Abstract
Purpose: Childhood brain tumor (BT) survivors experience significant neurocognitive sequelae that affect health-related quality of life (HRQOL). A model of neurodevelopmental late effects and family functioning in childhood cancer survivors suggests associations between survivor neurocognitive functioning, family functioning, and survivor HRQOL. This study examines the concurrent associations between survivor neurocognitive functioning, family functioning, and survivor emotional HRQOL, and the indirect effects of neurocognitive functioning on survivor emotional HRQOL through family functioning.
Methods: Participants included young adult-aged childhood BT survivors (18–30 years old; N=34) who were on average 16 years post-diagnosis, and their mothers. A brief neuropsychological battery assessed working and verbal memory, processing speed, and executive functioning. Survivors and mothers completed measures of family functioning, and mothers completed a proxy-report measure of survivor HRQOL.
Results: Spearman bivariate correlations examined the associations between indices of survivor neurocognitive functioning and concurrent family functioning and survivor emotional HRQOL. Poorer survivor processing speed, working memory, verbal memory, and executive function were significantly associated with worse survivor- and mother-reported family functioning (r's range: 0.36–0.58). Additionally, worse survivor processing speed and executive function were significantly associated with poorer survivor emotional HRQOL (r's range: 0.44–0.48). Bootstrapping analyses provided evidence for the indirect effects of neurocognitive functioning on survivor emotional HRQOL through family functioning.
Conclusion: These findings suggest that family functioning is an important variable that might mitigate the negative influence of neurocognitive late effects on survivors and is a potential target in future interventions.
Keywords: : brain tumor, neurocognitive late effects, families, quality of life
Improved survival rates for childhood brain tumors (BT) have led to more survivors aging into young adulthood and increased the need to address disease- and treatment-related sequelae. Young adult (YA) survivors of childhood BT may not attain expected developmental milestones due to significant medical1–3 and neurocognitive late effects.4 They have the poorest emotional health-related quality of life (HRQOL) among childhood cancer survivors5 and are less likely to be married, have a college degree, be employed, or live independently than controls.6,7 These psychosocial difficulties, combined with complex medical late effects,1–3 place significant demands on survivors' families.8–10
Neurocognitive deficits likely significantly contribute to these poor psychosocial outcomes. Childhood BT survivors experience neurocognitive late effects across multiple domains4,11 that often hinder survivor autonomy12,13 and are associated with poorer psychosocial functioning.14 In addition to declines in intellectual functioning (IQ),4 survivors demonstrate deficits in attention, memory, and processing speed.15–19 Factors such as age at diagnosis,20,21 tumor location,22–24 and the modality and toxicity of tumor-directed treatments11 influence the type and severity of late effects.
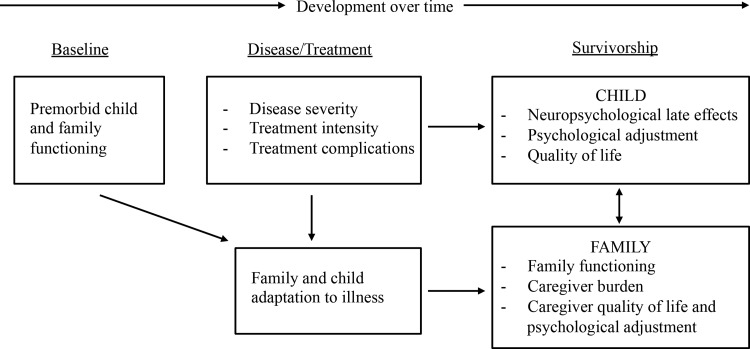
Consistent with a systems perspective of childhood cancer,25,26 the neurocognitive deficits of childhood BT survivors exist within a family context and may have associations with family outcomes. A theoretical model of neurodevelopmental late effects in childhood cancer highlights the importance of families and suggests associations between survivor neurocognitive late effects, family adaptation and functioning, and survivor HRQOL (Fig. 1).27 The model proposes bidirectional associations between survivor neurocognitive functioning and family functioning as well as associations between family functioning and survivor HRQOL. The model suggests that general family functioning variables (e.g., cohesion, communication) and family adaptation to late effects are related to survivor adjustment and HRQOL.
FIG. 1.
Model of neurodevelopmental late effects and family functioning in pediatric cancer survivors. Adapted from: Peterson CC, Drotar D. Family impact of neurodevelopmental late effects in survivors of pediatric cancer: review of research, clinical evidence, and future directions. Clin Child Psychol Psychiatry. 2006;11(3):349–66.
The associations between survivor neurocognitive late effects, family functioning, and HRQOL are likely complex. Survivor neurocognitive difficulties may increase family management of daily tasks and require close family involvement during adulthood.27 Although there may be direct associations between survivor neurocognitive late effects and survivor emotional HRQOL, the model in Figure 1 suggests indirect associations between survivor neurocognitive functioning and emotional HRQOL through family functioning. Positive family adaptation to survivor late effects and better overall family functioning may enhance the ability to successfully manage these demands and promote better survivor emotional HRQOL.28
Although research has examined the interrelations between child and family functioning in pediatric traumatic brain injury (TBI),29,30 little research has examined these associations in pediatric BT survivors in general and no studies have focused on YA survivors. One study of school-aged BT survivors found that a combination of illness and family factors, such as family stress level and family structure, best predicted child IQ.31
Given the strong presence of family systems in theoretical models of youth's health26,27 and the evidence highlighting family functioning in pediatric TBI,29,30 further research is warranted in YA survivors of childhood BTs. The purpose of the current study was to examine the concurrent associations between survivor neurocognitive functioning, family functioning, and survivor emotional HRQOL and test the indirect effects of survivor neurocognitive functioning on survivor emotional HRQOL through family functioning. Specific hypotheses were: (1) poorer survivor neurocognitive functioning will be associated with worse family functioning; (2) poorer survivor neurocognitive functioning will be related to lower survivor emotional HRQOL; and (3) survivor neurocognitive functioning will have significant indirect effects on survivor emotional HRQOL through family functioning.
Methods
Participants
Participants were pediatric BT survivors aged 18–30 at study and their mothers who were a subsample of participants who had participated in an earlier parent study on caregiving for pediatric BT survivors.32 Participation in the current study occurred approximately 18–24 months after the earlier study. Eligibility criteria included being more than 5 years post-diagnosis and more than 2 years from the end of treatment, and residing at least part-time with his/her mother. Exclusion criteria included cognitive deficits prior to the BT and inability to complete study tasks (e.g., blind).
Procedure
An institutional review board approved all study procedures.
Participant recruitment
Survivors and their mothers who participated in the earlier study on caregiving were recruited for the current study. Families were sent a letter and then contacted by phone to discuss the study and schedule a data collection appointment. Participants received a brief letter summarizing their performance on the neurocognitive battery. The sample from the larger study has been described elsewhere.32 Of the 71 mother–survivor dyads that participated in the earlier study and were available for this study, 23 were ineligible for the current study for a variety of reasons: no longer living part-time with his/her mother (n=11), not within age range (n=4), significant visual impairments (n=3), pre-existing cognitive deficits (n=2), and survivor was deceased (n=1) or had recurrence and resumed treatment (n=1). Fifteen of the potential mother–survivor dyads were never reached despite multiple attempts. No mother–survivor dyad actively declined participation.
The sample of mothers included a range of socioeconomic backgrounds and is considered representative of the original sample. There were no significant demographic differences between participants in the current sample and participants in the original sample32 or between those who participated in the current study and those who were eligible but did not participate.
Data collection
Data collection occurred either in survivors' homes or in the oncology clinic of a large children's hospital. Informed consent was obtained from both survivors and their mothers prior to beginning study procedures.
Measures
Survivor neurocognitive function
The Wechsler Adult Intelligence Scale, Fourth Edition (WAIS-IV)33 assessed survivor auditory working memory and processing speed. Survivors completed the Digit Span and Letter-Number Sequencing subtests (whose scaled scores combine to form the Working Memory Index score), as well as the Coding and Symbol Search subtests, which provided the Processing Speed Index score. The California Verbal Learning Test, Second Edition, Short Form (CVLT-II SF)34 assessed survivor auditory verbal memory. The long-delay recall standard score served as the measure of verbal memory in analyses.
Two stand-alone tests from the Delis–Kaplan Executive Function System (D-KEFS)35 assessed survivor executive function: the Trail Making Test and the Tower Test. The Trail Making Test measures flexibility of thinking on a visual-motor sequencing task; the scaled score from the switching task was used in analyses. The Tower Test is a measure of planning and problem-solving; the scaled achievement score from the Tower Test was used in analyses. Significant correlations with other widely used measures of executive function demonstrate the validity of these subtests.35
Emotional quality of life
Mothers completed the Pediatric Oncology Quality of Life Scale (POQOLS)36 as the measure of survivor emotional HRQOL (subsequently referred to as “HRQOL” in Analyses and Results). The POQOLS is a 21-item proxy measure of HRQOL that was validated in school-aged children with cancer who were both on and off treatment. Although not specifically designed for YAs, the POQOLS was chosen for the current study due to its use in the earlier parent study. The POQOLS includes three factors that comprise a total score: physical functioning, emotional distress, and response to medical treatments. This study focused only on the emotional distress subscale in analyses due to its theoretical associations with neurocognitive and family functioning.27 Scores for this scale range from 6–42, with higher scores indicating poorer emotional HRQOL. The emotional distress scale has been significantly related to both internalizing and externalizing behavior problems in children being treated for cancer.36 Internal consistency for the POQOLS emotional distress scale was 0.81.
Family functioning
Both survivors and mothers completed the 12-item General Functioning Scale from the Family Assessment Device (FAD GFS),37 a well-established measure of general family functioning with excellent psychometric properties.38 The FAD GFS encompasses the seven dimensions of McMaster's model of family functioning (e.g., problem-solving, communication, roles, affective responsiveness).39 Scores range from 0–4, with higher scores indicating higher levels of general family dysfunction. Scores above 2.0 indicate problematic family functioning. Internal consistencies for the survivors' and mothers' FAD GFS scores were 0.84 and 0.92, respectively.
The PedsQL Family Impact Module (PedsQL FIM)40 Family Functioning summary score assessed the impact of the survivor's health on family functioning. This scale is the average of the Daily Activities and Family Relationships scales. On these scales, mothers rated how much difficulty their families have with completing daily activities (e.g., household tasks) or with family relationships (e.g., communication or conflict) as a result of their survivors' health. Scores range from 0–100, with higher scores representing better family functioning. The PedsQL FIM Family Functioning scale has demonstrated strong psychometric properties in previous studies with chronic illness populations41,42 and has been related to functional disability in pediatric pain41 and to severity of ADHD.42 Internal consistency for the PedsQL FIM Family Functioning scale was 0.94.
Treatment intensity
As part of the earlier parent study,32 the Intensity of Treatment Rating scale43,44 was modified and pilot-tested to rate the intensity of tumor-directed treatments for pediatric BT survivors. For each survivor, two investigators rated the intensity of the treatment (inter-rater reliability κ=0.97) on a 5-point ordinal scale from the least intensive to the most intensive. Treatments were rated as: (1) minimal—resection only; (2) average—focal radiation and/or non-intensive chemotherapy; (3) moderate—moderate chemotherapy with or without focal radiation but no craniospinal radiation; (4) intensive—craniospinal radiation with or without moderate, non-intensive chemotherapy OR high-dose chemotherapy with stem cell rescue; and (5) most intensive—craniospinal radiation and intensive chemotherapy with stem cell rescue.
Data analyses
Spearman bivariate correlations examined associations between indices of survivor neurocognitive functioning and concurrent family functioning and survivor HRQOL due to the non-normal distributions of the data in some of the variables (working memory, processing speed, Trail Making Test switching, PedsQL FIM Family Functioning, and POQOLS emotional distress). Bootstrapping procedures for mediation directly tested the indirect effects of survivor neurocognitive functioning on survivor HRQOL through family functioning using bootstrap methods.45,46 Bootstrapping involves drawing repeated samples, or iterations, from the data in order to produce multiple estimates of indirect effects.47 This approach has been validated and is now the preferred method for estimating indirect effects45,46,48 and addresses some of the shortcomings associated with Baron and Kenny's multiple regression approach,49 including improving power and reducing the probability of Type I and II errors.47 Additionally, the bootstrapping procedure is a nonparametric approach that allows for non-normal distributions and smaller sample sizes.45,47,48,50 Each bootstrapping model used 10,000 iterations. Bootstrapping determines significant mediation by finding that the bias-corrected bootstrap 95% confidence intervals do not contain zero.
Results
There were 34 survivor–mother dyads. Participants were diagnosed between the ages of 0 and 15 years old and were an average 16 years from diagnosis. Survivors were generally evenly split across gender and were mainly Caucasian (73.5%). The sample included a variety of diagnoses, including primitive neuroectodermal tumors and low-grade tumors. Half of the sample received radiation therapy. Table 1 has information on sample characteristics.
Table 1.
Participant Demographics
| Measure | n | % | Mean | SD |
|---|---|---|---|---|
| Gender | ||||
| Male | 16 | 47.1 | ||
| Female | 18 | 52.9 | ||
| Race | ||||
| Caucasian | 25 | 73.5 | ||
| African American | 7 | 20.6 | ||
| Asian | 2 | 5.9 | ||
| Age at study | 23.53 | 3.36 | ||
| Age at diagnosis | 7.36 | 4.64 | ||
| Diagnosis | ||||
| PNET | 11 | 32.4 | ||
| Low-grade astrocytoma | 10 | 29.4 | ||
| Low-grade glioma | 7 | 20.6 | ||
| Craniopharyngioma | 4 | 11.8 | ||
| Other | 2 | 5.8 | ||
| Tumor location | ||||
| Infratentorial | 17 | 50.0 | ||
| Cortex (supratentorial) | 9 | 26.5 | ||
| Midline (supratentorial) | 8 | 23.5 | ||
| Received radiation therapy | 17 | 50.0 | ||
| Treatment intensitya | ||||
| 1. Minimal—resection only | 9 | 26.5 | ||
| 2. Average—focal radiation±non-intensive chemotherapy | 13 | 38.2 | ||
| 3. Moderate—moderate chemotherapy±focal radiation, but no craniospinal radiation | 1 | 2.9 | ||
| 4. Intensive—craniospinal radiation±moderate non-intensive chemotherapy OR high-dose chemotherapy with stem cell rescue | 10 | 29.4 | ||
| 5. Most intensive—craniospinal radiation and intensive chemotherapy and stem cell rescue | 1 | 2.9 | ||
| Household income | ||||
| <$40,000 | 8 | 23.5 | ||
| $40,000–$100,000 | 11 | 32.4 | ||
| >$100,000 | 15 | 44.1 | ||
| Survivor employment | ||||
| Full-time | 8 | 23.5 | ||
| Part-time | 8 | 23.5 | ||
| Unemployed | 18 | 52.9 | ||
| Attending school | 9 | 26.5 | ||
| Federal financial support | 14 | 41.2 | ||
| Maternal demographics | ||||
| Age | 53.74 | 5.67 | ||
| Partnered relationship | 22 | 64.7 | ||
Based on the Intensity of Treatment Rating scale.
PNET, primitive neuroectodermal tumor; SD, standard deviation.
Descriptive and preliminary analyses (Table 2)
Table 2.
Mean Scores for Primary Variables
| Variables | Range | Mean (SD) |
|---|---|---|
| Survivor HRQOL (POQOLS) | 6–28 | 12.91 (6.57) |
| FAD GFS—survivor report | 1–2.92 | 1.88 (0.49) |
| FAD GFS—mother report | 1–2.83 | 1.67 (0.52) |
| PedsQL FIM Family Functioning score | 25.83–100 | 74.21 (22.64) |
| WAIS-IV Processing Speed Index score | 50–122 | 80.41 (19.58) |
| WAIS-IV Working Memory Index score | 53–142 | 90.79 (19.85) |
| CVLT-II SF Long Delay z-score | −4.00–1.00 | −1.18 (1.18) |
| D-KEFS Trail Making Test switching scaled score | 1–14 | 5.88 (4.66) |
| D-KEFS Tower Test achievement scaled score | 2–13 | 7.97 (3.32) |
CVLT-II SF, California Verbal Learning Test, Second Edition, Short Form; D-KEFS, Delis-Kaplan Executive Function System; FAD GFS, Family Assessment Device General Functioning Scale; HRQOL, health-related quality of life; PedsQL FIM, PedsQL Family Impact Module; POQOLS, Pediatric Oncology Quality of Life Scale; SD, standard deviation; WAIS-IV, Wechsler Adult Intelligence Scale, Fourth Edition.
In general, mothers reported good survivor HRQOL. On the FAD GFS, both survivors and mothers reported levels of family functioning below the cutoff that would indicate problematic family functioning. Mothers reported moderate-to-low family functioning as a result of their survivor's health on the PedsQL FIM Family Functioning scale. Survivor working memory was in the average range, while survivor processing speed and long-term verbal memory were in the low-average range. Survivor scores on performance-based measures of executive function revealed borderline mental flexibility abilities (D-KEFS Trail Making Test switching scaled score) and low-average problem-solving abilities (D-KEFS Tower Test achievement scaled score).
Correlational analyses (Table 3)
Table 3.
Correlations Between Health-Related Quality of Life, Family Functioning, and Domains of Neurocognitive Function
| Variables | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|
| 1. Survivor HRQOL | 0.13 | 0.40* | −0.61** | −0.47** | −0.25 | −0.30 | −0.44** | −0.22 |
| 2. FAD GFS—survivor report | — | 0.09 | −0.38* | −0.42* | −0.42* | −0.35* | −0.25 | −0.39* |
| 3. FAD GFS—mother report | — | — | −0.50** | −0.05 | −0.25 | −0.14 | −0.17 | −0.07 |
| 4. PedsQL FIM Family Functioning score | — | — | — | 0.55** | 0.39* | 0.43* | 0.55** | 0.39* |
| 5. WAIS-IV Processing Speed Index score | — | — | — | — | 0.64** | 0.56** | 0.80** | 0.51** |
| 6. WAIS-IV Working Memory Index score | — | — | — | — | — | 0.52** | 0.77** | 0.54** |
| 7. CVLT-II Long Delay z-score | — | — | — | — | — | — | 0.57** | 0.34* |
| 8. D-KEFS Trail Making Test switching scaled score | — | — | — | — | — | — | — | 0.46** |
| 9. D-KEFS Tower Test achievement scaled score | — | — | — | — | — | — | — | — |
Note. *p<0.05; **p<0.01.
CVLT-II SF, California Verbal Learning Test, Second Edition, Short Form; D-KEFS, Delis-Kaplan Executive Function System; FAD GFS, Family Assessment Device General Functioning Scale; HRQOL, health-related quality of life; PedsQL FIM, PedsQL Family Impact Module; WAIS-IV, Wechsler Adult Intelligence Scale, Fourth Edition.
Worse survivor HRQOL was associated with worse mother-reported family functioning (r's range: 0.40–0.61) but not survivor-reported family functioning. Poorer survivor processing speed, working memory, long-term verbal memory, and executive functioning were significantly associated with worse survivor-reported family functioning (r's range: 0.35–0.53) and mother-reported family functioning (r's range: 0.39–0.55). Slower survivor processing speed (r=−0.47; p<0.01) and lower mental flexibility (r=−0.44; p<0.01) were related to poorer survivor HRQOL.
Bootstrapping analyses (Table 4)
Table 4.
Effects of Neurocognitive Function on Survivor Health-Related Quality of Life Through Family Functioninga
| Bootstrappingb95% CI | ||||
|---|---|---|---|---|
| Direct effect (c′) | Indirect effect (c) | Lower | Upper | |
| I. Effects of processing speed | −0.06 | −0.10 | −0.2272 | −0.0211 |
| II. Effects of working memory | 0.00 | −0.07 | −0.1738 | −0.0194 |
| III. Effects of long-term verbal memory | 0.67 | −1.74 | −3.7907 | −0.5426 |
| IV. Effects of executive function (D-KEFS Trail Making Test switching scaled score) | −0.22 | −0.40 | −0.9045 | −0.1061 |
| V. Effects of executive function (D-KEFS Tower Test achievement scaled score) | 0.08 | −0.50 | −1.2361 | −0.0898 |
PedsQL Family Impact Module Family Functioning score served as mediator in all analyses.
10,000 bootstrap samples.
BC, bias corrected; CI, confidence interval; D-KEFS, Delis–Kaplan Executive Function System.
Bootstrapping analyses examined the indirect effects of each of the five neurocognitive variables on survivor HRQOL through mother-reported family functioning using either the FAD GFS or the PedsQL FIM Family Functioning scale. Survivor-reported FAD GFS scores were not included in these models, since they were unrelated to survivor HRQOL in correlational analyses. Analyses using mother-reported FAD GFS scores did not support the indirect effects of the neurocognitive variables on survivor HRQOL through family functioning. However, the bias-corrected bootstrap 95% confidence intervals for all the bootstrapping models testing the neurocognitive domains' indirect effects on survivor HRQOL through mother-reported PedsQL FIM Family Functioning scores revealed significant indirect effects. The direct effects in Table 4 reflect the difference in survivor HRQOL when two survivors differ by one unit on the independent variable when scores on the mediator (the PedsQL FIM Family Functioning scale) are equal. The indirect effects indicate the difference in survivor HRQOL when two survivors differ by one unit on the independent variable as a result of the tendency for better neurocognitive functioning to be associated with better family functioning, which in turn is associated with better survivor HRQOL.
Discussion
Findings from this study emphasize the importance of broadening the framework to include family functioning when examining the emotional HRQOL and neurocognitive functioning of YA survivors of childhood BT. This study is one of the first to highlight the association between neurocognitive and family functioning and provides further evidence on their associations with survivor HRQOL. Poorer concurrent survivor neurocognitive functioning across all measured domains was related to worse survivor- and mother-reported family functioning and worse performance in two domains of neurocognitive functioning was associated with lower survivor HRQOL. Furthermore, this study offers preliminary evidence for the indirect effects of survivor neurocognitive functioning on survivor HRQOL through family functioning. These results have potential implications for improving survivor HRQOL by addressing family functioning and management of survivors' neurocognitive late effects. It is notable that these associations were evident in survivors who were an average of 16 years from diagnosis, suggesting a need for additional clinical and research attention focused on YA BT survivors.
Survivor performance on the neurocognitive measures was relatively consistent with other studies of long-term survivors of childhood BT and provides further evidence for chronic neurocognitive sequelae.51–53 The significant correlations between concurrent survivor neurocognitive functioning and family functioning support the proposed associations depicted in Peterson and Drotar's model of childhood cancer survivorship27 and provide further rationale for studying survivors within a family context.25,26 Survivors experiencing greater neurocognitive late effects reported worse global family functioning. Additionally, poorer survivor neurocognitive functioning was associated with greater mother-reported impact on family functioning. Notably, taking longer to accomplish everyday tasks or having difficulty switching attention during tasks may particularly strain families. These findings are consistent with studies of pediatric TBI that show the effects of injury on family functioning and caregiver burden,54 particularly among those children with severe TBI and likely greater impairments.55,56
Poorer survivor processing speed and mental flexibility were related to worse mother-rated survivor emotional HRQOL. This finding is consistent with an earlier study that demonstrated IQ as a strong determinant of HRQOL in long-term survivors of childhood BT.57 Additional research is needed to clarify the nature of the effects of poor neurocognitive functioning on survivor HRQOL, as there are likely numerous mediating variables that contribute to this association. For example, poor neurocognitive functioning may decrease attainment of numerous developmental tasks,14 thus leading to poorer HRQOL. Given the strong evidence for neurocognitive declines in this population,4 the present finding substantiates the vulnerability of this group for poor long-term psychosocial outcomes.14
This study provides initial evidence for the role of a survivor's family in explaining some of the associations between poor neurocognitive function and survivor HRQOL. All the domains of survivor neurocognitive functioning measured in this study had indirect effects on survivor HRQOL through family functioning. Survivors with more neurocognitive late effects may be at greater risk for having lower HRQOL due to the tendency for these survivors to have families that have difficulties related to completing family activities, conflict, and poor communication and problem-solving. In longitudinal studies of pediatric TBI, family and parenting variables have been shown to be important in promoting better outcomes.58,59
Despite being significantly correlated with mother-reported family functioning on the PedsQL FIM Family Functioning scale, models testing the indirect effects of neurocognitive function on survivor HRQOL through family functioning as measured by the FAD GFS were not significant. It is possible that the FAD GFS is too general to detect issues particular to families of childhood BT survivors; the Family Functioning scale of the PedsQL FIM might better reflect the experiences of families of youth with chronic conditions. Future research within pediatric BT survivorship should consider focusing on family management of survivor late effects instead of general family functioning, using measures such as the Family Management Measure.60
This study had some limitations. First, this cross-sectional study is unable to determine the causal associations between variables. It is likely that neurocognitive functioning and family functioning interact over time to influence survivor HRQOL. Longitudinal studies are needed to address this question. Second, this study relied solely on mothers' reports of survivor HRQOL, and the associations between survivor HRQOL and mother-rated family functioning may reflect single source bias. Third, the current sample was relatively small and consisted of survivors who live with their mothers at least part-time and participated in an earlier study. Therefore, the sample may reflect potential selection bias and may not be representative of the larger population of childhood BT survivors. However, survivors of childhood BT are more likely to live with their families of origin compared to controls.6,7
The current study offers novel data on the associations between neurocognitive and family functioning and HRQOL in YA survivors of childhood BT. This study used well-normed measures of neurocognitive functioning and obtained information on family functioning from multiple perspectives. The findings suggest two potential future directions. First, prospective studies that follow families from diagnosis are needed to specify family predictors of survivors' HRQOL and potential targets for family-based interventions. For example, communication or problem-solving might be particularly influential in mitigating the negative influence of neurocognitive deficits on survivors, and interventions that address these variables may enhance survivor and family functioning. Second, efforts to improve survivors' cognitive functioning61,62 may improve HRQOL and alleviate the burden on families. Developing interventions that target both survivor and family functioning for long-term BT survivors will be an important next step, as these factors have direct associations with both survivor HRQOL and caregiver competence.32
Conclusions
Poorer YA survivor neurocognitive functioning was associated with worse family functioning and poorer survivor HRQOL. Survivor neurocognitive functioning also had indirect effects on survivor HRQOL through family functioning. Family functioning may be an important variable in mitigating the negative influences of neurocognitive late effects in YA survivors of childhood BT and is a potential intervention target.
Acknowledgments
This research was supported by the National Institute of Nursing Research (NINR R01 NR009651-01A1; PI: Janet A. Deatrick, PhD) and the American Cancer Society (Grant PF-11-168-01-PCSM; PI: Matthew C. Hocking, PhD).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Gurney JG, Kadan-Lottick NS, Packer RJ, et al. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood Cancer Survivor Study. Cancer. 2003;97(3):663–73 [DOI] [PubMed] [Google Scholar]
- 2.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. New Eng J Med. 2006;355(15):1572–82 [DOI] [PubMed] [Google Scholar]
- 3.Packer RJ, Gurney JG, Punyko JA, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: Childhood Cancer Survivor Study. J Clin Oncol. 2003;21(17):3255–61 [DOI] [PubMed] [Google Scholar]
- 4.Robinson KE, Kuttesch JF, Champion JE, et al. A quantitative meta-analysis of neurocognitive sequelae in survivors of pediatric brain tumors. Pediatr Blood Cancer. 2010;55(3):525–31 [DOI] [PubMed] [Google Scholar]
- 5.Zeltzer L, Recklitis C, Buchbinder D, et al. Psychological status in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ness KK, Morris EB, Nolan VG, et al. Physical performance limitations among adult survivors of childhood brain tumors. Cancer. 2010;116(12):3034–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zebrack B, Gurney JG, Oeffinger K, et al. Psychological outcomes in long-term survivors of childhood brain cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2004;22(6):999–1006 [DOI] [PubMed] [Google Scholar]
- 8.Deatrick JA, Mullaney EK, Mooney-Doyle K. Exploring family management of childhood brain tumor survivors. J Pediatr Oncol Nurs. 2009;26(5):303–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foley B, Barakat LP, Herman-Liu A, et al. The impact of childhood hypothalamic/chiasmatic brain tumors on child adjustment and family functioning. Child Health Care. 2000;29(3):209–23 [Google Scholar]
- 10.Hutchinson KC, Willard VW, Hardy KK, Bonner MJ. Adjustment of caregivers of pediatric patients with brain tumors: a cross-sectional analysis. Psychooncology. 2009;18(5):515–23 [DOI] [PubMed] [Google Scholar]
- 11.Turner CD, Rey-Casserly C, Liptak CC, Chordas C. Late effects of therapy for pediatric brain tumor survivors. J Child Neurol. 2009;24(11):1455–63 [DOI] [PubMed] [Google Scholar]
- 12.Gurney JG, Krull KR, Kadan-Lottick N, et al. Social outcomes in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27(14):2390–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pang JW, Friedman DL, Whitton JA, et al. Employment status among adult survivors in the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2008;50(1):104–10 [DOI] [PubMed] [Google Scholar]
- 14.Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: a report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23(6):705–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiehna EN, Mulhern RK, Li C, et al. Changes in attentional performance of children and young adults with localized primary brain tumors after conformal radiation therapy. J Clin Oncol. 2006;24(33):5283–90 [DOI] [PubMed] [Google Scholar]
- 16.Mabbott DJ, Penkman L, Witol A, et al. Core neurocognitive functions in children treated for posterior fossa tumors. Neuropsychology. 2008;22(2):159–68 [DOI] [PubMed] [Google Scholar]
- 17.Mulhern RK, White HA, Glass JO, et al. Attentional functioning and white matter integrity among survivors of malignant brain tumors of childhood. J Int Neuropsychol Soc. 2004;10(2):180–9 [DOI] [PubMed] [Google Scholar]
- 18.Nagel BJ, Delis DC, Palmer SL, et al. Early patterns of verbal memory impairment in children treated for medulloblastoma. Neuropsychology. 2006;20(1):105–12 [DOI] [PubMed] [Google Scholar]
- 19.Palmer SL, Reddick WE, Gajjar A. Understanding the cognitive impact on children who are treated for medulloblastoma. J Pediatr Psychol. 2007;32(9):1040–9 [DOI] [PubMed] [Google Scholar]
- 20.Mulhern RK, Palmer SL, Reddick WE, et al. Risks of young age for selected neurocognitive deficits in medulloblastoma are associated with white matter loss. J Clin Oncol. 2001;19(2):472–9 [DOI] [PubMed] [Google Scholar]
- 21.Sands SA, Kellie SJ, Davidow AL, et al. Long-term quality of life and neuropsychologic functioning for patients with CNS germ-cell tumors: from the First International Germ-Cell Tumor Study. Neuro Oncol. 2001;3(3):175–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King TZ, Fennell EB, Williams L, et al. Verbal memory abilities of children with brain tumors. Child Neuropsychol. 2004;10(2):76–88 [DOI] [PubMed] [Google Scholar]
- 23.Mulhern RK, Hancock J, Fairclough D, Kun LE. Neuropsychological status of children treated for brain tumors: a critical review and integrative analysis. Med Pediatr Oncol. 1992;20(3):181–91 [DOI] [PubMed] [Google Scholar]
- 24.Papazoglou A, King TZ, Morris RD, Krawiecki NS. Cognitive predictors of adaptive functioning vary according to pediatric brain tumor location. Dev Neuropsychol. 2008;33(4):505–20 [DOI] [PubMed] [Google Scholar]
- 25.Kazak AE. Families of chronically ill children: a systems and social-ecological model of adaptation and challenge. J Consult Clin Psychol. 1989;57(1):25–30 [DOI] [PubMed] [Google Scholar]
- 26.Kazak AE, Simms S, Rourke MT. Family systems practice in pediatric psychology J Pediatr Psychol. 2002;27(2):133–43 [DOI] [PubMed] [Google Scholar]
- 27.Peterson CC, Drotar D. Family impact of neurodevelopmental late effects in survivors of pediatric cancer: review of research, clinical evidence, and future directions. Clin Child Psychol Psychiatry. 2006;11(3):349–66 [DOI] [PubMed] [Google Scholar]
- 28.Hocking MC, Hobbie WL, Deatrick JA, et al. Neurocognitive and family functioning and quality of life among young adult survivors of childhood brain tumors. Clin Neuropsychol. 2011;25(6):942–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor HG, Yeates KO, Wade SL, et al. Influences on first year recovery from traumatic brain injury in children. Neuropsychology. 1999;13(1):76–89 [DOI] [PubMed] [Google Scholar]
- 30.Taylor HG, Yeates KO, Wade SL, et al. Bidirectional child-family influences on outcomes of traumatic brain injury in children. J Int Neuropsychol Soc. 2001;7(6):755–67 [DOI] [PubMed] [Google Scholar]
- 31.Carlson-Green B, Morris RD, Krawiecki NS. Family and illness predictors of outcome in pediatric brain tumors. J Pediatr Psychol. 1995;20(6):769–84 [DOI] [PubMed] [Google Scholar]
- 32.Deatrick JA, Hobbie WL, Ogle S, et al. Competence in caregivers of adolescent and young adult childhood brain tumor survivors. Health Psychol. 2014;33(10):1103–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wechsler D. WAIS-IV administration and scoring manual. San Antonio, TX: Pearson; 2008 [Google Scholar]
- 34.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test—children's version (CVLT-C). San Antonio, TX: Pearson; 1994 [Google Scholar]
- 35.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS). San Antonio, TX: The Psychological Corporation; 2001 [Google Scholar]
- 36.Goodwin DAJ, Boggs SR, Graham-Pole J. Development and validation of the Pediatric Oncology Quality of Life Scale. Psychol Assess. 1994;6(4):321–8 [Google Scholar]
- 37.Epstein NB, Baldwin LM, Bishop DS. The McMaster Family Assessment Device. J Marital Fam Ther. 1983;9(2):171–80 [Google Scholar]
- 38.Alderfer MA, Fiese BH, Gold JI, et al. Evidence-based assessment in pediatric psychology: family measures. J Pediatr Psychol. 2008;33(9):1046–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller IW, Ryan CE, Keitner GI, et al. The McMaster approach to families: theory, assessment, treatment and research. J Fam Therapy. 2000;22(2):168–89 [Google Scholar]
- 40.Varni JW, Sherman SA, Burwinkle TM, et al. The PedsQL™ Family Impact Module: preliminary reliability and validity. Health Qual Life Outcomes. 2004;2:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jastrowski Mano KE, Khan KA, Ladwig RJ, Weisman SJ. The impact of pediatric chronic pain on parents' health-related quality of life and family functioning: reliability and validity of the PedsQL 4.0 Family Impact Module. J Pediatr Psychol. 2011;36(5):517–27 [DOI] [PubMed] [Google Scholar]
- 42.Limbers CA, Ripperger-Suhler J, Boutton K, et al. A comparative analysis of health-related quality of life and family impact between children with ADHD treated in a general pediatric clinic and a psychiatric clinic utilizing the PedsQL. J Atten Disord. 2011;15(5):392–402 [DOI] [PubMed] [Google Scholar]
- 43.Kazak AE, Hocking MC, Ittenbach RF, et al. A revision of the Intensity of Treatment Rating Scale: classifying the intensity of pediatric cancer treatment. Pediatr Blood Cancer. 2012;59(1):96–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Werba BE, Hobbie W, Kazak AE, et al. Classifying the intensity of pediatric cancer treatment protocols: the Intensity of Treatment Rating Scale 2.0 (ITR-2). Pediatr Blood Cancer. 2007;48(7):673–7 [DOI] [PubMed] [Google Scholar]
- 45.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. New York: Guilford Press; 2013 [Google Scholar]
- 46.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879–91 [DOI] [PubMed] [Google Scholar]
- 47.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36(4):717–31 [DOI] [PubMed] [Google Scholar]
- 48.Hayes AF, Scharkow M. The relative trustworthiness of inferential tests of the indirect effect in statistical mediation analysis: does method really matter? Psychol Sci. 2013;24(10):1918–27 [DOI] [PubMed] [Google Scholar]
- 49.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–82 [DOI] [PubMed] [Google Scholar]
- 50.Fritz MS, MacKinnon DP. Required sample size to detect the mediated effect. Psychol Sci. 2007;18(3):233–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maddrey AM, Bergeron JA, Lombardo ER, et al. Neuropsychological performance and quality of life of 10 year survivors of childhood medulloblastoma. J Neurooncol. 2005;72(3):245–53 [DOI] [PubMed] [Google Scholar]
- 52.Reddick WE, White HA, Glass JO, et al. Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer. 2003;97(10):2512–9 [DOI] [PubMed] [Google Scholar]
- 53.Reimers TS, Ehrenfels S, Mortensen EL, et al. Cognitive deficits in long-term survivors of childhood brain tumors: identification of predictive factors. Med Pediatr Oncol. 2003;40(1):26–34 [DOI] [PubMed] [Google Scholar]
- 54.Stancin T, Wade SL, Walz NC, et al. Traumatic brain injuries in early childhood: initial impact on the family. J Dev Behav Pediatr. 2008;29(4):253–61 [DOI] [PubMed] [Google Scholar]
- 55.Wade SL, Carey J, Wolfe CR. The efficacy of an online cognitive-behavioral family intervention in improving child behavior and social competence following pediatric brain injury. Rehabil Psychol. 2006;51(3):179–89 [Google Scholar]
- 56.Wade SL, Taylor HG, Drotar D, et al. A prospective study of long-term caregiver and family adaptation following brain injury in children. J Head Trauma Rehabil. 2002;17(2):96–111 [DOI] [PubMed] [Google Scholar]
- 57.Reimers TS, Mortensen EL, Nysom K, Schmiegelow K. Health-related quality of life in long-term survivors of childhood brain tumors. Pediatr Blood Cancer. 2009;53(6):1086–91 [DOI] [PubMed] [Google Scholar]
- 58.Kurowski BG, Taylor HG, Yeates KO, et al. Caregiver ratings of long-term executive dysfunction and attention problems after early childhood traumatic brain injury: family functioning is important. PM R. 2011;3(9):836–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yeates KO, Taylor HG, Walz NC, et al. The family environment as a moderator of psychosocial outcomes following traumatic brain injury in young children. Neuropsychology. 2010;24(3):345–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knafl K, Deatrick JA, Gallo A, et al. Assessment of the psychometric properties of the Family Management Measure. J Pediatr Psychol. 2011;36(5):494–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Butler RW, Copeland DR, Fairclough DL, et al. A multicenter, randomized clinical trial of a cognitive remediation program for childhood survivors of a pediatric malignancy. J Consult Clin Psychol. 2008;76(3):367–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hardy KK, Willard VW, Allen TM, Bonner MJ. Working memory training in survivors of pediatric cancer: a randomized pilot study. Psychooncology. 2013;22(8):1856–65 [DOI] [PMC free article] [PubMed] [Google Scholar]