Abstract
Background
Less invasive fusion approaches, such as extreme lateral interbody fusion (XLIF), have proliferated, but few reports have critically assessed fusion rates. To date, no studies have reported computed tomography (CT) documented fusion rates following XLIF.
Methods
An institutional review board-approved prospective radiographic and CT assessment of minimally disruptive anterior lumbar interbody fusion (mini-ALIF) fusions performed through the XLIF approach. Sixty-six patients (88 operative levels) were examined 12 months after XLIF to determine the rate and quality of anterior lumbar fusion.
Results
Eighty five of the 88 levels (96.6%) were judged fused by CT. Sixty-four of the 66 patients (97.0%) were judged fused by CT. Patient satisfaction at 12 months after surgery was high, with 89.4% reportedly “satisfied or very satisfied” with their results. No revisions were necessary for pseudarthrosis.
Conclusion
Mini-ALIF using an XLIF approach reliably results in anterior lumbar fusion.
Keywords: Minimally invasive, Fusion, Lateral, Anterior, Computed tomography
Anterior fusion of the lumbar spine is a well-established technique for the treatment of developmental, traumatic, neoplastic, and degenerative conditions.1–3 As technology has improved, techniques have been developed that purport to allow fusion through less invasive and minimally disruptive approaches (mini-ALIF).4–6 Among these options for anterior interbody fusion is extreme lateral interbody fusion (XLIF).7, 8 We have reported our experience with this technique previously, with regard to complications9 and as applied to difficult reconstructive situations10, 11 and patient populations.12, 13 Despite the justified interest in surgery through less-disruptive approaches, very few of the previous reports on less-invasive techniques have dealt specifically with the fundamental question that must be posed to any new fusion technology: does the operative segment fuse?
Traditional open approaches to anterior lumbar fusion have shown excellent fusion rates, regularly 95% or better.14–21 In evaluating fusion, it has been well-documented that, while plain and dynamic radiography can delineate motion (and thus indicate a failure to fuse), it cannot assess definitively the presence of bridging bone across the operative segment; computed tomography (CT) has been used as the gold standard for assessing fusion formation in most of the recent studies.22–32
In this report, the CT fusion rate is described for mini-ALIF using an XLIF approach.
Materials and methods
Study design
After obtaining Institutional Review Board approval, patients returning for follow-up 12 months after mini-ALIF using XLIF were prospectively consented to undergo CT assessment of fusion status, in addition to fusion assessment by plain and dynamic radiographs. Fusion status was assessed by an independent reviewer.
All patients had been treated with extreme lateral interbody fusion using standard techniques, as have been described elsewhere.8 The graft material consisted of local bone harvested from the central vertebral bodies and augmented by demineralized bone matrix and cancellous allograft (Optecure + CCC; Exactech, Gainesville, FL) reconstituted with bone marrow aspirated from the iliac crest.
Anteroposterior, lateral, and flexion-extension lateral radiographs were obtained on all patients. On radiographs, fusion was defined as bridging bone connecting the adjacent vertebrae and angular motion less than 5° and less than 3 mm of translation between levels with flexion and extension. Fusion was considered solid on plain radiograph, only if all 3 criteria were met. The quality of bridging bone was graded using modified Lenke criteria.33 Additionally, radiographs were compared to preoperative and postoperative films to assess disk height and listhesis and the maintenance of correction over time.
Thin-slice (1 mm) CT scans with sagittal reconstructions were reviewed. The scans were assessed for the presence of trabecular bone traversing the operative disk space, either through or adjacent to the implant. In addition, the quantitative volume of traversing bone was assessed and graded as follows: Grade 1, less than 25% of the operative level; Grade 2, 25-50% of the operative level; Grade 3, greater than 50% of the operative level. Only Grade 3 scans were considered definitively fused.
Sixty-six patients (25 male, 41 female; average age, 62.2 years; average BMI, 30.4) underwent CT. A total of 88 disc levels had been treated operatively (6 3-level fusions, 10 2-level fusions, and 50 single-level fusions). Supplemental posterior instrumentation was used in 61 cases (56 pedicle screw constructs, 5 transfacet fusions) and lateral instrumentation in 4. One 3-level standalone fusion was performed.
Results
A synopsis of the radiographic outcomes from the series in presented in Table 1. On average, there was an 80% reduction in pain from before surgery to 12 months after surgery. Average disk height increased over 4 mm after surgery, with an average loss of 1 mm over the course of 12 months. Listhesis improved by 75%, and this reduction was maintained. At 12 months, patient satisfaction was high, with 89.4% reportedly “satisfied” or “very satisfied” with their results, and an equal number reporting they “likely” or “definitely” would elect to undergo the procedure again.
Table 1.
Clinical and radiographic data on the patient series
| Preop | Postop | 3 mos. | 6 mos. | 12 mos. | |
|---|---|---|---|---|---|
| VAS | 8.6 | 2.5 | 1.7 | 1.7 | |
| Disk Height (mm) | 6.2 | 10.3 | 9.7 | 9.4 | 9.3 |
| Slip (mm) | 4.3 | 0.8 | 0.8 | 0.9 | 0.8 |
| Lenke | 2.1 | 1.3 | 1.1 |
At 12 months after surgery, 6 patients were felt to have incomplete bridging bone by plain radiographs (5 cases with modified Lenke score 2, and 1 modified Lenke score 3). Five of the 6 would be classified as “probably fused” by modified Lenke criteria.33 Only 1 of these patients showed motion on flexion-extension lateral radiographs – possibly because all the other patients had adjunctive instrumentation used to stabilize the fused segment. The patient with evidence of motion on radiographs had been treated with a standalone 3-level XLIF.
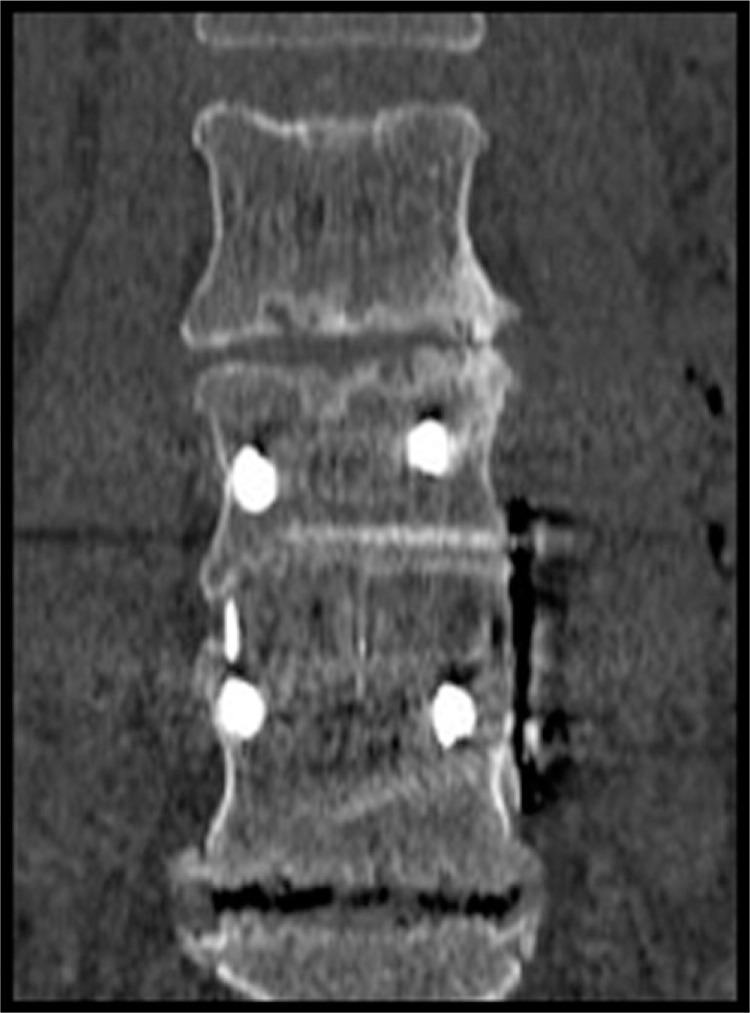
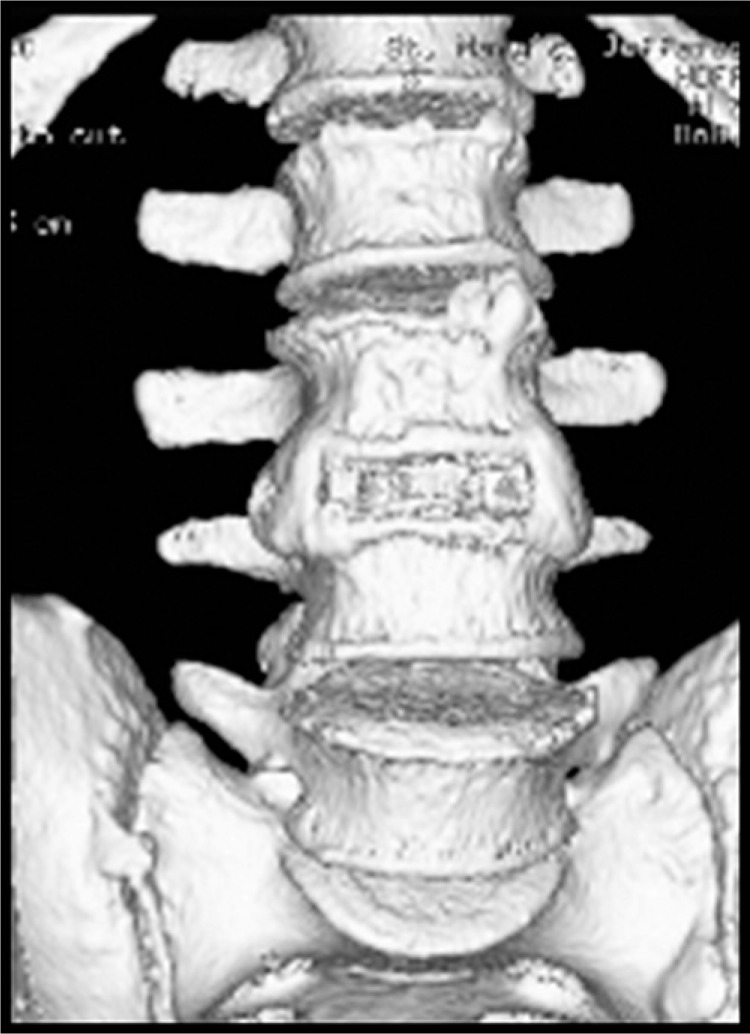
Computed tomographic analysis of the 88 operative levels showed evidence of complete bridging in 85 levels (Grade 3). Three of the levels (in 2 patients) were judged to be Grade 2, and thereby interpreted as not fused. Thus, by plain radiograph criteria, 98.4% of the patients were judged as “fused” or “probably fused” due to the presence of bridging bone and the absence of motion on dynamic radiographs. By CT critieria, 64 of 66 patients (97.0%) were fused and 85 of 88 levels (96.6%) were Grade 3 (Figures 1 and 2).
Fig. 1.
Computed tomography demonstrates bridging bone across fusion site.
Fig. 2.
Three-dimensional reconstruction showing fusion formation.
Two of the Grade 2 levels (in 1 patient) were part of a 3-level standalone XLIF in an 87-year-old female. The third Grade 2 level was one of a 3-level instrumented XLIF in a 69-year-old female. Both patients had improved pain scores and rated themselves as satisfied with their clinical outcomes.
There were no reoperations due to pseudarthrosis.
Discussion
Anterior fusion of the lumbar spine has been performed routinely for the better part of a half century, and with reliable progression toward fusion of the operated levels.1–3 Using modern approaches fusion rates have approached 100%14–21, 34 even when rigorously analyzed by computed tomography, the current gold standard for fusion assessment.26–32
Burkus et al,34 in discussing the largest series of ALIF fusions (679 procedures) in the literature, reported a CT-documented and motion radiograph-confirmed fusion rate of 92-97% at 12 months after surgery, depending on fusion technique and graft material. These procedures were standalone ALIFs using threaded cages inserted either through an open approach or laparoscopically, using either iliac crest autograft or or bone morphogenic protein (rh-BMP) in the cages.
A smaller, more recent study35 compared open ALIF and minimally invasive TLIF with both groups having allograft chips contained within interbody cages and both groups stabilized with transpedicular instrumentation. This study is pertinent because the majority of our patients were also stabilized with posterior pedicle screw instrumentation. Kim et al found fusion in 95.8% of the ALIF group assessed by plain radiography. The present results are very similar to those reported in these earlier series with an overall fusion rate of 96%, as assessed by independently-reviewed CT scans and motion radiographs.
Despite the success in achieving fusion using ALIF through traditional approaches, concerns remain about the morbidity associated with open surgery. In response to these concerns, less invasive technologies are revolutionizing the care of patients needing thoracolumbar spinal fusion. More rapid recovery is facilitated by decreased tissue trauma. We have previously reported our clinical outcomes and improved complications profile when applying mini-ALIF through an XLIF technique.9–13 Hospitalization in these larger reports averaged 1.2 days; as noted in this report, fusion rates at 12 months after surgery are equal to the large series reported by Burkus et al.34 As an extremely technology-driven and expensive speciality, spinal surgery, in general, has recently been subjected to long-overdue scrutiny regarding outcomes and costs.36–38 Even though surgery for spondylolisthesis has been shown to be more effective than nonoperative care, recent interpretations of the Spine Patient Outcomes Research Trial (SPORT) have questioned the cost effectiveness of fusion surgery compared to decompression alone for degenerative stenosis with spondylolisthesis.39 This study noted a quality-adjusted life year (QALY) gain of 0.23 in the fusion cohort, but this came at a cost of $115,600 per QALY gained. No breakdown of the 344 fusion surgeries (269 with instrumentation) by type of procedure was provided; however, based on the time frame of the study, it may be inferred that the vast majority of those fusions were performed using traditional open techniques.
As shown in previous reports,9–13 the complications associated with XLIF fusion are notably less than the complications reported with traditional open approaches. It stands to reason that less invasive fusion options, like XLIF, would be expected to yield a markedly decreased dollar cost per QALY gained, because these techniques require shorter hospital stays and result in fewer expensive complications, assuming that these newer technologies can be shown to yield reliable spinal fusion. As was noted some years ago by Ackerman et al,40 new technologies, even if initially more costly, may prove to have a societal cost savings if they result in decreases in the use of other healthcare resources through decreases in morbidity and more rapid return to function.
The question of cost-effectiveness becomes more important, because many newer technologies are often grouped together in a single treatment setting. These costs then become additive and thus offset some of the savings resulting from improved morbidity profiles. Although not the subject of this report, the graft material composite used in this study (demineralized bone matrix reconstituted with iliac crest bone marrow aspirate, cancellous allograft chips, and local autograft harvested from the central vertebral bodies) carries a significantly reduced price compared to the more expensive rh-BMP products. The use of demineralized bone matrix as a graft extender dates back 4 decades,41, 42 and its use in spinal surgery has been reported43–45 using other techniques. The present data would suggest that some newer less invasive technologies that yield structural anterior column support, like XLIF, may allow the use of less expensive grafting alternatives in some situations.
Early in the last century, E.A. Codman wrote, “Give me something different for there is a chance of it being better.”2 Much has changed in the ensuing years, but the search for better treatment alternatives continues. Newer is not necessarily better; it must first be shown to be equivalent. The present data suggest that minimally disruptive ALIF performed through an XLIF approach reliably results in anterior column arthrodesis at least as well as traditional open techniques. Experience with this technique in larger series9–13 would lead to a belief that, considering improved morbidity and complications profiles, newer may indeed be better.
References
- 1.Flynn JC, Hogue MA. Anterior fusion of the lumbar spine with long-term follow-up. J Bone Joint Surg Am. 1979;61:1143–50. [PubMed] [Google Scholar]
- 2.Sacks S. Anterior interbody fusion of the lumbar spine. J Bone Joint Surg Am. 1965;47B:211–23. [PubMed] [Google Scholar]
- 3.Stauffer RN, Coventry MB. Anterior interbody lumbar spine fusion: analysis of Mayo Clinic series. J Bone Joint Surg Am. 1972;54:756–68. [PubMed] [Google Scholar]
- 4.Bergey DL, Villavicencio AT, Goldstein T, et al. Endoscopic lateral transpsoas approach to the lumbar spine. Spine. 2004;29:1681–88. doi: 10.1097/01.brs.0000133643.75795.ef. [DOI] [PubMed] [Google Scholar]
- 5.Eck JC, Hodges S, Humphreys SC. Minimally invasive lumbar spinal fusion. J Am Acad Orthop Surg. 2007;15:321–9. doi: 10.5435/00124635-200706000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Foley KT, Langston TH, Schwender JD. Minimally invasive lumbar fusion. Spine. 2003;28:526–35. doi: 10.1097/01.BRS.0000076895.52418.5E. [DOI] [PubMed] [Google Scholar]
- 7.Ozgur BM, Aryan HE, Pimenta L, et al. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;26:435–43. doi: 10.1016/j.spinee.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Rodgers WB, Cox CS, Gerber EJ. Experience and early results with a minimally invasive technique for anterior column support through eXtreme Lateral Interbody Fusion: XLIF®. US Musculoskeletal Review. 2007;1:28–32. [Google Scholar]
- 9.Rodgers WB, Gerber EJ, Patterson JR. Intraoperative and early post-operative complications in extreme lateral interbody fusion (XLIF): An analysis of 600 cases; Submitted for publication. [DOI] [PubMed] [Google Scholar]
- 10.Rodgers WB, Gerber EJ, Patterson JR. Grade 2 spondylolisthesis at L4-5 treated by XLIF: safety and mid-term results in the “worst case scenario.”; Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodgers WB, Cox CS, Gerber EJ. Minimally invasive treatment (XLIF) of adjacent segment disease after prior lumbar fusions. Int J Minimally Invasive Spinal Technol. 2009;4:1–7. [Google Scholar]
- 12.Rodgers WB, Cox CS, Gerber EJ. Early complications of extreme lateral interbody fusion in the obese; J Spinal Disord Tech; In press. [DOI] [PubMed] [Google Scholar]
- 13.Rodgers WB, Gerber EJ. Lumbar fusion in octogenarians: the promise of minimally invasive surgery; Submitted for publication. [DOI] [PubMed] [Google Scholar]
- 14.Boden SD, Zdeblick TA, Harvinder SS, et al. The use of rhBMP-2 in interbody fusion cages. Spine. 2000;25:376–81. doi: 10.1097/00007632-200002010-00020. [DOI] [PubMed] [Google Scholar]
- 15.Burkus JK, Gornet MF, Dickman CA, et al. Anterior lumbar interbody fusion using rh-BMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337–49. doi: 10.1097/00024720-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Burkus JK, Transfeldt EE, Kitchel SH, et al. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine. 2002;27:2396–408. doi: 10.1097/00007632-200211010-00015. [DOI] [PubMed] [Google Scholar]
- 17.Burkus JK. Interbertebral fixation: clinical results with anterior cages. Orthop Clin N Am. 2002;33:349–57. doi: 10.1016/s0030-5898(01)00012-8. [DOI] [PubMed] [Google Scholar]
- 18.Burkus JK. Bone morphgenetic proteins in anterior lumbar interbody fusion: old techniques and new technologies. J Neurosurg Spine. 2004;3:254–60. doi: 10.3171/spi.2004.1.3.0254. [DOI] [PubMed] [Google Scholar]
- 19.Burkus JK, Schuler TC, Gornet MF, et al. Anterior lumbar interbody fusion for the management of chronic lower back pain: current strategies and concepts. Orthop Clin N Am. 2004;35:25–32. doi: 10.1016/S0030-5898(03)00053-1. [DOI] [PubMed] [Google Scholar]
- 20.Burkus JK, Harvinder S, Sandhu MF, et al. Use of rhBMP-2 in combination with structural cortical allografts: clinical and radiographic outcomes in anterior lumbar spinal surgery. J Bone Joint Surg Am. 2005;87:1205–12. doi: 10.2106/JBJS.D.02532. [DOI] [PubMed] [Google Scholar]
- 21.Burkus JK, Gornet MF, Schuler TC, et al. Six-year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphgenetic protein-2. J Bone Joint Surg Am. 2009;91:1181–9. doi: 10.2106/JBJS.G.01485. [DOI] [PubMed] [Google Scholar]
- 22.Bono CM, Khanda A, Vadapalli S, et al. Residual sagittal motion after lumbar fusion. Spine. 2007;32:417–22. doi: 10.1097/01.brs.0000255201.74795.20. [DOI] [PubMed] [Google Scholar]
- 23.Burkus JK, Foley K, Haid R, et al. Surgical interbody research group – radiographic assessment of interbody fusion devices: fusion criteria for anterior lumbar interbody surgery. Neurosurg Focus. 2001;10:1–9. doi: 10.3171/foc.2001.10.4.12. [DOI] [PubMed] [Google Scholar]
- 24.Burkus JK, Dorchak JD, Sanders DL. Radiographic assessment of interbody fusion using recombinant human bone morphogenetic protein type 2. Spine. 2003;28:372–7. doi: 10.1097/01.BRS.0000048469.45035.B9. [DOI] [PubMed] [Google Scholar]
- 25.Chafetz N, Cann CE, Morris JM, et al. Pseudarthrosis following lumbar fusion: detection by direct coronal CT scanning. Radiology. 1981;162:803–5. doi: 10.1148/radiology.162.3.3809497. [DOI] [PubMed] [Google Scholar]
- 26.Cook SD, Patron LP, Christakis PM. Comparison of methods for determining the presence and extent of anterior lumbar interbody fusion. Spine. 2004;29:1118–23. doi: 10.1097/00007632-200405150-00013. [DOI] [PubMed] [Google Scholar]
- 27.Larsen JM, Rimoldi RL, Capen DA, et al. Assessment of pseudarthrosis in pedicle screw fusion: a prospective study comparing plain radiographs, flexion/extension radiographs, CT scanning and bone scintigraphy with operative findings. J Spinal Disord Tech. 1996;9:117–20. [PubMed] [Google Scholar]
- 28.Lebhowl NH. Radiographic evaluation of the postoperative interbody fusion patient: is CT the study of choice? Editorial: AJNR Am J Neuroradiol. 2005;26:1885–7. [PMC free article] [PubMed] [Google Scholar]
- 29.McAfee PC. Symposium: A critical discrepancy – A criteria of successful arthrodesis following interbody spinal fusions. Spine. 2001;26:320–34. doi: 10.1097/00007632-200102010-00020. [DOI] [PubMed] [Google Scholar]
- 30.Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 4: radiographic assessment of fusion. J Neurosurg Spine. 2005;2:653–7. doi: 10.3171/spi.2005.2.6.0653. [DOI] [PubMed] [Google Scholar]
- 31.Santos ER, Gross DG, Morcom RK, et al. Radiologic assessment of interbody fusion using carbon fiber cages. Spine. 2003;28:997–1001. doi: 10.1097/01.BRS.0000061988.93175.74. [DOI] [PubMed] [Google Scholar]
- 32.Williams AL, Gornet MF, Burkus JK. CT evaluation of lumbar interbody fusion: current concepts. AJNR Am J Neuroradiol. 2005;26:2057–66. [PMC free article] [PubMed] [Google Scholar]
- 33.Bridwell KH, Lenke LG, McEnery KW, et al. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine. 1995;20:1359–67. [PubMed] [Google Scholar]
- 34.Burkus JK, Heim SE, Gornet MF, et al. Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using LT-CAGE lumbar tapered fusion device. J Spinal Disord Tech. 2003;16:113–22. doi: 10.1097/00024720-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Kim JS, Kang BU, Lee SH, et al. Mini-transoraminal lumbar interbody fusion versus anterior lumbar interbody fusion augmented by percutaneous pedicle screw fixation: a comparison of surgical outcomes in adult low-grade isthmic spondylolisthesis. J Spinal Disord Tech. 2009;22:114–21. doi: 10.1097/BSD.0b013e318169bff5. [DOI] [PubMed] [Google Scholar]
- 36.Pearson AM, Lurie JD, Blood EA, et al. Spine Patient Outcomes Trial: Radiographic predictors of clinical outcomes after operative or non-operative treatment of degenerative spondylolisthesis. Spine. 2008;33:2759–66. doi: 10.1097/BRS.0b013e31818e2d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. New Engl J Med. 2007;356:2257–70. doi: 10.1056/NEJMoa070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. Four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am. 2009;91:1295–304. doi: 10.2106/JBJS.H.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tosteson ANA, Lurie JD, Tosteson TD, Skinner JS, Herkowitz H, Albert T, et al. Surgical treatment of spinal stenosis with and without degenerative spondylolisthesis: Cost-effectiveness after 2 years. Ann Intern Med. 2008;149:845–53. doi: 10.7326/0003-4819-149-12-200812160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ackerman SJ, Mafilios MS, Polly DW. Economic evaluation of bone morphogenetic protein versus autogenous iliac crest bone graft in single-level anterior lumbar fusion. An evidence-based modeling approach. Spine. 2002;27:594–9. doi: 10.1097/00007632-200208151-00017. [DOI] [PubMed] [Google Scholar]
- 41.Urist MR, Silverman BF, Buring K, et al. The bone induction principle. Clin Orthop Relat Res. 1967;53:243–83. [PubMed] [Google Scholar]
- 42.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 43.Frenkel SR, Moskovich R, Spivak J, et al. Demineralized bone matrix. Enhancement of spinal fusion. Spine. 1993;18:1634–9. doi: 10.1097/00007632-199309000-00011. [DOI] [PubMed] [Google Scholar]
- 44.Martin GJ, Jr, Boden SD, Titus L, Scarborough NL. New formulations of demineralized bone matrix as a more effective graft alternative in experimental posterolateral lumbar spine arthrodesis. Spine. 1999;24:637–45. doi: 10.1097/00007632-199904010-00005. [DOI] [PubMed] [Google Scholar]
- 45.Cammisa FP, Jr, Lowery G, Garfin SR, et al. Two-year fusion rate equivalency between Grafton DBM gel and autograft in posterolateral spine fusion: a prospective controlled trial employing a side-by-side comparison in the same patient. Spine. 2004;29:660–6. doi: 10.1097/01.brs.0000116588.17129.b9. [DOI] [PubMed] [Google Scholar]