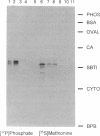
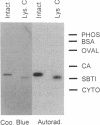
Abstract
Although the actions of insulin and a number of growth factors that signal via protein-tyrosine kinase receptors are believed to involve increased phosphorylation of key intracellular proteins, relatively few of the downstream phosphoproteins have been identified. In this report we describe a cDNA encoding one of the most prominent insulin-stimulated phosphoproteins in rat adipocytes. The cDNA encodes a protein, designated PHAS-I, which has 117 amino acids and a M(r) of 12,400. When translated in vitro and subjected to SDS/PAGE, PHAS-I migrates anomalously, having an apparent M(r) of 21,000. The predicted amino acid composition is interesting in that approximately 45% of the PHAS-I protein is accounted for by only four amino acids--serine, threonine, proline, and glycine. The PHAS-I gene is expressed in a variety of tissues, although the highest levels of mRNA are present in fat and skeletal muscle, two of the most insulin-responsive tissues. The nucleotide and deduced amino acid sequences of PHAS-I differ from any that have been reported, and homology screening provided no clues concerning the function of the protein. However, in view of its tissue distribution and the fact that the protein is phosphorylated in response to insulin, we speculate that PHAS-I is important in insulin action.
Full text
PDF

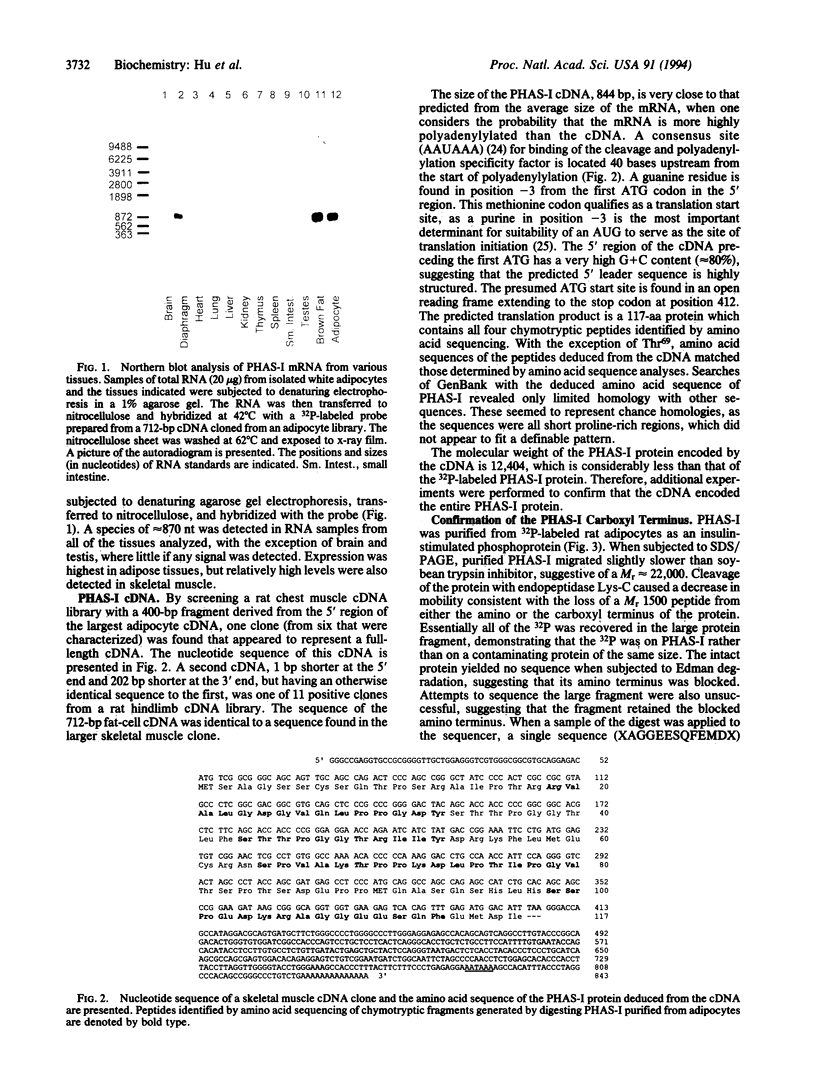


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. G. MAP kinases--ubiquitous signal transducers and potentially important components of the cell cycling machinery in eukaryotes. Cell Signal. 1992 May;4(3):239–246. doi: 10.1016/0898-6568(92)90063-e. [DOI] [PubMed] [Google Scholar]
- Avruch J., Alexander M. C., Palmer J. L., Pierce M. W., Nemenoff R. A., Blackshear P. J., Tipper J. P., Witters L. A. Role of insulin-stimulated protein phosphorylation in insulin action. Fed Proc. 1982 Aug;41(10):2629–2633. [PubMed] [Google Scholar]
- Avruch J., Leone G. R., Martin D. B. Effects of epinephrine and insulin on phosphopeptide metabolism in adipocytes. J Biol Chem. 1976 Mar 10;251(5):1511–1515. [PubMed] [Google Scholar]
- Avruch J., Tornqvist H. E., Gunsalus J. R., Price D. J., Kyriakis J. M., Yurkow E. J., Ahmad M. F., Banerjee P. The role of tyrosine-and serine-threonine-protein phosphorylation in insulin action. Adv Second Messenger Phosphoprotein Res. 1990;24:295–300. [PubMed] [Google Scholar]
- Benjamin W. B., Singer I. Actions of insulin, epinephrine, and dibutyryl cyclic adenosine 5'-monophosphate on fat cell protein phosphorylations. Cyclic adenosine 5'-monophosphate dependent and independent mechanisms. Biochemistry. 1975 Jul 29;14(15):3301–3309. doi: 10.1021/bi00686a003. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Nemenoff R. A., Avruch J. Insulin and growth factors stimulate the phosphorylation of a Mr-22000 protein in 3T3-L1 adipocytes. Biochem J. 1983 Jul 15;214(1):11–19. doi: 10.1042/bj2140011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear P. J., Nemenoff R. A., Avruch J. Preliminary characterization of a heat-stable protein from rat adipose tissue whose phosphorylation is stimulated by insulin. Biochem J. 1982 Jun 15;204(3):817–824. doi: 10.1042/bj2040817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cobb M. H., Boulton T. G., Robbins D. J. Extracellular signal-regulated kinases: ERKs in progress. Cell Regul. 1991 Dec;2(12):965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews C. M., Erikson R. L. Extracellular signals and reversible protein phosphorylation: what to Mek of it all. Cell. 1993 Jul 30;74(2):215–217. doi: 10.1016/0092-8674(93)90411-i. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Brownsey R. W., Belsham G. J. A partial view of the mechanism of insulin action. Diabetologia. 1981 Oct;21(4):347–362. doi: 10.1007/BF00252681. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Midgley P. J., Rutter G. A., Thomas A. P., McCormack J. G. Studies into the mechanism whereby insulin activates pyruvate dehydrogenase complex in adipose tissue. Ann N Y Acad Sci. 1989;573:285–296. doi: 10.1111/j.1749-6632.1989.tb15005.x. [DOI] [PubMed] [Google Scholar]
- Diggle T. A., Denton R. M. Comparison of the effects of insulin and adrenergic agonists on the phosphorylation of an acid-soluble 22 kDa protein in rat epididymal fat-pads and isolated fat-cells. Biochem J. 1992 Mar 15;282(Pt 3):729–736. doi: 10.1042/bj2820729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle T. A., Schmitz-Peiffer C., Borthwick A. C., Welsh G. I., Denton R. M. Evidence that insulin activates casein kinase 2 in rat epididymal fat-cells and that this may result in the increased phosphorylation of an acid-soluble 22 kDa protein. Biochem J. 1991 Oct 15;279(Pt 2):545–551. doi: 10.1042/bj2790545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J., DeMott M., Atherton D., Mische S. M. Internal protein sequence analysis: enzymatic digestion for less than 10 micrograms of protein bound to polyvinylidene difluoride or nitrocellulose membranes. Anal Biochem. 1992 Mar;201(2):255–264. doi: 10.1016/0003-2697(92)90336-6. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka T., Cooper D. R., Farese R. V. Insulin stimulates the translocation of protein kinase C in rat adipocytes. FEBS Lett. 1989 Nov 6;257(2):337–340. doi: 10.1016/0014-5793(89)81565-0. [DOI] [PubMed] [Google Scholar]
- Kennelly P. J., Krebs E. G. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991 Aug 25;266(24):15555–15558. [PubMed] [Google Scholar]
- Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991 Nov;115(4):887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larner J. Insulin and the stimulation of glycogen synthesis. The road from glycogen structure to glycogen synthase to cyclic AMP-dependent protein kinase to insulin mediators. Adv Enzymol Relat Areas Mol Biol. 1990;63:173–231. doi: 10.1002/9780470123096.ch3. [DOI] [PubMed] [Google Scholar]
- Lawrence J. C., Jr, Hiken J. F., James D. E. Phosphorylation of the glucose transporter in rat adipocytes. Identification of the intracellular domain at the carboxyl terminus as a target for phosphorylation in intact-cells and in vitro. J Biol Chem. 1990 Feb 5;265(4):2324–2332. [PubMed] [Google Scholar]
- Lawrence J. C., Jr, James C. Activation of glycogen synthase by insulin in rat adipocytes. Evidence of hormonal stimulation of multisite dephosphorylation by glucose transport-dependent and -independent pathways. J Biol Chem. 1984 Jun 25;259(12):7975–7982. [PubMed] [Google Scholar]
- Lawrence J. C., Jr Signal transduction and protein phosphorylation in the regulation of cellular metabolism by insulin. Annu Rev Physiol. 1992;54:177–193. doi: 10.1146/annurev.ph.54.030192.001141. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Ramakrishna S., Benjamin W. B. Insulin stimulates phosphorylation of a heat-stable protein in rat adipose tissue. Biochem Biophys Res Commun. 1983 Dec 28;117(3):758–764. doi: 10.1016/0006-291x(83)91662-5. [DOI] [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Sachs A., Wahle E. Poly(A) tail metabolism and function in eucaryotes. J Biol Chem. 1993 Nov 5;268(31):22955–22958. [PubMed] [Google Scholar]
- Sevetson B. R., Kong X., Lawrence J. C., Jr Increasing cAMP attenuates activation of mitogen-activated protein kinase. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10305–10309. doi: 10.1073/pnas.90.21.10305. [DOI] [PMC free article] [PubMed] [Google Scholar]