Abstract
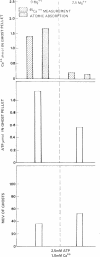
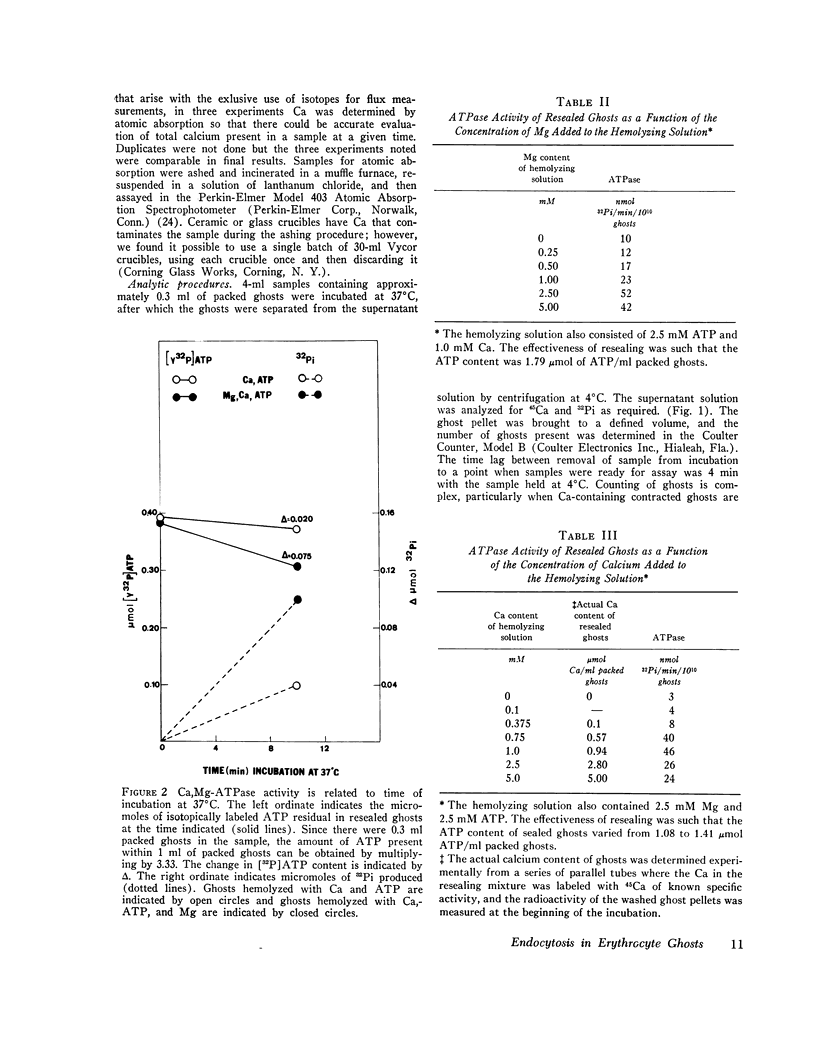
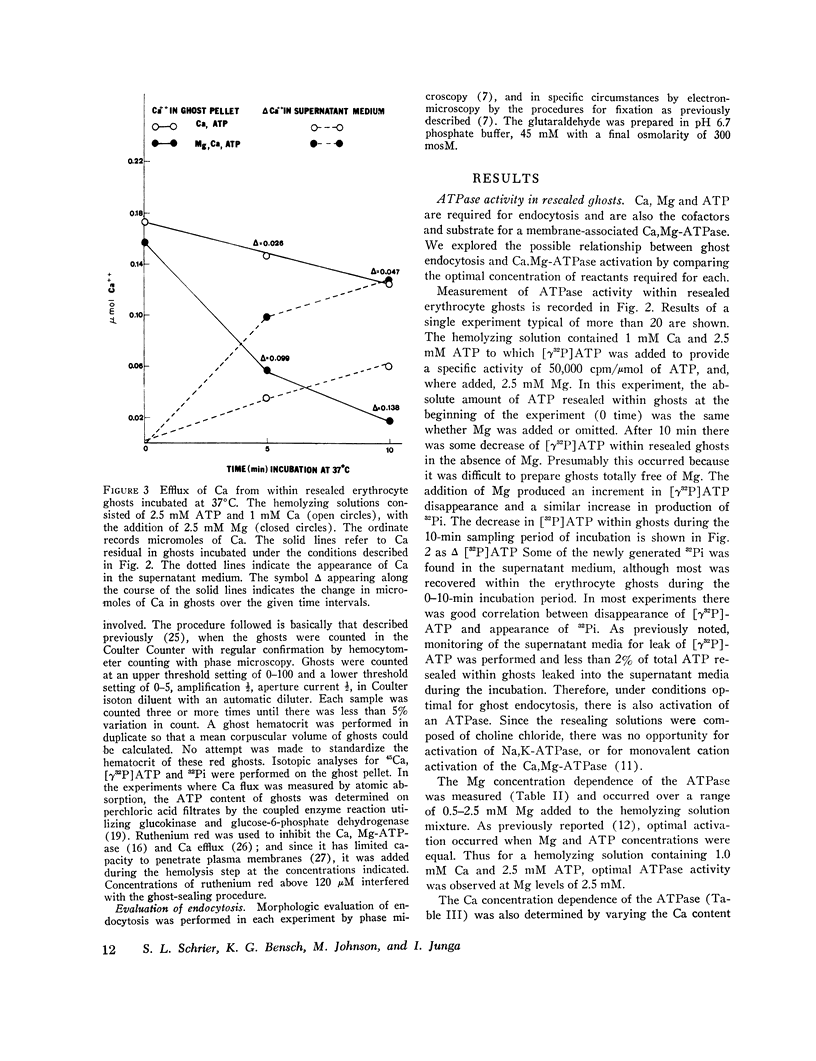
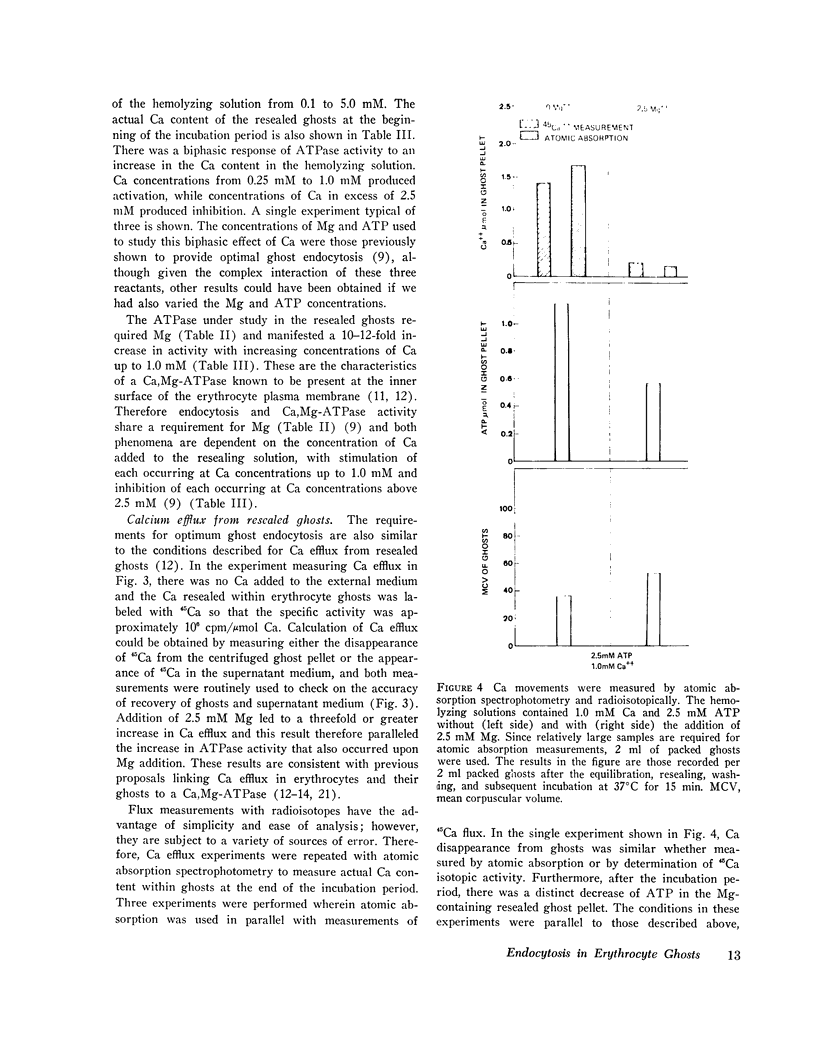
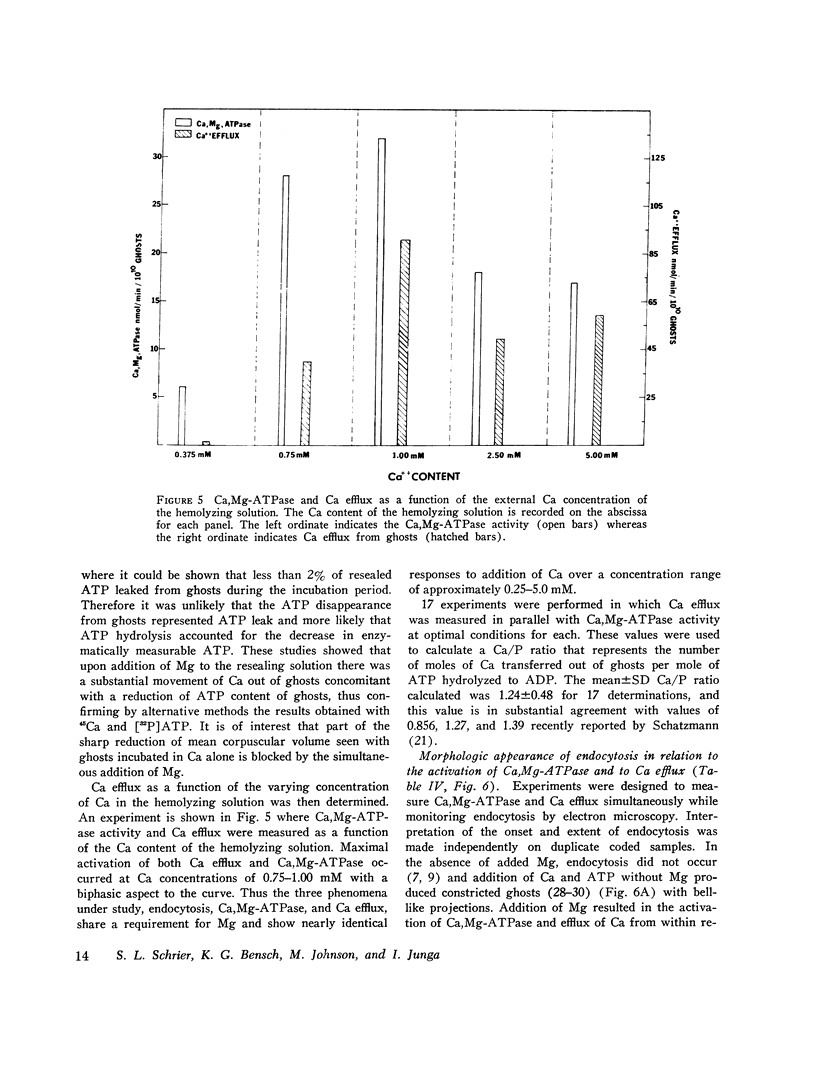
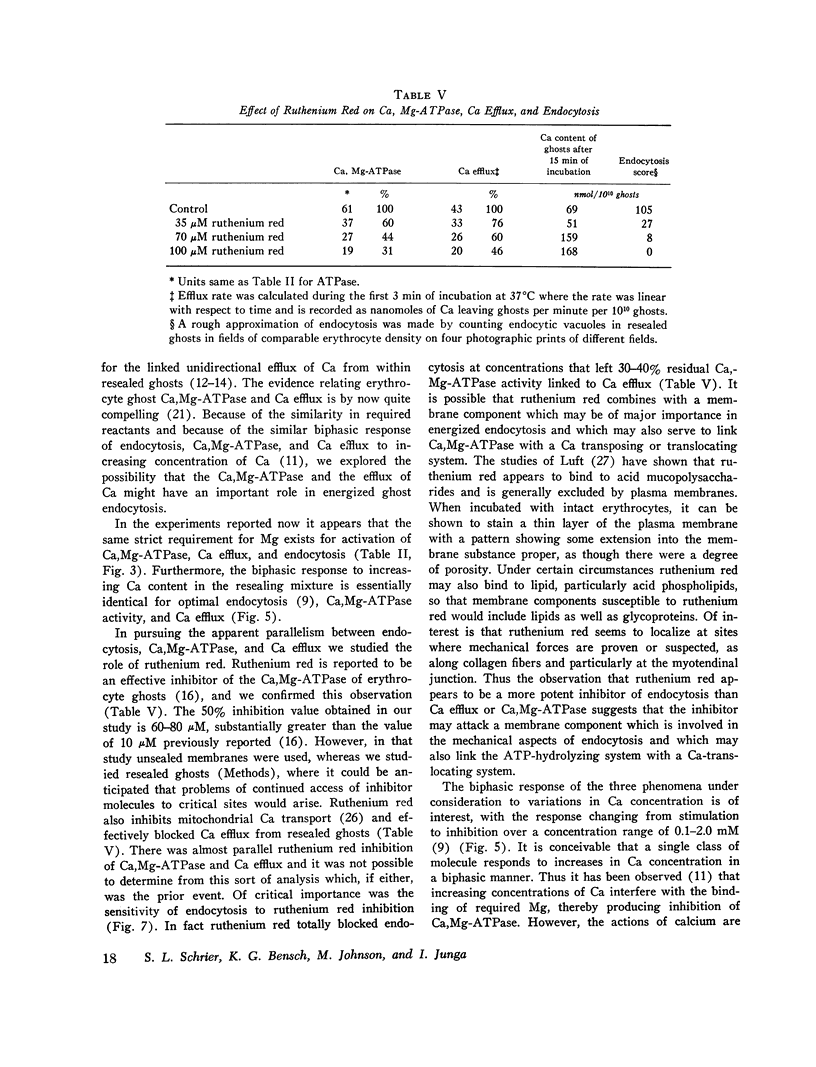
The mechanism of endocytosis in resealed human erythrocyte ghosts was studied. The energy for endocytosis or micropinocytosis appears to be derived from Mg-ATP, and membrane internalization is preceded by activation of a membrane-associated Ca,Mg-ATPase and by the active efflux of Ca. Endocytosis, Ca,Mg-ATPase activity, and active Ca efflux all require the presence of Mg. Furthermore, these three phenomena, endocytosis, Ca,Mg-ATPase activity, and active Ca extrusion, all have a concentration dependence on Ca such that low concentrations stimulate and higher concentrations inhibit the phenomena. The optimal concentration of Ca is identical for endocytosis, active Ca efflux, and Ca,Mg-ATPase. Morphologic studies indicated that while active Ca efflux and activation of the Ca,Mg-ATPase activity occurred promptly upon onset of incubation, there was a significant time delay before endocytosis occurred, which suggests that endocytosis additionally involved a more slowly functioning mechanicochemical mechanism. Ruthenium red, a specific inhibitor of Ca,Mg-ATPase and Ca transport, inhibited endocytosis in a concentration-related manner. Prostaglandins E1 and E2 had no measurable effect on ghost endocytosis, active Ca efflux, or Ca,Mg-ATPase activity.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. E., Valeri C. R. Prostaglandins in hematology. Arch Intern Med. 1974 Jan;133(1):86–96. [PubMed] [Google Scholar]
- Ben-Bassat I., Bensch K. G., Schrier S. L. Drug-induced erythrocyte membrane internalization. J Clin Invest. 1972 Jul;51(7):1833–1844. doi: 10.1172/JCI106985. [DOI] [PMC free article] [PubMed] [Google Scholar]
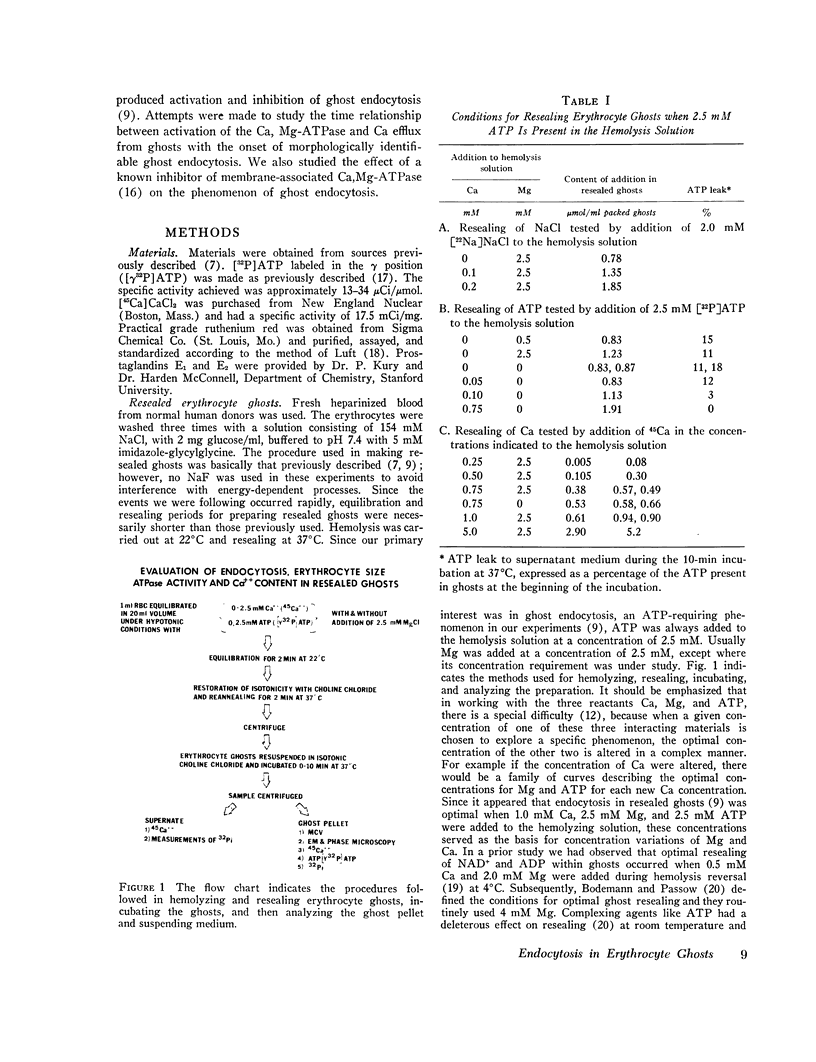
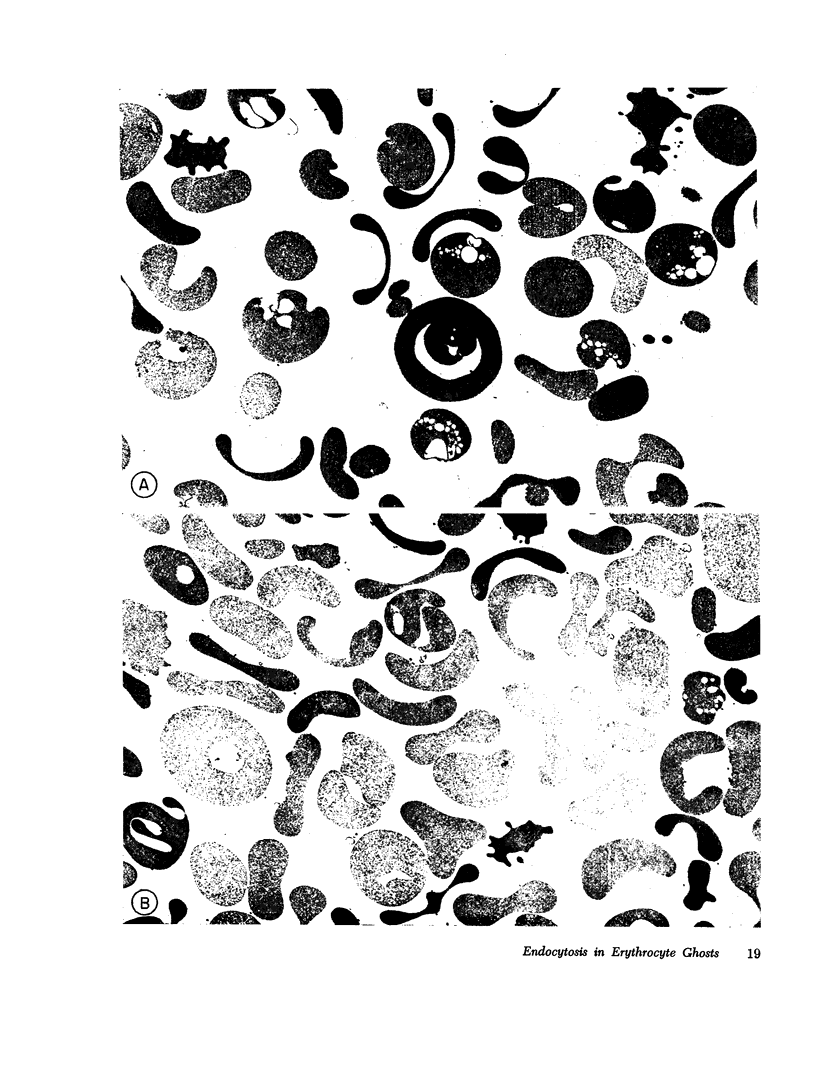
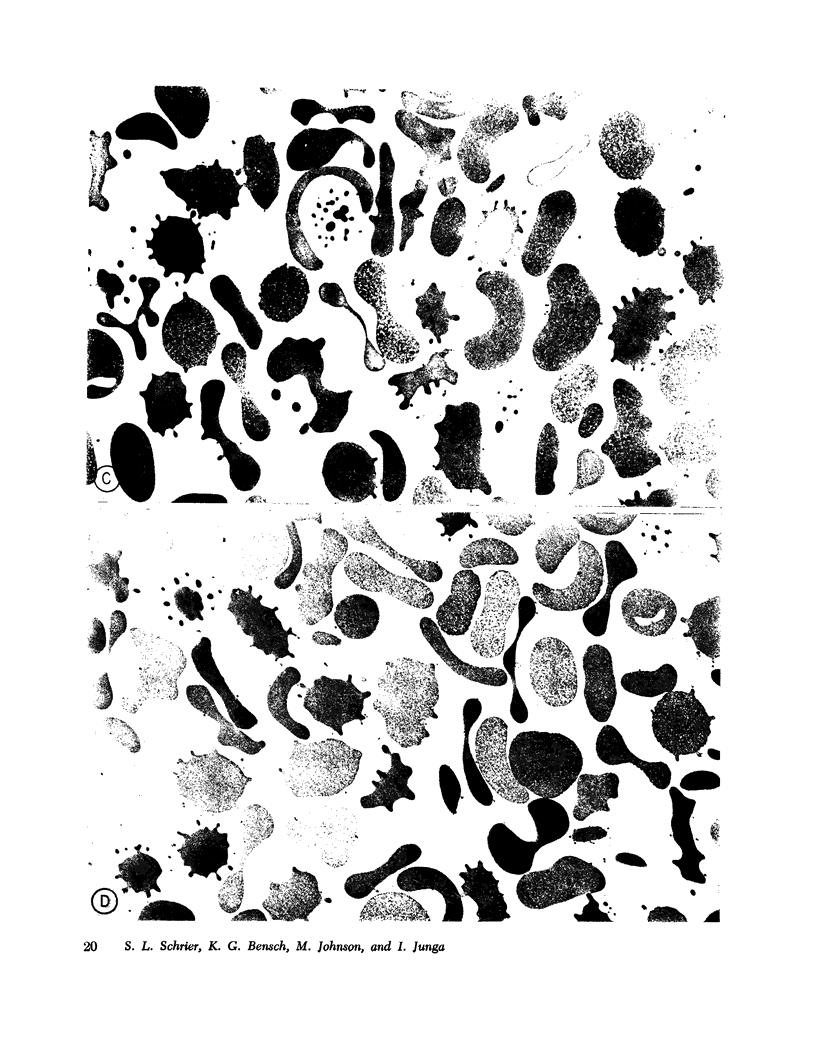
- Bodemann H., Passow H. Factors controlling the resealing of the membrane of human erythrocyte ghosts after hypotonic hemolysis. J Membr Biol. 1972;8(1):1–26. doi: 10.1007/BF01868092. [DOI] [PubMed] [Google Scholar]
- Bramley T. A., Coleman R. Effects of inclusion of Ca 2+ , Mg 2+ , EDTA or EGTA during the preparation of erythrocyte ghosts by hypotonic haemolysis. Biochim Biophys Acta. 1972 Dec 1;290(1):219–228. doi: 10.1016/0005-2736(72)90065-x. [DOI] [PubMed] [Google Scholar]
- Fujii T., Sato T., Hanzawa T. Calcium and magnesium contents of mammalian erythrocyte membranes. Chem Pharm Bull (Tokyo) 1973 Jan;21(1):171–175. doi: 10.1248/cpb.21.171. [DOI] [PubMed] [Google Scholar]
- Ginn F. L., Hochstein P., Trump B. F. Membrane alterations in hemolysis: Internalization of plasmalemma induced by primaquine. Science. 1969 May 16;164(3881):843–845. doi: 10.1126/science.164.3881.843. [DOI] [PubMed] [Google Scholar]
- Harrison D. G., Long C. The calcium content of human erythrocytes. J Physiol. 1968 Dec;199(2):367–381. doi: 10.1113/jphysiol.1968.sp008658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyde C. P., Gardner F. H. Acquisition of autophagic vacuoles by human erythrocytes. Physiological role of the spleen. Blood. 1970 Nov;36(5):566–575. [PubMed] [Google Scholar]
- Holroyde C. P., Oski F. A., Gardner F. H. The "pocked" erythrocyte. Red-cell surface alterations in reticuloendothelial immaturity of the neonate. N Engl J Med. 1969 Sep 4;281(10):516–520. doi: 10.1056/NEJM196909042811002. [DOI] [PubMed] [Google Scholar]
- Katsumata Y., Asai J. Ultrastructural changes of erythrocyte ghosts having no connection with hydrolysis of ATP. Arch Biochem Biophys. 1972 May;150(1):330–332. doi: 10.1016/0003-9861(72)90043-4. [DOI] [PubMed] [Google Scholar]
- Kent G., Minick O. T., Volini F. I., Orfei E. Autophagic vacuoles in human red cells. Am J Pathol. 1966 May;48(5):831–857. [PMC free article] [PubMed] [Google Scholar]
- Kury P. G., Ramwell P. W., McConnell H. M. The effect of prostaglandins E1 and E2 on the human erythrocyte as monitored by spin labels. Biochem Biophys Res Commun. 1974 Jan 23;56(2):478–483. doi: 10.1016/0006-291x(74)90867-5. [DOI] [PubMed] [Google Scholar]
- LaCelle P. L. Alteration of membrane deformability in hemolytic anemias. Semin Hematol. 1970 Oct;7(4):355–371. [PubMed] [Google Scholar]
- Lee K. S., Shin B. C. Studies on the active transport of calcium in human red cells. J Gen Physiol. 1969 Dec;54(6):713–729. doi: 10.1085/jgp.54.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. II. Fine structural localization in animal tissues. Anat Rec. 1971 Nov;171(3):369–415. doi: 10.1002/ar.1091710303. [DOI] [PubMed] [Google Scholar]
- Moore C. L. Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochem Biophys Res Commun. 1971 Jan 22;42(2):298–305. doi: 10.1016/0006-291x(71)90102-1. [DOI] [PubMed] [Google Scholar]
- Palek J., Curby W. A., Lionetti F. J. Size dependence of ghosts from stored erythrocytes on calcium and adenosine triphosphate. Blood. 1972 Aug;40(2):261–275. [PubMed] [Google Scholar]
- Palek J., Stewart G., Lionetti F. J. The dependence of shape of human erythrocyte ghosts on calcium, magnesium, and adenosine triphosphate. Blood. 1974 Oct;44(4):583–597. [PubMed] [Google Scholar]
- Penniston J. T., Green D. E. The conformational basis of energy transformations in membrane systems. IV. Energized states and pinocytosis in erythrocyte ghosts. Arch Biochem Biophys. 1968 Nov;128(2):339–350. doi: 10.1016/0003-9861(68)90040-4. [DOI] [PubMed] [Google Scholar]
- Poste G., Allison A. C. Membrane fusion. Biochim Biophys Acta. 1973 Dec 28;300(4):421–465. doi: 10.1016/0304-4157(73)90015-4. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J. Dependence on calcium concentration and stoichiometry of the calcium pump in human red cells. J Physiol. 1973 Dec;235(2):551–569. doi: 10.1113/jphysiol.1973.sp010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzmann H. J., Vincenzi F. F. Calcium movements across the membrane of human red cells. J Physiol. 1969 Apr;201(2):369–395. doi: 10.1113/jphysiol.1969.sp008761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier S. L. ATP synthesis in human erythrocyte membranes. Biochim Biophys Acta. 1967 Sep 9;135(4):591–598. doi: 10.1016/0005-2736(67)90091-0. [DOI] [PubMed] [Google Scholar]
- Schrier S. L., Giberman E., Danon D., Katchalski E. Studies on ATPase in sheared micro vesicles of human erythrocyte membranes. Biochim Biophys Acta. 1970;196(2):263–273. doi: 10.1016/0005-2736(70)90014-3. [DOI] [PubMed] [Google Scholar]
- Schrier S. L., Junga I., Seeger M. Vacuole formation in human erythrocyte ghosts. Proc Soc Exp Biol Med. 1973 Jun;143(2):565–567. doi: 10.3181/00379727-143-37367. [DOI] [PubMed] [Google Scholar]
- Schrier S. L. Organization of enzymes in human erythrocyte membranes. Am J Physiol. 1966 Jan;210(1):139–145. doi: 10.1152/ajplegacy.1966.210.1.139. [DOI] [PubMed] [Google Scholar]
- Triplett R. B., Wingate J. M., Carraway K. L. Calcium effects on erythrocyte membrane proteins. Biochem Biophys Res Commun. 1972 Nov 15;49(4):1014–1020. doi: 10.1016/0006-291x(72)90313-0. [DOI] [PubMed] [Google Scholar]
- Watson E. L., Vincenzi F. F., Davis P. W. Ca 2+ -activated membrane ATPase: selective inhibition by ruthenium red. Biochim Biophys Acta. 1971 Dec 3;249(2):606–610. doi: 10.1016/0005-2736(71)90140-4. [DOI] [PubMed] [Google Scholar]
- Weed R. I., LaCelle P. L., Merrill E. W. Metabolic dependence of red cell deformability. J Clin Invest. 1969 May;48(5):795–809. doi: 10.1172/JCI106038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner M. L., Lee K. S. Active calcium ion uptake by inside-out and right side-out vesicles of red blood cell membranes. J Gen Physiol. 1972 Apr;59(4):462–475. doi: 10.1085/jgp.59.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L., Bryan J., Ruby A., Mazia D. Precipitation of proteins by vinblastine and calcium ions. Proc Natl Acad Sci U S A. 1970 Jul;66(3):807–814. doi: 10.1073/pnas.66.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wins P., Schoffeniels E. ATP+ca++-linked contraction of red cell ghosts. Arch Int Physiol Biochim. 1966 Nov;74(5):812–820. doi: 10.3109/13813456609059954. [DOI] [PubMed] [Google Scholar]
- Wins P., Schoffeniels E. Studies on red-cell ghost ATPase systems: properties of a (Mg2+ + Ca2+)-dependent ATPase. Biochim Biophys Acta. 1966 Jul 13;120(3):341–350. doi: 10.1016/0926-6585(66)90301-3. [DOI] [PubMed] [Google Scholar]