Abstract
Objective: The aim of this study was to determine if living in a lower income neighborhood is associated with mortality of patients with bronchopulmonary dysplasia (BPD) on home ventilation.
Methods: Patients were divided into two groups by their ZIP code–based annual household income (Z-AHI), their year of birth, and the median state household income. Survival, liberation from ventilation, and decannulation rates were analyzed between the groups.
Results: Over 27 years, 94 patients met our inclusion criteria: 58 (61.7%) were in the group with lower Z-AHI, and 36 (38.3%) were in the group with the Z-AHI above the median state household. Of the patients who died, 14/15 were in the lower Z-AHI group (p=0.003). Survival probability at 60 months of age showed no significant difference between the two groups: 81% [95% CI 70.9, 91.1] for the group with the Z-AHI below the median state household, and 100% [95% CI 100.0, 90.3] for the group with higher Z-AHI (p=0.31).
Conclusions: The results of this study are descriptive, as the cause of the association between mortality rate and living in an area with lower household income is not yet understood. The difference in mortality rates between groups above and below the median state income suggests a serious health disparity, which warrants further study. Additional understanding of this effect requires more complete and direct measurement of socioeconomic status and individual characteristics, and better understanding of local environmental conditions.
Introduction
Health disparities (i.e., preventable differences in the burden of disease or opportunities to achieve optimal health) are experienced by millions of people in socially disadvantaged populations every year.1 Health disparities occur in groups often marginalized by racial, ethnic, sex, disability status, rural residence, and socioeconomic classifications.2 Such disparities can sometimes occur as a result of the quality of healthcare received. Factors such as insurance coverage and the cultural awareness of healthcare providers influence treatment and can create disparities, leading to inequality in treatment between certain groups of patients. Furthermore, the differences in environmental factors are particularly divisive along socioeconomic status (SES) lines.3
Indicators of SES provide information about an individual's access to social and economic resources.4 SES is a complex construct, but most often these indicators are income, education, and occupation. While education and occupation reveal individually based dimensions of SES, household income is the most indicative of standard of living and of advantages household members experience through sharing goods and services.4
While higher income is associated with improved health in children, with findings documented across a range of diseases, including asthma, obesity, and injury,5–7 low SES has been repeatedly established as a risk factor for many negative health outcomes, independent of known biologic risk factors. Low SES has even been cited as a cause of death.8–10
Accessibility, affordability, and quality of childcare available to families in the neighborhood represents an institutional resource that may act as a mediator of neighborhood effects on young children's outcomes. The characteristics of childcare available in the community have implications for children's learning experiences, behavioral functioning, and physical health. Children with bronchopulmonary dysplasia (BPD) who develop chronic respiratory failure, and consequently require chronic ventilation at home, are at increased risk for comorbidities due to their fragile state.11 The aim of this study was to determine the association between median household income, assessed at the ZIP code level, and the long-term outcome of patients with BPD who were discharged on home ventilation via tracheostomy. Thus, while evidence exists of the effects of low SES on children and children with chronic diseases,10 the goal of this study was to consider health disparities in a very specific, at-risk population of pediatric patients. This is the first study to address the health disparities on the health outcomes of these fragile children.
Methods
Patients
We conducted a retrospective cohort study of patients who carried the diagnosis of BPD that required chronic ventilator support at home. These patients were followed in the Pediatric Pulmonology Clinic—Home Ventilator Program (HVP), at Riley Hospital for Children (Indiana University, Indianapolis, IN). Due to the complexities of each child's medical condition, all families received at least 8 hours of skilled home nursing/respite services per day. Excluded were patients with other comorbidities that could contribute to the development of chronic respiratory failure: congenital cardiac disease (requiring surgery and/or medication) other than patent ductus arteriosis; chest surgery; chromosomal abnormalities; anatomical abnormalities (agenesis of one lung, tracheal-esophageal fistula, and congenital diaphragmatic hernia); and metabolic disease and congenital neurological abnormalities (Chiari malformation, myelomeningocele). An additional exclusion criterion included being a resident of a chronic care facility, since the guardian's address was not known, and because environmental factors affecting low SES families may not be applicable to residents at chronic care facilities. Those patients who were not followed at the authors' institution in the last 3 years were considered lost to follow-up. The study was approved by the Institutional Review Board of Indiana University.
Data collection
One author (A.I.C.) reviewed the complete medical chart for each child from the hospital's electronic medical record (Careweb™, Regenstrief Institute, Cerner Corporation, Kansas City, MO) and from the paper charts that were part of the HVP. Data elements abstracted included sex, race, gestational age at birth, birth weight, length of neonatal intensive care unit (NICU) hospitalization, the date when the patient became ventilator independent, the date of decannulation, the ZIP code of the discharge address, and health insurance coverage.
Statistical analysis
The household's contextual SES has been previously defined by the median income of the patient's ZIP code of residence. ZIP codes and U.S. census data frequently serve as an SES figure in research.12–17 In order to determine the median household income, patients' residential postal ZIP codes at initial discharge from the hospital and the census data were utilized to determine the ZIP code–based annual household income (Z-AHI). The 1990 census data were used for patients born before 1995, the 2000 census data for those patients born between 1995 and 2004, and the 2010 census data for those born after 2004.18 Patients were divided into two groups based on the median state household income in three different years: 1990 ($28,797), 2000 ($41,567), and 2010 ($47,697). Demographic and clinical variables were analyzed to test for differences between the two income groups. Data were reported as medians and interquartile ranges (IQR) for continuous variables and frequency (%) for categorical variables; p-values resulted from the Wilcoxon or chi square test respectively.
Kaplan–Meier estimates were calculated for each group for time from birth to (age at) death, age at liberation from ventilation, and age at decannulation. Log-rank tests were used to compare the differences between these time-to-event outcomes. Survival time, liberation from ventilation, and decannulation were obtained for each group with a 95% confidence interval. Due to the low number of death events, median time to death was not estimable. Instead, the percentage of survival probability at 60 months of age was calculated. The survival probability at 60 months of age was calculated for each group by using the method described by Klein et al.19 Results are reported as survival probability with 95% confidence intervals.
Differences in follow-up hospitalization rates between the two groups were also analyzed based on the main diagnosis at discharge. The main diagnoses were classified into the following groups: respiratory and nonrespiratory (renal, cardiac, social, gastrointestinal, infections, neurology, and surgeries). Follow-up hospitalization rates were determined by taking the number of hospitalizations and dividing by the appropriate time interval. Pre-decannulation interval was the time period from the discharge from initial hospital stay to event (decannulation or current age). Post-decanulation interval was the time period from decannulation to current age. Data were analyzed using the Wilcoxon nonparametric test. Rate descriptive data was reported as medians with ranges. All analyses were performed with SAS v9.3 (SAS Institute, Cary, NC). A p-value<0.05 was selected for statistical significance.
Results
Between 1984 and 2010, 628 children were cared for in the HVP at the authors' institution. Of these, 94 (14.9%) met the inclusion criteria and were enrolled in this study. All of these patients carried the diagnosis of BPD and were receiving chronic ventilation via tracheostomy at initial discharge from the hospital, and were in the care of their biological or foster parents. Baseline characteristics of these patients are described in Table 2.
Table 2.
Baseline Characteristics and Outcomes of 94 Children with BPD Who Were Ventilator Dependent via Tracheostomy at Home
| Characteristics | All patients (n=94) | Z-AHI below the median state household (n=58; 61.7%) | Z-AHI above the median state household (n=36; 38.3%) | p-Value |
|---|---|---|---|---|
| Status: | ||||
| Alive | 76 (80.9) | 44 (75.9) | 32 (88.9) | |
| Deceased | 15 (15.9) | 14 (24.1) | 1 (2.8) | 0.003 |
| Lost to follow-up | 3 (3.2) | 0 (0) | 3 (8.3) | |
| Male | 55 (58.5) | 34 (58.6) | 21 (58.3) | 0.98 |
| Race: | ||||
| White | 65 (69.1) | 43 (74.1) | 22 (61.1) | |
| Black | 22 (23.4) | 11 (19.0) | 11 (30.6) | 0.39 |
| Other (Hispanic, Asian) | 7 (7.5) | 4 (6.9) | 3 (8.3) | |
| Birth weight, gramsa | 760.0b (650–920) | 725.0c (653.8–930.0) | 780.0d (640.0–905.0) | 0.46 |
| Gestational age, weeksa | 26 (25–27) | 26.0 (25.0–27.0) | 26.0 (25.0–27.0) | 0.90 |
| Health insurance: | ||||
| Medicaid | 57 (60.6) | 37 (63.8) | 20 (55.6) | |
| Private/Medicaid | 17 (18.1) | 9 (15.5) | 8 (22.2) | 0.66 |
| Private | 20 (21.3) | 12 (20.7) | 8 (22.2) | |
| Initial hospital stay length (months)a | 10e (8–12) | 10.8f (8–13) | 9.4g (7.0–11.5) | 0.92 |
| Caregivers: | ||||
| Biological parents | 81 (86.2) | 52 (89.7) | 29 (80.6) | 0.21 |
| Foster parents | 13 (13.8) | 6 (10.3) | 7 (19.4) | |
Results reported as median (interquartile range).
Birth weight was missing for seven patients.
Birth weight was missing for four patients.
Birth weight was missing for three patients.
Date of discharge was missing for eight patients.
Date of discharge was missing for five patients.
Date of discharge was missing for three patients.
Z-AHI, ZIP code-based annual household income.
Morbidity during the initial Neonatal Intensive Care Unit (NICU) hospitalization included the following: 94 (100%) patients received a gastrostomy tube (37.2% also had a Nissen fundoplication); 40 (42.5%) were diagnosed with retinopathy of prematurity; 22 (23.4%) had severe intraventricular hemorrhage that necessitated a ventriculo-peritoneal shunt; 13 (13.8%) had seizures; 26 (27.7%) had documented sepsis; 20 (21.2%) had inguinal hernia repair; 18 (19.1%) had pulmonary hypertension; 15 (15.9%) had systemic hypertension; 13 (13.8%) were diagnosed with necrotizing enterocolitis; and 18 (19.1%) had patent ductus arteriosus, of which 10 needed surgical ligation. Secondary data analysis revealed no significant differences in the neonatal comorbidities between the groups (data not shown).
The majority (58 subjects; 61.7%) lived in an area with a Z-AHI below the state median household income, leaving 36 subjects (38.3%) in an area with Z-AHI above the state median household income (Table 1). All but one of the patients who died were in the group with lower Z-AHI. There were no other significant differences between the two groups. Sex, race, birth weight, gestational age, health insurance, length of hospital stay, and caregivers were all comparable (Table 2). No significant differences were found between the two groups as far as median time to liberation from ventilation (26 months [IQR: 20.0, 33.0] for low Z-AHI; 23 months [IQR: 18.0, 29.0] for high Z-AHI; p=0.18); and median time to decannulation (39 months [IQR: 32.0, 46.5] for low Z-AHI; 34 months [IQR: 25.5, 43.0] for high Z-AHI; p=0.11).
Table 1.
Median Household Income and Distribution of 94 Children with BPD Who Were Ventilator Dependent via Tracheostomy at Home
| Patients born before 1995 (n=29) | Patients born between 1995 and 2004 (n=33) | Patients born after 2004 (n=32) | |
|---|---|---|---|
| Year and median state household income | 1990, $28,797 | 2000, $41,567 | 2010, $47,697 |
| Income range | $17,417–48,696 | $19,819–70,715 | $27,664–93,835 |
| Number above state median | 6 | 13 | 17 |
| Number below state median | 23 | 20 | 15 |
BPD, bronchopulmonary dysplasia.
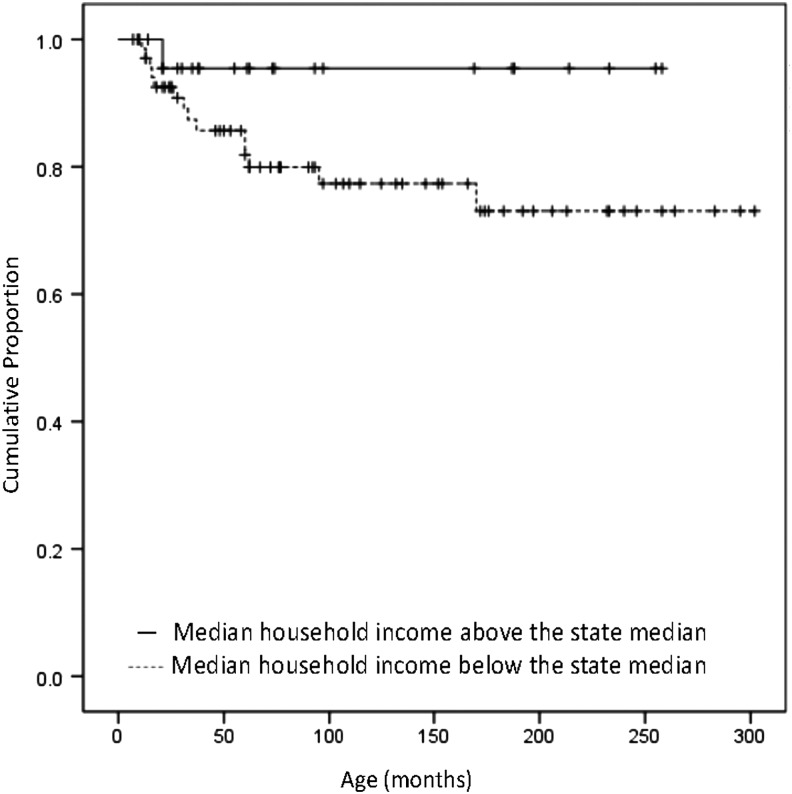
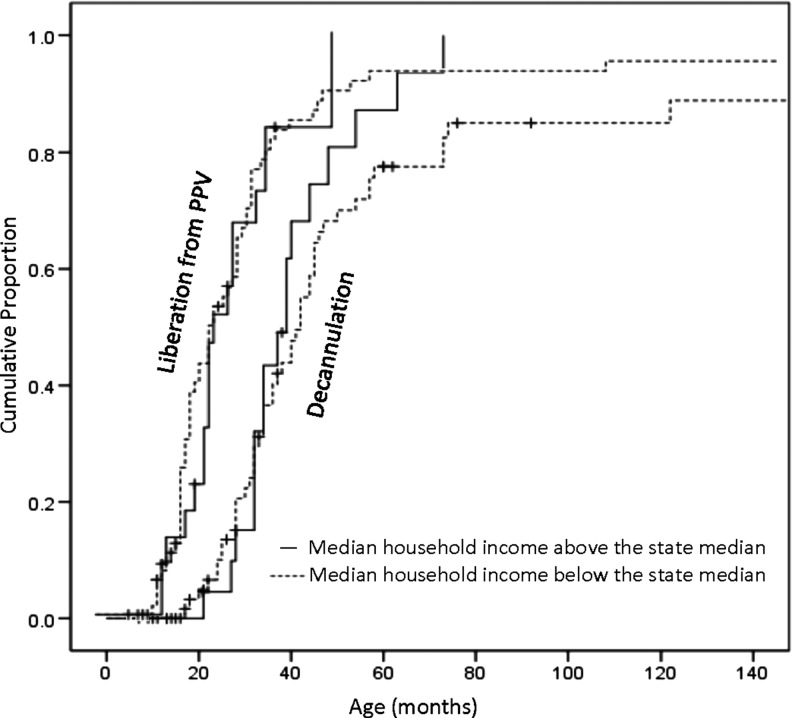
Every patient but one who died was from the group with Z-AHI below the median state household income (Fig. 1). Probability of liberation from ventilation at 60 months of age showed no significant difference between the two groups: 84.5% [95% CI 72.6, 92.6] for the group with Z-AHI below the median state household, and 86.1% [95% CI 70.5, 95.3] for the group with higher Z-AHI (p=0.99). Probability of decannulation at 60 months showed no significant difference between the two groups: 72.4% [95% CI 59.1, 83.3] for the group with Z-AHI below the median state household, and 77.8% [95% CI 60.8, 89.9] for the group with higher Z-AHI (p=0.97) (Fig. 2). Using the Klein method,19 with this small sample size, survival probability at 60 months of age showed no significant difference between the two groups: 81.0% [95% CI 70.9, 91.1] for the group with Z-AHI below the median state household, and 100.0% [95% CI 100.0, 90.3] for the group with higher Z-AHI (p=0.31). Secondary analysis using Cox proportional hazard models (including the covariates birth weight, sex, and race) revealed that the child's risk of death did not differ with age (results not included) (Fig. 2).
FIG. 1.
Kaplan–Meier curve for survival stratified by household income in regard to the median state household.
FIG. 2.
Kaplan–Meier curve for liberation from ventilation and decannulation stratified by median household income in regard to the median state household.
Follow-up hospitalization rates per year for each group before and after decannulation were analyzed, based on the main diagnosis at discharge (Table 3). There were no significant differences between the two groups for each individual diagnosis group.
Table 3.
Follow-up Hospitalization Ratesa per Year for 94 Children with BPD Who Were Initially Ventilator Dependent via Tracheostomy at Home, Before and After Decannulation
| Diagnosis group | Z-AHI below the median state household | Z-AHI above the median state household | p-Value |
|---|---|---|---|
| Before decannulation | |||
| Respiratory | 0.92 (0–60) | 0.55 (0–16) | 0.75 |
| Nonrespiratory | 0.26 (0–4.8) | 0.21 (0–2.57) | 0.94 |
| After decannulation | |||
| Respiratory | 0 (0–6) | 0 (0–0.48) | 0.81 |
| Nonrespiratory | 0 (0–1.41) | 0.09 (0–12) | 0.09 |
Values represent median values (range); p-values were calculated using the Wilcoxon test.
Amongst the deceased patients within the group with Z-AHI below the state median household income, 10 patients died while ventilator dependent; four others were tracheostomy collar dependent. The median age at death was 28.5 months (IQR 15.7–60). The circumstances of death were not known for the majority of these patients, as most of them died at home, and no autopsies were performed. For those who had a documented cause of death in their medical records, one death was tracheostomy related (accidental decannulation), two deaths were expected (the patients had “do not resuscitate” orders), and two other deaths were secondary to cardiorespiratory arrest. The sole patient within the group with Z-AHI higher than the state median household income died at 95 months of age. Although he was liberated from ventilation, his death was tracheostomy related (accidental decannulation). None of these deaths were related to trauma.
Discussion
This study describes the association of Z-AHI on the long-term outcome of patients with BPD who were discharged on home ventilation via tracheostomy. An association between median household income and mortality rate is demonstrated among these patients, as nearly all of patients who died were part of families (biological or foster) who lived in areas with Z-AHI below the state median household income. This relationship is consistent with previous studies.15,20–22 The association between Z-AHI and mortality has been previously described in pediatric and adult literature for chronic diseases such as cystic fibrosis,16 lupus nephritis, and systemic lupus erythematous.23
In this small sample size, no other differences were found between the two groups. While lower birth weight and premature birth have been previously associated with lower SES,3 in our study no differences were found between the two groups with regard to gestational age or birth weight. In addition, sex, race, and health insurance coverage (Medicaid or private health insurance) were similar between the two groups. Furthermore, our analysis (using Klein et al.) did not identify low household income as a risk factor for survival probability at 60 months for patients with BPD. While the nonsignificance is probably due to the small number of death events (only one death in the high income group), the difference between the results of univariate and multivariate analyses may be due to differences in unmeasured patient characteristics that act as confounders. Regardless of the causal pathway, more children discharged on home ventilation died when living in areas with ZIP incomes below the poverty level.
The cohort described in this study represents a group of patients with very serious chronic illness, requiring extensive caregiving both at home and in the hospital setting. Usually, due to the severity of their condition, children with BPD who are ventilator dependent via tracheostomy receive professional nursing care at home. All patients enrolled in this study had received at least 8 hours per day of nursing assistance. A high rate of rehospitalization was found for these children, which is not unexpected, given the severity of their disease. However, no differences were found in the number of hospitalizations between the income groups, both overall and by main diagnosis class at discharge.
This study has limitations that warrant consideration. First, the cause of death for most of these children is unknown, as most of them died at home. Without additional details, it is not possible to gauge how environmental context relating to low SES may have related to the child's death. Another important limitation of our study relates to the use of a preexisting database, which did not allow direct SES to be evaluated through parent-reported income, number of people in the household, number of people contributing to the family income, parental educational attainment level, or current occupation. Furthermore, birth certificates were not reviewed, as these may identify maternal age and education level that are often associated with child's health outcome. While use of Z-AHI provides an indicator of probable household income, more specific detail would have been useful. ZIP code measures usually cover a larger area and have more within-unit variation than the smaller census tracts, but they are easily obtained and may be more stable because they are computed from larger populations. However, because of the range of incomes within Z-AHI, it is impossible to know how far above or below the state median income each individual subject's household may fall. It is not known how many, if any, individuals lived in individual households below the poverty line. While these patients were followed in the HVP, demographic changes were not captured in the medical charts, so it was not possible to identify if any of these patients crossed over from a lower to a higher income group.
Despite these limitations, the results of this study raise important contextual concerns about the harm caused by health disparities. The difference between a 2.8% mortality rate for children living in ZIP codes with higher median incomes versus a 24.1% mortality rate for children living in ZIP codes with lower median incomes may illustrate the disadvantage a low SES can have on health outcomes in young patients. Families with higher incomes may have access to the resources required for children with severe chronic respiratory illnesses such as BPD. Income provides access to safer housing in better neighborhoods, which in turn provides the ability to avoid negative environmental conditions.2,3 Indoor environmental toxins, such as cigarettes, low-quality heating and cooking sources, and outdoor air pollution, are also more likely to be factors in low-income homes.24,25
Several conceptual frameworks evaluating the neighborhood effect on children's health outcomes have been described. For example, Anderson and Aday modeled healthcare access and service use as a function of a person's predisposition to use healthcare services, factors that enable or impede use, and the need for healthcare.26 Predisposing factors included demographic and social characteristics (e.g., age, race/ethnicity, sex, education, and marital status). Enabling and impeding characteristics included individual and family resources (income and employment) and community healthcare resources (supply of providers in the individual's county of residence) and economic circumstances (county unemployment level). A person's need for services is measured by his or her health status.
Access to medical services is another community resource that may mediate neighborhood effects on children's and adolescents' physical and mental health. Most researchers examining the link between health outcomes, such as low birth weight, injury, and maltreatment, and neighborhood sociodemographic characteristics do not include the extent of medical (and social) services available in the community in their analyses. A previous Infant Health and Development Program study27 found that across the first 3 years of life, residence in poor and middle-income neighborhoods was associated with more emergency room visits than residence in affluent neighborhoods, and families in middle-income neighborhoods reported more doctor visits than families in poor or affluent neighborhoods. These findings suggest that access to particular types of medical services may vary by neighborhood SES (effects were found controlling for family characteristics, including income).
In addition to the impact of a low SES on the child patient directly, SES may also play a part in negative health outcomes in children because of the impact the low SES has on parents and families as a whole. While these children should be receiving nursing support at home, the availability of home nurses is limited. If the nurses cannot come to fulfill their duties, then the parents must stay home with the vent-dependent child. However, for many employed parents, taking time off to care for their vent-dependent child means losing income or, worse, risking their job.28 Low-income parents are more likely to have health problems themselves, which could inhibit their abilities to provide the sort of attention required for BPD patients. They are also very likely to have high levels of stress, but lack time or resources to seek healthy stress relief, which also may affect their child's quality of care.7,24
The presence of a chronic illness can also add extra burdens to families, which can be expressed in a variety of psychological, social, and financial ways.29,30 Coupled with a low SES, which leads to both emotional and practical challenges for adults,3 caring for a child with a severe chronic illness may be overwhelming. Parents with fewer resources may have less access to community and social supports, which may lead to providing less than optimal care to their children.31 Meanwhile, parents with a higher income may be able to afford resources that would assist in caring for their child with BPD, such as higher quality in-home care or additional support, such as childcare for the patient's siblings. These sorts of contextual variables may bridge the connection between low SES and increased mortality in children with chronic illnesses such as BPD.
Conclusions
The results of this study are descriptive, as the cause of the association between mortality rate and living in an area with lower household income is not yet understood. The difference in mortality rates between groups above and below the median state income suggests a serious health disparity, which warrants further study. Additional understanding of this effect will require more complete and direct measurement of the multidimensional SES and a better understanding of how SES correlates to treatment adherence, local environmental conditions, and especially the care of these patients during the early years of life. Also, in future studies, including other data sources such as birth certificates or mapping residential addresses to block groups will provide a more precise evaluation of household income.
Acknowledgment
Dr. Cristea was supported by an NIH training grant (T32 HS 017588-04).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.CDC. Community Health and Program Services (CHAPS): Health Disparities Among Racial/Ethnic Populations. Atlanta: U.S. Department of Health and Human Services, 2008 [Google Scholar]
- 2.2020 HP. Disparities. Healthy People 2020 2010; 2014 [Google Scholar]
- 3.Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Affairs 2002;21:60–76 [DOI] [PubMed] [Google Scholar]
- 4.Daly MC, Duncan GJ, McDonough P, Williams DR. Optimal indicators of socioeconomic status for health research. Am J Public Health 2002;92:1151–1157 [Erratum appears in Am J Public Health 2002;92:1212] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen E, Martin AD, Matthews KA. Understanding health disparities: the role of race and socioeconomic status in children's health. Am J Public Health 2006;96:702–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown RL. Epidemiology of injury and the impact of health disparities. Curr Opin Pediatr 2010;22:321–325 [DOI] [PubMed] [Google Scholar]
- 7.Case A, Paxson C. Parental behavior and child health. Health Affairs 2002;21:164–178 [DOI] [PubMed] [Google Scholar]
- 8.Smith GD, Neaton JD, Wentworth D, Stamler R, Stamler J. Socioeconomic differentials in mortality risk among men screened for the Multiple Risk Factor Intervention Trial: I. White men. Am J Public Health 1996;86:486–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith GD, Wentworth D, Neaton JD, Stamler R, Stamler J. Socioeconomic differentials in mortality risk among men screened for the Multiple Risk Factor Intervention Trial: II. Black men. Am J Public Health 1996;86:497–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aber JL, Bennett NG, Conley DC, Li J. The effects of poverty on child health and development. Ann Rev Public Health 1997;18:463–483 [DOI] [PubMed] [Google Scholar]
- 11.Cristea AI, Carroll AE, Davis SD, Swigonski NL, Ackerman VL. Outcomes of children with severe bronchopulmonary dysplasia who were ventilator dependent at home. Pediatrics 2013;132:e727–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vila PM, Swain GR, Baumgardner DJ, Halsmer SE, Remington PL, Cisler RA. Health disparities in Milwaukee by socioeconomic status. WMJ 2007;106:366–372 [PubMed] [Google Scholar]
- 13.Franks P, Fiscella K. Effect of patient socioeconomic status on physician profiles for prevention, disease management, and diagnostic testing costs. Med Care 2002;40:717–724 [DOI] [PubMed] [Google Scholar]
- 14.Thomas AJ, Eberly LE, Davey Smith G, Neaton JD; Multiple Risk Factor Intervention Trial Research Group. ZIP-code-based versus tract-based income measures as long-term risk-adjusted mortality predictors. Am J Epidemiol 2006;164:586–590 [DOI] [PubMed] [Google Scholar]
- 15.Colvin JD, Zaniletti I, Fieldston ES, et al. Socioeconomic status and in-hospital pediatric mortality. Pediatrics 2013;131:e182–190 [DOI] [PubMed] [Google Scholar]
- 16.O'Connor GT, Quinton HB, Kneeland T, et al. Median household income and mortality rate in cystic fibrosis. Pediatrics 2003;111:e333–339 [DOI] [PubMed] [Google Scholar]
- 17.Powell LM, Slater S, Mirtcheva D, Bao Y, Chaloupka FJ. Food store availability and neighborhood characteristics in the United States. Prev Med 2007;44:189–195 [DOI] [PubMed] [Google Scholar]
- 18.www.census.gov/geo/ZCTA/zcta.html Accessed September21, 2012
- 19.Klein JP, Logan B, Harhoff M, Andersen PK. Analyzing survival curves at a fixed point in time. Stat Med 2007;26:4505–4519 [DOI] [PubMed] [Google Scholar]
- 20.Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children's health: how and why do these relationships change with age? Psychol Bull 2002;128:295–329 [DOI] [PubMed] [Google Scholar]
- 21.Larson K, Halfon N. Family income gradients in the health and health care access of US children. Matern Child Health J 2010;14:332–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: what patterns tell us. Am J Public Health 2010;100:S186–S194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jolly M, Mikolaitis RA, Shakoor N, Fogg LF, Block JA. Education, ZIP code-based annualized household income, and health outcomes in patients with systemic lupus erythematosus. J Rheumatol 2010;37:1150–1157 [DOI] [PubMed] [Google Scholar]
- 24.Pampel FC, Krueger PM, Denney JT. Socioeconomic disparities in health behaviors. Ann Rev Sociol 2010;36:349–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med 2000;343:1742–1749 [DOI] [PubMed] [Google Scholar]
- 26.Andersen R, Aday LA. Access to medical care in the U.S.: realized and potential. Med Care 1978;16:533–546 [DOI] [PubMed] [Google Scholar]
- 27.Klebanov PK, Brooks-Gunn J, McCarton C, McCormick MC. The contribution of neighborhood and family income to developmental test scores over the first three years of life. Child Dev 1998;69:1420–1436 [PubMed] [Google Scholar]
- 28.Schuster MA, Chung PJ. Time off to care for a sick child—why family-leave policies matter. N Engl J Med 2014;371:493–495 [DOI] [PubMed] [Google Scholar]
- 29.Perrin JM.Shane M, Bloom SR. Home and Community Care for Chronically Ill Children. New York: Oxford Press, 1993 [Google Scholar]
- 30.Stein R, ed. Caring for Children with Chronic Illness: Issues and Strategies. New York: Springer, 1989 [Google Scholar]
- 31.Hashima PY, Amato PR. Poverty, social support, and parental behavior. Child Dev 1994;65:394–403 [PubMed] [Google Scholar]