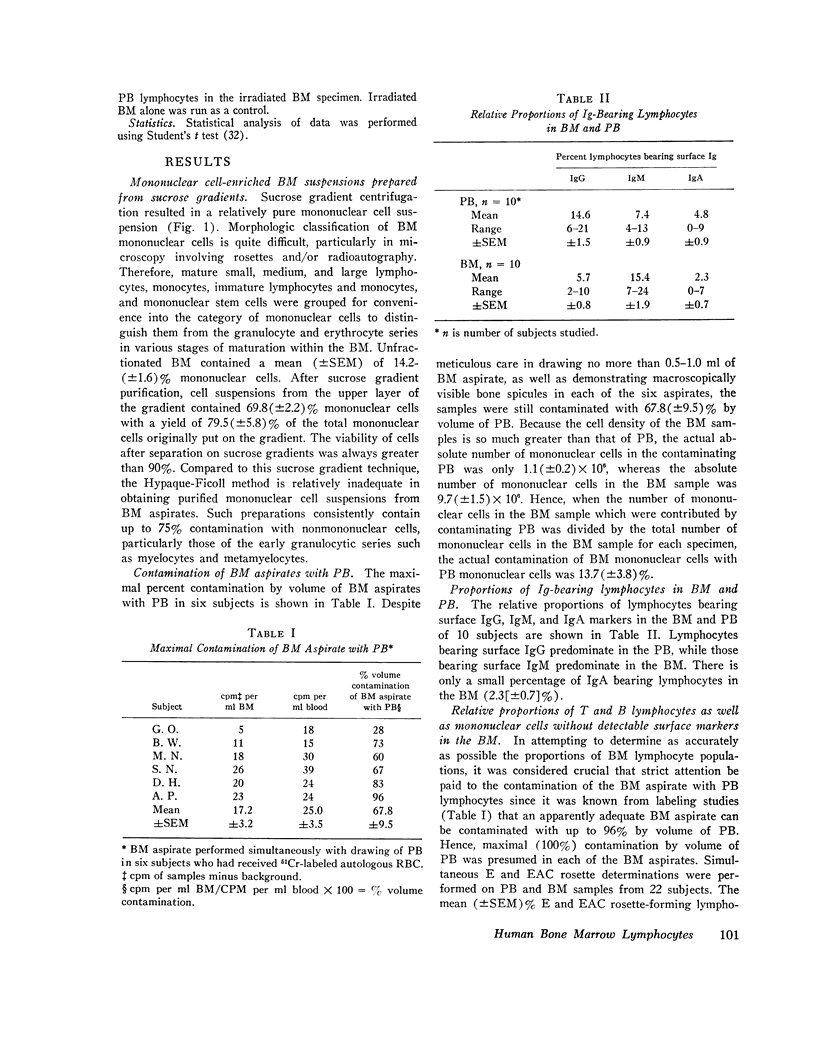
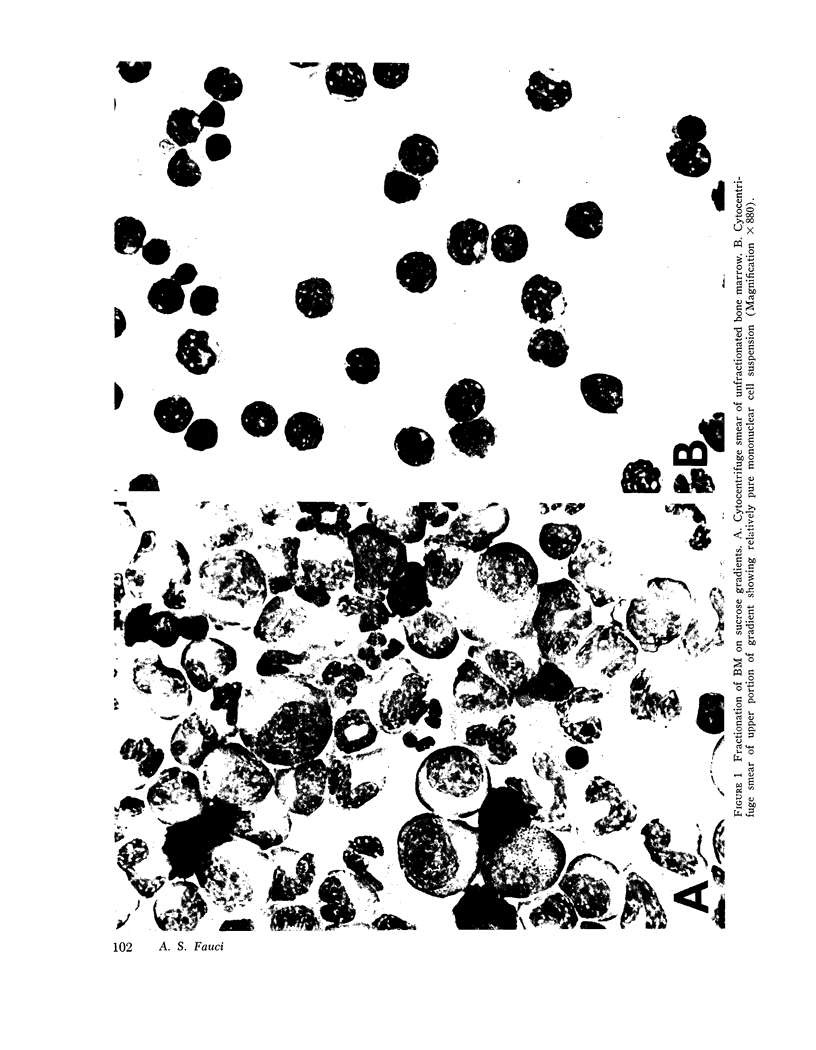
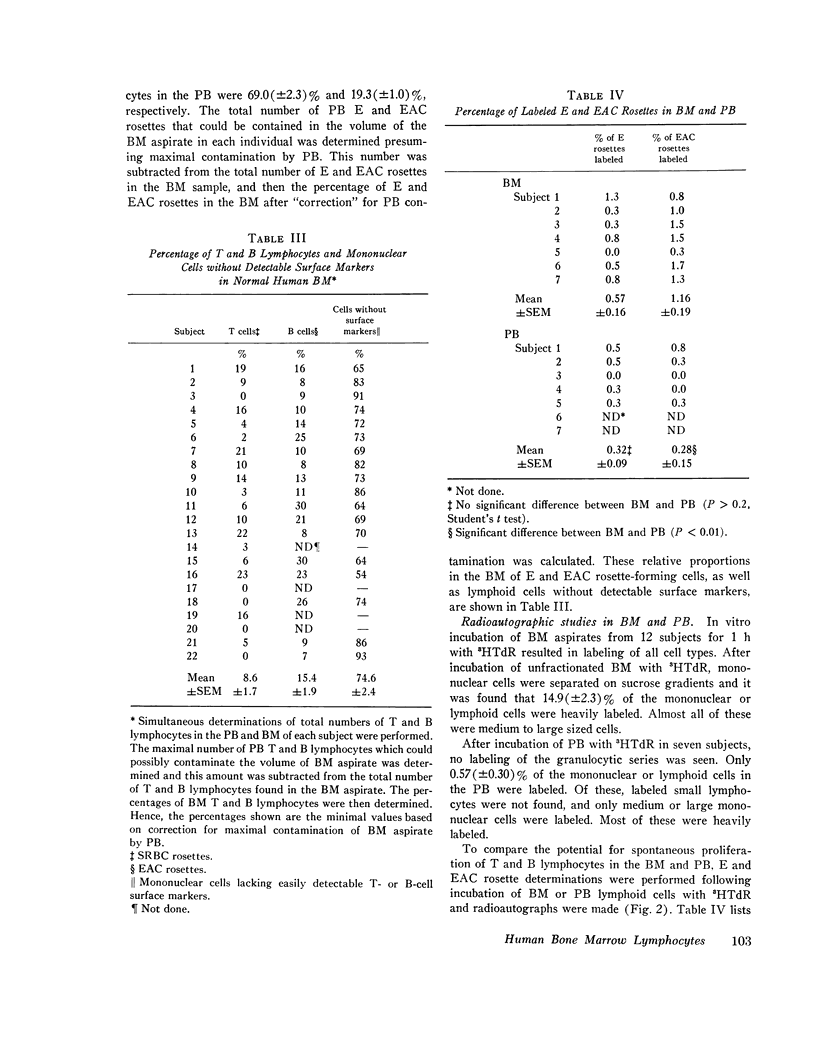
Abstract
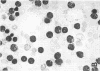
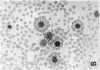
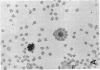
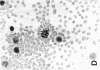
This study was undertaken to determine the proportions and in vitro immune capacities of lymphocyte populations in the bone marrows of normal humans. Relatively pure mononuclear cell suspensions were obtained from bone marrow aspirates by linear sucrose gradient centrifugations. Simultaneous peripheral blood and bone marrow specimens from each individual were assayed for lymphocyte surface markers and mitogen responsiveness. Maximal possible contamination of bone marrow aspirates by peripheral blood was determined by performing aspirates on individuals who had received 51chromium-labeled autologous erythrocytes. Rhymus-derived (T) lymphocytes, as determined by the sheep red blood cell (E) rosette assay, comprised 8.6-(plus or minus 1.6)% of the total bone marrow lymphocyte pool. Bone marrow-derived (B) lymphocytes, as determined by the presence of a complement receptor, made up 15.4-(plus or minus 1.9)% of the lymphocyte pool whereas 74.6 (plus or minus 2.4)% of mononuclear cells lacked easily detectable surface markers. These findings could not be explained by contamination with peripheral blood lymphocytes since contamination was corrected for in the calculations. Lymphocyte-enriched suspensions of bone marrow cells responded to stimulation with phytohemagglutinin, concanalin A, and particularly pokeweed mitogen. In vitro incubations of bone marrow and peripheral blood lymphocytes with tritiated thymidine followed by determinations of E and erythrocyte antibody complement (EAC) rosettes were performed. Simultaneous rosetteradioautographs demonstrated that the proliferative potential of bone marrow B lymphocytes was greater than peripheral blood B lymphocytes (P less than 0.01). On the other hand, the proliferative potential of bone marrow T lymphocytes was the same as that of peripheral blood T lymphocytes. These findings demonstrate that in addition to containing B lymphocytes the normal bone marrow contains a small fraction of T lymphocytes similar to the mature T lymphocyte pool found in the peripheral blood. These T cells most probably enter the bone marrow parenchyma as part of the normal recirculating lymphocyte pool.
Full text
PDF


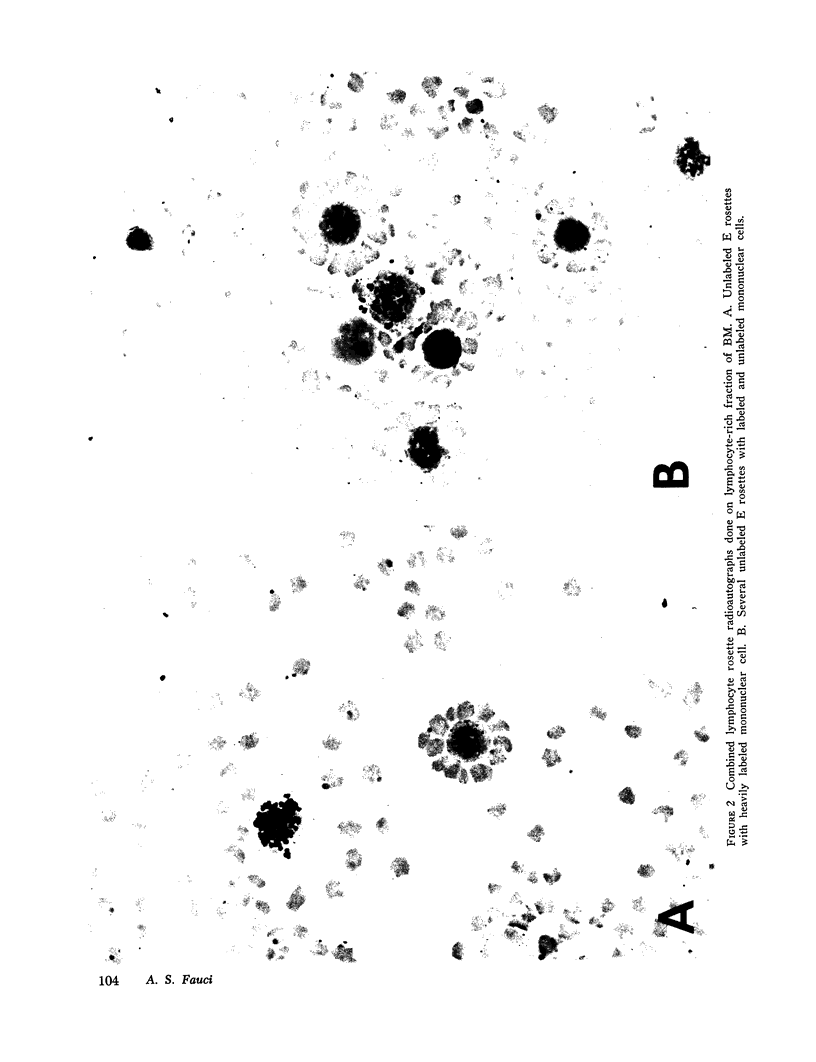

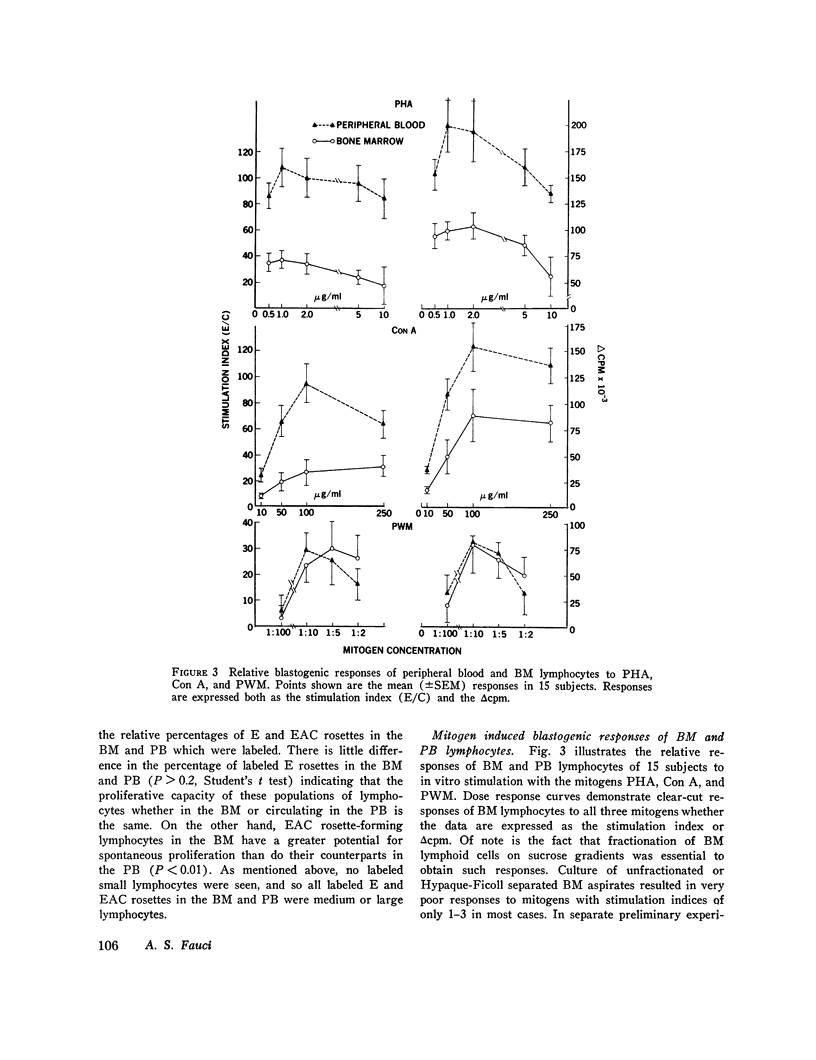
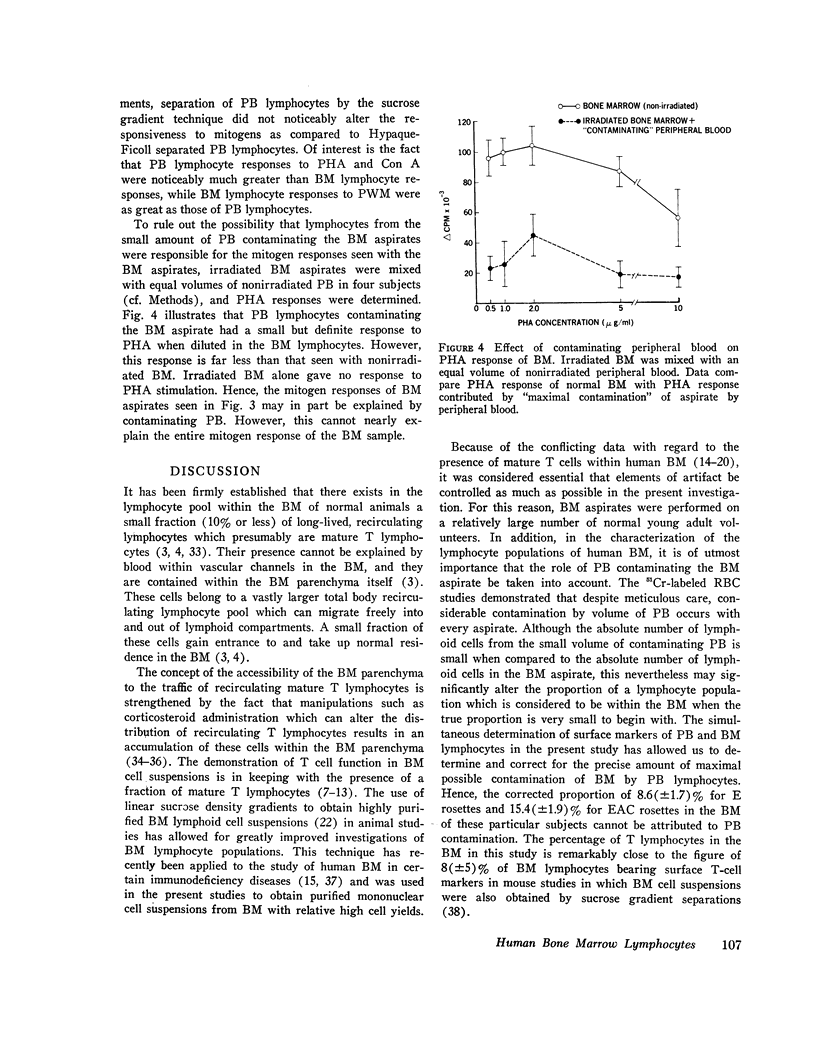



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Casella S. R., Abdou N. L., Abrahamsohn I. A. Comparative study of bone marrow and blood B cells in infantile and acquired agammaglobulinemia. Possible role of circulating anti-IgM in pathogenesis. J Clin Invest. 1973 Sep;52(9):2218–2224. doi: 10.1172/JCI107407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdou N. I., Richter M. The role of bone marrow in the immune response. Adv Immunol. 1970;12:201–270. doi: 10.1016/s0065-2776(08)60170-4. [DOI] [PubMed] [Google Scholar]
- Asherson G. L., Zembala M., Mayhew B. Passive transfer of contact sensitivity by bone marrow cells and evidence for their origin from immunized lymph nodes. Int Arch Allergy Appl Immunol. 1974;46(2):256–260. doi: 10.1159/000231128. [DOI] [PubMed] [Google Scholar]
- BOND V. P., CRONKITE E. P., FLIEDNER T. M., SCHORK P. Deoxyribonucleic acid synthesizing cells in peripheral blood of normal human beings. Science. 1958 Jul 25;128(3317):202–203. doi: 10.1126/science.128.3317.202. [DOI] [PubMed] [Google Scholar]
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borella L., Green A. A. Sequestration of PHA-responsive cells (T-lymphocytes) in the bone marrow of leukemic children undergoing long-term immunosuppressive therapy. J Immunol. 1972 Nov;109(5):927–932. [PubMed] [Google Scholar]
- Borella L., Sen L. T cell surface markers on lymphoblasts from acute lymphocytic leukemia. J Immunol. 1973 Oct;111(4):1257–1260. [PubMed] [Google Scholar]
- Borella L., Sen L. The distribution of lymphocytes with T- and B-cell surface markers in human bone marrow. J Immunol. 1974 Feb;112(2):836–843. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- CRONKITE E. P., BOND V. P., FLIEDNER T. M., RUBINI J. R. The use of tritiated thymidine in the study of DNS synthesis and cell turnover in hemopoietic tissues. Lab Invest. 1959 Jan-Feb;8(1):263–277. [PubMed] [Google Scholar]
- CRONKITE E. P., FLIEDNER T. M., BOND V. P., RUBINI J. R. Dynamics of hemopoietic proliferation in man and mice studied by H3-thymidine incorporation into DNA. Ann N Y Acad Sci. 1959 Jun 25;77:803–820. doi: 10.1111/j.1749-6632.1959.tb36943.x. [DOI] [PubMed] [Google Scholar]
- Chen M. G., Price G. B., Makinodan T. Incidence of delayed mortality (secondary disease) in allogeneic radiation chimeras receiving bone marrow from aged mice. J Immunol. 1972 May;108(5):1370–1378. [PubMed] [Google Scholar]
- Claman H. N. Bone marrow T cells. I. Response to the T cell mitogens, phytohemagglutinin and concanavalin A. J Immunol. 1974 Mar;112(3):960–964. [PubMed] [Google Scholar]
- Cohen J. J. Hydrocortisone resistance of activated initiator cells in graft versus host reactions. Nature. 1971 Jan 22;229(5282):274–275. doi: 10.1038/229274a0. [DOI] [PubMed] [Google Scholar]
- Cohen J. J. Thymus-derived lymphocytes sequestered in the bone marrow of hydrocortisone-treated mice. J Immunol. 1972 Mar;108(3):841–844. [PubMed] [Google Scholar]
- DONOHUE D. M., GABRIO B. W., FINCH C. A. Quantitative measurement of hematopoietic cells of the marrow. J Clin Invest. 1958 Nov;37(11):1564–1570. doi: 10.1172/JCI103749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C. Alternate-day prednisone therapy and human lymphocyte subpopulations. J Clin Invest. 1975 Jan;55(1):22–32. doi: 10.1172/JCI107914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. Mechanisms of corticosteroid action on lymphocyte subpopulations. I. Redistribution of circulating T and b lymphocytes to the bone marrow. Immunology. 1975 Apr;28(4):669–680. [PMC free article] [PubMed] [Google Scholar]
- Ford W. L., Gowans J. L. The traffic of lymphocytes. Semin Hematol. 1969 Jan;6(1):67–83. [PubMed] [Google Scholar]
- Frank M. M., Gaither T. The effect of temperature on the reactivity of guinea-pig complement with gamma G and gamma M haemolytic antibodies. Immunology. 1970 Dec;19(6):967–974. [PMC free article] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Geha R. S., Gatien J. G., Parkman R., Crain J. D., Rosen F. S., Merler E. Discontinuous density gradient analysis of human bone marrow: presence of alloantigen--responsive, PHA--unresponsive cells in norman bone marrow; absence of B lymphocytes in the bone marrow of patients with X--linked agammaglobulinemia. Clin Immunol Immunopathol. 1974 Apr;2(3):404–415. doi: 10.1016/0090-1229(74)90058-0. [DOI] [PubMed] [Google Scholar]
- Haas R. J., Bohne F., Fliedner T. M. On the development of slowly-turning-over cell types in neonatal rat bone marrow (studies utilizing the complete tritiated thymidine labelling method complemented by C-14 thymidine administration). Blood. 1969 Dec;34(6):791–805. [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Wigzell H., Aiuti F. Human lymphocyte subpopulations: classification according to surface markers and-or functional characteristics. Transplant Rev. 1973;16:163–195. doi: 10.1111/j.1600-065x.1973.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Levine M. A., Claman H. N. Bone marrow and spleen: dissociation of immunologic properties by cortisone. Science. 1970 Mar 13;167(3924):1515–1517. doi: 10.1126/science.167.3924.1515. [DOI] [PubMed] [Google Scholar]
- Papamichail M., Brown J. C., Holborow E. J. Immunoglobulins on the surface of human lymphocytes. Lancet. 1971 Oct 16;2(7729):850–852. doi: 10.1016/s0140-6736(71)90224-8. [DOI] [PubMed] [Google Scholar]
- Pegrum G. D., Ready D., Thompson E. The in vitro effect of phytohaemagglutinin on separated human bone marrow cells. Br J Haematol. 1968 Oct;15(4):377–380. doi: 10.1111/j.1365-2141.1968.tb01553.x. [DOI] [PubMed] [Google Scholar]
- Rosse C. Migration of long-lived lymphocytes to the bone marrow and to other lymphomyeloid tissues in normal parabiotic guinea pigs. Blood. 1972 Jul;40(1):90–97. [PubMed] [Google Scholar]
- Ryser J. E., Vassalli P. Mouse bone marrow lymphocytes and their differentiation. J Immunol. 1974 Sep;113(3):719–728. [PubMed] [Google Scholar]
- Röpke C., Everett N. B. Migration of small lymphocytes in adult mice demonstrated by parabiosis. Cell Tissue Kinet. 1974 Mar;7(2):137–150. doi: 10.1111/j.1365-2184.1974.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Singhal S. K., Richter M. Cells involved in the immune response. IV. The response of normal and immune rabbit bone marrow and lymphoid tissue lymphocytes to antigens in vitro. J Exp Med. 1968 Nov 1;128(5):1099–1128. doi: 10.1084/jem.128.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touraine J. L., Incefy G. S., Touraine F., Rho Y. M., Good R. A. Differentiation of human bone marrow cells into T lymphocytes by in vitro incubation with thymic extracts. Clin Exp Immunol. 1974 May;17(1):151–158. [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Till J. E., Siminovitch L., McCulloch E. A. Cytological evidence for a relationship between normal hemotopoietic colony-forming cells and cells of the lymphoid system. J Exp Med. 1968 Mar 1;127(3):455–464. doi: 10.1084/jem.127.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Osmond D. G. Blastogenic response of lymphocytes separated from bone marrow to allogeneic lymphoid cells in vitro. Immunology. 1971 Nov;21(5):767–779. [PMC free article] [PubMed] [Google Scholar]