved OPSG indices in the majority of obese children with OSA.
Citation:
Com G, Carroll JL, Tang X, Melguizo MS, Bower C, Jambhekar S. Characteristics and surgical and clinical outcomes of severely obese children with obstructive sleep apnea. J Clin Sleep Med 2015;11(4):467–474.
Keywords: adenotonsillectomy, children, obesity, sleep apnea, positive airway pressure treatment, uvulopharyngopalatoplasty
Obstructive sleep apnea (OSA) in children is characterized by recurrent episodes of partial upper airway obstruction and/or intermittent airflow cessation during sleep and usually is accompanied by oxygen desaturation, hypercapnia, and arousals.1,2 An overnight polysomnography (OPSG) is considered the gold standard for the diagnosis of OSA in children.1,3 Adenotonsillar enlargement is the most common risk factor for pediatric OSA; however, other conditions likely impact airway patency during sleep. The prevalence of obesity among children in the US has increased at an alarming rate over the past decades. Obese individuals are at increased risk for developing OSA, and the degree of OSA is proportionate to the degree of obesity.1,4
OSA is associated with significant adverse outcomes, including poor school performance, hyperactivity, cognitive dysfunction, depressive symptoms, and excessive daytime sleepiness; therefore, effective treatment is important.1,5 Studies suggest that OSA improves after adenotonsillectomy (AT) in the majority of children with no comorbidities; however, 19% to 73% of children, especially those with obesity, may continue to have residual OSA.1,2,6,7 This large variability in the frequency of residual OSA following AT among studies is likely related to the fact that most of the studies did not differentiate between non-obese and obese children, had few subjects, included children with different age groups, and had different definitions of cure in each study. Studies evaluating the efficacy of AT in obese children with OSA indicate that apnea-hypopnea index (AHI) improves following AT.8–11 A recent meta-analysis concluded that AT improves but does not resolve OSA in the majority of obese children.12 However, more studies are needed to assess the effectiveness of AT in older children and adolescents with severe obesity.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Literature about severely obese children with obstructive sleep apnea (OSA) and their outcomes is limited. The efficacy of airway surgeries other than adenotonsillectomy for the treatment of OSA in this population is unknown.
Study Impact: Airway surgery yields significant improvement in severely obese children with OSA. Adenotonsillectomy is associated with significant improvement in AHI, and the success rate of surgery depends on the history of previous surgery, tonsil size, and OSA severity. Adherence with positive airway pressure treatment is poor.
Surgeries other than AT have been used for the treatment of OSA. Although the effectiveness of uvulopharyngopalatoplasty (UPPP) for the treatment of OSA is well studied in adults, the role of UPPP and other airway surgeries in pediatric population is not clear. The few published reports of UPPP in children are limited to children with neurodisability.13–15 Positive airway pressure (PAP) is the treatment of choice for most children with residual OSA and is effective when applied properly, although adherence to treatment is relatively poor.1,16–19
In this study, we aimed to describe the characteristics and surgical outcomes of a large case series of severely obese children and adolescents with OSA followed by our Pediatric Sleep Disorders Center. We specifically investigated the impact of AT alone on the severity of OPSG indices in obese children with OSA. We also studied the factors associated with the success rate of surgery. We investigated the efficacy of UPPP alone or in addition to AT for the treatment of OSA. Finally, we examined the adherence to PAP treatment in children with residual OSA.
METHODS
Study Subjects
This retrospective study was approved by the Institutional Review Board at the University of Arkansas for Medical Sciences. All obese children with OSA on a diagnostic OPSG (2000 through 2010) were included. Severe obesity was classified as a BMI z-score ≥ 2.33.20 Patients with comorbidities such as neuro-muscular disorders, dysmorphic syndromes, or chronic cardio-pulmonary problems were excluded. Demographics included age, gender, and ethnicity. History of previous upper airway surgery and follow-up in the weight management clinic was recorded.
Overnight Polysomnography (OPSG)
OPSG indices were measured using standard diagnostic criteria.21 Sleep architecture was scored by standard techniques.22 Respiratory events and arousals were defined according to the American Academy of Sleep Medicine Scoring Manual.21 OPSG variables included SpO2 nadir, percentage of time spent while SpO2 < 90%, peak end-tidal CO2 (PETCO2) levels, arousal index (AI), and obstructive apnea-hypopnea index (AHI). Obstructive AHI was defined as the number of obstructive apneas and hypopneas per hour of total sleep time. Mild OSA was defined as AHI ≥ 1.5 < 5/h, moderate OSA was defined as AHI ≥ 5 < 10/h, and severe OSA was defined as AHI ≥ 10/h. We defined cure as postsurgical obstructive AHI < 1.5/h, based on the recommended normative OPSG values for children aged 1 to 18 years in the review article of Beck and Marcus.23–25
Upper Airway Surgeries
Each patient with OSA was evaluated by a pediatric otolaryngologist. Tonsils were graded as absent, 1+ to 2+, and 3+ to 4+ based on the scheme proposed by Brodsky.26 Adenoid size was determined by the surgeon during surgery as the percentage of airway obstruction (absent, < 50%, and > 50%). Performed airway surgeries included tonsillectomy; AT; adenoidectomy + UPPP; UPPP alone; and UPPP + AT, turbinate trim, and tongue base suspension. Indications of these surgeries were not standardized and were based on the individual otolaryngologist's clinical decision. Surgical ineligibility was defined as either a patient with a previous history of AT or a physical examination showing absent or minimal adenotonsillar hypertrophy and/or elongated palate. The decision for specific surgery was made based on physical findings.
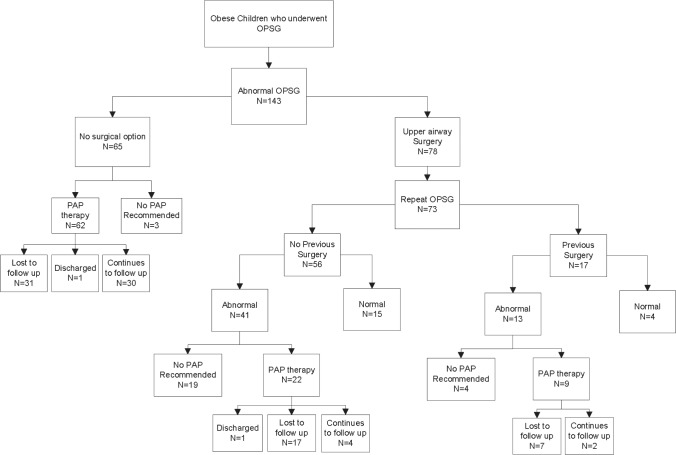
Patients with residual OSA who were recommended PAP were identified (Figure 1). PAP adherence was defined as the percentage of nights that PAP was used > 4 h. Based on current Medicare guidelines, adequate PAP adherence was defined as > 4 h PAP use/night for > 70% of nights.27 PAP adherence was objectively assessed with data from secure data memory cards in the PAP device.28 We classified adherence as “good” at > 4 h of nightly PAP use on > 70% of nights, as “borderline” at > 4 h of nightly PAP use on 50% to 70% of nights, and as “poor” with > 4 h of nightly PAP use on < 50% of nights.
Figure 1. The flowchart of the study description.
Sixty five of 143 children with OSA were not eligible for surgery and recommended PAP therapy except 3 children with mild OSA. Of 78 children who underwent airway surgery, 73 had a postsurgery OPSG. PAP therapy was recommended to 31 of 54 children with residual OSA.
Statistical Analysis
Data were analyzed using statistical software R version 2.15 (Vienna, Austria). Continuous variables are presented as medians, interquartile ranges, means, and standard deviations. Categorical variables are presented with frequencies and percentages. Spearman correlation coefficients were used to test the relationships between BMI and OPSG variables. Kruskal-Wallis test was used to compare continuous variables by the severity of OSA on the demographic variables. Wilcoxon and the Stuart-Maxwell tests were used to assess the significance of change in variables following surgery. Multivariable linear regression model was used to test the relationship between the postsurgical AHI and tonsils and adenoid size, age, and BMI z-scores. A paired two-sample t-test was used to assess the effect of a previous surgery on the AHI for patients with a previous surgery and for patients without a previous surgery. A p value < 0.05 was considered statistically significant.
RESULTS
Study Subjects
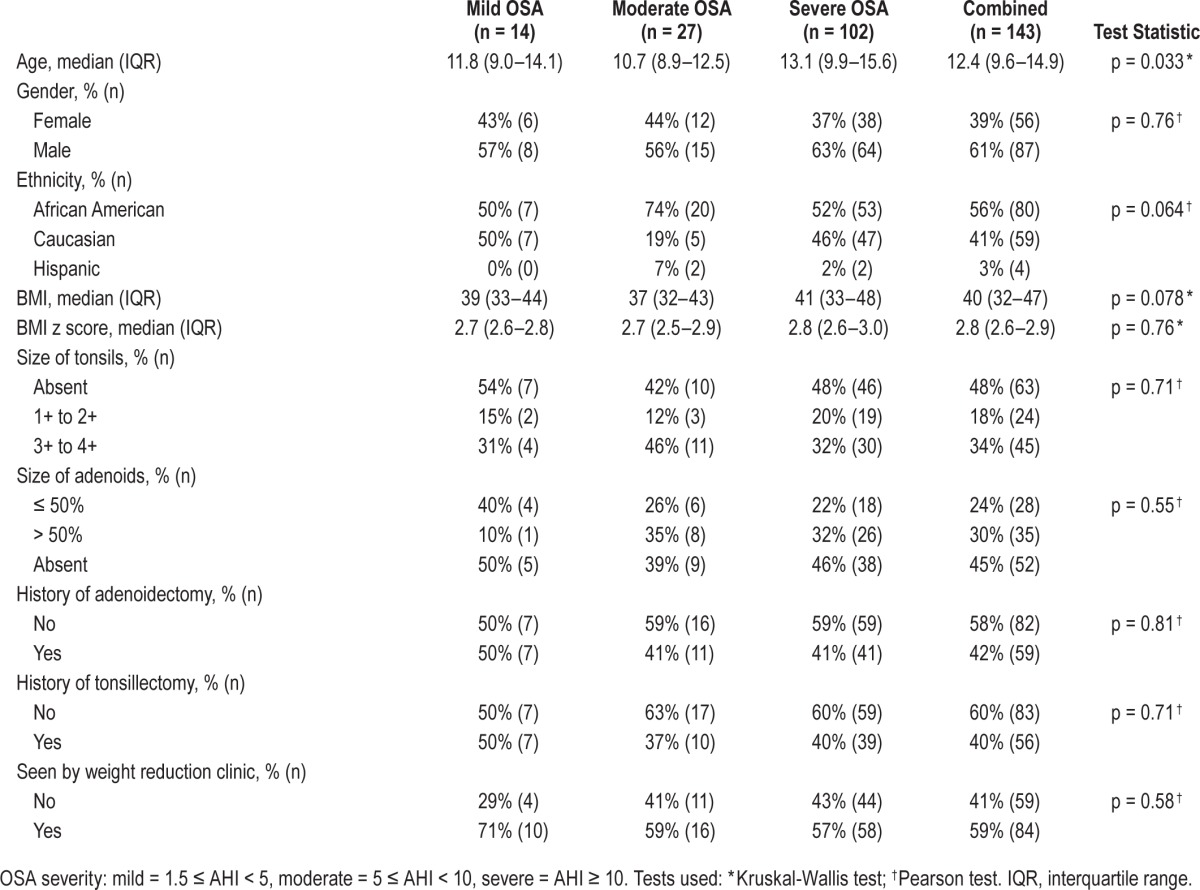
During the study period, 143 obese children (median age 12.4 years [interquartile range (IQR) 9.6–14.9] and 57 (40%) female) were diagnosed with OSA (Table 1). Of these children, 56% were African American, 41% Caucasian, and 3% Hispanic. Histories indicated 60 (42%) children had an adenoidectomy and 56 (39%) children had a tonsillectomy. Nineteen percent had a single BMI measurement, and 81% had multiple BMI measurements. The median BMI was 40 kg/m2 (IQR 32–47), and median BMI z-score was 2.8 (IQR 2.6–2.9). The median follow up time was 11 months (IQR 5 months – 3 years). Our weight management clinic followed 84 (59%) of the children, and of these children, 44 (52%) had dyslipidemia, 21 (25%) insulin resistance, and 18 (21%) nonalcoholic fatty liver. During the study period, only 2 children lost weight.
Table 1.
Characteristics of obese children diagnosed with mild to severe obstructive sleep apnea (OSA) after initial overnight polysomnography.
Overnight Polysomnography (OPSG)
We classified the 143 patients based on OSA severity: 14 (10%) with mild OSA, 27(19%) with moderate OSA and 102 (71%) with severe OSA (Table 1). The initial median AHI was 17/h (IQR 8.2–37.2), mean AHI was 30.7 ± 33.8, SpO2 nadir was 84% (IQR 77–89), and PETCO2 was 54 mm Hg (IQR 50–57). Although children with higher BMI tended to have more severe OSA, the association did not reach a significant level (p = 0.078). There was no association between the severity of AHI and gender, size of tonsils and adenoids, or ethnicity, but a positive association between AHI and older age (p = 0.033) existed.
Upper Airway Surgeries
Of 143 children with OSA, 65 children were not eligible for surgery. Of the 65 children not eligible for surgery, PAP was recommended for 62, and the remaining 3, who had mild OSA, were treated medically (Figure 1).
Surgery was performed on 78 of the children with OSA. Of these children, 17 (22%) had a history of previous airway surgery, 23 (30%) had (3+/4+) tonsils, and 23 (30%) had > 50% nasopharyngeal occlusion by adenoids observed during surgery. The median age at surgery was 12 years (IQR 9–15). One child had tonsillectomy alone, one had tonsillectomy and UPPP, 23 had AT (3 had previous adenoidectomy), 27 had AT + UPPP (1 had previous tonsillectomy), 11 had adenoidectomy + UPPP (5 had previous tonsillectomy), 8 had UPPP alone (7 had previous tonsillectomy ± adenoidectomy), and 7 had combined surgeries of adenoidectomy ± tonsillectomy ± turbinate trim ± tongue base suspension (1 had previous tonsillectomy).
Postsurgery OPSG was conducted for 73 of 78 (94%) children. Only 19 (26%) children were cured after surgery (AHI < 1.5/h). Although the majority of the children had residual OSA, the severity of OSA (AHI) decreased significantly after surgery (p < 0.001; Table 2). Similarly, the AI, PETCO2, and SpO2 nadir improved significantly (p < 0.002, p = 0.019, p < 0.001, respectively). Thirty-nine (53%) children had post-surgery AHI ≥ 5/h, and their AI and SpO2 did not improve significantly following surgery. The success rate of surgery was higher in children with mild and moderate OSA than severe OSA (p < 0.001). There was no significant improvement in AHI following additional surgery in 17 children with previous history of surgery (p = 0.1578), whereas AHI improved significantly in 61 children who did not have previous surgery (p < 0.0001; Figure 2). Improvement in AHI following surgery depended on the tonsil size (p = 0.02) but not on the adenoid size (p = 0.70).
Table 2.
Summary of overnight polysomnography variables before and after airway surgery (n = 73).
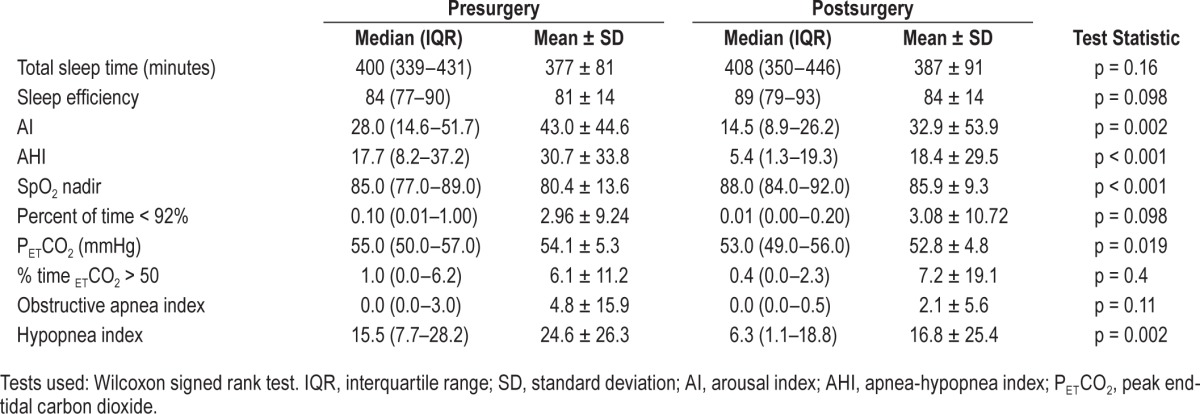
Figure 2. Figure compares presurgery and postsurgery AHI in children with no history of previous surgery (left) and with a history of previous upper airway surgery (right).
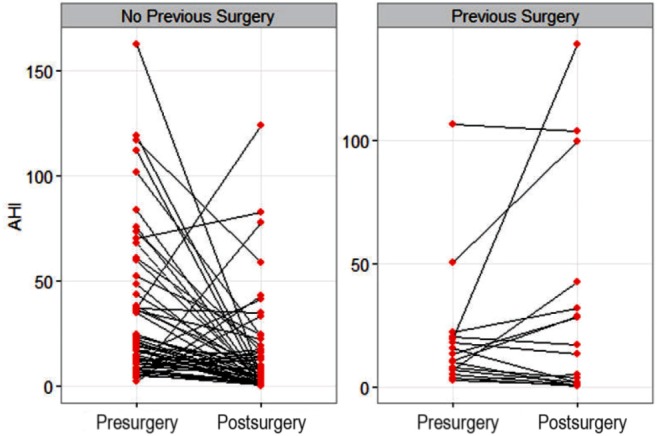
Postsurgery AHI depends on the presence of a previous surgery (p = 0.003).
When we investigated the impact of different surgeries on the severity of OSA, AT was associated with significant improvement in the severity of AHI (p = 0.008; Table 3). However, there was no difference in the cure rate (AHI < 1.5/h) between surgeries (p = 0.52). The cure rate was 12.5% in UPPP, 35% in AT, 18% in adenoidectomy + UPPP, and 18.5% in AT + UPPP.
Table 3.
Efficacy of different airway surgeries on the severity of obstructive sleep apnea (OSA) in obese pediatric patients.
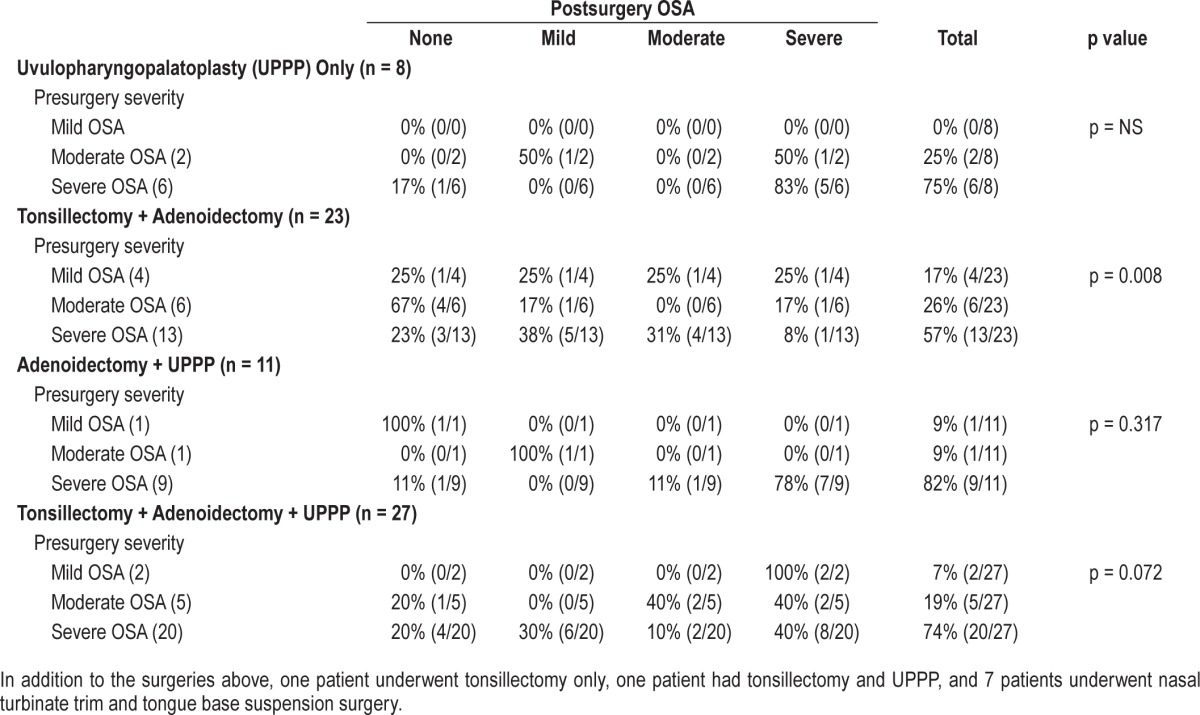
UPPP was mostly performed in children who had a history of previous surgery; 88% of children who underwent UPPP alone and 45% of children who had adenoidectomy + UPPP had a previous history of surgery. To investigate if UPPP was performed in addition to AT in children with smaller tonsil/ adenoid size, we compared children who had AT (n = 23) to children who had AT + UPPP (n = 27). There was no difference in demographics and the size of tonsils and adenoids between the two cohorts, except AT + UPPP was performed in older children (p = 0.02).
PAP Adherence
PAP was recommended to 93 children: 62 following the initial OPSG and 31 after the second OPSG (Figure 1). Of the children with PAP recommended, 50% of children receiving PAP recommendation after initial OPSG and 86% of children receiving PAP recommendation following surgery were lost to follow-up (16% within 3 months, 34% within a year, and 53% within 2 years; Figure 3). We found no difference in demographics, BMI, attendance to weight reduction clinic, and severity of OSA among children who continued follow-up versus lost to follow-up. Of 36 children who continued follow-up, only 11% had good PAP adherence. We found no association between adherence and ethnicity. Among children with good adherence, 67% were African American and 33% were Caucasian.
Figure 3. Patients with residual OSA and lost to follow up.
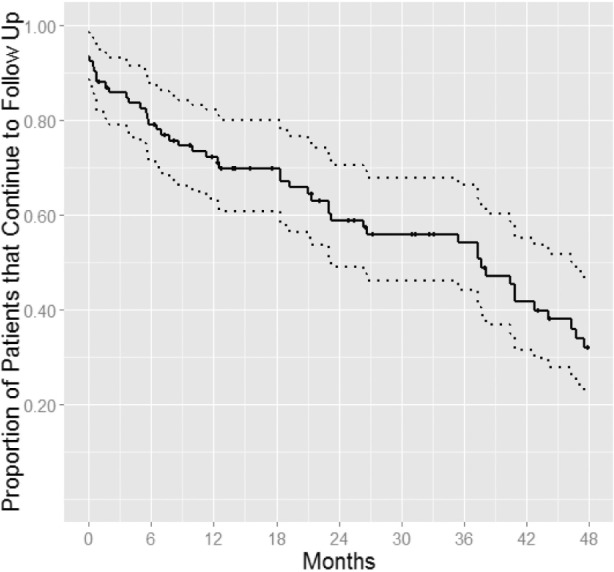
Kaplan-Meier curve shows the percentage of patients who were recommended PAP treatment and lost to follow-up over time.
DISCUSSION
This study shows that airway surgery yields significant improvement in OPSG indices in severely obese children and adolescents with OSA. Although surgery cured only 26% of children, if a cutoff AHI of < 5/h was used to define cure instead of < 1.5/h, almost half of the children would have been cured by surgery. The success rate of surgery was higher in children with mild and moderate OSA, children without history of previous surgery, and in children with enlarged tonsils. The current study was not designed to examine surgical efficacy; however, we found that among different types of surgeries, AT was associated with significant improvement in AHI. While UPPP alone was not effective for treatment of OSA in morbidly obese children, an added benefit of UPPP to AT could not be demonstrated by this study. Adherence with PAP was poor, and the majority of children with residual OSA for whom PAP was recommended were lost to follow-up.
The prevalence of obesity among adults and children in US has increased at an alarming rate.29 The epidemic is even more pronounced in south-central states, with obesity rates among children and adults exceeding the national average for obesity rates.30 Obesity is an independent risk factor for snoring and OSA.1 The risk of OSA among obese children has increased four- to five-fold, and for every increment in BMI of 1 kg/m2 beyond the mean BMI for age and gender, the risk of OSA increases by 12%.4 In our study, we did not find a significant association between BMI and AHI, probably because the majority of our subjects were severely obese. A few studies have demonstrated significant improvement in the severity of OSA in obese adolescents who lost weight either in a residential weight loss program31 or following bariatric surgery.32 In our study, none of the 84 subjects who attended a weight reduction clinic lost weight.
Hypertrophy of adenotonsillar tissue is a major contributor to the development of OSA in children, and AT is the primary treatment of this condition.1 However, the outcome of AT may not be as favorable as expected, particularly when OSA is severe and/or when obesity is present.2,32,33 Studies examining efficacy and outcome of AT in pediatric OSA have demonstrated large variability in the frequency of residual OSA after AT, ranging from 19% to 73%.10,32–36 Most of these studies did not differentiate between non-obese and obese children, had few subjects, and had variable results depending on the definition of cure.1 Shine et al. studied 19 obese patients with OSA who underwent AT; although OSA improved significantly, only 37% were cured (AHI < 5/h).9 A meta-analysis investigated 110 obese children with OSA from four studies (mean sample size, 27.5; mean BMI z-score, 2.81).12 The mean pre- and post-adenotonsillectomy AHIs were 29.4/h and 10.3/h, respectively. The criterion for cure was AHI < 5/hour in 3 of 4 studies, and 88% of children would have evidence of residual OSA if AHI < 1/hour had been used.1 Using an AHI < 1/h as the cutoff, a retrospective multi-center study showed that only 157 children (mean BMI z-score 1.35 ± 1.74; age 6.9 ± 3.8 years) of 578 (27.2%) normalized their breathing patterns during sleep. The study also found that age, BMI, and pre-AT AHI were factors influencing the postsurgery AHI.2 Although our subjects were severely obese (median z-score 2.8) and older (median age of surgery 12 y), beyond the age when adenotonsillar hypertrophy prevalence is high, AT resulted in complete resolution (AHI < 1.5/h) of OSA in 26% children, and in conversion of severe OSA to mild to moderate OSA in another 29%. If we had used an AHI < 5/h as the definition of “complete resolution,” our cohort's cure rate would have been 46%, higher than rates reported in previous studies. Similar to the study by Bhattacharjee et al.,2 we found that the severity of OSA was an important factor influencing postsurgical AHI. In addition, we also found that tonsil size and history of prior surgery also influenced the surgical outcome.
Our data support the current recommendation of AT as the first-line treatment of OSA for even severely obese children and adolescents. There are few reports investigating the efficacy of airway surgeries other than AT for the treatment of OSA in children.13–15 Combined surgeries of AT, turbinectomy and/ or septoplasty, tongue-hyoid advancement, uvulopalatoplasty, conventional mandibular advancement, distraction osteogenesis of the mandible, and tongue reduction in 18 neurologically compromised children showed a significant improvement of OSA following surgery.14 A recent report of tongue base suspension surgery in children with cerebral palsy showed that moderate to severe OSA in this population may safely benefit from the added technique of tongue base suspension.15 UPPP has been successfully performed in adults with OSA,37,38 but there are limited data available for this procedure in children. Our study suggests that UPPP alone may not be beneficial for the treatment of OSA in obese children. The reason for this observation may well be selection of patients as our children who underwent UPPP were older, had a history of previous surgery, and had more severe OSA. In addition, the sample size was small. On the other hand, although all of our children were severely obese, were older, and had severe OSA, our cure rate of 26% is very similar to the study of 578 non-obese children with OSA who underwent AT resulting in a cure rate of 27.2%.2 Since the majority of our children had UPPP ± AT, we can speculate that adding UPPP to AT may be beneficial in selective cases. Prospective studies with high number of patients are needed to study the effect of UPPP in pediatric patients with OSA ± obesity.
PAP is considered the second line of treatment in children with unresolved OSA after AT.1 While this intervention appears to be safe in children, extensive behavioral conditioning is needed to achieve adequate adherence. Data from adult studies indicate that 5% to 50% of patients discontinue PAP therapy in the first week of treatment.27 Long-term adherence has been reported to range widely from 17% to 71%, with a cutoff point of 4 hours of use per night.39,40 Adherence with PAP in children varies depending on the population studied, definitions used, and technique used for measuring adherence.16 Adherence rate is particularly low when PAP is prescribed for adolescents with OSA.41 Reports have shown that African American adults and children are less likely to use their PAP therapy.40 However, we did not find an association between ethnicity and poor adherence. The low adherence rate in our subjects is compatible with previous studies. It is interesting that 30 of 62 children (48%) with “no surgical option” and recommended PAP continued to follow up in the clinic, whereas in the surgery group only 6 of 31 children (19%) who were recommended PAP continued to follow up. This significant difference can be partially explained by possible bias of the family/child that surgery would cure the OSA. Patients/families are probably more inclined to accept PAP if they know surgery is not an option. In addition, since OPSG indices (AHI, AI, and SpO2) improved following surgery in the majority of children, these children might have functioned better following surgery and did not feel that PAP was necessary. Future prospective studies might investigate quality of life or neurocognitive outcomes as measures of success of surgery knowing that 100% cure based on AHI < 1.5/h is hard to achieve and the PAP adherence is low.
Limitations
Our study was not designed to examine surgical efficacy but rather to describe a case series of obese children with OSA treated in our institution. Although patients who underwent AT appeared to be associated with the highest cure rate compared to other surgeries, the cure rates were not found to be significantly different among the four surgery groups (p = 0.52); this may be due to inadequate power of the study related to the limited sample size. Although our dataset included all severely obese children with OSA over 10 years, we still observed a relatively small sample size, particularly in the UPPP alone group. Our children were evaluated by several surgeons, and there was no standardized approach to identify children eligible for specific surgeries. UPPP was performed in older children and in the ones with severe OSA suggesting selection bias. A prospective multicenter study with sufficient sample size is needed for validating our results and further conclusion.
CONCLUSION
Airway surgery improves AHI in severely obese children and adolescents with OSA, although complete resolution of OSA occurs in only 26% of patients. AT is associated with significant improvement in AHI. The success rate of surgery depends on history of previous surgery, size of tonsils, and severity of OSA. Although an added benefit of UPPP in addition to AT cannot be demonstrated by this study, it may be effective in selected cases. Adherence with PAP is poor similar to the non-obese population. Our findings of low adherence and failure in weight management despite follow-up in a specialized center suggest that future efforts should focus on prevention of obesity and related complications and improving adherence.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. The work was performed at Arkansas Children's Hospital, Little Rock, AR.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AT
adenotonsillectomy
- BMI
body mass index
- OPSG
overnight polysomnography
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- UPPP
uvulopharyngopalatoplasty
REFERENCES
- 1.Marcus CL, Brooks LJ, Draper KA, et al. American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:e714–55. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. 2010;182:676–83. doi: 10.1164/rccm.200912-1930OC. [DOI] [PubMed] [Google Scholar]
- 3.Jambhekar S, Carroll JL. Diagnosis of pediatric obstructive sleep disordered breathing: beyond the gold standard. Expert Rev Respir Med. 2008;2:791–809. doi: 10.1586/17476348.2.6.791. [DOI] [PubMed] [Google Scholar]
- 4.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children: associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–32. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 5.Gozal D, Kheirandish-Gozal L. Neurocognitive and behavioral morbidity in children with sleep disorders. Curr Opin Pulm Med. 2007;13:505–9. doi: 10.1097/MCP.0b013e3282ef6880. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell RB, Kelly J. Outcomes of adenotonsillectomy for obstructive sleep apnea in obese and normal-weight children. Otolaryngol Head Neck Surg. 2007;137:43–8. doi: 10.1016/j.otohns.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Friedman M, Wilson M, Lin HC, Chang HW. Updated systematic review of tonsillectomy and adenoidectomy for treatment of pediatric obstructive sleep apnea/hypopnea syndrome. Otolaryngol Head Neck Surg. 2009;140:800–8. doi: 10.1016/j.otohns.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 8.Apostolidou MT, Alexopoulos EI, Chaidas K, et al. Obesity and persisting sleep apnea after adenotonsillectomy in Greek children. Chest. 2008;134:1149–55. doi: 10.1378/chest.08-1056. [DOI] [PubMed] [Google Scholar]
- 9.Shine NP, Lannigan FJ, Coates HL, Wilson A. Adenotonsillectomy for obstructive sleep apnea in obese children: effects on respiratory parameters and clinical outcome. Arch Otolaryngol Head Neck Surg. 2006;132:1123–7. doi: 10.1001/archotol.132.10.1123. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell RB, Kelly J. Adenotonsillectomy for obstructive sleep apnea in obese children. Otolaryngol Head Neck Surg. 2004;131:104–8. doi: 10.1016/j.otohns.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien LM, Sitha S, Baur LA, Waters KA. Obesity increases the risk for persisting obstructive sleep apnea after treatment in children. Int J Pediatr Otorhinolaryngol. 2006;70:1555–60. doi: 10.1016/j.ijporl.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Costa DJ, Mitchell R. Adenotonsillectomy for obstructive sleep apnea in obese children: a meta-analysis. Otolaryngol Head Neck Surg. 2009;140:455–60. doi: 10.1016/j.otohns.2008.12.038. [DOI] [PubMed] [Google Scholar]
- 13.Kosko J R, Derkay CS. Uvuloalatopharyngoplasty: treatment of obstructive sleep apnea in neurologically impaired pediatric patients. Int J Pediatr Otorhinolaryngol. 1995;32:241–6. doi: 10.1016/0165-5876(95)01178-e. [DOI] [PubMed] [Google Scholar]
- 14.Cohen SR, Lefaivre JF, Burstein FD, et al. Surgical treatment of obstructive sleep apnea in neurologically compromised patients. Plast Reconstr Surg. 1997;99:638–46. doi: 10.1097/00006534-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Hartzell LD, Guillory RM, Munson PD, et al. Tongue base suspension in children with cerebral palsy and obstructive sleep apnea. Int J Pediatr Otorhinolaryngol. 2013;77:534–7. doi: 10.1016/j.ijporl.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Harford KL, Jambhekar S, Com G, et al. Behaviorally based adherence program for pediatric patients treated with positive airway pressure. Clin Child Psychol Psychiatry. 2013;18:151–63. doi: 10.1177/1359104511431662. [DOI] [PubMed] [Google Scholar]
- 17.Marcus CL, Rosen G, Ward SL, et al. Adherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apnea. Pediatrics. 2006;117:e442–51. doi: 10.1542/peds.2005-1634. [DOI] [PubMed] [Google Scholar]
- 18.O'Donnell AR, Bjornson CL, Bohn SG, Kirk VG. Compliance rates in children using noninvasive continuous positive airway pressure. Sleep. 2006;29:651–8. [PubMed] [Google Scholar]
- 19.Uong EC, Epperson M, Bathon SA, Jeffe DB. Adherence to nasal positive airway pressure therapy among school-aged children and adolescents with obstructive sleep apnea syndrome. Pediatrics. 2007;120:e1203–11. doi: 10.1542/peds.2006-2731. [DOI] [PubMed] [Google Scholar]
- 20.Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, Curtin LR. Characterizing extreme values of body mass index-for-age by using the 2000 Centers for Disease Control and Prevention growth charts. Am J Clin Nutr. 2009;90:1314–20. doi: 10.3945/ajcn.2009.28335. [DOI] [PubMed] [Google Scholar]
- 21.Iber C, Ancoli-Israel S, Chesson A, Quan S. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM Manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 22.Rechtschaffen A, Kales A. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 23.Beck SE, Marcus CL. Pediatric polysomnography. Sleep Med Clin. 2009:393–406. doi: 10.1016/j.jsmc.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R. Polysomnographic values in children 2-9 years old: additional data and review of the literature. Pediatr Pulmonol. 2005;40:22–30. doi: 10.1002/ppul.20236. [DOI] [PubMed] [Google Scholar]
- 25.Witmans MB, Keens TG, Davidson Ward SL, Marcus CL. Obstructive hypopneas in children and adolescents: normal values. Am J Respir Crit Care Med. 2003;168:1540. doi: 10.1164/ajrccm.168.12.954. [DOI] [PubMed] [Google Scholar]
- 26.Ng SK, Lee DL, Li AM, Wing YK, Tong MC. Reproducibility of clinical grading of tonsillar size. Arch Otolaryngol Head Neck Surg. 2010;136:159–62. doi: 10.1001/archoto.2009.170. [DOI] [PubMed] [Google Scholar]
- 27.Weaver TE. Adherence to positive airway pressure therapy. Curr Opin Pulm Med. 2006;12:409–13. doi: 10.1097/01.mcp.0000245715.97256.32. [DOI] [PubMed] [Google Scholar]
- 28.Jambhekar SK, Com G, Tang X, et al. Role of a respiratory therapist in improving adherence to positive airway pressure treatment in a pediatric sleep apnea clinic. Respir Care. 2013;58:2038–44. doi: 10.4187/respcare.02312. [DOI] [PubMed] [Google Scholar]
- 29.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet. 2002;360:473–82. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- 30.Raczynski JM, Thompson JW, Phillips MM, Ryan KW, Cleveland HW. Arkansas Act 1220 of 2003 to reduce childhood obesity: its implementation and impact on child and adolescent body mass index. J Public Health Policy. 2009;30:S124–40. doi: 10.1057/jphp.2008.54. [DOI] [PubMed] [Google Scholar]
- 31.Verhulst SL, Franckx H, Van Gaal L, De Backer W, Desager K. The effect of weight loss on sleep-disordered breathing in obese teenagers. Obesity (Silver Spring) 2009:1178–83. doi: 10.1038/oby.2008.673. [DOI] [PubMed] [Google Scholar]
- 32.Kalra M, Inge T, Garcia V, et al. Obstructive sleep apnea in extremely overweight adolescents undergoing bariatric surgery. Obes Res. 2005;13:1175–9. doi: 10.1038/oby.2005.139. [DOI] [PubMed] [Google Scholar]
- 33.Tauman R, Gulliver TE, Krishna J, et al. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J Pediatr. 2006;149:803–8. doi: 10.1016/j.jpeds.2006.08.067. [DOI] [PubMed] [Google Scholar]
- 34.Brietzke SE, Gallagher The effectiveness of tonsillectomy and adenoidectomy in the treatment of pediatric obstructive sleep apnea/hypopnea syndrome: a meta-analysis. Otolaryngol Head Neck Surg. 2006;134:979–84. doi: 10.1016/j.otohns.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell RB. Adenotonsillectomy for obstructive sleep apnea in children: outcome evaluated by pre- and postoperative polysomnography. Laryngoscope. 2007;117:1844–54. doi: 10.1097/MLG.0b013e318123ee56. [DOI] [PubMed] [Google Scholar]
- 36.Guilleminault C, Li K, Quo S, Inouye RN. A prospective study on the surgical outcomes of children with sleep-disordered breathing. Sleep. 2004;27:95–100. [PubMed] [Google Scholar]
- 37.Millman RP, Carlisle CC, Rosenberg C, Kahn D, McRae R, Kramer NR. Simple predictors of uvulopalatopharyngoplasty outcome in the treatment of obstructive sleep apnea. Chest. 2000;118:1025–30. doi: 10.1378/chest.118.4.1025. [DOI] [PubMed] [Google Scholar]
- 38.Boot H, van Wegen R, Poublon RM, Bogaard JM, Schmitz PI, van der Meché FG. Long-term results of uvulopalatopharyngoplasty for obstructive sleep apnea syndrome. Laryngoscope. 2000;110:469–75. doi: 10.1097/00005537-200003000-00027. [DOI] [PubMed] [Google Scholar]
- 39.Stuck BA, Leitzbach S, Maurer JT. Effects of continuous positive airway pressure on apnea-hypopnea index in obstructive sleep apnea based on long-term compliance. Sleep Breath. 2012;16:467–71. doi: 10.1007/s11325-011-0527-8. [DOI] [PubMed] [Google Scholar]
- 40.Sawyer AM, Gooneratne NS, Marcus CL, et al. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;6:343–56. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beebe DW, Byars KC. Adolescents with obstructive sleep apnea adhere poorly to positive airway pressure (PAP), but PAP users show improved attention and school performance. PLoS One. 2011;6:e16924. doi: 10.1371/journal.pone.0016924. [DOI] [PMC free article] [PubMed] [Google Scholar]