Abstract
Background
Endoscopic spine surgery has evolved gradually through improvements in endoscope design, instrumentation, and surgical techniques. The ability to visualize and treat painful pathology endoscopically through the foramen has opened the door for the diagnosis and treatment of degenerative conditions of the lumbar spine (from T10 to S1). Other endoscopic techniques for treating a painful disc have been focused on a posterior approach and has been compared with micro–lumbar discectomy. These procedures have not been more effective than open microdiscectomy but are less invasive, have less surgical morbidity, and allow for more rapid surgical recovery. Spinal decompression and fusion was the fallback procedure when nonsurgical treatment or discectomy failed to relieve sciatica and back pain. Foraminal endoscopic surgery, however, provides a truly minimally invasive alternative approach to the pathoanatomy of the lumbar spine because it preserves the multifidus muscle, maintains motion, and eliminates or, at worst, delays the need for fusion.
Methods
The following developments helped facilitate the evolution of a transforaminal endoscopic surgery procedure for disc herniations from a foraminal disc decompression, also known as percutaneous endoscopic lumbar discectomy, to a more complete foraminal surgical technique that can address spinal stenosis and spinal instability. This expanded capability gives foraminal endoscopic surgery distinct advantages and flexibility for certain painful degenerative conditions compared with open surgery. Advancement of the technique occurred when needle trajectory and placement was refined to better target each type of herniation with precise needle and cannula positioning directed at the herniation. New instrumentation and inclusion of a biportal technique also facilitated removal of extruded, migrated, and sequestered disc herniations. The further development of foraminoscopes with larger working channels and high speed burrs to remove bone more efficiently, along with recognition of foraminal pathoanatomy in the foramen, led to the identification and treatment of other painful degenerative conditions of the lumbar spine such as failed back surgery syndrome, recurrent disc herniations, lateral foraminal stenosis, degenerative spondylolisthesis, and isthmic spondylolisthesis.
A summary of the endoscopic techniques currently used and trademarked by the author as the YESS technique include: (1) a published protocol for optimal needle and instrument placement calculated by lines drawn on the skin from the C-arm image; (2) evocative chromodiscography by the operating surgeon with nonionic radiologic contrast and indigo carmine dye to confirm concordant pain production and to stain tissue in contact with the injectate; (3) selective endoscopic discectomy, which targets the removal of loose degenerative nucleus stained differentially by indigo carmine dye; (4) thermal annuloplasty, a visualized radiofrequency thermal modulation of disc and annular defects guided by vital tissue staining; (5) endoscopic foraminoplasty, a decompression of the lateral and subarticular recess, including disc and foraminal degenerative and isthmic spondylolisthesis; (6) visually and radiologically guided exploration of the epidural space; (7) probing the hidden zone of MacNab for normal nerves (and branches of spinal nerves known as furcal nerves) versus anomalous autonomic nerves in the foramen; and (8) a uniportal and biportal technique for inside-out removal of extruded and sequestered nucleus pulposus.
Results
Endoscopic foraminal surgical procedures are not limited to disc decompression. The approaches and techniques allow access to the lumbar spine for treatment of conditions ranging from discogenic pain to failed back surgery syndrome (most commonly caused by residual or recurrent disc herniation and lateral recess stenosis). More than 3000 patients have undergone endoscopic posterolateral surgical exploration and decompression by the author since 1991. The first 80 patients reported formed the basis for expansion of techniques as new instruments and adjunctive therapy methods were added to selective endoscopic discectomy and thermal annuloplasty. New anatomic and pathoanatomic conditions were reported as they were encountered.
Conclusions
New skills will become desirable and necessary for the spine surgeon to keep up with endoscopic technology in spine care. The emphasis is on visualization of painful pathoanatomy and preservation of mobility. A new focus is on nucleus replacement, annular repair, annular reinforcement, biologics, and even transforaminal interbody fusion as the procedure of last resort. The transforaminal surgical approach to the lumbar spine can allow for minimally invasive access without negatively affecting and destabilizing the multifidus muscle.
Keywords: Chymopapain, arthroscopic microdiscectomy, laser disc decompression, evocative chromodiscography, selective endoscopic discectomy, endoscopic thermal annuloplasty, endoscopic foraminoplasty
INTRODUCTION
A form of minimally invasive surgical technique in the lumbar spine that has been scientifically evaluated with level I and II studies is chemonucleolysis. Two large randomized doubleblind and 30 cohort studies1, 2 confirmed its efficacy compared with placebo. Endoscopic spine surgery has similarly been studied and promoted by Hermantin et al,3 and Mayer and Brock4 with prospective level III studies. Other techniques continue to be introduced, but many fall out of favor when surgeons compare them with the more familiar open microdiscectomy. This failure to catch on may be attributable to decreased popularity, the need to overcome the learning curve of a new and unfamiliar procedure that is more technically difficult than open discectomy, and the narrowed indications for minimally invasive procedures brought about by new endoscopic procedures, where the technique is dependent on a single surgical tool or on meticulous stratification of patient-inclusion criteria. It may also be attributable to the technical difficulty of learning a new technique that is not routinely taught in academic programs. In my surgical experience, the adjunctive utilization of minimally invasive technologies such as chymopapain and laser5–8 to augment disc decompression has improved surgical results and helped evolve endoscopic disc and foraminal surgery.
The hypothesis for this study was that the inclusion of minimally invasive methods, when combined with endoscopic surgical visualization, offers advantages over the isolated fluoroscopically guided technique. Adjunctive therapy has helped provide improved endoscopic visualization and treatment of foraminal pathoanatomy that consequently expanded the strict and stratified patient-selection criteria needed for each technique. I had experience with chymopapain injections, automated percutaneous lumbar discectomy, and laser disc decompression for contained disc herniations before I learned Kambin's arthroscopic microdiscectomy. The spine arthroscope finally allowed visualization of the disc and foramen in a way that provided a means to study and record the pathoanatomy of this area of the lumbar spine.
At the time I adopted arthroscopic microdiscectomy, claims were being made that laser use may cause scar formation intradiscally, a condition found by Yonezawa experimentally in rabbits, which, if also present in humans, may help stabilize the disc.
This was never confirmed in humans or in the first 80 patients (introduced in the Methods section) on whom the potassiumtitanyl-phosphate (KTP) laser (Laserscope, San Jose, California) was used as an adjunct to arthroscopic microdiscectomy. The combination of scar tissue and chemical digestion of the residual nucleus was hypothesized to improve results and decrease recurrence. Adding direct endoscopic visualization would offer a means to study the effects of each technique and the pathophysiology of discogenic pain. As adjunctive therapy was added to spinal endoscopy, the incorporation of a combination of minimally invasive technologies enhances each individual technique. The combination of technologies and the ability to operate on painful conditions in a conscious patient made it more surgically intuitive. The trend of combining technologies should enhance our ability to study and evolve new and innovative methods. Such is the case for endoscopic transforaminal disc surgery, which begins with endoscopic disc excision through the foramen.
Selective endoscopic discectomy is a trademarked term that describes my surgical technique for endoscopic discectomy (the Yeung Endoscopic Spine System [YESS]; Richard Wolf Surgical Instrument Company, Vernon Hills, Illinois).9 (The trademark is important to differentiate this procedure from endoscopic techniques that are similar but more limited in scope and efficacy.) Other surgeons performing endoscopic discectomy who do not include all the steps outlined here have used similar names such as percutaneous endoscopic lumbar discectomy (PELD). Kambin10, 11 first introduced arthroscopic microdiscectomy in 1973 simultaneously with Hijikata.12 Kambin's procedure evolved from a central nuclectomy to a posterior fragmentectomy as surgical experience and tools aided the mechanical removal of an increasing variety of disc herniations. I adopted Kambin's arthroscopic microdiscectomy (AMD) procedure at about the same time minimally invasive procedures for nuclectomy came into favor through the introduction of chymopapain, automated percutaneous discectomy, and percutaneous laser disc decompression (PLDD). As interest in PLDD peaked, AMD was also gaining interest. It was a natural process to use arthroscopic tools to try to visualize the effect of the laser on the nucleus. Thus, I discovered that combining the potassium-titanyl-phosphate laser (the first laser to be approved by the Food and Drug Administration for disc decompression) with arthroscopic microdiscectomy allowed for better visualization and better relief of back pain than discectomy alone. A retrospective review of my first endoscopic cases was first analyzed by independent reviewers Farouq Al Hamdan (F.A.H.) and Alex Hadjipavlou of the University of Texas Medical Branch at Galveston in 1996.13
MATERIALS AND METHODS
The first 80 consecutive patients retrospectively studied (1991–1995) came to surgery with Kambin's AMD technique, combined with PLDD (using a Laserscope KTP laser).13 The mean age was 41.9 years (range, 25 to 69 years). The distribution of the operated levels was as follows: L2–L3 (n = 2); L3–L4 (n = 12); L4–L5 (n = 28); L5–S1 (n = 21); and L4–L5 and L5–S1 (n = 17). I operated on all patients, but all cases were independently evaluated by F.A.H. via follow-up examination and questionnaire. Pain questionnaires were conducted preoperatively as well. Inclusion criteria in this study were (1) lumbar disc herniation confirmed by history and clinical examination with neurological deficit and/or positive tension sign in the lower extremity; (2) radiological findings of lumbar disc herniation in magnetic resonance imaging (MRI), computed tomography and discography; and (3) failed conservative treatment for at least 6 weeks after onset of unrelenting symptoms. Patients with predominant back pain were included as long as there was associated sciatica, either unilateral or bilateral. No exclusion was made for herniation size or extruded or migrated fragments within reach of the endoscopic discectomy instruments.
Follow-up MRI as part of the study was ordered on 28 postoperative patients to compare with the preoperative films. All large herniations with satisfactory results had measurable decrease in herniation size, whereas small herniations with protrusions of 3 mm or less often showed no significant change, even when results were good or excellent. Noncontained herniations were included in this study. Noncontainment criteria were excluded by the criteria set for laser disc decompression, but not chymopapain injection. The addition of chymopapain as an adjunctive technique was reserved for extruded herniations where there was a possibility that the extruded herniation could not be removed endoscopically because of technical and anatomical considerations in each individual patient. The literature on chymopapain included patients with extruded disc material that could come in contact with the enzyme injected into the disc. A separate follow-up review of this population of patients with extruded herniated nucleus pulposus revealed a 10% higher success rate when chymopapain was used in conjunction with endoscopic discectomy than without.
It was recognized early in this series of extruded herniations that when the height of the herniation was greater than the width at the base of the herniation, fragments were more likely to be incompletely extracted with the YESS “inside-out technique.” When chymopapain was used as an adjunct to endoscopic extraction of these extrusion patterns on MRI, it was visually easier to pull the herniation back into the disc with flexible pituitary forceps.8
As my experience with arthroscopic microdiscectomy combined with laser disc decompression increased, the arthroscopic microdiscectomy technique was further modified with the use of unipolar, then bipolar, radiofrequency for hemostasis and tissue modulation in lieu of laser. An institutional review board–approved study of 50 patients was initiated in 1997 that used a temperature-controlled flexible probe by Oratec Interventions Inc (Menlo Park, California). Two complications of temporary severe dysesthesia with sympathetic dystrophy, including one patient with residual foot-drop, caused me to abandon the temperature-controlled probe in favor of a steerable, bipolar, high-frequency (4.0 MHZ) flexible probe (Ellman International Inc, Hewett, New York).
Laser continued to play a vital role with transition from KTP to holmium: yttrium-aluminum-garnet (Ho:YAG) laser (Trimedyne, Irvine, California) for soft tissue and bone ablation. The Ho:YAG laser with a side-firing tip and a straight fiber guided with a nitinol curved tube was useful for ablation of osteophytes and for releasing herniated disc fragments attached to the annulus and endplate. In 1996–1997 I broke away from the Kambin system and opted for multiple cannula configurations designed to accommodate the different anatomic situations presented by each patient as opposed to using an oval cannula favored by Kambin. New instruments were developed for annular dilation, including differently-configured hinged pituitary disc rongeurs for more efficient discectomy. Concomitant availability of flexible pituitary rongeurs and shavers (Endius Inc, Plainville, Massachusetts) improved the efficiency of nuclectomy. The bipolar flexible radiofrequency probes provided tissue modulation, hemostasis, and the ability to better probe spinal nerves in the epidural space.
In 1997 I received Food and Drug Administration 510(k) approval for a new endoscopic spine system, YESS,9 that included a set of complementary tools to enhance safety as well as visualization of the endoscopic procedure. A multichannel operating scope with distal irrigation provided unparalleled visualization. Microendoscopic anatomy could now be routinely seen. The smaller disc herniations had visible annular tears that could be probed endoscopically after the nucleus was resected. The visualized tears often contained granulation tissue, interpositional disc material, or evidence of neovascularization. This condition allowed for therapeutic thermal modulation treatment of annular tears that can be compared to intradiscal electrothermal therapy (IDET), but is more effective because of the ability to visualize the pathology to be treated.14–17
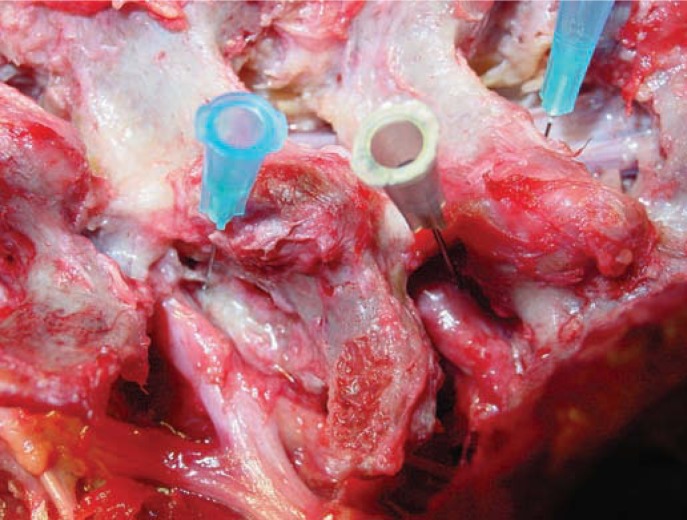
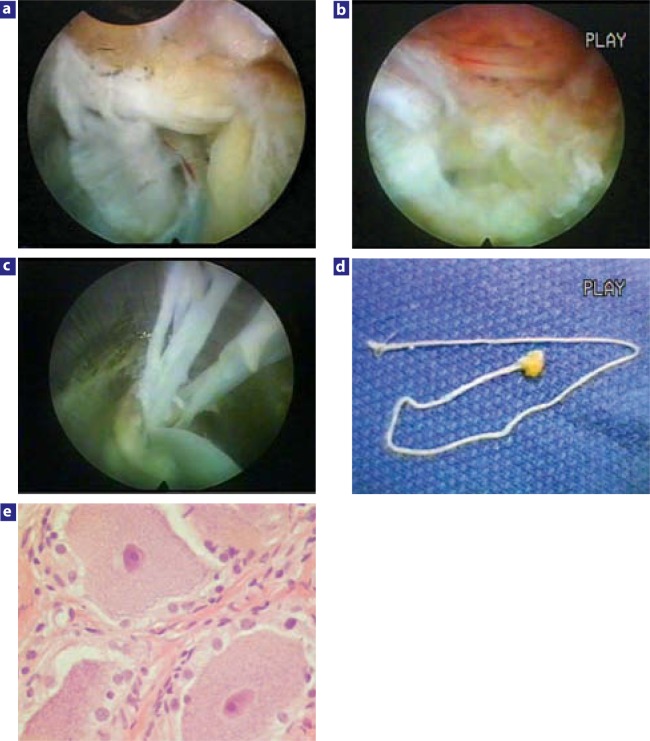
The use of radiofrequency modulation was even more effective than laser in relieving back pain in patients with painful degenerative discs diagnosed by positive evocative discography. In large disc herniations, the decompressed spinal nerve is easily confirmed in the epidural space after the disc fragment is removed, and the annular defect allows indirect and direct visualization of the epidural space. Anomalous anatomy such as furcal nerves and conjoined nerves not usually seen with traditional approaches are readily seen with the endoscope, especially when the furcal (forked) nerve is seen as a split branch arising from the exiting nerve (Figure 1).18 I report here an autonomic nerve found in the foramen lateral to the traversing nerve. A pathology slide of the specimen identified ganglion cells at its end, and the pathologist reported the nerve as an autonomic nerve.18
Figure 1.
Foraminal view of cadaver dissection at L4–5 and L5–S1. Note the tiny furcal nerve branch speared by the blue hubbed needle that branches from exiting L4 nerve at L4–L5. The nerve seen in Figure 5b would be what a small furcal branch looks like, but in this instance, excisional biopsy confirmed by pathology slide revealed the nerve to be an autonomic nerve because of the ganglia seen on the slide. These furcal nerves in the “hidden zone” of MacNab vary in size and can cause postoperative dysesthesia. When the nerve is equivalent in size to the spinal nerve, it can be interpreted as a conjoined nerve if it cannot be traced back to its origin.
Early Surgical Technique
Patients were operated in the prone position in accordance with Kambin's technique, with or without sedation. Only local anesthetic (xylocaine 1%) was injected into the skin, subcutaneous tissue, needle tract, and muscle fascia. The annulus was anesthetized topically with a xylocaine-soaked cottonoid through the cannula. An intravenous antibiotic (1 g of cephalosporin) was given immediately prior to the procedure, and the antibiotic was also put in the irrigation fluid at 1 ampoule of epinephrine per liter of fluid. Fluoroscopic control verified exact needle placement in the center or posterior quadrant of the involved disc via the posterolateral approach.
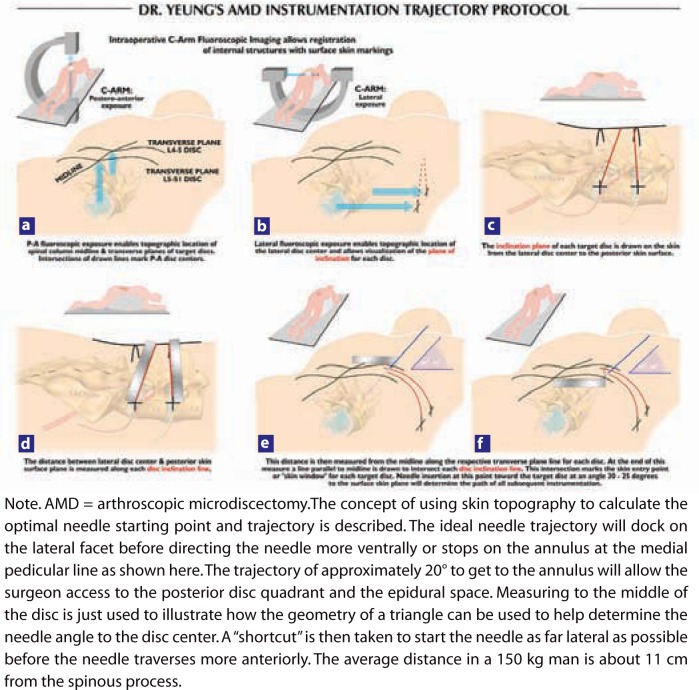
Later, a standardized method was developed (known as the YESS technique, mentioned earlier) for initial needle placement determined by lines drawn on the skin from the C-arm (Figure 2). Key points of the YESS technique in aiding exact needle and cannula placement include (1) preoperative patient positioning to place the dorsal spinous process exactly in the middle between the pedicles in the posterior-anterior (PA) view; (2) drawing a line parallel to the disc to bisect the disc space at the operative level in the PA view; (3) obtaining a perfect lateral C-arm image to eliminate parallax error; (4) drawing a line that bisects the disc space in the perfect lateral projection; (5) selecting a needle starting point caudal, at, or cephalad to the PA line as determined by the lateral line that bisects the disc space; (6) starting the needle far laterally on the back in the middle of the arc where the skin window curves anteriorly instead of laterally; and (7) directing the needle at a 20° trajectory to dock on the lateral facet, then walking the needle ventrally to the foramen. This protocol identifies the skin window and targets the foraminal window at the base of the disc herniation and the epidural space.19, 20 With this technique, it was safe to enter the disc by palpation, and avoiding injury to the exiting nerve. Diluted indigo carmine dye 10% to 20% was mixed with iopamidol 200 or 300 and injected into the disc space as part of evocative chromodiscography. Patients with only severe concordant back pain reproduced without sciatica were still considered surgical candidates if the pain reproduction was severe and the discogram pattern was abnormal.
Figure 2.
Protocol for drawing lines for optimal skin window in foraminal endoscopic surgery: (a–e) The procedure is carried out in an operating room under aseptic conditions, with use of only local anesthesia and conscious sedation. (Do not use propofol!) Free hand C-arm biplane guidance method is used. Two basic C-arm projected images are utilized, postero-anterior and lateral projections. Both views require parallelism of the X-ray beam to target the endplates. When the intervertebral disc is not perpendicular to the floor, the C-arm tube is tilted until the beam (dotted line) is parallel to the disc endplates, i.e., at L5–S1 (Ferguson view). The skin window is estimated by measuring the distance from the anterior disc to the skin or by placing the skin window as far lateral on the back as possible before the skin window goes directly anteriorly. (f) From the skin window aim the approach needle toward the posterior disc quadrant at approximately 20° in the frontal plane. The path is medial to the quadratus lumborum. This avoids inadvertent visceral penetration. The trajectory places the posterior third of the disc and the epiannular space within reach of the operating tools. Use a more vertical angle if an epidural procedure is not needed. Enter the disc through the annular window and place the needle tip in the midline. In the lateral projection, the needle need not pass the midpoint of the disc. Evocation contrast or indigo carmine discography is performed in this position.
The KTP laser disc ablation was started first by delivering energy at 10 watts (1 second on, 1 second off, to permit heat dissipation). Lasering was paused if the patient reported discomfort from the heat created by the laser. A maximum of 1800 joules (limited by the Laserscope protocol) was utilized per disc. An adequate, bloodless, intradiscal working cavity was thus created.
The technique of arthroscopic microdiscectomy and posterior fragmentectomy began with the introduction of the scope into the disc space after measuring the appropriate angle of entry. Prior to annular fenestration, the scope visualized the triangular working zone structures to be certain that the sharp 3 mm and 5 mm trephine would not injure the nerve root or dura. The scope was then introduced into the created intradiscal cavity. Deflecting sheath and flexible forceps were used to target the herniation fragment.
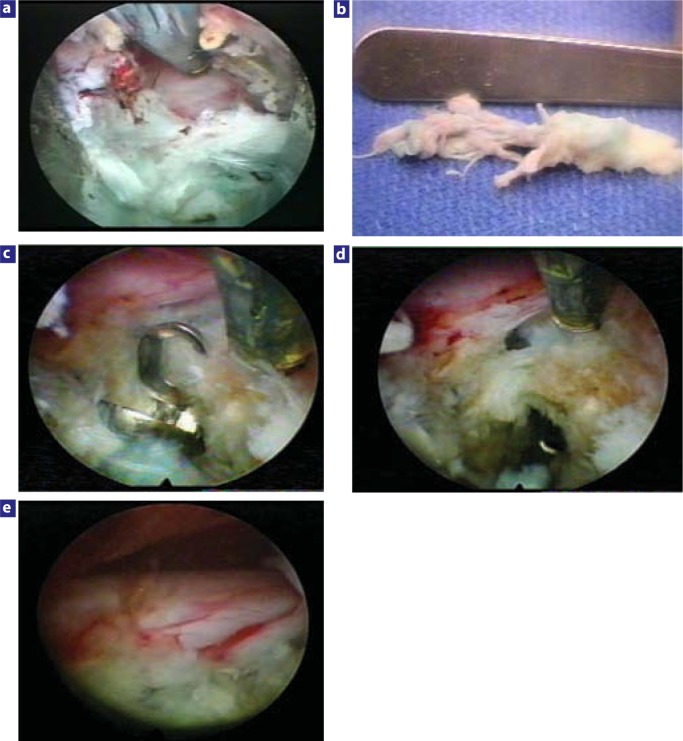
The indigo carmine dye stained the degenerative nuclear material blue-green. The pigment also enhanced the effect of the KTP laser on degenerative nuclear disc material, while intact annular fibers in a contained herniation remained relatively unstained. If the herniation breaches the annulus in extruded herniations, the vital dye will stain both the disc material and annular collagen, visually guiding the surgeon on the extent of discectomy and posterior fragmentectomy needed. If the surgeon was unable to reach the extruded fragment, a biportal approach was established to try to reach the fragment from the opposite foramen. After the herniated fragment was retrieved, the nerve root and epidural space was probed or visualized, and the disc space was further inspected for any loose fragments (Figure 3). The patient was then transferred to the recovery room with relief of preoperative leg pain noted, allowed to ambulate, and then discharged home on the same day.
Figure 3.
Examples of nerve decompression for extruded disc herniations with the uniportal and biportal technique: (a) Foraminal view of the decompressed L5 nerve after endoscopic extraction of a large extruded disc fragment at L4–L5. The posterior longitudinal ligament and posterior annulus are still shielding the ventral aspect of the traversing nerve. (b) Foraminal fragment extracted from beneath and lateral to the traversing L5 nerve seen in Figure 3a. The fragment was extracted through a uniportal approach, but large paracentral and central herniations are indications for a biportal approach. (c) Biportal removal of a paracentral herniated nucleus pulposus with a flexible pituitary forceps. (d) Probing an additional residual sequestered subligamentous fragment beneath the traversing nerve in this same patient. It is possible to probe the epidural space and the nerves with flexible instruments. (e) Final inspection of a completely decompressed traversing nerve in this same patient.
In the first 80 consecutive patients, follow-up evaluation and examination were performed by an independent reviewer (F.A.H.).13 Ninety percent of these patients were operated with a uniportal approach. Patients were personally and individually interviewed and examined. Follow-up time averaged 3 years and ranged from 2 to 4.5 years. Clinical examination, chart review, X-rays, MRIs, and computed tomography scans were also reviewed for each patient. MacNab criteria (excellent, good, fair, poor) were used (Table 1). The visual analogue scale (0–10) was also utilized for preoperative and postoperative pain evaluation, severity of symptoms, and the dominant presenting complaint of lower-back pain or leg pain was noted. Improvement was considered immediate if the majority of the patient's symptoms improved within 24 hours, or delayed if there was gradual resolution of symptoms over a period of days or weeks.
Table 1.
Modified MacNab Criteria
| Assessment | Definition |
|---|---|
| Excellent | No pain, no functional restrictions |
| Good | Occasional back/leg pain, brief or slight functional restriction |
| Fair | Improved overall function, permanent work and activities of daily living restrictions |
| Poor | No improvement in pain/functional level or reoperation at index level |
RESULTS
Subjective evaluation and postoperative satisfaction for the procedure and whether they would recommend it to other patients with a similar condition were also assessed by questionnaire or personal phone call. Sixty-nine patients (89%) were satisfied with the procedure and would recommend it to others. MacNab criteria “good” or “excellent” results were found in 56 (70%) patients. Predominant leg pain patients had similar success in 80%, whereas those with predominant back pain had a lower good/excellent success rate of 63%. This population included similar numbers of workers’ compensation patients, litigation patients, and private patients. Twenty-four patients (30%) were rated “fair” or “poor,” because they required subsequent surgery within the 2-year follow-up period or still required prescription pain medication and did not return to work.
Eight patients (10%) who did not improve after the procedure and continued to have significant symptoms underwent subsequent open discectomy. All procedures were for extruded fragments that were incompletely removed endoscopically, and all subsequently had a good/excellent result after laminectomy and discectomy. This population of patients was recognized to have disc extrusion in which the height of the herniation was greater than the length of the base.8 In subsequent patients, this MRI pattern caused me to either recommend a standard lumbar microdiscectomy or augmentation with chymopapain. The technical causes of failure in the 8 patients were either failure to completely remove an extruded fragment or unrecognized foraminal stenosis.
Postoperative-to preoperative pain improvement difference on the visual analogue scale averaged 7.2. Twenty-eight patients (50% of the good-or excellent-rated group) had immediate improvement of symptoms, while the remaining 28 patients had gradual resolution of symptoms over a period of days or weeks. Postoperative complications included 2 cases of transient dysesthesia of the lower extremity. Both cases resolved spontaneously. No patient was worse after the surgery.
DISCUSSION
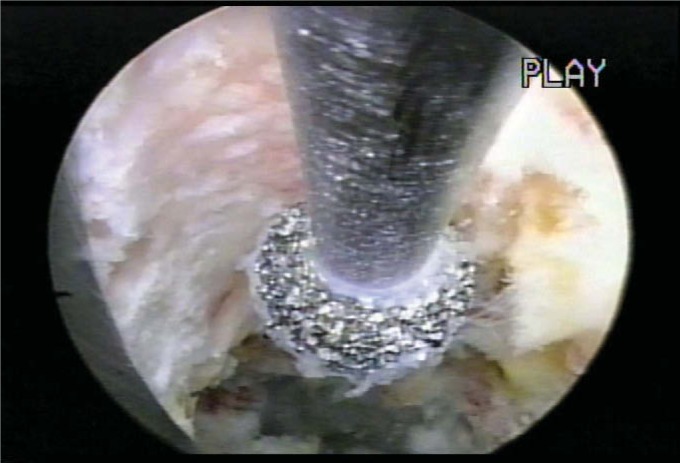
The lessons learned from this initial series of patients treated with arthroscopic microdiscectomy combined with KTP laser disc decompression prompted a gradual expansion of the spectrum of disc herniations suitable for endoscopic extraction. Chymopapain was seen to soften nucleus pulposus, making it easier to remove with mechanical extraction and with endoscopic suction. Extruded fragments caught in the annular defect were easier to extract through the cannula. The desire for direct visualization then prompted the more frequent use of biportal techniques when flexible shavers and pituitary rongeurs further enhanced the technique. The availability of a side-firing, then a straight-firing Ho:YAG laser directed with a nitinol directional guide further aided bone and soft tissue ablation. The straight firing fiber was especially helpful in releasing the collagenized fragment from the annulus. The YESS system then added a foraminal scope (renamed Vertibris) with a 4.2 operating channel. This expanded the development of adjunctive tools such as basket forceps, kerrisons, articulated pituitary rongeurs through the working channel, along with high-speed hooded cutting and nonhooded diamond burrs (Figure 4). Now it became possible and safe to treat foraminal stenosis and impingement from foraminal osteophytes. The endoscope was also utilized to remove pedunculated synovial cysts and to explore foraminal pathology, including misdirected pedicle screws.19
Figure 4.
Endoscopic foraminal decompression of the superior facet with a diamond burr.
In spite of the high learning curve involved and the paucity of training available in academic institutions, the movement toward motion preservation has renewed interest in endoscopic spine procedures. In its early introduction, endoscopic techniques were limited to contained disc herniations, including central herniations that do not usually present with the classic leg-pain-greater-than-back-pain scenario.21–23 Many patients with symptomatic herniations that present with predominant back pain and bilateral sciatica who were not offered surgical options benefited from endoscopic decompression and thermal annuloplasty. Electrothermal procedures such as IDET were developed to address chronic discogenic back pain but not central disc herniation. Endoscopic nuclectomy with thermal annuloplasty was very effective for central disc herniations. The results of IDET have not been as good as hoped, and it has suffered a fate similar to that of many other nonvisualized intradiscal techniques. But the effect of a hybrid procedure that combined adjunctive surgical techniques provided better results for back pain as well as sciatica with very low morbidity. Recurrent herniation rates were demonstrated to be lower.6, 7
Historically, techniques that promoted an isolated method such as chemonucleolysis, percutaneous lumbar discectomy, automated percutaneous lumbar discectomy, PLDD, AMD, and IDET all have touted minimal invasiveness, but because all the techniques were introduced as a single isolated technique or device, patient selection criteria had to be very narrow and stringent—more critical than with traditional discectomy. When successful, however, these procedures shared advantages over traditional open discectomy. It was also possible to incorporate the technique earlier in the painful degenerative process than with traditional discectomy.21–31 This initial study of the combined technique of PLDD and AMD as a laser-assisted, percutaneous discectomy technique that enhanced intraoperative visualization, also had an effect on postoperative back pain relief.13 However, dysesthesia continues to be a risk in spite of careful monitoring and meticulous technique. At this time, it is apparently unavoidable. We have documented possible causes from anomalous anatomy such as autonomic nerves, the presence of furcal nerves and conjoined nerves in the foramen (Figure 5), and speculation that the ablation of inflammatory and granulation tissue may cause irritation of the dorsal root ganglion.32 Dysesthesia may even occur as a normal consequence of improved circulation and recovery secondary to the decompression of an ischemic nerve.
Figure 5.
Anomalous nerves in the foramen. (a) Large bifurcation of the right L4 exiting nerve in the foramen at L4–L5. The head is cephalad at 3 o'clock. This size furcal nerve will cause complications of dysesthesia, profound weakness, and numbness if resected or ablated. Ablation or removal of smaller furcal nerves 1 mm or smaller will only produce temporary dysesthesia. Gradual improvement should then occur because the main nerve is still intact. Surgeons must recognize furcal nerves in the foramen and avoid cutting them if more than a millimeter in size, but sometimes it is not possible to get into the disc without affecting these furcal nerve branches. Surgeons who use the transcanal approach for decompression of the subarticular and lateral recess may be removing furcal nerves without realizing it. (b) Anomalous autonomic nerve in foraminal zone. This anomalous autonomic nerve in the foraminal zone is easy to mistake for a furcal nerve. (c) Surgical removal of the nerve shown in Figure 5b during visualized removal of the foraminal herniation. (d) Excisional biopsy of this nerve identified it as an autonomic nerve. Autonomic nerves can be part of an anomalous trunk of nerves that to my knowledge has not been identified in the foramen or mentioned previously in the peer-reviewed literature. This anomalous nerve was extracted inadvertently from the axilla between the exiting and traversing nerve in the patient in Figure 5b during the removal of a foraminal disc herniation. The nerve was lateral to the traversing nerve and away from the dura and could not have been cauda equina. The patient had dysesthesia, but responded to foraminal epidural blocks and sympathetic blocks. Dysesthesia is usually temporary, but residuals are possible. Removal of these small nerves cannot always be avoided and is a known risk of foraminal surgery. (e) Photomicrograph of the nerve shown in Figure 5b. This nerve, thought to be a furcal nerve, contained ganglion cells. The presence of ganglion cells differentiates an autonomic nerve from a myelinated furcal or conjoined nerve.
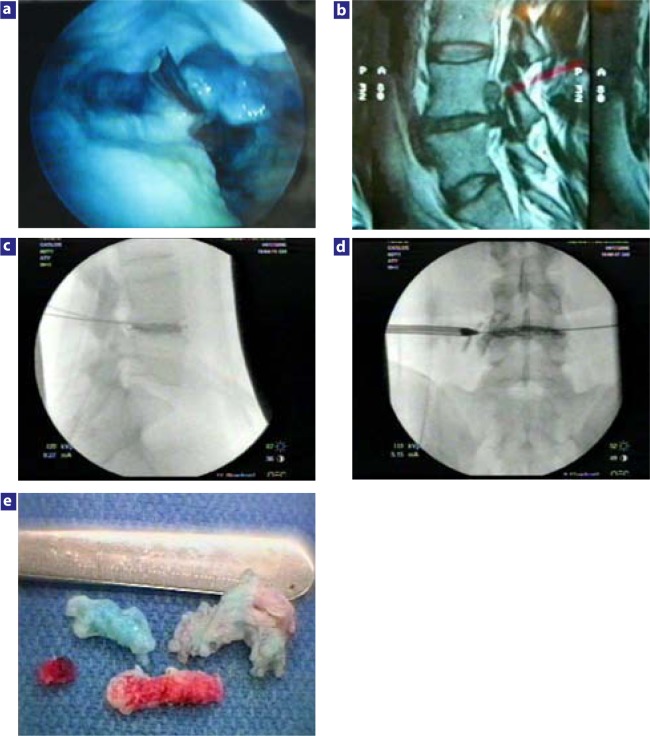
Each minimally invasive technique, therefore, had its own procedure-related limitations and complications. Some of the complication fears such as with chymopapain are overly hyped and were not justified when a thorough review of causes of complications was studied. Smith29 reported enzymatic dissolution of nucleus pulposus (with chymopapain injection) in humans in 1964. Significant concern arose about complications including anaphylaxis, postoperative back pain, stiffness, and neurological complications. The utilization of lower dosage, the ability to directly-visualize the effect of chymopapain via endoscopic visualization of the nucleus pulposus, the incorporation of perioperative discography to monitor the spread of chymopapain, and its use in combination with endoscopic discectomy have mitigated much of the risk (Figure 6).6, 8 Recent analysis of chymopapain revealed impurities that could be responsible for some of the allergic reactions, but since the advent of an antibody test for chymopapain, a more pure chymopapain will likely eliminate the concern about allergy. Chymopapain is still the most studied minimally invasive procedure, which makes its current decline—or even demise— unwarranted. It has been demonstrated to be even more versatile and effective when combined with discectomy.6, 8
Figure 6.
The effect of chymopapain on nucleus pulposus. (a) A biportal technique was used to inject 1000 units of chymopapain into the indigo carmine–stained nucleus pulposus before endoscopic shavers were used to evacuate the partially digested nucleus pulposus. Note the presence of the spinal needle used to inject the chymopapain, and that some of the nucleus is still stained by the indigo carmine dye. The consistency of the nucleus pulposus was made softer and easier for the shaver to evacuate the nucleus, leaving the cavity shown. To perform a thorough nuclectomy, however, the surgeon must also include manual discectomy with pituitary forceps. In selective endoscopic discectomy, the goal is to remove as much of the loose fragments that are stained blue as feasible. More-normal disc tissue will resist staining. The use of chymopapain to help with nuclectomy may play an important role in some nucleus replacement implants, because residual normal noncollagenized nuclear material may be more difficult to remove mechanically with traditional tools. If used in conjunction with mechanical discectomy, the chymopapain-treated nucleus is slippery, aiding its manual extraction, especially if the fragment is also bathed by chymopapain that has leaked out of the annular defect. (b) This extruded sequestered disc herniation is aided by the injection of chymopapain into the disc before manual extraction. When chromodiscography follows, the discogram pattern helps the surgeon target the disc fragment(s) to be removed. The chymopapain treated is softer and more slippery, making it easier to remove from the epidural space. (c, d) Chromodiscography demonstrates that the dye and extruded fragment are likely extruded and can be found in the axilla between the traversing and exiting nerve, making removal of the extruded fragment(s) probable. In the lateral view there is a void in the discogram pattern suggestive of a collagenous fragment and the lateral view demonstrates leakage of dye to the exiting nerve. The combined pattern suggests fragments in the axilla and epidural space. This is confirmed by the fragments removed. (e) Fragments visualized and extracted from the foramen with the endoscope. Note the differential staining, including the inflamed fragment(s) from the epidural space. The blue-stained fragment was found mostly inside the disc, whereas the partially stained fragment was partially trapped in the annular defect and the unstained inflamed fragment was loose in the epidural space. Chymopapain, when used as an adjunctive procedure, helps the surgeon with mechanical extraction and will help digest any small extruded fragment inadvertently left behind.
The use of thermal energy may play a major role in sealing annular tears and reduces not only radicular symptoms from chemical irritation, but also back pain by depopulating sensitized pain nociceptors in the annulus. The combination of percutaneous techniques enables the operating surgeon to visualize annular tears better and, when associated with disc herniations, to extract, by visualization, larger and extruded herniations by targeting the herniation site under direct vision.33, 34 The back pain component of internal disc derangement can be ameliorated by radiofrequency probes and intradiscal electrothermal catheters.
Lasers have been in clinical use for the treatment of herniated lumbar intervertebral discs for more than a decade. Only Ho: YAG and KTP lasers are approved by the Food and Drug Administration for this use. The lasers produce photothermal action on tissues and body fluids. The energy of the KTP laser is transmitted by monochromic synchronized light beam and is absorbed by the disc tissues and converted to heat. Heat causes coagulation of proteins at temperatures higher than 45°C, shrinking of collagen at 65°C, and boiling of water at 100°C.35–38 Higher temperature will result in vaporization, ablation, carbonization, and charring. Heterogeneity of biological tissues results in variable absorption of laser energy, so visualized monitoring of tissue response is desired. The KTP laser wavelength (0.532 microns) is in the blue-green visible light spectrum and has been used in ophthalmology to treat retinal lesions and in dermatology to treat pigmented and vascular skin lesions. The absorption is significantly enhanced by the presence of pigments such as hemoglobin or melanin.5 Absorption also increases in presence of indigo carmine; a blue-green vital dye, which is taken preferentially by the degenerative nucleus pulposus while normal tissues remain relatively unstained.5 Removal of stained degenerative disc tissue primarily allows the surgeon to be more selective in targeting the herniation site under direct vision, hence the term “selective endoscopic discectomy.”
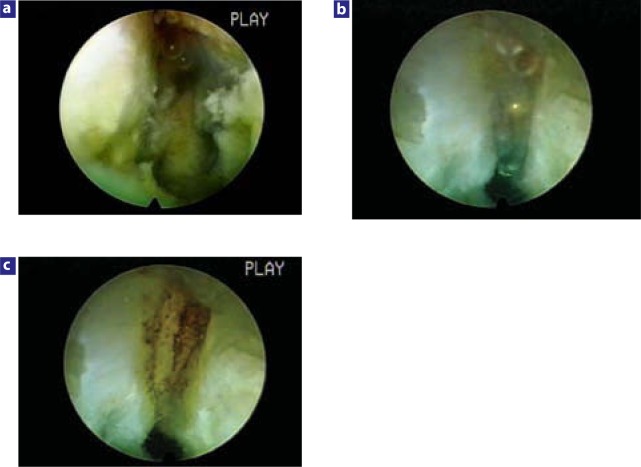
Degenerated discs have circumferential and radial fissures, which provide communication between the nuclear space and outer annular layers. In noncontained disc herniation, the discogram shows clear and direct seepage of the dye into the epidural space. Inflammatory mediators from inside the disc space can follow similar paths. Compressed, irritated, and inflamed nerve roots are very sensitive to stretching or mobilization, which causes severe pain. Dorsal root ganglia, usually located in the neural foramen region, are especially susceptible to such effects. The dorsal root ganglia have more extensive response when inflamed, compressed, or chemically irritated and fire spontaneously for prolonged periods of time even when the original stimulation has been removed. Any irritation of the dorsal root ganglion may result in transient dysesthesia that, in the 2 patients (out of the 80) who experienced it, resolved spontaneously. Adequate removal, ablation, and heating of the nuclear tissue may eliminate the inflammatory mediator source from the nucleus pulposus, including phospholipase A2, prostaglandin E2, and interleukin 6, and consequently remove the chemical component of neural irritation. Laser is currently used for tissue ablation, but thermal modulation of the annulus is accomplished by a flexible bipolar radiofrequency probe (Figure 7).
Figure 7.
Thermal annuloplasty sealing an annular tear with a bipolar high frequency flexible probe. (a) Annular tear visualized with a 20-degree spine endoscope. Note bleeding coming from the tear in the posterior annulus paracentrally after extraction of nuclear material that was keeping the tear open. (b) Bipolar flexible radiofrequency probe thermally shrinking the annular tear under direct vision. A biportal approach is utilized here. (c) Sealed annular tear. Clinical result was excellent. At 1-year follow-up, the patient still had complete resolution of sciatica and back pain. Single quadrant tears respond very well with thermal annuloplasty, whereas 360° tears in a degenerated, narrowed disc may still get relief, but back pain may eventually increase.
Five mechanisms explain the resolution of back and leg pain in patients with a herniated lumbar disc who underwent selective endoscopic discectomy and thermal annuloplasty: (1) decompression of the disc space, (2) removal and ablation of the source of the inflammatory mediators, (3) coagulation and depopulation of the free nerve endings in the annulus, (4) sealing of the annular fissures, and (5) removal of the disc herniation that was pressing on the sensitive spinal nerve. The transforaminal endoscopic approach is feasible and desirable not only for the treatment of lumbar disc herniations,39 but also for a wide spectrum of painful conditions of the lumbar spine.
Combination of adjunctive techniques in the evolution of foraminal endoscopic discectomy and foraminal spine decompression provides a surgical success rate comparable to traditional surgery. It allows for earlier intervention without “burning bridges” for subsequent surgical treatment. It has a high patient satisfaction rate with low morbidity. As surgeon experience and surgical skills improve, the learning curve decreases, results improve, and restrictions on patient selection lessen because of surgical accessibility of pathology. Evolving methodology in endoscopic discectomy techniques and the reapplication of adjunctive treatment learned in past attempts at minimal invasiveness can be reintroduced to further advance the field of minimally invasive disc surgery.
Although this paper is presented to historically demonstrate the value of using adjunctive techniques to enhance endoscopic foraminal discectomy, we have continued to advance and evolve endoscopic transforaminal surgery for many degenerative conditions of the lumbar spine. New scopes and instrumentation have enabled the surgeon who specializes in endoscopic lumbar surgery to improve diagnosis and treatment of painful conditions of the lumbar spine, such as foraminal decompression of lateral and even mild central stenosis. Dysesthesia and neuropraxia continue to be risks in spite of careful monitoring and meticulous technique. At this time, these risks apparently are unavoidable, because of anomalous anatomical features such as autonomic nerves in the foramen, the presence of furcal nerves, neoneurogenesis in the inflammatory membrane, conjoined nerves, and unavoidable changes in circulation around the dorsal root ganglion in the foramen following foraminal decompression.18
Biography
The author receives a licensing fee and royalties for sales of the Yeung Endoscopic Spine System (Richard Wolf Surgical Instrument Company, Vernon Hills, Illinois.). No funds were received in direct support of this study.
REFERENCES
- 1.Dabezies EJ, Langford K, Morris J, Shields CB, Wilkinson HA. Safety and efficacy of chymopapain (Discase) in the treatment of sciatica due to a herniated nucleus pulposus. Results of a randomized, double-blind study. Spine. 1988;13:561–565. doi: 10.1097/00007632-198805000-00022. [DOI] [PubMed] [Google Scholar]
- 2.Javid MJ, Nordby EJ, Ford LT, et al. Safety and efficacy of chymopapain (Chymodiactin) in herniated nucleus pulposus with sciatica. Results of a randomized, double-blind study. JAMA. 1983;249:2489–2494. [PubMed] [Google Scholar]
- 3.Hermantin FU, Peters T, Quartararo L, Kambin P. A prospective, randomized study comparing the results of open discectomy with those of video-assisted arthroscopic microdiscectomy. J Bone Joint Surg Am. 1999;81:958–965. doi: 10.2106/00004623-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Mayer HM, Brock M. Percutaneous endoscopic discectomy: surgical technique and preliminary results compared to microsurgical discectomy. J Neurosurg. 1993;78:216–225. doi: 10.3171/jns.1993.78.2.0216. [DOI] [PubMed] [Google Scholar]
- 5.Yeung AT. Spine: State of the Art Reviews. Vol. 7. Spine: State of the Art Reviews; 1993. Considerations for use of the KTP laser for disc decompression and ablation; pp. 67–93. [Google Scholar]
- 6.Hoogland T, Shubert M, Miklitz B, Ramirez A. Transforaminal posterolateral endoscopic discectomy with or without the combination of a low-dose chymopapain: a prospective randomized study in 280 consecutive cases. Spine. 2006;31:E890–E897. doi: 10.1097/01.brs.0000245955.22358.3a. [DOI] [PubMed] [Google Scholar]
- 7.Sagher O, Szabo TA, Chenelle AG, Jane JA. Intraoperative chemonucleolysis as an adjunct to lumbar discectomy. Spine. 1995;20:1923–1927. doi: 10.1097/00007632-199509000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Yeung AT. Intra-operative chemoneucleolysis as an adjunct to arthroscopic microdiscectomy. Presented at: International Intradiscal Therapy Society Annual Meeting; Naples, Florida: 1997. May 29, [Google Scholar]
- 9.Yeung AT. Minimally invasive disc surgery with the Yeung Endoscopic Spine System (YESS) Surg Technol Int. 2000;VIII:267–277. [PubMed] [Google Scholar]
- 10.Kambin P. Posterolateral percutaneous lumbar discectomy and decompression: arthroscopic microdiscectomy; Arthroscopic Microdiscectomy Minimal Intervention in Spinal Surgery; Baltimore, Maryland, and Munich, Germany: Urban & Schwarzenberg; 1991. pp. 67–99. [Google Scholar]
- 11.Kambin P, Zhou L. History and current status of percutaneous arthroscopic disc surgery. Spine. 1996;21(24 suppl):57s–61s. doi: 10.1097/00007632-199612151-00006. [DOI] [PubMed] [Google Scholar]
- 12.Hijikata S, Yamagishi M, Nakayama T, et al. Percutaneous nucleotomiemenus behandlung der diskushernie. J Toden Hosp. 1975;5:5–13. [Google Scholar]
- 13.Yeung AT. Enhancement of KTP/532 LDD and AMD procedures with a vital dye. Presented as a scientific exhibit at the American Academy of Orthopaedic Surgeons Annual Meeting; San Francisco, California: 1992. Feb 18–23, [Google Scholar]
- 14.Yeung AT, Morrison P, Felts M, Carter J. Intradiscal thermal therapy for discogenic low back pain, in the practice of minimally invasive spinal technique. In: Savitz MH, Chiu J, Yeung AT, editors. The Practice of Minimally Invasive Spinal Technique. Richmond, Virginia: AAMISMS Education; 2000. pp. 237–248. [Google Scholar]
- 15.Yeung AT. Factors affecting IDET outcome: an endoscopic analysis of IDET. Presented at the Western Orthopedic Association Annual Meeting; San Francisco, California: 2001. Sep 22, [Google Scholar]
- 16.Tsou PM, Alan Yeung T, Yeung AT. Posterolateral transforaminal selective endoscopic discectomy and thermal annuloplasty for chronic lumbar discogenic pain: a minimal access visualized intradiscal surgical procedure. Spine J. 2004;4:564–573. doi: 10.1016/j.spinee.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Yeung AT, Yeung CA. Microtherapy in low back pain. In: Mayer HM, editor. Minimally Invasive Spine Surgery: A Surgical Manual. Springer Verlag: Heidelberg; 2005. [Google Scholar]
- 18.Yeung AT, Yeung CA. In vivo endoscopic visualization of pathoanatomy in painful degenerative conditions of the lumbar spine. Surgical Technology International XV; San Francisco, California: Universal Medical Press; 2006. pp. 243–256. [PubMed] [Google Scholar]
- 19.Yeung AT, Yeung CA. Advances in endoscopic disc and spine surgery: foraminal approach. Surgical Technology International XI; San Francisco, California: Universal Medical Press; 2002. pp. 253–261. [PubMed] [Google Scholar]
- 20.Yeung AT, Yeung CA. Posterolateral selective endoscopic discectomy: the YESS Technique. In: Kim D, Fessler R, Regan J, editors. Endoscopic Spine Surgery and Instrumentation: Percutaneous Procedures. New York, New York: Thieme Medical Publishers; 2005. [Google Scholar]
- 21.Onik G, Helms G. Percutaneous lateral discectomy using a new aspiration probe. Am J Neuroradiology. 1985;6:290. [Google Scholar]
- 22.Regan JJ, Guyer RD. Endoscopic technique in spinal surgery. Clin Orthop. 1997;335:122–139. [PubMed] [Google Scholar]
- 23.Kambin P, Gillman H. Percutaneous lateral discectomy of the lumbar spine. A preliminary report. Clin Orthop. 1983;174:127–132. [Google Scholar]
- 24.Karasek M, Bogduk N. Twelve-month follow-up of a controlled trial of intradiscal thermal annuloplasty for back pain due to internal disc disruption. Spine. 2000;25:2601–2607. doi: 10.1097/00007632-200010150-00010. [DOI] [PubMed] [Google Scholar]
- 25.Quigley MR, Maroon JC. Laser discectomy: a review. Spine. 1994;19:53–56. doi: 10.1097/00007632-199401000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Quigley MR, Maroon JC. Automated percutaneous discectomy. Neurosurg Clin N Am. 1996;7:29–35. [PubMed] [Google Scholar]
- 27.Saal JS, Saal JA. Management of chronic discogenic low back pain with a thermal intradiscal catheter. A preliminary report. Spine. 2000;25:382–388. doi: 10.1097/00007632-200002010-00021. [DOI] [PubMed] [Google Scholar]
- 28.Saal JA, Saal JS. Intradiscal electrothermal treatment for chronic discogenic low back pain: a prospective outcome study with minimum 1-year follow-up. Spine. 2000;25:2622–2627. doi: 10.1097/00007632-200010150-00013. [DOI] [PubMed] [Google Scholar]
- 29.Smith L. Enzyme dissolution of the nucleus pulposus in humans. JAMA. 1964;187:137–140. doi: 10.1001/jama.1964.03060150061016. [DOI] [PubMed] [Google Scholar]
- 30.Choy DS, Ascher PW, Ranu HS, et al. Percutaneous laser disc decompression. A new therapeutic modality. Spine. 1992;17:949–956. doi: 10.1097/00007632-199208000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Quigley MR. Percutaneous laser discectomy. Neurosurg Clin N Am. 1996;7:37–42. [PubMed] [Google Scholar]
- 32.Yeung AT, Savitz MH. Complications of percutaneous spinal surgery. In: Vacarro A, editor. Complications in Adult and Pediatric Spine Surgery. New York, New York: Marcel Dekker; 2004. [Google Scholar]
- 33.Yeung AT. Minimally invasive disc surgery with the Yeung Endoscopic Spine System (YESS). Surgical Technology International VIII; San Francisco, California: Universal Medical Press; 1999. pp. 267–277. [PubMed] [Google Scholar]
- 34.Yeung AT. The evolution of percutaneous spinal endoscopy and discectomy: state of the art. Mt Sinai J Med. 2000;67:327–332. [PubMed] [Google Scholar]
- 35.Dew DK. Laser biophysics for the orthopaedic surgeon. Clin Orthop Relat Res. 1995;310:6–13. [PubMed] [Google Scholar]
- 36.Dew DK, Supik L, Darrow CR, Price GF. Tissue repair using lasers: a review. Orthopedics. 1993;16:581–587. doi: 10.3928/0147-7447-19930501-11. [DOI] [PubMed] [Google Scholar]
- 37.Bass LS, Moazami N, Pocsidio J, Oz MC, LoGerfo P, Treat MR. Changes in type I collagen following laser welding. Lasers Surg Med. 1992;12:500–505. doi: 10.1002/lsm.1900120508. [DOI] [PubMed] [Google Scholar]
- 38.Vangsness CT, Mitchell W, Nimni M, Erlich M, Saadat V, Schmotzer H. Collagen shortening. An experimental approach with heat. Clin Orthop Relat Res. 1997;337:267–271. doi: 10.1097/00003086-199704000-00030. [DOI] [PubMed] [Google Scholar]
- 39.Yeung AT, Tsou PM. Posterolateral endoscopic excision of lumbar disc herniation: surgical technique, outcome, and complications in 307 consecutive cases. Spine. 2002;27:722–731. doi: 10.1097/00007632-200204010-00009. [DOI] [PubMed] [Google Scholar]