Abstract
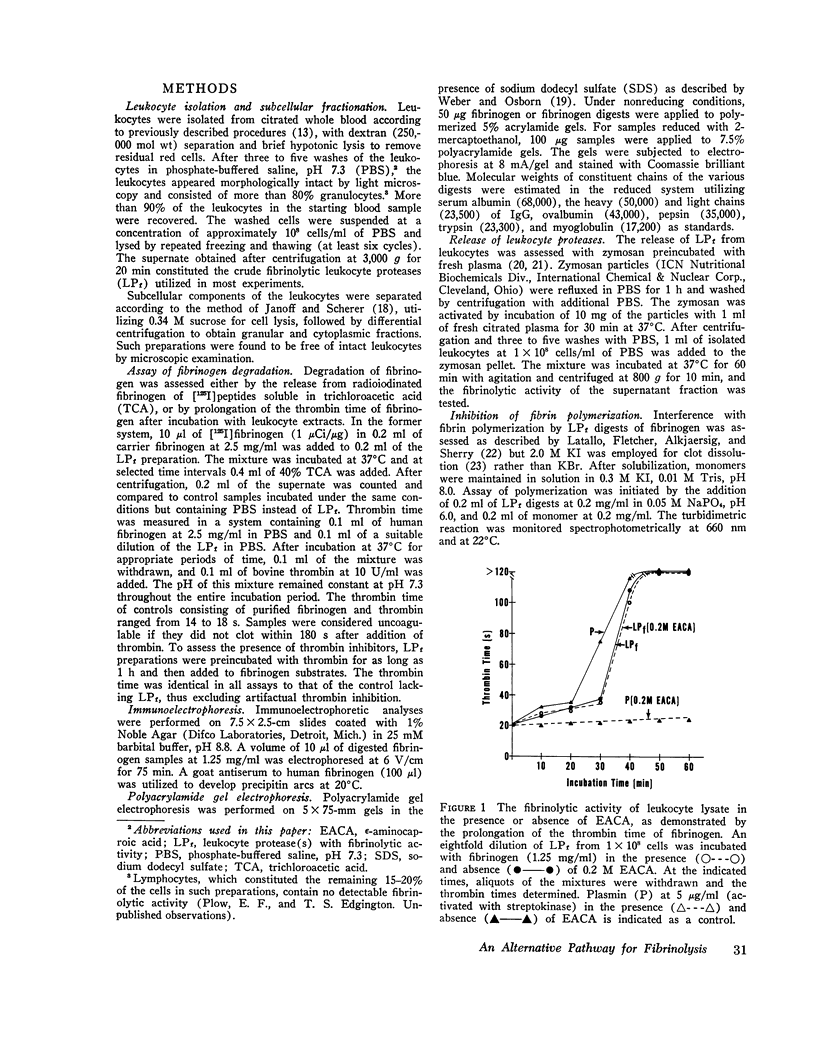
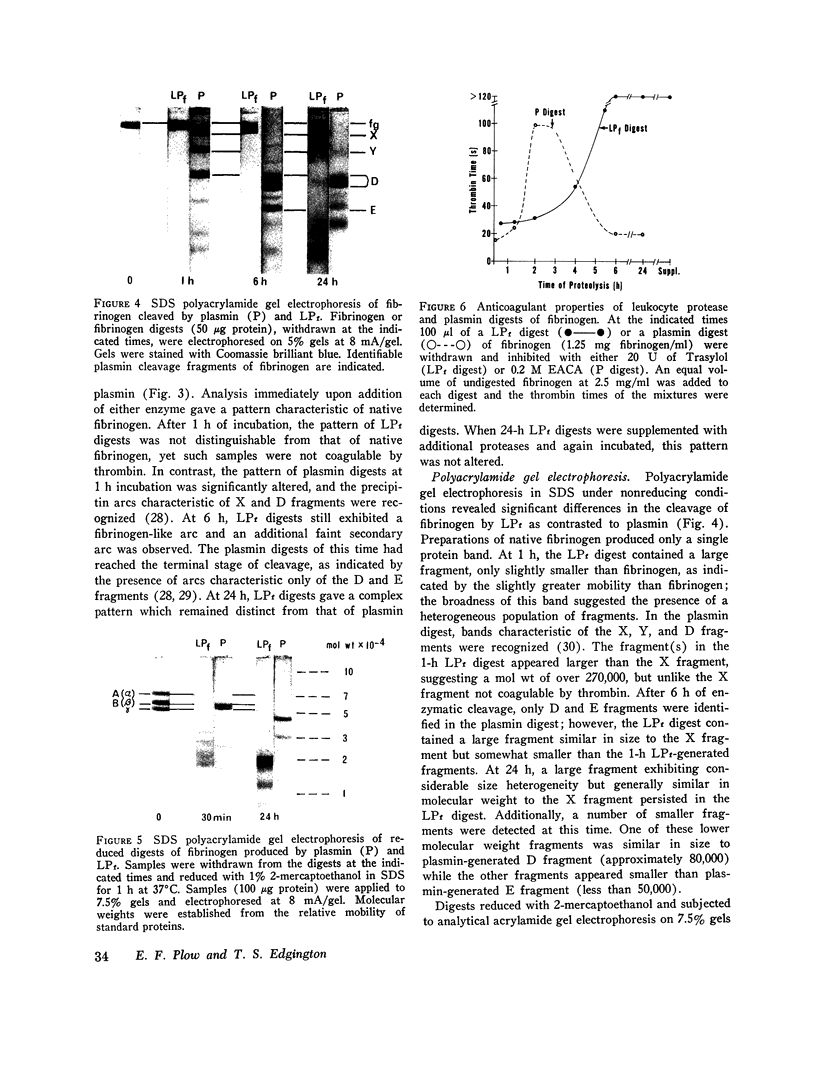
An alternative fibrinolytic system, active at physiological pH, is present in peripheral blood leukocytes. The fibrinolytic proteases localized predominantly in the leukocyte granules are capable of degrading both fibrinogen and fibrin, and plasmin activity does not contribute significantly to this proteolytic event. The specificity of the alternative fibrinolytic proteases for fibrinogen and the characteristics of the derivative cleavage fragments are clearly distinguishable from the classical plasmin system. The high molecular weight derivatives of fibrinogen, generated by the alternative system, under physiological conditions, are larger than the plasmin-generated X fragment, exhibit immunoelectrophoretic mobility comparable to native fibrinogen, and are not coagulable by thrombin. Analysis of the constituent polypeptide chains of the fragments reveals cleavage of the Aalpha, Bbeta, and gamma chains of fibrinogen. The lower molecular weight derivatives of fibrinogen, generated by the alternative system, are structurally distinct from previously described fibrinogen degradation products and exhibit potent anticoagulant activity. This anticoagulant activity can be attributed to interference with normal fibrin polymerization. The proteases of the alternative fibrinolytic systems are actively secreted by leukocytes when stimulated to undergo a nonlytic release reaction. These results provide direct evidence for a fibrinolytic system resident in leukocyte granules that is associated with the leukocyte release reaction and is capable of generating unique fibrinogen cleavage fragments.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnhart M. I. Importance of neutrophilic leukocytes in the resolution of fibrin. Fed Proc. 1965 Jul-Aug;24(4):846–853. [PubMed] [Google Scholar]
- Budzyński A. Z., Stahl M., Kopeć M., Latallo Z. S., Wegrzynowicz Z., Kowalski E. High molecular weight products of the late stage of fibrinogen proteolysis by plasmin and their structural relation to the fibrinogen molecule. Biochim Biophys Acta. 1967 Oct 23;147(2):313–323. doi: 10.1016/0005-2795(67)90409-6. [DOI] [PubMed] [Google Scholar]
- CAVIEZEL O., VOLLERY M., VANNOTTI A. FIBRINOLYSE LEUCOCYTAIRE. Schweiz Med Wochenschr. 1964 Jul 18;94:1016–1020. [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Schubert D., Schwartz S. A. Amino acid sequence studies on artiodactyl fibrinopeptides. I. Dromedary camel, mule deer, and cape buffalo. Arch Biochem Biophys. 1967 Feb;118(2):456–467. doi: 10.1016/0003-9861(67)90374-8. [DOI] [PubMed] [Google Scholar]
- Furlan M., Beck E. A. Plasmic degradation of human fibrinogen. I. Structural characterization of degradation products. Biochim Biophys Acta. 1972 May 18;263(3):631–644. doi: 10.1016/0005-2795(72)90044-x. [DOI] [PubMed] [Google Scholar]
- GANS H. FIBRINOLYTIC PROPERTIES OF PROTEASES DERIVED FROM HUMAN, DOG AND RABBIT LEUKOCYTES. Thromb Diath Haemorrh. 1964 Jan 1;10:379–389. [PubMed] [Google Scholar]
- Gay R. J., McComb R. B., Bowers G. N., Jr Optimum reaction conditions for human lactate dehydrogenase isoenzymes as they affect total lactate dehydrogenase activity. Clin Chem. 1968 Aug;14(8):740–753. [PubMed] [Google Scholar]
- HENRY R. L. LEUKOCYTES AND THROMBOSIS. Thromb Diath Haemorrh. 1965 Mar 15;13:35–46. [PubMed] [Google Scholar]
- Henson P. M. Interaction of cells with immune complexes: adherence, release of constituents, and tissue injury. J Exp Med. 1971 Sep 1;134(3 Pt 2):114s–135s. [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 1971 Dec;107(6):1535–1546. [PubMed] [Google Scholar]
- Hermann G., Miescher P. A. Differentiation of leukocytic fibrinolytic enzymes from plasmin by the use of plasmatic proteolytic inhibitors. Int Arch Allergy Appl Immunol. 1965;27(6):346–354. doi: 10.1159/000229618. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Paddock R. J., George W. J. Hormonal control of neutrophil lysosomal enzyme release: effect of epinephrine on adenosine 3',5'-monophosphate. Science. 1974 Mar 1;183(4127):855–857. doi: 10.1126/science.183.4127.855. [DOI] [PubMed] [Google Scholar]
- Janoff A., Scherer J. Mediators of inflammation in leukocyte lysosomes. IX. Elastinolytic activity in granules of human polymorphonuclear leukocytes. J Exp Med. 1968 Nov 1;128(5):1137–1155. doi: 10.1084/jem.128.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay A. B., Pepper D. S., Ewart M. R. Generation of chemotactic activity for leukocytes by the action of thrombin on human fibrinogen. Nat New Biol. 1973 May 9;243(123):56–57. [PubMed] [Google Scholar]
- LATALLO Z. S., FLETCHER A. P., ALKJAERSIG N., SHERRY S. Influence of pH, ionic strength, neutral ions, and thrombin on fibrin polymerization. Am J Physiol. 1962 Apr;202:675–680. doi: 10.1152/ajplegacy.1962.202.4.675. [DOI] [PubMed] [Google Scholar]
- MOUNTER L. A., ATIYEH W. Proteases of human leukocytes. Blood. 1960 Jan;15:52–59. [PubMed] [Google Scholar]
- Marder V. J., Shulman N. R., Carroll W. R. High molecular weight derivatives of human fibrinogen produced by plasmin. I. Physicochemical and immunological characterization. J Biol Chem. 1969 Apr 25;244(8):2111–2119. [PubMed] [Google Scholar]
- Marder V. J., Shulman N. R. High molecular weight derivatives of human fibrinogen produced by plasmin. II. Mechanism of their anticoagulant activity. J Biol Chem. 1969 Apr 25;244(8):2120–2124. [PubMed] [Google Scholar]
- Mills D. A. A molecular model for the proteolysis of human fibrinogen by plasmin. Biochim Biophys Acta. 1972 May 18;263(3):619–630. doi: 10.1016/0005-2795(72)90043-8. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Finlayson J. S., Galanakis D. K. The essential covalent structure of human fibrinogen evinced by analysis of derivatives formed during plasmic hydrolysis. J Biol Chem. 1973 Nov 25;248(22):7913–7929. [PubMed] [Google Scholar]
- Ohlsson K. Properties of leucocytic protease. Clin Chim Acta. 1971 May;32(3):399–405. doi: 10.1016/0009-8981(71)90441-4. [DOI] [PubMed] [Google Scholar]
- PAZ R. A., SPECTOR W. G. The mononuclear-cell response to injury. J Pathol Bacteriol. 1962 Jul;84:85–103. doi: 10.1002/path.1700840111. [DOI] [PubMed] [Google Scholar]
- Pizzo S. V., Schwartz M. L., Hill R. L., McKee P. A. The effect of plasmin on the subunit structure of human fibrinogen. J Biol Chem. 1972 Feb 10;247(3):636–645. [PubMed] [Google Scholar]
- Plow E. F., Edgington T. S. Discriminating neoantigenic differences between fibrinogen and fibrin derivatives. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1169–1173. doi: 10.1073/pnas.70.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F., Hougie C., Edgington T. S. Neoantigenic expressions engendered by plasmin cleavage of fibrinogen. J Immunol. 1971 Nov;107(5):1496–1500. [PubMed] [Google Scholar]
- Plow E., Edgington T. S. Immunobiology of fibrinogen. Emergence of neoantigenic expressions during physiologic cleavage in vitro and in vivo. J Clin Invest. 1973 Feb;52(2):273–282. doi: 10.1172/JCI107183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopowicz J. Distribution of fibrinolytic and proteolytic enzymes in subcellular fractions of human granulocytes. Thromb Diath Haemorrh. 1968 Mar 31;19(1):84–93. [PubMed] [Google Scholar]
- Prokopowicz J. Purification of plasminogen from human granulocytes using DEAE-Sephadex column chromatography. Biochim Biophys Acta. 1968 Jan 22;154(1):91–95. doi: 10.1016/0005-2795(68)90262-6. [DOI] [PubMed] [Google Scholar]
- RIDDLE J. M., BARNHART M. I. ULTRASTRUCTURAL STUDY OF FIBRIN DISSOLUTION VIA EMIGRATED POLYMORPHONUCLEAR NEUTROPHILS. Am J Pathol. 1964 Nov;45:805–823. [PMC free article] [PubMed] [Google Scholar]
- Triantaphyllopoulos D. C., Chandra S. Digestion of fibrin by thrombin. Biochim Biophys Acta. 1973 Nov 11;328(1):229–232. doi: 10.1016/0005-2795(73)90349-8. [DOI] [PubMed] [Google Scholar]
- Wasi S., Murray R. K., Macmorine D. R., Movat H. Z. The role of PMN-leucocyte lysosomes in tissue injury, inflammation and hypersensitivity. II. Studies on the proteolytic activity of PMN-leucocyte lysosomes of the rabbit. Br J Exp Pathol. 1966 Aug;47(4):411–423. [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weissmann G., Zurier R. B., Spieler P. J., Goldstein I. M. Mechanisms of lysosomal enzyme release from leukocytes exposed to immune complexes and other particles. J Exp Med. 1971 Sep 1;134(3 Pt 2):149s–165s. [PubMed] [Google Scholar]