Abstract
Purpose: Cancer-related fatigue in adults has been the subject of considerable recent research, confirming its importance as a common and debilitating symptom, and establishing a number of evidence-based interventions. There has, however, been limited focus on the fatigue suffered by teenagers and young adults with cancer, a group recognized as having unique experiences and developmental needs. We have undertaken a systematic review of the literature to provide a comprehensive overview of studies evaluating fatigue in this younger patient group in order to guide clinical practice and future research.
Method: We searched MEDLINE, EMBASE, PsycINFO, and CINAHL databases for literature containing data relating to any aspect of fatigue in patients aged 13–24 at cancer diagnosis or treatment.
Results: Sixty articles were identified, of which five described interventional clinical trials. Cancer-related fatigue was consistently one of the most prevalent, severe, and distressing symptoms, and it persisted long-term in survivors. It was associated with a number of factors, including poor sleep, depression, and chemotherapy. There was little evidence for the effectiveness of any intervention, although exercise appears to be the most promising. Importantly, fatigue was itself a significant barrier to physical and social activities.
Conclusion: Cancer-related fatigue is a major and disabling problem in young cancer patients. Effective management strategies are needed to avoid compounding the dependence and social isolation of this vulnerable patient group. Future research should focus on providing evidence for the effectiveness of interventions, of which activity promotion and management of concurrent symptoms are the most promising.
Keywords: : fatigue, prevalence, impact, intervention
Cancer is the leading cause of disease-related death in teenagers and young adults (TYAs) in the United States and Europe.1,2 Each year, more than 11,000 TYA patients in the United States and over 2000 in the United Kingdom are diagnosed with cancer, and incidence rates are rising.2,3 It is increasingly recognized that such patients have experiences and needs that differ significantly from those of children and adults.4 As well as having to negotiate the physical, cognitive, emotional, and behavioral changes that occur in adolescence, young people can suffer from a different cancer profile, longer periods of cancer treatment, a worse prognosis, and a particularly devastating sense of despair and isolation.3,5,6 There has been an international drive to develop age-appropriate specialist care and to generate an evidence base addressing the specific needs of this patient group.4,7
Cancer-related fatigue is a “persistent, subjective sense of tiredness related to cancer or cancer treatment that interferes with usual functioning.”8 It is a multidimensional symptom with physical, affective, and cognitive components. The range of perceptions may include a feeling of weakness and inability to perform tasks, decreased motivation and low mood, and difficulty in thinking clearly.9 It differs from fatigue felt by healthy individuals in that it is of greater magnitude, disproportionate to the level of exertion, and incompletely relieved by rest.
Fatigue appears to be both the most common and the most distressing symptom experienced by adult patients with cancer.10,11 Many surveys suggest a prevalence of over 75%, with rates increasing in conjunction with oncological treatment.12,13 Adult patients report that fatigue is the symptom which has the greatest negative impact on their quality of life.11
Despite the size of the problem, cancer-related fatigue has traditionally been neglected both in clinical and research terms, with healthcare staff and patients tending to consider it an unavoidable consequence of cancer and its treatment.10,14 However, over the last two decades, a substantial evidence base has been developing in the adult literature. Systematic reviews of research in older adults has shown that—apart from treating reversible underlying causes—the most promising approaches are exercise15 and psychosocial interventions such as education.16
Recognition of the particular importance of cancer-related fatigue in younger cancer patients has only emerged over the last 5–10 years. Lack of energy has been shown to be the most common symptom in children with cancer, with children placing emphasis on a sense of physical weakness, associating fatigue with disruption of sleep.17 TYA cancer patients also appear to suffer from significant fatigue,18–20 unsurprising given that even healthy teenagers and young adults have a propensity to experience fatigue. The developmental need for longer sleep during this important phase of brain maturation is hindered by circadian rhythm shifts and a tendency to develop unhelpful sleep habits.21,22 TYAs perceive fatigue in both cognitive and physical terms.5 Fatigue is believed to have a particularly negative impact on quality of life in this group, as it hinders many of the key developmental needs of this age, such as autonomy and the formation of close peer relationships.23 Many TYA cancer patients remain or return to being dependent on their parents at a time when they would have been expecting to achieve independence. It is well recognized clinically that the parents of fatigued TYAs bear a considerable burden.
In one previous review of fatigue in teenagers in cancer published a decade ago,20 Erikson found there was minimal research focusing on fatigue in this age range. Given the number of articles published since that review, it was decided to systematically appraise the current evidence base with a focus on the TYA age group. The review was designed to be broad to provide a comprehensive overview of studies investigating any aspect of cancer-related fatigue in patients diagnosed or treated for cancer while aged 13–24 years old. The research questions were:
1. What is the prevalence and severity of cancer-related fatigue in TYA patients during and after treatment?
2. What is the impact of fatigue on TYA cancer patients?
3. What is the experience of parents of fatigued TYA cancer patients?
4. What are the correlates of cancer-related fatigue in this patient group?
5. How effective are interventions to manage fatigue in TYA patients?
Methods
Literature search strategy
The literature was searched within MEDLINE, EMBASE, PsycINFO, and CINAHL databases for January 1981 through October 2013. Preliminary searches had suggested that there was no literature of relevance published prior to 1981. The search strategy is detailed in Table 1. Reference and citation searches were also undertaken, with manual searching of all issues of a key journal—the Journal of Adolescent and Young Adult Oncology (issues 1–4 of both volume 1 and volume 2)—as well as the proceedings of the 2012 Teenage Cancer Trust International Conference. Related systematic reviews were searched, including reviews evaluating symptoms experienced by teenagers with cancer,24 fatigue in lymphoma patients,25 and interventions for fatigue in children and adults.26,27 Experts in teenage and young adult cancer-related fatigue were also contacted.
Table 1.
Search Terms
| #1 | exp fatigue/ |
| #2 | fatigue* or tire* or exhaust* or lethargy* (title or abstract) |
| #3 | exp neoplasm/ |
| #4 | neoplasm* or cancer* or carcinoma* or lymphoma* or leukaemia* or leukemia* (title or abstract) |
| #5 | 1 or 2 |
| #6 | 3 or 4 |
| #7 | 5 and 6 |
| #8a | limit 7 to “adolescent” or “young adult” |
| #9 | tya* or teenage* or “young adult”* (title or abstract) |
| #10 | 7 and 9 |
| #11 | 8 or 10 |
Both “adolescent” and “young adult” limiting terms were available in the MEDLINE and CINAHL database searches, but only “adolescent” was available for the EMBASE and PsycINFO searches.
Selection criteria
The key inclusion criteria were that all study participants had malignant disease, and that either the majority were aged 13–24 years old at the time of cancer diagnosis or treatment or the results for this age subgroup were presented separately. Included studies could investigate any aspect of cancer-related fatigue, use any outcome measure, and employ quantitative or qualitative methods. Exclusion criteria included non-English language publication, absence of original empirical data, phase I/II clinical trials, trials involving fewer than 10 patients, case reports, and retrospective case note reviews. Studies of TYA-aged survivors of pediatric cancer were not included.
Quality assessment and data analysis
Gough's Weight of Evidence Framework28 was employed to assess article quality, relevance, and bias, and to generate an overall judgment about contribution. This framework includes analysis of “fitness for purpose” and relevance to the research question, providing a more applied synthesis of evidence than simply assessing the generic quality of each article. Four scores of “low,” “medium,” or “high” are given for each of the following:
• Weight of Evidence A: The integrity of the evidence in its own terms
• Weight of Evidence B: The appropriateness of method for answering the review questions
• Weight of Evidence C: The appropriateness of the focus or relevance for answering the review questions
• Weight of Evidence D: The overall rating generated by combining the Weight of Evidence A, B, and C scores
All articles, irrespective of relevance and quality, were included, but those rated “medium” and “high” were given greater weight in the analysis.
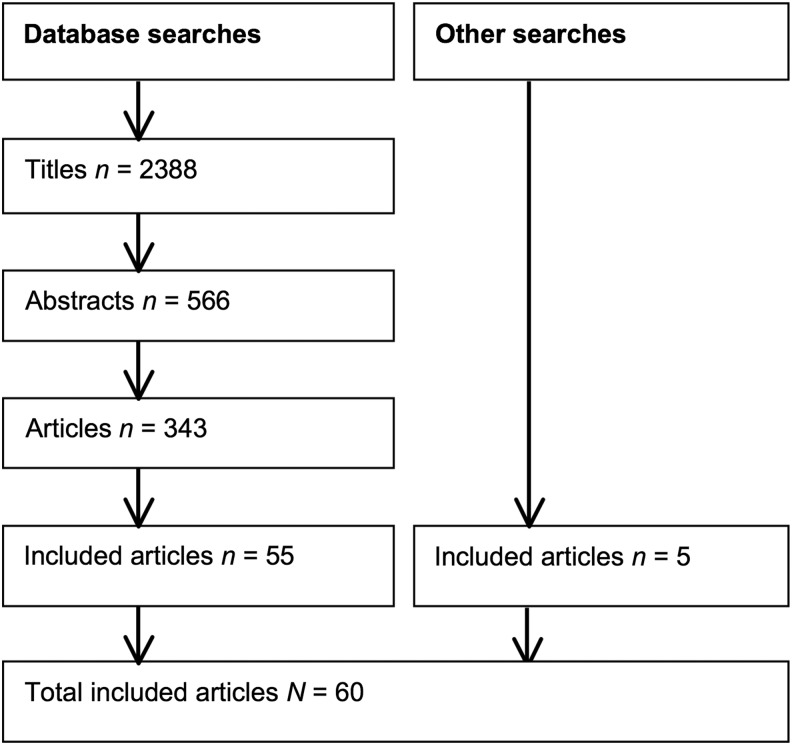
The searches produced 2388 unique titles that were initially screened by one researcher (AS). After 566 abstracts were reviewed by two researchers (AS and SG), 343 full articles were read by one researcher (AS), who excluded most of them on the basis of the participants' ages. Two researchers (AS and SB) read the remaining articles, with disagreements resolved by discussion. The final number of included articles was 60 (Fig. 1). A narrative synthesis was undertaken in relation to each of the research questions.
FIG. 1.
PRISMA flowchart of included articles. Note. Although four pairs of articles and two groups of three articles were publications describing different aspects of the same study's data set, because of the distinct areas of focus, the 60 articles have been viewed as 60 separate studies. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Results
Description of articles
Thirty-seven of the 60 included articles were from the United States or Canada, eight were from Norway or Sweden, and three were from the United Kingdom. All were published between 1992 and 2013. The 60 articles encompassed a total of 52 separate studies, as four pairs of articles23,29–35 and two groups of three articles36–41 were publications describing different aspects of the same study's data set. It was decided to handle these as different studies, because of their distinct areas of focus. Therefore, for the purposes of this review, the 60 articles were viewed as 60 separate studies.
Thirty-six studies were cross-sectional and observational. Of the 24 prospective longitudinal studies, five were interventional clinical trials. Most studies involved only quantitative methods, though seven were qualitative and eight employed a mixed-methods design. Most were rated as medium or poor on Gough's Weight of Evidence Framework. Although 13 were of high quality on Weight of Evidence A (integrity of the evidence in its own terms),17,30,40,42–51 only three were judged to be of overall high quality by Weight of Evidence D.17,30,47
All participants (aside from those in some of the control groups) had a cancer diagnosis. Five studies only recruited patients with lymphoma and/or leukemia,31,32,45,52,53 and one had only patients with extremity bone tumors.54 All of the remaining studies investigated more than one cancer type. The majority of participants were in the 13–24 years old age range in 46 studies; subgroup data for this age range was presented separately in the remaining 14 papers. The number of patients within each study ranged from 855 to 199,56 and the median or mean time since diagnosis ranged from 2 months57 to 20 years.32
Fatigue was the study's focus and the first outcome measure described in the results for only 18 studies;5,18,19,23,30–32,35,36,42,46,47,50,54,58–61 two of these involved validating symptom measures46,59 and none were interventional. Across the 60 included studies, the most commonly used fatigue outcome measures were the Fatigue Scale-Adolescent (FS-A),59 the Multidimensional Fatigue Scale (MFS),62 and the Memorial Symptom Assessment Scale (MSAS 10–18),63 used in 14,12, and 8 studies, respectively. Eleven studies used a range of other validated fatigue measures, including the Chalder Fatigue Questionnaire, Piper Fatigue Scale, and the Functional Assessment in Chronic Illness Therapy fatigue scale. Eight studies used unvalidated measures, and the remaining seven studies were entirely qualitative in design. Table 2 provides further detail on the more commonly used measures.
Table 2.
Summary of Key Fatigue Outcome Measures
| Name of measure | Description |
|---|---|
| Chalder Fatigue Questionnaire64 | 11-item multidimensional fatigue scale developed for use in adult epidemiological studies of patients with chronic disease, and to find fatigue “cases.” It is scored using a 4-point verbal rating scale, with a case is defined as a score of ≥4 dichotomized bimodal scoring. |
| Fatigue Scale-Adolescent59 | 14-item multidimensional scale developed specifically to assess cancer-related fatigue in adolescents 13–18 years old. It is scored using a 5-point verbal rating scale, and parent and staff proxy versions have been developed. |
| Functional Assessment in Chronic Illness Therapy fatigue scale100 | Initially developed as a multidimensional measure of fatigue in adult oncology patients with anemia, its use has been widened to include fatigue assessment in chronic illness. It is a standalone scale within the wider FACIT measurement system. It has 13 items, scored using a 5-point verbal rating scale. |
| Memorial Symptom Assessment Scale17 | 32-item scale developed to assess the frequency, severity, and associated distress of 32 common symptoms, (including fatigue) in adult cancer patients. Each symptom is measured with 4-point (frequency and severity) or 5-point (distress) numerical rating scales. It has been modified for use for patients 10–18 (30-item) and 7–12 (8-item) years old; a number of other revised versions also exist. |
| Multidimensional Fatigue Scale62 | 18-item scale developed to measure fatigue in pediatric cancer patients and now used as a generic multidimensional measure in all pediatric patient populations. It is a module of the PedsQL measurement model, which assesses pediatric quality of life. Versions are available for patients 5–7, 8–12, 13–18, and 18–24 years old, with associated parent proxy versions. |
| Piper Fatigue Scale101 | Multidimensional fatigue scale developed for use in adult cancer patients. A number of versions exist, including the original 40-item scale and a revised 22-item scale. Each item is scored with a 0–10 numerical rating scale. It assesses four domains of fatigue—behavioral/severity, affective meaning, sensory, and cognitive/mood—and generates a score for each domain as well as a total score. |
| Symptom Distress Scale102 | 11-item scale that measures distress related to symptoms (including fatigue) scored using a 5-point verbal rating scale. It was developed specifically to identify the concerns of adult patients receiving cancer treatment. |
Fatigue prevalence
Twenty-four studies investigated the prevalence of TYA cancer-related fatigue (Table 3). Fatigue was measured during treatment in 14 studies, after treatment in five studies, and in a mixed population of patients both during and after treatment in five studies. A “fatigue case” was most commonly defined as fatigue being scored as “present” using the MSAS, a score of anything other than “no fatigue” on a 5-point Likert scale, or a dichotomized score of ≥4 on the Chalder Fatigue Scale.64
Table 3.
Studies Evaluating Fatigue Prevalence, Grouped by Relationship to Cancer Treatment
| Study | Aim(s) | Participants (mixed tumors unless stated) | Study design and fatigue assessments | Definition of fatigue “case” | Fatigue prevalence (rank compared to other symptoms) | Weight of Evidence D score |
|---|---|---|---|---|---|---|
| DURING TREATMENT | ||||||
| Ameringer et al. 201357 | Examine the trajectory of symptoms across a chemotherapy cycle | • N=9 • Mean age: 15.3 years • Mean since diagnosis: 2.6 months |
• Longitudinal observational pilot • FS-A |
Not described | • All participants experienced some fatigue at every time point | Low |
| Atay et al. 201273 | Determine symptom prevalence 1, 2, and 3 months after diagnosis | • N=54 • 61% 13–18 years |
• Longitudinal observational • MSAS 10–18 |
Presence of “lack of energy” in last week on MSAS | • Month 1: 66.7% (5 of 30) • Month 2: 75.9% (2 of 30) • Month 3: 63% (joint 2 of 30) |
Medium |
| Baggott et al. 201071 | Describe changes in symptoms at weekly intervals from D1 | • N=66 • Mean age: 14.8 years • Mean since diagnosis: 16.3 months |
• Longitudinal observational • Revised MSAS 10–18 |
Presence of “lack of energy” in last week on MSAS | • Week 0: 75.8% (1 of 31) • Week 1: 70.5% (2 of 31) • Week 2: 57.4% (2 of 31) • Significant linear decrease over time |
Medium |
| Baggott et al. 201265 | Describe the usefulness of eDiary to record symptoms over a 3-week trial | • N=10 • Mean age: 18.2 years • Mean since diagnosis: 12.2 months |
• Longitudinal observational • 2 questions from FS-A in a VAS format |
VAS >30 on 0–100 scale on at least 1 day | • Reported fatigue-physical and fatigue-mental: 100% (1 of 11) • % days that VAS >30 on 0–100 scale, 64% and 62%, respectively (1 of 11) |
Low |
| Baggott et al. 201270 | Evaluate symptom clusters | • N=131 • Mean age: 14.8 years • Median since diagnosis: 3.3 months |
• Cross-sectional on D0 of ≥cycle 2 of chemotherapy • Revised MSAS 10–18 |
Presence of “lack of energy” in last week on MSAS | • 75.6% (1 of 31) | Medium |
| Corey et al. 200856 | Describe the relationship between support and symptom distress | • N=72 (ARM 2) • Mean age: 14.8 years (ARM 2) • Mean since diagnosis: 3.75 years (ARM 1 and 2) |
• Secondary analysis of data from 2 studies • Symptom Distress Scale |
3–5 on 1–5 Likert scale | • ARM 2 (recent diagnosis): 42.5% | Medium |
| Enskar et al. 200766 | Evaluate distress, coping support, and care | • N=54 • Mean age: 16.0 years • 32 were <3 months of diagnosis |
• Cross-sectional • LSS-A |
Anything other than “not at all”/“do not agree at all” on 1–5 VRS | • For 15 patients on treatment: 93%; significantly more than patients off treatment (p<0.05) | Medium |
| Erickson et al. 201023 | Describe fatigue patterns during month of chemotherapy | • N=20 • Mean age: 16.1 years • Mean since starting chemotherapy: 8.71 weeks |
• Longitudinal mixed methods • Daily fatigue NRS, MFS |
>0 on 0–10 NRS | • Experienced fatigue at some point, including during “days immediately following chemotherapy”: 100% | Medium |
| Erickson et al. 201135 | Describe relationship between fatigue and sleep-wake disturbances | • N=20 • Mean age: 16.1 years • Mean since starting chemotherapy: 8.71 weeks |
• Longitudinal observational • Weekly MFS from D1 for 5 weeks |
“Feeling tired” “sometimes,” “often,” or “almost always” | • At some point over study period: 75% | Medium |
| Hedstrom et al. 200540 | Investigate perceptions of distress amongst recently diagnosed adolescents | • N=56 • Age (years): 13–15: n=35; 16–19: n=21 |
• Cross-sectional, mixed methods • Fatigue assessed as an aspect of distress using Likert scale |
3–5 on 0–5 Likert scale | • 62% (4 of 20) | Medium |
| Mandrell et al. 201147 | Calculate the revised FS-A score that defines a fatigue “case” meriting clinical intervention | • N=138 • Mean age: 14.83–16.27 years across all studies |
• Analysis of data from 9 studies • FS-A and FS-P |
>31 on scale of 13–65 | • ALL: 15%; 7–14%/26–33% (ALL off/on DXM) • Solid tumor or AML: 10% (D1), 28% (D2), 50% (D3–4) • Mixed diagnoses: 36% (D1); 56% (final day); 54% (1 week after end) |
High |
| Miller et al. 201172 | Describe prevalence, frequency, severity, and distress of multiple symptoms | • N=39 • Mean age: 13.5 years |
• Longitudinal observational • MSAS 10–18 daily for 5 days evaluating symptoms from “past day” |
Presence of “lack of energy” in last week on MSAS | • 49.6% (2 of 31) | Medium |
| Walker et al. 201068 | Describe symptoms before (T1) and 1 week after (T2) chemotherapy | • N=51 • Mean age: 14.2 years • Mean since diagnosis: 6 months |
• Longitudinal observational in 2 centers • MSAS 7–12 |
Presence of “tiredness” in last week on MSAS 7–12 | • T1: 54.3% (1 of 9) • T2: 67.4% (1 of 9) |
Medium |
| Williams et al. 201269 | Calibrate the Therapy-Related Symptom Checklist-Children | • N=163 • Subgroup of 385 participants in age range 12–17 years |
• Cross-sectional • TRSC-C |
Severity of “feeling sluggish” of more than 0 on 0–4 Likert | • 81% (1 of 31) | Medium |
| AFTER TREATMENT | ||||||
| Adams et al. 200452 | Evaluate cardiovascular function after mediastinal RT | • N=48 HD survivors • Median age at diagnosis: 14.2 years • Median since diagnosis: 14.3 years |
• Cross-sectional • General Health Survey (unvalidated tool) |
≥1 on 0–4 Likert scale of fatigue severity | • 67% | Medium |
| Aksnes et al. 200754 | Examine fatigue, mental distress, and QOL in EBT survivors compared to matched controls | • N=57 EBT survivors • Mean age at diagnosis across subgroups: 16–25 years • Mean since diagnosis: 9–14 years |
• Case-control (non-contemporaneous controls HD, TC, NORMS) • CFQ |
Sum of ≥4 using dichotomized (0, 0, 1, 1) 4-point Likert scale | • EBT survivors: 14% • HD survivors: 21% • TC survivors: 16% • NORMS: 10%; p=0.3 EBT vs. NORMS |
Medium |
| Enskar et al. 200766 | Evaluate distress, coping support, and care | • N=54 • Mean age: 16.0 years • 32 were <3 months of diagnosis |
• Cross-sectional • LSS-A |
Anything other than “not at all” on 1–5 VRS | • For 39 patients after treatment: 67%, less than on treatment (p<0.05) | Medium |
| Hamre et al. 201332 | Determine prevalence of chronic fatigue in leukemia/lymphoma survivors | • N=92 (subgroup of 290); all HD • Mean age diagnosis: 14.6 years • Mean age at survey: 35.0 years |
• Cross-sectional • CFQ |
Sum of≥4 using dichotomized (0, 0, 1, 1) 4-point Likert scale | • 35% vs. 8% in control group (note control group age=19–50 years) • Adjusted odds ratio of fatigue in HD vs. control: 5.9 |
Low |
| Mulrooney et al. 200850 | Describe prevalence and risk factors for fatigue and sleep disturbance | • N=631 (subgroup of 1897) • Diagnosed age: 15–21 years • >5 years from diagnosis |
• 26-center cohort study • FACIT-fatigue |
Score below 10th percentile for sibling cohort | • 8.6% | Medium |
| MIXED DURING AND AFTER TREATMENT | ||||||
| Collins et al. 200017 | Determine symptom prevalence, characteristics, and distress | • N=160 • 70 had chemotherapy within last 2–4 weeks, 58 >4 months ago • Mean age: 14.0 years |
• Cross-sectional • MSAS 10–18 |
Presence of “lack of energy” in last week on MSAS | • Overall: 49.7% (1 of 30) • CNS tumor: 66.7% (1 of 30) • Lymphoma: 50% (1 of 30) • Leukemia: 43.8% (2 of 30) • Solid tumor: 53.7% (3 of 30) |
Medium |
| Corey et al. 200856 | Describe relationship between distress and three sources of support | • N=127 (ARM 1) • Mean age: 16.4 years (ARM 1) • Mean since diagnosis: 3.75 years (ARM 1 and 2) |
• Secondary analysis of data from 2 studies • Symptom Distress Scale |
3–5 on 1–5 Likert scale | • ARM 1 (mixed population): 31.4% • 1-year increase in age increased odds of fatigue by 1.23–1.25 |
High |
| Enskar et al. 199767 | Evaluate adolescents' experience of areas of life affected by the disease | • N=10 • Age 13–16 years at diagnosis n=8 • Age at interview: 15–20 years |
• Cross-sectional observational • 5-point NRS (non-validated questionnaire) |
≥2 on 1–5 NRS | • 100% | Medium |
| Ream et al. 200618 | Investigate impact of fatigue on adolescents | • N=22 • Age: 13–20 years |
• Longitudinal, mixed methods • Daily diary entry and completion of fatigue NRS |
Mention of fatigue in diary entries | • Mentioned fatigue during treatment: 32; 30% in early remission (1–2 years after treatment), 10% in late remission (>5 years) | Medium |
| Yeh et al. 2008,33 200934 | Assess symptoms in older Taiwanese children | • N=144 • Mean age: 14.2 years • For 108 on treatment, mean since diagnosis: 21.2 months |
• Cross-sectional observational • MSAS 10–18 |
Presence of “lack of energy” in last week on MSAS | • 52% (1 of 30) • On/off treatment: 52.8%, 50.0% • Leukemia: 57.3% • Lymphoma: 46.2% • Solid tumor: 46.5% |
Medium |
ALL, acute lymphatic leukemia; AML, acute myeloid leukemia; ARM 1, Adolescent Resilience Model study 1; ARM 2, Adolescent Resilience Model study 2; CFQ, Chalder Fatigue Questionnaire; CNS, central nervous system; D1, D2, etc., day 1, day 2, etc.; DXM, dexamethasone; EBT, extremity bone tumor; FACIT-fatigue, Functional Assessment of Chronic Illness Therapy-Fatigue Scale; FS-A, Fatigue Scale-Adolescent; FS-P, Fatigue Scale-Parent; HD, Hodgkin disease; LSS-A, Life Situation Scale for Adolescents; MFS, Multidimensional Fatigue Scale; MSAS (7–12, 10–18), Memorial Symptom Assessment Scale (for 7–12 and 10–18 age range); NORMS, healthy controls; NRS, Numerical Rating Scale; QOL, quality of life; RT, radiotherapy; T1, T2, etc., time point 1, time point 2, etc.; TC, testicular cancer; TRSC-C, Therapy-Related Symptom Checklist-Children; VAS, Visual Analogue Scale; VRS, Verbal Rating Scale.
The heterogeneity of study populations, outcome measures, and definitions of “fatigue case” hindered comparison of prevalence data between studies and prevented meta-analysis. Fatigue prevalence ranged from 7%47 to 100%65 during treatment, 9%50 to 67%66 after treatment, and between 31%56 and 100%67 in the mixed populations. As detailed in Table 3, the prevalence of fatigue and other symptoms were compared on 20 occasions in 10 studies; it was the most prevalent symptom on 11 occasions17,33,34,65,68–71 and the second-most prevalent on six occasions.17,71–73
Two studies—both with survivor populations—included a control group; both used non-contemporaneous controls. Aksnes et al. found a fatigue prevalence of 14% in extremity bone tumor survivors, which was not significantly different from that of age- and gender-matched cases from healthy population surveys (p=0.30).54 In contrast, Hamre et al. reported a fatigue prevalence of 34% in survivors of Hodgkin lymphoma, compared to 8% in an unmatched healthy control population (p<0.001).32
Fatigue severity
The severity of fatigue was measured in 32 instances: 20 studies during treatment, five studies after treatment, six studies with a mixed cohort of patients both during and after treatment, and one study that provided data on two separate patient cohorts during and after treatment, respectively. Seven studies reported relative severity of fatigue in comparison to other symptoms: fatigue was most severe in four studies,34,52,68,69 second-70 and third-most72 severe in one study each, and fourth-most severe in two studies.40,67 Fourteen different outcome measures were used across these 30 studies. The most commonly used outcome measures were the FS-A, MFS, and MSAS, in nine, eight, and four studies each, respectively. Comparison and synthesis of severity scores was again not possible due to heterogeneity of study populations, methods of reporting the data (for example, absolute score or percentage with score above a defined value), or measures used.
Three studies incorporated a control group, all of which compared fatigue severity in patients during treatment to that in survivors (Table 4). Controls ranged from contemporaneous recruits to unmatched reference data. All three studies showed significantly greater fatigue severity in the cancer groups compared to the controls.
Table 4.
Controlled Studies Evaluating Fatigue Severity
| Study | Aim(s) | Participants (mixed tumors unless stated) | Study design and fatigue assessments | Fatigue severity | Weight of Evidence D score |
|---|---|---|---|---|---|
| Aksnes et al. 200754 | Examine fatigue, mental distress, and QOL in EBT survivors compared to matched controls | • N=57 EBT survivors • Mean age at diagnosis within different cancer type subgroups: 16–25 years • Mean since diagnosis: 9–14 years |
• Case-control (non-contemporaneous age- and gender-matched controls HD, TC, NORMS) • CFQ |
• Mean total fatigue: • EBT survivors: 13.2 • HD survivors: 13.4 • TC survivors: 13.4 • NORMS: 11.8 • EBT survivors had significantly more fatigue than NORMS (p=0.003), but not more than other survivor groups |
Medium |
| Daniel et al. 201330 | Compare adolescent and parent reports of fatigue in patients with and without cancer | • N=102 with cancer on treatment • Mean age: 15.75 years • Mean since diagnosis: 20.4 months • N=97 controls; mean age: 15.55 years |
• Cross-sectional observational (contemporaneous controls) • MFS |
• MFS score in cancer and control groups, respectively: 58.54 and 71.72 (p<0.001) • Total fatigue, general fatigue, and sleep/rest fatigue worse, but not cognitive fatigue |
High |
| Smith et al. 201351 | Examine HRQOL of AYA patients and associated health-related characteristics | • N=159 • Age: 15–25 years • Subgroup of large AYA HOPE study with 523 cancer patients aged 15–39 at diagnosis • Since diagnosis: 6–14 months • 80% not on treatment |
• Cross-sectional observational (compared to age-range matched population norms) • MFS |
• Mean MFS scores: • Ages 18–25: 61.3, significantly worse than reference healthy population score of 71.0 (p=0.001) • Ages 15–17: 59.8, not significantly different from ages 18–25 (no reference data for this age range) |
Medium |
AYA, adolescent and young adult; AYA HOPE, Adolescent & Young Adult Health Outcomes & Patient Experience study; CFQ, Chalder Fatigue Questionnaire; EBT, extremity bone tumor; HD, Hodgkin disease; HRQOL, health-related quality of life; MFS, Multidimensional Fatigue Scale; NORMS, healthy controls; QOL, quality of life; TC, testicular cancer.
Eight studies compared fatigue severity between younger children and teenagers treated for cancer, of which five measured fatigue with both the Fatigue Scale-Adolescent (FS-A) and the Fatigue Scale-Child (FS-C).37,53,60,74,75 The FS-A and FS-C76 each use 14 age-appropriate questions and are validated for 13–18 and 7–12 year olds, respectively. Fatigue was reported as being more severe in adolescents than in younger children in all but one of the eight studies.53
Many of the studies that used the FS-A outcome also measured parent reports of fatigue,37,38,47,49,59,60,74,75,77 but patient and parent scores are not directly comparable. One study did directly compare the fatigue scores of TYA patients with proxy scores from their caregivers, and found that their caregivers tended to overestimate their fatigue severity.30
Fatigue severity was assessed over time in 11 longitudinal studies; all involved participants currently receiving chemotherapy.23,35,37,57,60,68,71,75,77–79 In general, fatigue scores were worse in the two weeks after receiving chemotherapy and then improved until the next cycle. No longitudinal studies investigated fatigue severity over the longer period from treatment into survivorship. One small cross-sectional, observational study of three groups of adolescents during treatment (n=8), 1–2 years after treatment (n=6), and five or more years after treatment (n=8) found that fatigue scores were highest during treatment, lowest during early remission, and higher again during late remission.18
Impact of fatigue
Twenty-two studies reported the impact of fatigue on patients.17–19,23,33,34,37,39–43,48,55,68,72,73,80–84 Distress caused by fatigue was the most commonly described impact, reported in 11 studies. The MSAS, a scale that allows comparison of the level of distress caused by each symptom, was used in six of these studies.17,33,34,68,72,73 When symptoms were ranked in order of distress, fatigue was in the top half with only one exception.17 One study reported that distress was correlated with the frequency and severity of fatigue.34 The remaining five studies used diverse measures of distress,40,43,55,82,83 and fatigue was one of the top four most distressing conditions in four of these studies.40,43,82,83
The second-most frequently described impact of fatigue was that it was a barrier to physical activity or exercise, which was reported in six studies.18,23,42,80,81,84 Fatigue was the first-80 and second-most84 significant barrier in one study each. Being unable to take part in exercise led to frustration and loss of confidence, with parents becoming “overprotective” and preventing their adolescents from taking part in activities that demanded energy.42 Four studies reported fatigue as a barrier to other social activities,18,23,42,48 including returning to school.48 A negative impact on affective state, mood, or anxiety was described in three studies.19,23,37
Experience of parents
Although 10 studies collected parent proxy reports of fatigue severity,30,37,38,47,49,59,60,74,75,77 none investigated the experiences of parents of adolescent teenagers or young adults with cancer-related fatigue. Parent proxy reports of fatigue severity correlated more closely with those of their children for parents of cancer patients than for parents of healthy controls, which was attributed to the cancer patients' parents being “physically closer” and “more attuned to the needs” of their children.30,59,61 However, cancer patients' parents appeared to be less adept than the patients themselves at perceiving changes in fatigue over time.60,77 One study collected staff proxy reports of patient fatigue as well as parent proxy reports, and found that staff reports correlated less tightly than parent reports with patients' self-reported fatigue.59
Fatigue correlates
Factors correlating with the presence or severity of fatigue were reported in 27 studies, including five of the six scoring highest on the Gough's Weight of Evidence Framework.30,38,47,49,51
Eleven studies highlighted an association between fatigue and physical symptoms, including poor sleep,35,37,57,61,74,75 being part of a symptom cluster,33,70,73 and nausea.29,61 Almost all participants in the three studies that examined symptom clusters were receiving chemotherapy;33,70,73 although there was no consistency in the specific symptoms found to cluster with fatigue, the symptoms tended to be chemotherapy-related. While receiving chemotherapy23,36,44,47,60,66 or dexamethasone45 both correlated with increasing fatigue, the evidence for a correlation between fatigue and hospital admission47,60,72 or anemia46,60 was conflicting. Even though patients associated fatigue with “doing too many things” or “being too active,”5,23 there was a correlation between improved performance status and lower fatigue scores.46,78
Depression or low mood correlated with fatigue in five studies;30,38,59,61,85 negative affect,29 global distress,72 anxiety,33,73 and non-specific psychological conditions44,72 were identified as correlates in a further five publications. Consistent with the link with physical and psychological symptoms, a high correlation with poor quality of life or satisfaction with life was reported in four studies.23,30,66,85
Although some of these correlations are intuitively likely to represent causal relationships—such as the relationship between fatigue and poor sleep quality—no studies addressed causality. However, one longitudinal study that observed a predictable fluctuation in fatigue during chemotherapy regimens of varying frequency23 and a study involving planned periods on and off dexamethasone45 both suggested causal relationships with fatigue.
Interventions to manage fatigue
Table 5 details the five interventional trials for TYA cancer patients in which fatigue was used as an outcome measure.43,74,79,86,87 Fatigue was not stated to be the primary outcome measure in any of the five. Three studies were uncontrolled79,86,87 and three were feasibility studies.74,79,87 Four involved evaluation of a structured activity intervention, and one investigated a self-care coping intervention. All of the interventions were standardized with a degree of individualization in accordance with each patient's exercise capacity. Two uncontrolled trials found a statistically significant benefit from their interventions,79,86 which involved intensive structured exercise in a gymnasium at weekly intervals for more than two months. The remaining two physical activity intervention studies involved bringing portable gym equipment to the patient's hospital room and did not find any significant effect.74,87 The self-care coping intervention was also ineffective.43
Table 5.
Studies with Fatigue Interventions
| Study | Aim(s) | Participants (mixed tumors unless stated) | Study design and intervention | Fatigue assessments | Results | Weight of Evidence D score |
|---|---|---|---|---|---|---|
| Atkinson et al. 201286 | Determine the impact of a structured exercise intervention | • N=55 • Age: 15–25 years |
• Uncontrolled trial • 2–3 sessions/week individual structured exercise in a hospital/private gymnasium with an exercise physiologist over 10 weeks |
• Assessed pre- and post-intervention • Revised PFS, Ferrans and Powers QLI, measures of functional fitness |
• Improvement in fatigue (p≤0.0001), as well as QOL and functional assessments • PFS scores decreased by 32% |
Medium |
| Hinds et al. 200043 | Evaluate effects of an educational intervention designed to facilitate self-care coping | • N=78 • Mean age: 16.0 years |
• Two-center randomized controlled trial • 40-minute intervention between T1 (1–12 days after diagnosis) and T2 (5–7 weeks after): information on self-care coping, video with strategies, and rehearsal of strategies • Control had equal time to discuss any topic |
• Assessed at T1, T2, 3 months, and 6 months after diagnosis • Six outcome measures, including SDS (includes 5-point fatigue severity VRS) |
• No difference in SDS score between groups at any time point • Fatigue 1 of 4 most distressing symptoms at every time point |
Low |
| Hinds et al. 200774 | Evaluate the feasibility of using an enhanced physical activity intervention | • N=11 • Subgroup age: 13–18 years |
• Two-center pilot randomized controlled trial • Pedaling a stationary bicycle exerciser for 30 minutes twice daily for 2–4 days of hospitalization with equipment brought to hospital room • Control spent equal time with researcher |
• Assessed daily on days 1, 2, and 3 • Patient, parent, and staff fatigue reports (FS-C, -A, -P, -S), wrist actigraphy, parent sleep diary, hemoglobin |
• No difference between groups | Medium |
| Keats et al. 200879 | Assess feasibility of a physical activity intervention | • N=10 • Age: 14–18 years • Mean since diagnosis: 62.5 months |
• Uncontrolled feasibility study • Weekly 90 minutes of group education and training for 8 weeks in gymnasium, then variety of non-competitive activities over 16 weeks |
• Assessed at baseline, week 8, week 16, 3 months, and 1 year • PedsQL, MFS, Leisure Score Index, FitnessGram (a physical fitness test) |
• Improvement in fatigue between baseline and 3 months (p=0.01), but post-intervention benefits not sustained at 3 months and 1 year | Medium |
| Rosenhagen et al. 201187 | Investigate feasibility and acceptability of sports therapy | • N=13 • Mean age: 15.3 years |
• Uncontrolled feasibility study (control group gave acceptability data only) • Individualized exercise using ergometer in hospital room during isolation phase of SCT |
• Assessed on days 1 and 14 and on discharge after SCT • QOL self-assessment, MFS |
• Non-significant trend for fatigue improvement pre- and post-intervention | Low |
FS-A, Fatigue Scale-Adolescent; FS-C, Fatigue Scale-Child; FS-P, Fatigue Scale-Parent; FS-S, Fatigue Scale-Staff; MFS, Multidimensional Fatigue Scale; PedsQL, Pediatric Quality of Life Inventory; PFS, Piper Fatigue Scale; QLI, quality of life index; QOL, quality of life; SDS, Symptom Distress Scale; SCT, stem cell transplant; T1, T2, etc., time point 1, time point 2, etc.; VRS, Verbal Rating Scale.
Discussion
This review provides evidence that fatigue is one of the most prevalent and severe symptoms experienced by teenagers and young adults suffering from cancer, occurring in the majority of patients and particularly prevalent during cancer treatment. This finding is consistent with evidence in adults with cancer, for whom fatigue is now accepted as the most prevalent symptom.11
There were no studies comparing TYA patients' fatigue with that of older adults with cancer. However, a number of publications compared fatigue in TYA patients to that in young children, with fatigue being more severe in the TYA population. This is consistent with the developmental sleep changes of adolescence. Healthy teenagers tend to develop fatigue and daytime sleepiness related to inadequate sleep.21 At this age, longer sleep is needed, yet shifts in circadian rhythms result in later bed times; the amount of time spent sleeping is further limited by unhelpful sleep habits, such as caffeine consumption and social engagements.21 Controlled studies have shown that fatigue in teenaged and young adult cancer patients is even higher than that in healthy controls.30,32,51,54 The dual risk factors for fatigue development—having cancer and being within or soon after the teenaged years—appear to combine to create a particularly significant problem in this vulnerable patient group.
Cancer-related fatigue is not only present during or soon after treatment: several studies of TYA cancer survivors showed long-term continuation of fatigue many years after cancer diagnosis and treatment.32,52,54 Given that TYA cancer patients have an approximately 80% five-year survival rate,6 this means that large numbers of young people are contending with ongoing morbidity while attempting to rebuild their lives after a cancer diagnosis.
Not only is fatigue prevalent, severe, and persistent, there is consistent evidence that fatigue causes significant distress, with a negative impact on quality of life. Fatigue may be a particularly distressing symptom in teenagers and young adults because of its impact on functioning at an age when independence and social interactions are high priorities. The inherent social isolation resulting from the diagnosis of a serious disease at a young age that can require years of burdensome treatment is further compounded by the presence of fatigue. The level of distress may also reflect the developmental stage of teenagers and young adults, who are more able than children to understand the significance of their symptoms and underlying cancer, yet may be less able to control and rationalize their emotions than older adults.
Despite the increasingly strong and consistent evidence base confirming the magnitude of the problem, there are no published studies evaluating interventions whose primary aim is to treat or prevent fatigue. The few interventional studies to date are mostly uncontrolled or feasibility studies investigating physical activity. Exercise is recognized as an effective treatment in adults with cancer-related fatigue. The presence of fatigue hinders activity, which then leads to deconditioning (or loss of “fitness”). This in turn worsens the fatigue, so leading to a vicious cycle that perpetuates the symptom. Exercise or activity is believed to work, at least in part, by preventing deconditioning and the development of this vicious cycle. Although two of the four physical activity interventional studies did report significant findings, both were uncontrolled. Given the well-established placebo effect that occurs with subjective symptoms such as fatigue,88 the strength of existing evidence for intervention effectiveness in the TYA age group is, as yet, poor.
In the context of physical activity being the only intervention for fatigue with any—albeit limited—supporting evidence in the TYA literature, a significant finding of this review is that fatigue itself is a key barrier to activity. There is mounting evidence that resting is perceived by patients, parents, and healthcare professionals as the best approach to managing fatigue. Studies have found that young patients feel that “being too active” or “doing too many things” may worsen fatigue;5,23 that parents encourage rest;42 and that in a mixed child and teenage population, healthcare professionals' most commonly recommended treatment for fatigue was rest and relaxation.89
There is evidence that activity levels tend to decline during the teenaged years due to conflicting priorities, fear of injury, and a sense of embarrassment.90 It is conceivable that TYA cancer patients may be particularly prone to such decline in activity; there may be perceived increased vulnerability to injury, and disease- or treatment-related bodily changes that could potentially cause self-consciousness.90 Cancer-related fatigue, in combination with these normal teenage inhibitions, can therefore present a formidable barrier to activity, paradoxically the very intervention that appears to have the greatest potential to improve their fatigue. Furthermore, at this formative age, life-long habits are developed. Maladaptive behaviors such as inactivity are therefore even more likely to persist, perpetuating fatigue and its associated disability and adverse psychosocial sequelae long-term.
Strengths and limitations
The main strength of this review is its breadth: studies were eligible if they investigated any aspect of cancer-related fatigue in TYA-aged patients, and even those with only a subgroup of patients aged 13–24 were included. The database search strategy was effective, with five articles found from other searches. The included studies, however, were heterogeneous and of relatively low quality, limiting the strength of the findings. Although experts in the field were contacted, literature not formally published in the form of journal articles (so-called “gray” literature) may have been missed. Exclusion of articles not in English may also have led to the omission of relevant articles.
Implications for clinical practice
Clinicians should be aware of the prevalence and severity of fatigue in TYA cancer patients, as well as the significant distress it causes. It is possible that a degree of therapeutic nihilism has developed due the lack of clearly effective pharmacological interventions for fatigue.91 Simply inquiring about the presence of fatigue in each clinical encounter may in itself be helpful, as this can openly acknowledge the problem and provide the possibility for peer and professional support.
Although the evidence base for exercise interventions is very limited, it is clear that encouragement of physical activity is likely to be helpful. In adults, it is well established that exercise improves cancer-related fatigue.15,92 The wider benefits of keeping active during and after cancer treatment include increased well-being, functioning, and quality of life, as well as reduced cancer recurrence and mortality.93–96 Clinicians, potentially lacking the time and knowledge to counsel TYA cancer patients about physical activity, should develop the skills to educate and address any misconceptions of TYA patients and their parents about the benefits of activity, should including confirming its potential to ameliorate rather than—as intuitively expected—worsen fatigue.
The correlation between fatigue and concurrent symptoms, such as poor sleep, is consistent with the evidence in adults that the management of sleep disorders97 and other symptoms98 can improve cancer-related fatigue. Rigorous control of concurrent symptoms, including education about sleep needs and habits in adolescence, may have a positive impact on fatigue.
Implications for future research
This review has revealed many gaps in the literature. Further prevalence studies are needed with concurrent controls, using a longitudinal design to evaluate changes in fatigue and fatigue-related distress within a cohort from treatment into long-term survivorship. It would be valuable to compare fatigue severity and associated distress in TYAs and older adults. Future studies could usefully attempt to establish the directionality of relationships between fatigue and other factors Determination of factors that cause, rather than are simply associated with, fatigue could helpfully guide fatigue management. Evidence for this review came from only five countries; further global research into TYA cancer-related fatigue is needed.
Use of dexamethasone in the TYA population appears to worsen fatigue by reducing sleep efficiency and increasing night-time wakenings.45 Conversely, in adults, this drug may improve fatigue.99 This conflicting evidence may indicate a genuine difference in the reaction to steroids of adolescents compared to older adults, and is worthy of further exploration.
Current research has established that cancer-related fatigue is a prevalent and distressing symptom in TYA cancer patients. It is essential that future research now focus on the development of interventions to manage fatigue, in order to limit the long-term suffering of this already vulnerable and burdened group of cancer patients. Physical activity—including determining its optimal forms and frequency—is a key research priority, as is assessing the impact of improving sleep hygiene and concurrent symptom control. Research considering parents' perspectives and experiences is also needed given that parental protectiveness may hinder the physical activity that could help ameliorate a TYA patient's fatigue. A deeper of understanding of parents' views and attitudes could facilitate meaningful education of parents that, in turn, effectively increases the activity of TYA cancer patients.
Conclusion
The fatigue experienced by teenagers and young adults with cancer is prevalent, persistent, and distressing. It has a negative impact on quality of life and social functioning that is particularly problematic at this formative age. The magnitude of the problem is established—it is now time to intervene.
Acknowledgments
SB and AS were funded by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care East of England at Cambridgeshire and Peterborough NHS Foundation Trust.
Disclaimer
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Office of National Statistics. Mortality statistics: deaths registered in England and Wales (Series DR, 2012). Accessed October16, 2014 from: www.ons.gov.uk/ons/rel/vsob1/mortality-statistics–deaths-registered-in-england-and-wales–series-dr-/2012/index.html
- 2.Cancer Research UK. Teenage and young adult cancer incidence statistics. Accessed October16, 2014 from: www.cancerresearchuk.org/cancer-info/cancerstats/teenage-and-young-adult-cancer/incidence/
- 3.Bleyer A, O'Leary M, Barr R, Ries LAG. (Eds). Cancer epidemiology in older adolescents and young adults 15 to 29 years of age, including SEER incidence and survival: 1975–2000 (NIH Publication No. 06-5767). Bethesda, MD: National Cancer Institute; 2006 [Google Scholar]
- 4.Smith S, Case L, Waterhouse K, et al. A blueprint of care for teenagers and young adults with cancer. London: Teenage Cancer Trust; 2012 [Google Scholar]
- 5.Hinds PS, Hockenberry-Eaton M, Gilger E, et al. Comparing patient, parent, and staff descriptions of fatigue in pediatric oncology patients. Cancer Nurs. 1999;22(4):277–88 [DOI] [PubMed] [Google Scholar]
- 6.O'Hara C, Moran A, TYA National Cancer Intelligence Advisory Group. Survival in teenagers and young adults with cancer in the UK. London: National Cancer Intelligence Network; August2012 [Google Scholar]
- 7.National Institute for Health and Clinical Excellence. Guidance on cancer services: improving outcomes in children and young people with cancer. London: National Institute for Health and Clinical Excellence; August2005 [Google Scholar]
- 8.Mock V, Atkinson A, Barsevick A, et al. NCCN practice guidelines for cancer-related fatigue. Oncology (Williston Park). 2000;14(11A):151–61 [PubMed] [Google Scholar]
- 9.Glaus A, Crow R, Hammond S. A qualitative study to explore the concept of fatigue/tiredness in cancer patients and in healthy individuals. Support Care Cancer. 1996;4(2):82–96 [DOI] [PubMed] [Google Scholar]
- 10.Curt GA. Fatigue in cancer. BMJ. 2001;322(7302):1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofman M, Ryan JL, Figueroa-Moseley CD, et al. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4–10 [DOI] [PubMed] [Google Scholar]
- 12.Vogelzang NJ, Breitbart W, Cella D, et al. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: results of a tripart assessment survey. The Fatigue Coalition. Semin Hematol. 1997;34(Suppl 1):4–12 [PubMed] [Google Scholar]
- 13.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5(5):353–60 [DOI] [PubMed] [Google Scholar]
- 14.Stone P, Richardson A, Ream E, et al. Cancer-related fatigue: inevitable, unimportant and untreatable? Results of a multi-centre patient survey. Cancer Fatigue Forum. Ann Oncol. 2000;11(8):971–5 [DOI] [PubMed] [Google Scholar]
- 15.Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2012;11:CD006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goedendorp MM, Gielissen MF, Verhagen CA, Bleijenberg G. Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database Syst Rev. 2009;1:CD006953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins JJ, Byrnes ME, Dunkel IJ, et al. The measurement of symptoms in children with cancer. J Pain Symptom Manage. 2000;19(5):363–77 [DOI] [PubMed] [Google Scholar]
- 18.Ream E, Gibson F, Edwards J, et al. Experience of fatigue in adolescents living with cancer. Cancer Nurs. 2006;29(4):317–26 [DOI] [PubMed] [Google Scholar]
- 19.Gibson F, Mulhall AB, Richardson A, et al. A phenomenologic study of fatigue in adolescents receiving treatment for cancer. Oncol Nurs Forum. 2005;32(3):651–60 [DOI] [PubMed] [Google Scholar]
- 20.Erickson JM. Fatigue in adolescents with cancer: a review of the literature. Clin J Oncol Nurs. 2004;8(2):139–45 [DOI] [PubMed] [Google Scholar]
- 21.Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8(6):602–12 [DOI] [PubMed] [Google Scholar]
- 22.Dahl RE, Lewin DS. Pathways to adolescent health sleep regulation and behavior. J Adolesc Health. 2002;31(6 Suppl):175–84 [DOI] [PubMed] [Google Scholar]
- 23.Erickson JM, Beck SL, Christian B, et al. Patterns of fatigue in adolescents receiving chemotherapy. Oncol Nurs Forum. 2010;37(4):444–55 [DOI] [PubMed] [Google Scholar]
- 24.Erickson JM, MacPherson CF, Ameringer S, et al. Symptoms and symptom clusters in adolescents receiving cancer treatment: a review of the literature. Int J Nurs Stud. 2013;50(6):847–69 [DOI] [PubMed] [Google Scholar]
- 25.Daniëls LA, Oerlemans S, Krol AD, et al. Persisting fatigue in Hodgkin lymphoma survivors: a systematic review. Ann Hematol. 2013;92(8):1023–32 [DOI] [PubMed] [Google Scholar]
- 26.McMillan EM, Newhouse IJ. Exercise is an effective treatment modality for reducing cancer-related fatigue and improving physical capacity in cancer patients and survivors: a meta-analysis. Appl Physiol Nutr Metab. 2011;36(6):892–903 [DOI] [PubMed] [Google Scholar]
- 27.Chang CW, Mu PF, Jou ST, et al. Systematic review and meta-analysis of nonpharmacological interventions on fatigue in children and adolescents with cancer. Worldviews Evid Based Nurs. 2013;10(4):208–17 [DOI] [PubMed] [Google Scholar]
- 28.Gough D. Weight of Evidence: a framework for the appraisal of the quality and relevance of evidence. Res Paper Educ. 2007;22(2):213–28 [Google Scholar]
- 29.Wesley KM, Zelikovsky N, Schwartz LA. Physical symptoms, perceived social support, and affect in adolescents with cancer. J Psychosoc Oncol. 2013;31(4):451–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daniel LC, Brumley LD, Schwartz LA. Fatigue in adolescents with cancer compared to healthy adolescents. Pediatr Blood Cancer. 2013;60(11):1902–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamre H, Zeller B, Kanellopoulos A, et al. Serum cytokines and chronic fatigue in adults surviving after childhood leukemia and lymphoma. Brain Behav Immun. 2013;30:80–7 [DOI] [PubMed] [Google Scholar]
- 32.Hamre H, Zeller B, Kanellopoulos A, et al. High prevalence of chronic fatigue in adult long-term survivors of acute lymphoblastic leukemia and lymphoma during childhood and adolescence. J Adolesc Young Adult Oncol. 2013;2(1):2–9 [Google Scholar]
- 33.Yeh CH, Chiang YC, Chien LC, et al. Symptom clustering in older Taiwanese children with cancer. Oncol Nurs Forum. 2008;35(2):273–81 [DOI] [PubMed] [Google Scholar]
- 34.Yeh CH, Wang CH, Chiang YC, et al. Assessment of symptoms reported by 10- to 18-year-old cancer patients in Taiwan. J Pain Symptom Manage. 2009;38(5):738–46 [DOI] [PubMed] [Google Scholar]
- 35.Erickson JM, Beck SL, Christian BR, et al. Fatigue, sleep-wake disturbances, and quality of life in adolescents receiving chemotherapy. J Pediatr Hematol Oncol. 2011;33(1):e17–25 [DOI] [PubMed] [Google Scholar]
- 36.Hockenberry MJ, Hooke MC, Gregurich M, McCarthy K. Carnitine plasma levels and fatigue in children/adolescents receiving cisplatin, ifosfamide, or doxorubicin. J Pediatr Hematol Oncol. 2009;31(9):664–9 [DOI] [PubMed] [Google Scholar]
- 37.Hockenberry MJ, Hooke MC, Gregurich M, et al. Symptom clusters in children and adolescents receiving cisplatin, doxorubicin, or ifosfamide. Oncol Nurs Forum. 2010;37(1):E16–27 [DOI] [PubMed] [Google Scholar]
- 38.Hockenberry MJ, Hooke MC, McCarthy K, Gregurich MA. Sickness behavior clustering in children with cancer. J Pediatr Oncol Nurs. 2011;28(5):263–72 [DOI] [PubMed] [Google Scholar]
- 39.Hedström M, Haglund K, Skolin I, von Essen L. Distressing events for children and adolescents with cancer: child, parent, and nurse perceptions. J Pediatr Oncol Nurs. 2003;20(3):120–32 [DOI] [PubMed] [Google Scholar]
- 40.Hedström M, Ljungman G, von Essen L. Perceptions of distress among adolescents recently diagnosed with cancer. J Pediatr Hematol Oncol. 2005;27(1):15–22 [DOI] [PubMed] [Google Scholar]
- 41.Hedström M, Skolin I, von Essen L. Distressing and positive experiences and important aspects of care for adolescents treated for cancer. Adolescent and nurse perceptions. Eur J Oncol Nurs. 2004;8(1):6–17 [DOI] [PubMed] [Google Scholar]
- 42.Chiang Y, Yeh CH, Wang KW, Yang CP. The experience of cancer-related fatigue in Taiwanese children. Eur J Cancer Care (Engl). 2009;18(1):43–9 [DOI] [PubMed] [Google Scholar]
- 43.Hinds P, Quargnenti A, Bush AJ, et al. An evaluation of the impact of a self-care coping intervention on psychological and clinical outcomes in adolescents with newly diagnosed cancer. Eur J Oncol Nurs. 2000;4(1):6–17 [DOI] [PubMed] [Google Scholar]
- 44.Hinds PS, Nuss SL, Ruccione KS, et al. PROMIS pediatric measures in pediatric oncology: valid and clinically feasible indicators of patient-reported outcomes. Pediatr Blood Cancer. 2013;60(3):402–8 [DOI] [PubMed] [Google Scholar]
- 45.Hinds PS, Hockenberry MJ, Gattuso JS, et al. Dexamethasone alters sleep and fatigue in pediatric patients with acute lymphoblastic leukemia. Cancer. 2007;110(10):2321–30 [DOI] [PubMed] [Google Scholar]
- 46.Lai JS, Cella D, Kupst MJ, et al. Measuring fatigue for children with cancer: development and validation of the pediatric Functional Assessment of Chronic Illness Therapy-Fatigue (pedsFACIT-F). J Pediatr Hematol Oncol. 2007;29(7):471–9 [DOI] [PubMed] [Google Scholar]
- 47.Mandrell BN, Yang J, Hooke MC, et al. Psychometric and clinical assessment of the 13-item reduced version of the Fatigue Scale–Adolescent instrument. J Pediatr Oncol Nurs. 2011;28(5):287–94 [DOI] [PubMed] [Google Scholar]
- 48.McLoone JK, Wakefield CE, Butow P, et al. Returning to school after adolescent cancer: a qualitative examination of Australian survivors' and their families' perspectives. J Adolesc Young Adult Oncol. 2011;1(2):87–94 [DOI] [PubMed] [Google Scholar]
- 49.Meeske K, Katz ER, Palmer SN, et al. Parent proxy-reported health-related quality of life and fatigue in pediatric patients diagnosed with brain tumors and acute lymphoblastic leukemia. Cancer. 2004;101(9):2116–25 [DOI] [PubMed] [Google Scholar]
- 50.Mulrooney DA, Ness KK, Neglia JP, et al. Fatigue and sleep disturbance in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study (CCSS). Sleep. 2008;31(2):271–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith AW, Bellizzi KM, Keegan TH, et al. Health-related quality of life of adolescent and young adult patients with cancer in the United States: the Adolescent and Young Adult Health Outcomes and Patient Experience study. J Clin Oncol. 2013;31(17):2136–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adams MJ, Lipsitz SR, Colan SD, et al. Cardiovascular status in long-term survivors of Hodgkin's disease treated with chest radiotherapy. J Clin Oncol. 2004;22(15):3139–48 [DOI] [PubMed] [Google Scholar]
- 53.Zupanec S, Jones H, Stremler R. Sleep habits and fatigue of children receiving maintenance chemotherapy for ALL and their parents. J Pediatr Oncol Nurs. 2010;27(4):217–28 [DOI] [PubMed] [Google Scholar]
- 54.Aksnes LH, Hall KS, Jebsen N, et al. Young survivors of malignant bone tumours in the extremities: a comparative study of quality of life, fatigue and mental distress. Support Care Cancer. 2007;15(9):1087–96 [DOI] [PubMed] [Google Scholar]
- 55.Azizi N, Mansour L, Tahmassian K, Mosavi F. Determining, ranking and comparing treatment stressors in children and adolescents with cancer in Tehran. Iranian J Cancer Prevent. 2012;5(3):138–43 [PMC free article] [PubMed] [Google Scholar]
- 56.Corey AL, Haase JE, Azzouz F, Monahan PO. Social support and symptom distress in adolescents/young adults with cancer. J Pediatr Oncol Nurs. 2008;25(5):275–84 [DOI] [PubMed] [Google Scholar]
- 57.Ameringer S, Elswick RK, Jr, Shockey DP, Dillon R. A pilot exploration of symptom trajectories in adolescents with cancer during chemotherapy. Cancer Nurs. 2013;36(1):60–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gibson F, Edwards J, Sepion B, Richardson A. Cancer-related fatigue in children and young people: survey of healthcare professionals' knowledge and attitudes. Eur J Oncol Nurs. 2006;10(4):311–6 [DOI] [PubMed] [Google Scholar]
- 59.Hinds PS, Hockenberry M, Tong X, et al. Validity and reliability of a new instrument to measure cancer-related fatigue in adolescents. J Pain Symptom Manage. 2007;34(6):607–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perdikaris P, Merkouris A, Patiraki E, et al. Evaluating cancer related fatigue during treatment according to children's, adolescents' and parents' perspectives in a sample of Greek young patients. Eur J Oncol Nurs. 2009;13(5):399–408 [DOI] [PubMed] [Google Scholar]
- 61.Whitsett SF, Gudmundsdottir M, Davies B, et al. Chemotherapy-related fatigue in childhood cancer: correlates, consequences, and coping strategies. J Pediatr Oncol Nurs. 2008;25(2):86–96 [DOI] [PubMed] [Google Scholar]
- 62.Varni JW, Burwinkle TM, Katz ER, et al. The PedsQL in pediatric cancer: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer. 2002;94(7):2090–106 [DOI] [PubMed] [Google Scholar]
- 63.Portenoy RK, Thaler HT, Kornblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A(9):1326–36 [DOI] [PubMed] [Google Scholar]
- 64.Chalder T, Berelowitz G, Pawlikowska T, et al. Development of a fatigue scale. J Psychosom Res. 1993;37(2):147–53 [DOI] [PubMed] [Google Scholar]
- 65.Baggott C, Gibson F, Coll B, et al. Initial evaluation of an electronic symptom diary for adolescents with cancer. JMIR Res Protoc. 2012;1(2):e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Enskär K, von Essen L. Prevalence of aspects of distress, coping, support and care among adolescents and young adults undergoing and being off cancer treatment. Eur J Oncol Nurs. 2007;11(5):400–8 [DOI] [PubMed] [Google Scholar]
- 67.Enskär K, Carlsson M, Golsäter M, Hamrin E. Symptom distress and life situation in adolescents with cancer. Cancer Nurs. 1997;20(1):23–33 [DOI] [PubMed] [Google Scholar]
- 68.Walker AJ, Gedaly-Duff V, Miaskowski C, Nail L. Differences in symptom occurrence, frequency, intensity, and distress in adolescents prior to and one week after the administration of chemotherapy. J Pediatr Oncol Nurs. 2010;27(5):259–65 [DOI] [PubMed] [Google Scholar]
- 69.Williams PD, Williams AR, Kelly KP, et al. A symptom checklist for children with cancer: the Therapy-Related Symptom Checklist-Children. Cancer Nurs. 2012;35(2):89–98 [DOI] [PubMed] [Google Scholar]
- 70.Baggott C, Cooper BA, Marina N, et al. Symptom cluster analyses based on symptom occurrence and severity ratings among pediatric oncology patients during myelosuppressive chemotherapy. Cancer Nurs. 2012;35(1):19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baggott C, Dodd M, Kennedy C, et al. Changes in children's reports of symptom occurrence and severity during a course of myelosuppressive chemotherapy. J Pediatr Oncol Nurs. 2010;27(6):307–15 [DOI] [PubMed] [Google Scholar]
- 72.Miller E, Jacob E, Hockenberry MJ. Nausea, pain, fatigue, and multiple symptoms in hospitalized children with cancer. Oncol Nurs Forum. 2011;38(5):E382–93 [DOI] [PubMed] [Google Scholar]
- 73.Atay S, Conk Z, Bahar Z. Identifying symptom clusters in paediatric cancer patients using the Memorial Symptom Assessment Scale. Eur J Cancer Care (Engl). 2012;21(4):460–8 [DOI] [PubMed] [Google Scholar]
- 74.Hinds PS, Hockenberry M, Rai SN, et al. Clinical field testing of an enhanced-activity intervention in hospitalized children with cancer. J Pain Symptom Manage. 2007;33(6):686–97 [DOI] [PubMed] [Google Scholar]
- 75.Orsey AD, Wakefield DB, Cloutier MM. Physical activity (PA) and sleep among children and adolescents with cancer. Pediatric Blood Cancer. 2013;60(11):1908–13 [DOI] [PubMed] [Google Scholar]
- 76.Hockenberry MJ, Hinds PS, Barrera P, et al. Three instruments to assess fatigue in children with cancer: the child, parent and staff perspectives. J Pain Symptom Manage. 2003;25(4):319–28 [DOI] [PubMed] [Google Scholar]
- 77.Hinds PS, Hockenberry M, Rai SN, et al. Nocturnal awakenings, sleep environment interruptions, and fatigue in hospitalized children with cancer. Oncol Nurs Forum. 2007;34(2):393–402 [DOI] [PubMed] [Google Scholar]
- 78.Hooke MC, Garwick AW, Gross CR. Fatigue and physical performance in children and adolescents receiving chemotherapy. Oncol Nurs Forum. 2011;38(6):649–57 [DOI] [PubMed] [Google Scholar]
- 79.Keats MR, Culos-Reed SN. A community-based physical activity program for adolescents with cancer (project TREK): program feasibility and preliminary findings. J Pediatr Hematol Oncol. 2008;30(4):272–80 [DOI] [PubMed] [Google Scholar]
- 80.Arroyave WD, Clipp EC, Miller PE, et al. Childhood cancer survivors' perceived barriers to improving exercise and dietary behaviors. Oncol Nurs Forum. 2008;35(1):121–30 [DOI] [PubMed] [Google Scholar]
- 81.Cassano J, Nagel K, O'Mara L. Talking with others who “just know”: perceptions of adolescents with cancer who participate in a teen group. J Pediatr Oncol Nurs. 2008;25(4):193–9 [DOI] [PubMed] [Google Scholar]
- 82.Griffith KC, Hart LK. Characteristics of adolescent cancer survivors who pursue postsecondary education. Cancer Nurs. 2000;23(6):468–76 [DOI] [PubMed] [Google Scholar]
- 83.Hinds PS, Quargnenti AG, Wentz TJ. Measuring symptom distress in adolescents with cancer. J Pediatr Oncol Nurs. 1992;9(2):84–6 [DOI] [PubMed] [Google Scholar]
- 84.Lockwood R, Smith C. Physical activity during and after treatment for cancer: what do teenagers and young adults want from a service? Poster presented at the Teenage Cancer Trust 7th International Conference on Teenage and Young Adult Cancer Medicine, June 25–26, 2012, in London, United Kingdom [Google Scholar]
- 85.Ruccione K, Lu Y, Meeske K. Adolescents' psychosocial health-related quality of life within 6 months after cancer treatment completion. Cancer Nurs. 2013;36(5):E61–72 [DOI] [PubMed] [Google Scholar]
- 86.Atkinson M, Osborn M. A structured ten-week exercise intervention is associated with improvements in quality of life, fatigue, and functional status in adolescents and young adults with cancer. Asia Pac J Clin Oncol. 2012;8(Suppl 3):abstract 321 [Google Scholar]
- 87.Rosenhagen A, Bernhörster M, Vogt L, et al. Implementation of structured physical activity in the pediatric stem cell transplantation. Klin Padiatr. 2011;223(3):147–51 [DOI] [PubMed] [Google Scholar]
- 88.de la Cruz M, Hui D, Parsons HA, Bruera E. Placebo and nocebo effects in randomized double-blind clinical trials of agents for the therapy for fatigue in patients with advanced cancer. Cancer. 2010;116(3):766–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gibson F, Garnett M, Richardson A, et al. Heavy to carry: a survey of parents' and healthcare professionals' perceptions of cancer-related fatigue in children and young people. Cancer Nurs. 2005;28(1):27–35 [DOI] [PubMed] [Google Scholar]
- 90.Grieser M, Vu M, Bedimo-Rung A, et al. Physical activity attitudes, preferences, and practices in African American, Hispanic, and Caucasian girls. Health Educ Behav. 2006;33(1):40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spathis A, Fife F, Blackhall F, et al. Modafinil for the treatment of fatigue in lung cancer: results of a placebo-controlled, double-blinded, randomized trial. J Clin Oncol. 2014;32(18):1882–8 [DOI] [PubMed] [Google Scholar]
- 92.Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2010;28(3):753–65 [DOI] [PubMed] [Google Scholar]
- 93.Meyerhardt JA, Giovannucci EL, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24(22):3527–34 [DOI] [PubMed] [Google Scholar]
- 94.Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21(9):1653–9 [DOI] [PubMed] [Google Scholar]
- 95.Speck RM, Courneya KS, Mâsse LC, et al. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87–100 [DOI] [PubMed] [Google Scholar]
- 96.Holmes MD, Chen WY, Feskanich D, et al. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479–86 [DOI] [PubMed] [Google Scholar]
- 97.Zee PC, Ancoli-Israel S. Does effective management of sleep disorders reduce cancer-related fatigue? Drugs. 2009;69(Suppl 2):29–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de Raaf PJ, de Klerk C, Timman R, et al. Systematic monitoring and treatment of physical symptoms to alleviate fatigue in patients with advanced cancer: a randomized controlled trial. J Clin Oncol. 2013;31(6):716–23 [DOI] [PubMed] [Google Scholar]
- 99.Yennurajalingam S, Frisbee-Hume S, Palmer JL, et al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol. 2013;31(25):3076–82 [DOI] [PubMed] [Google Scholar]
- 100.Yellen S, Cella D, Webster K, et al. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63–74 [DOI] [PubMed] [Google Scholar]
- 101.Piper BF, Dibble SL, Dodd MJ, et al. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum. 1998;25(4):677–84 [PubMed] [Google Scholar]
- 102.McCorkle R. The measurement of symptom distress. Semin Oncol Nurs. 1987;3(4):248–56 [DOI] [PubMed] [Google Scholar]