Abstract
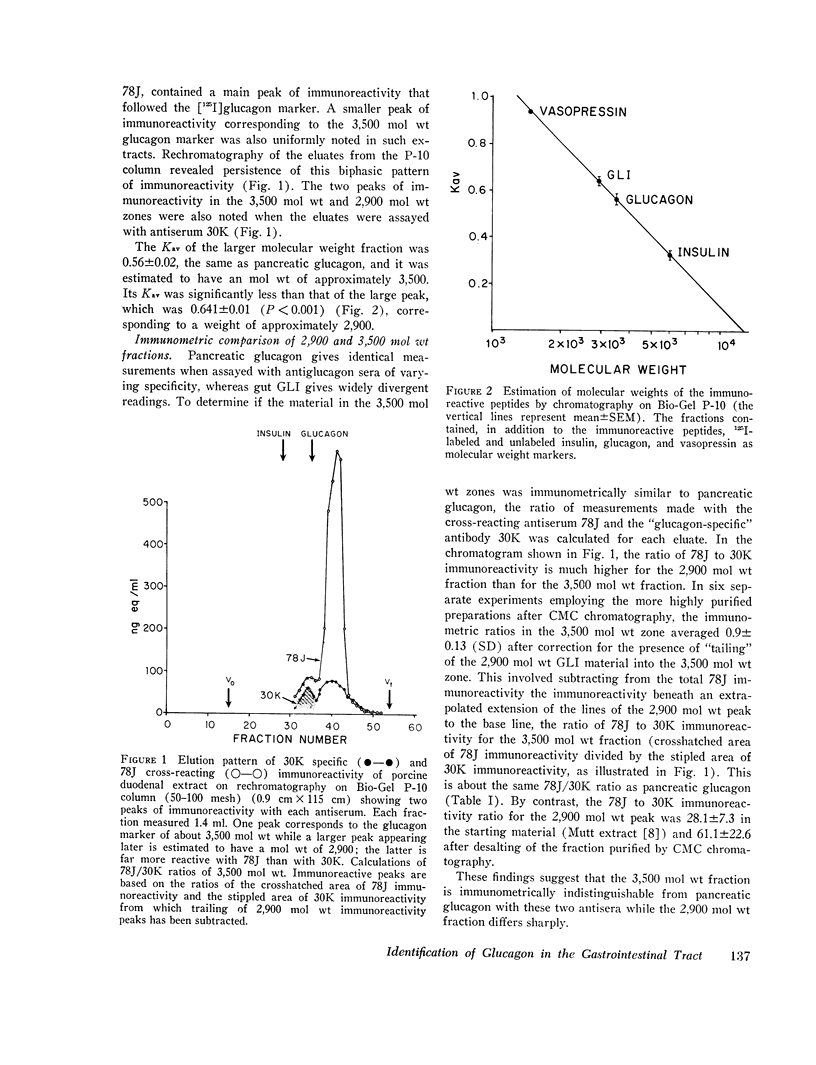
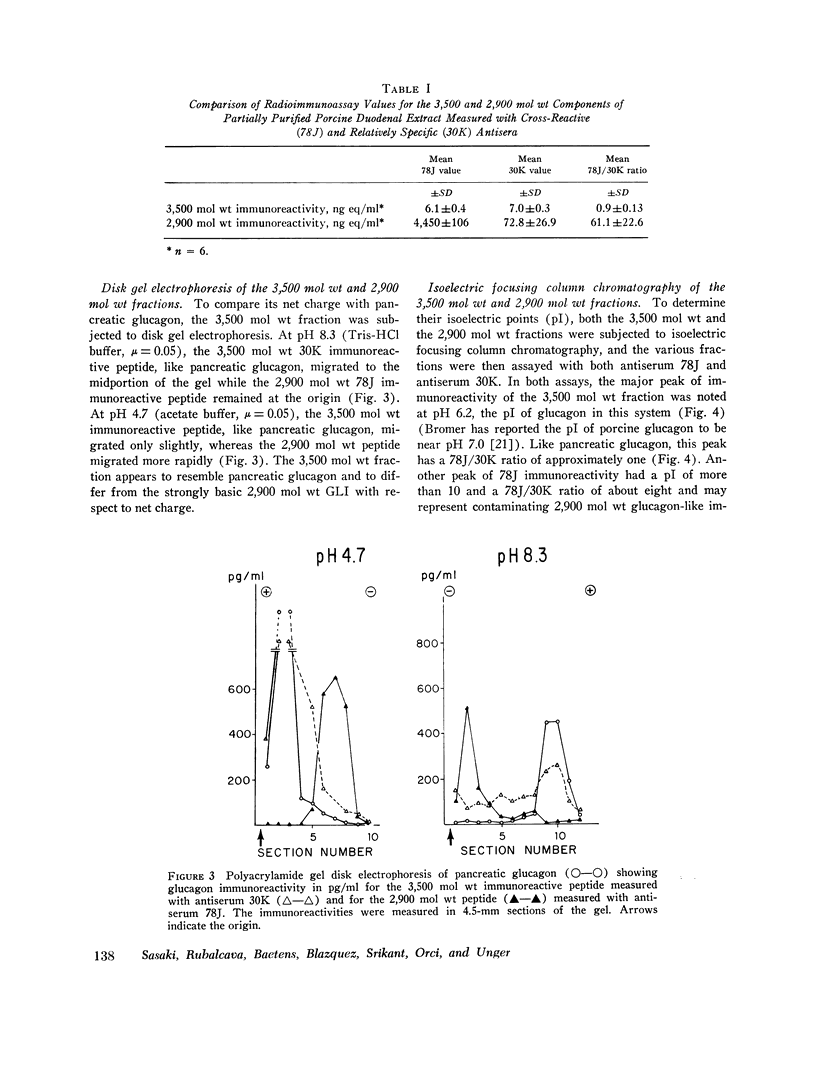
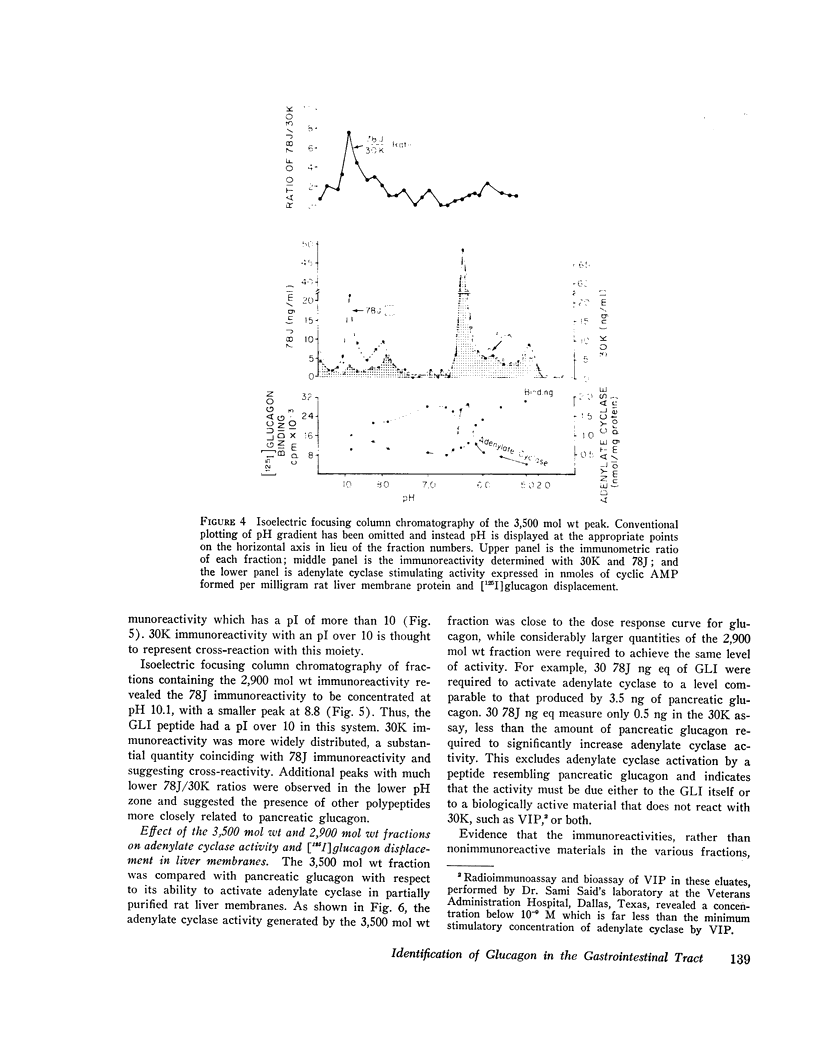
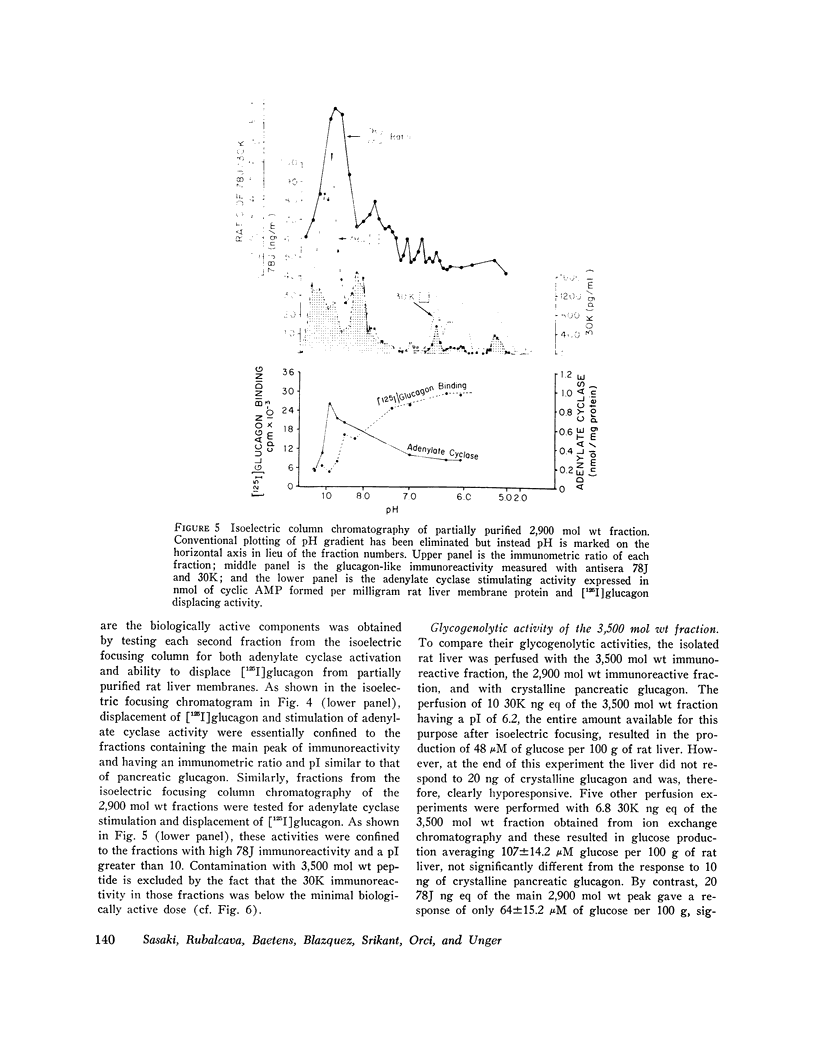
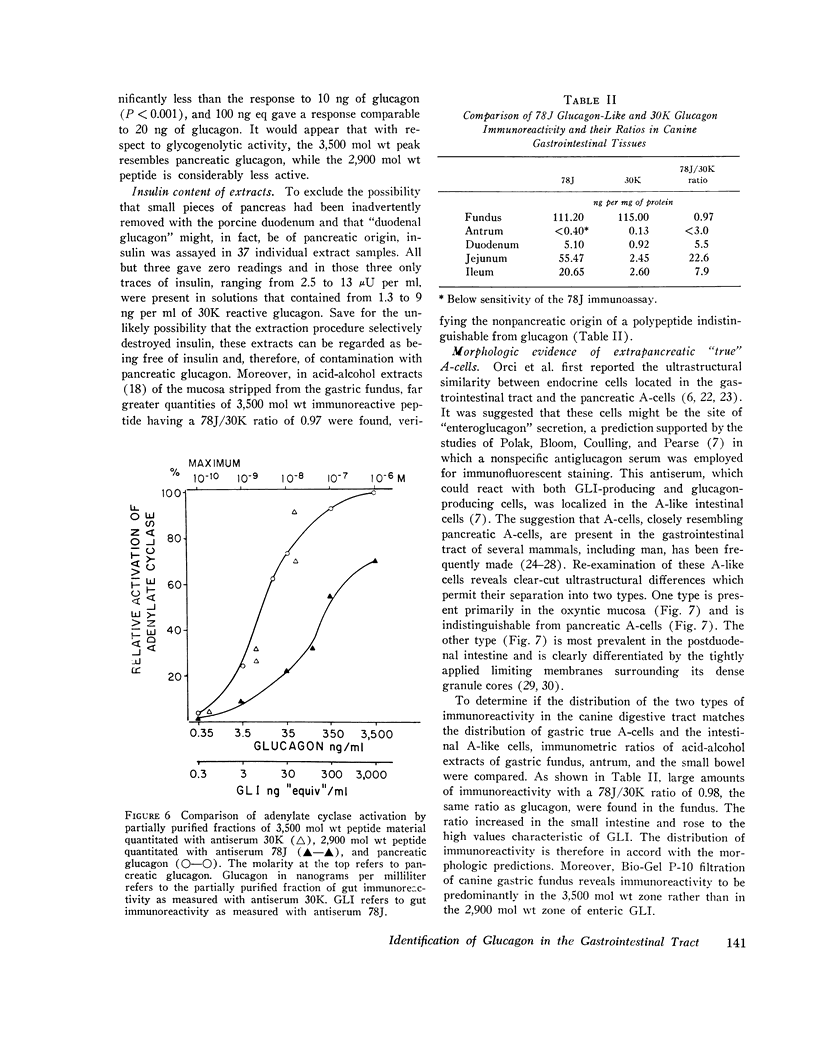
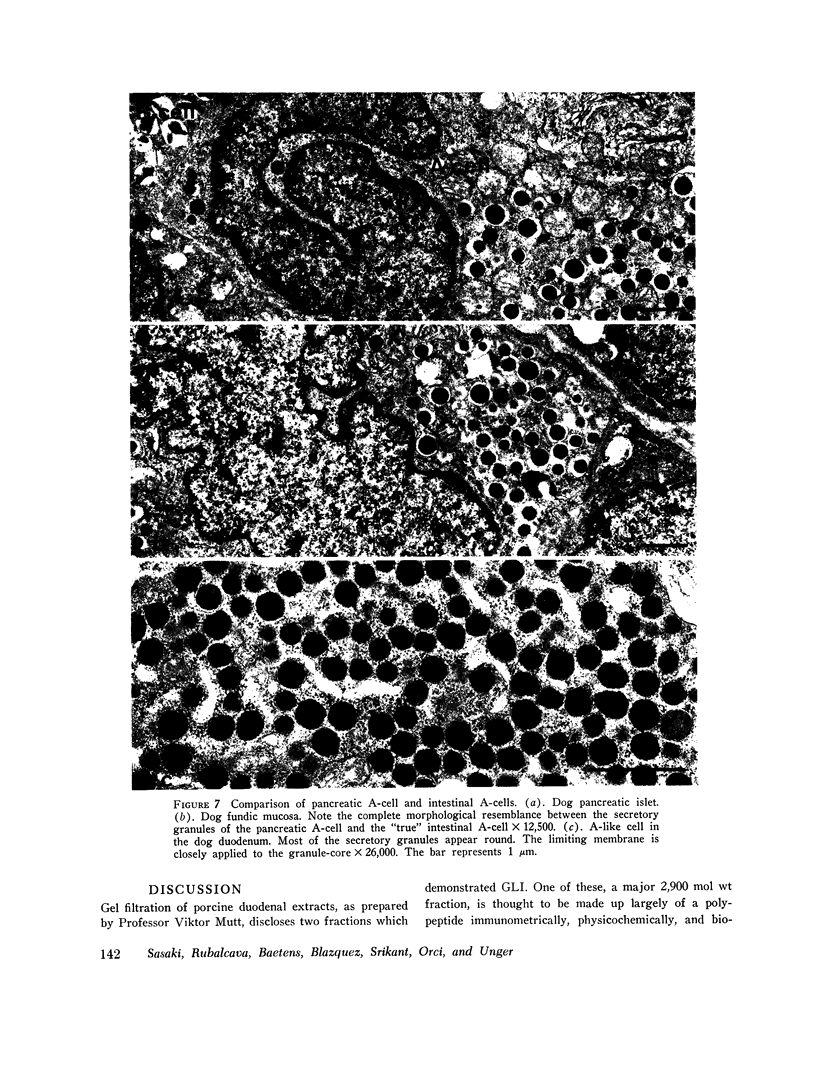
Gel filtration studies on Bio-Gel P-10 columns of a 50-fold purified porcine duodenal extract revealed a main peak of glucagon-like immunoreactivity (GLI) in the 2,900 mol wt zone and a smaller peak in the 3,500 mol wt zone, the same zone as the pancreatic glucagon marker. Like pancreatic glucagon, samples of 3,500 mol wt material gave essentially identical measurements in radioimmunoassays employing the pancreatic glucagon-specific antiserum 30K and the GLI crossreacting antiserum 78J, whereas the 2,900 mol wt peptide gave 60-fold higher readings in the 78J assay. On disk gel electrophoresis, the 3,500 mol wt fraction, like pancreatic glucagon, migrated at pH 8.3, whereas the 2,900 mol wt peptide remained at the origin; at pH 4.7, the 2,900 mol wt peptide migrated while the 3,500 mol wt immunoreactive peptide and glucagon remained at the origin. Isoelectric focusing revealed the 3,500 mol wt moiety to have an isoelectric point (pI) of 6.2, the same as pancreatic glucagon, whereas the 2,900 mol wt peptide had an pI greater than 10. The glycogenolytic activity of the 3,500 mol wt peptide in the perfused rat liver did not differ significantly from glucagon, and its adenylate cyclase stimulating activity in partially purified liver cell membranes was comparable to that of glucagon; the 2,900 mol wt peptide had less than 20% of these activities. In samples of 3,500 mol wt material subjected to isoelectric focusing, adenylate cyclase-stimulating activity was confirmed to fractions containing 30K immunoreactivity with a pI of 6.2. In samples of 2,900 mol wt material subjected to isoelectric focusing, adenylate cyclase-stimulating activity was confined to fractions containing 78J immunoreactivity with an pI greater than 10. Displacement of [125-I]glucagon from the membranes was limited to these two biologically active fractions. However, the affinity of both pancreatic glucagon and the 3,500 mol wt peptide was an order of magnitude greater than of the 2,900 mol wt peptide. Thus, by all of several biologic, physiocochemical, and immunometric techniques, the 3,500 mol wt gut immunoreactive peptide could not be distinguished from pancreatic glucagon, while the 2,900 mol wt peptide was readily differentiated by all these techniques. "True" A-cells, ultrastructurally indistinguishable from pancreatic A-cells but differing from the A-like cells of the lower bowel, were identified in the gastric fundus of dogs. Their distribution corresponded to that of the 3,500 mol wt immunoreactivity resembling pancreatic glucagon, while the distribution of "A-like cells" in the lower small intestine corresponded to that of GLI.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar-Parada E., Eisentraut A. M., Unger R. H. Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci. 1969 Jun;257(6):415–419. doi: 10.1097/00000441-196906000-00008. [DOI] [PubMed] [Google Scholar]
- Alberti K. G., Christensen N. J., Christensen S. E., Hansen A. P., Iversen J., Lundbaek K., Seyer-Hansen K., Orskov H. Inhibition of insulin secretion by somatostatin. Lancet. 1973 Dec 8;2(7841):1299–1301. doi: 10.1016/s0140-6736(73)92873-0. [DOI] [PubMed] [Google Scholar]
- Bataille D. P., Freychet P., Kitabgi P. E., Rosselin G. E. Gut glucagon: A common receptor site with pancreatic glucagon in liver cell plasma membranes. FEBS Lett. 1973 Mar 1;30(2):215–218. doi: 10.1016/0014-5793(73)80654-4. [DOI] [PubMed] [Google Scholar]
- Bataille D., Freychet P., Rosselin G. Interactions of glucagon, gut glucagon, vasoactive intestinal polypeptide and secretin with liver and fat cell plasma membranes: binding to specific sites and stimulation of adenylate cyclase. Endocrinology. 1974 Sep;95(3):713–721. doi: 10.1210/endo-95-3-713. [DOI] [PubMed] [Google Scholar]
- Braaten J. T., Faloona G. R., Unger R. H. The effect of insulin on the alpha-cell response to hyperglycemia in long-standing alloxan diabetes. J Clin Invest. 1974 Apr;53(4):1017–1021. doi: 10.1172/JCI107638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolati G., Capella C., Solcia E., Vassallo G., Vezzadini P. Ultrastructural and immunofluorescent investigations on the secretin cell in the dog intestinal mucosa. Histochemie. 1971;26(3):218–227. doi: 10.1007/BF00305655. [DOI] [PubMed] [Google Scholar]
- Böttger I., Faloona G. R., Unger R. H. The effect of calcium and other salts upon the release of glucagon-like immunoreactivity from the gut. J Clin Invest. 1972 Apr;51(4):831–836. doi: 10.1172/JCI106878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dobbs R., Sakurai H., Sasaki H., Faloona G., Valverde I., Baetens D., Orci L., Unger R. Glucagon: role in the hyperglycemia of diabetes mellitus. Science. 1975 Feb 14;187(4176):544–547. doi: 10.1126/science.1089999. [DOI] [PubMed] [Google Scholar]
- Frankel B. J., Gerich J. E., Hagura R., Fanska R. E., Gerritsen G. C., Grodsky G. M. Abnormal secretion of insulin and glucagon by the in vitro perfused pancreas of the genetically diabetic Chinese hamster. J Clin Invest. 1974 Jun;53(6):1637–1646. doi: 10.1172/JCI107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- KENNY A. J. Extractable glucagon of the human pancreas. J Clin Endocrinol Metab. 1955 Sep;15(9):1089–1105. doi: 10.1210/jcem-15-9-1089. [DOI] [PubMed] [Google Scholar]
- Katsilambros N., Rahman Y. A., Hinz M., Fussgänger R., Schröder K. E., Straub K., Pfeiffer E. F. Action of streptozotocin on insulin and glucagon responses of rat islets. Horm Metab Res. 1970 Sep;2(5):268–270. doi: 10.1055/s-0028-1095056. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiter K., Harding P. E., Chou M., Mashiter G. D., Stout J., Diamond D., Field J. B. Persistent pancreatic glucagon but not insulin response to arginine in pancreatectomized dogs. Endocrinology. 1975 Mar;96(3):678–693. doi: 10.1210/endo-96-3-678. [DOI] [PubMed] [Google Scholar]
- Matsuyama T., Foà P. P. Plasma glucose, insulin, pancreatic, and enteroglucagon levels in normal and depancreatized dogs. Proc Soc Exp Biol Med. 1974 Oct;147(1):97–102. doi: 10.3181/00379727-147-38288. [DOI] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Aguilar-Parada E., Unger R. H. Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med. 1970 Jul 16;283(3):109–115. doi: 10.1056/NEJM197007162830301. [DOI] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Unger R. H. The effect of experimental insulin deficiency on glucagon secretion. J Clin Invest. 1971 Sep;50(9):1992–1999. doi: 10.1172/JCI106691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr Isolation of an organ specific protein antigen from cell-surface membrane of rat liver. Biochim Biophys Acta. 1968 Apr 9;154(3):540–552. doi: 10.1016/0005-2795(68)90014-7. [DOI] [PubMed] [Google Scholar]
- Orci L., Forssmann W. G., Rouiller C. Zur Ultrastruktur der endokrinen Zellen im Epithel des Magendarmtraktes. Verh Anat Ges. 1969;63:187–194. [PubMed] [Google Scholar]
- Orci L., Pictet R., Forssmann W. G., Renold A. E., Rouiller C. Structural evidence for glucagon producing cells in the intestinal mucosa of the rat. Diabetologia. 1968 Jan;4(1):56–67. doi: 10.1007/BF01241034. [DOI] [PubMed] [Google Scholar]
- Polak J. M., Bloom S., Coulling I., Pearse A. G. Immunofluorescent localization of enteroglucagon cells in the gastrointestinal tract of the dog. Gut. 1971 Apr;12(4):311–318. doi: 10.1136/gut.12.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J. B., Jr, Takeshige K., Max M. H., Voorhees A. B., Jr Glucagon as the portal factor modifying hepatic regeneration. Surgery. 1972 Jul;72(1):74–82. [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld J. F., Heding L. G. Increased release of gut glucagon in reactive hypoglycaemia. Br Med J. 1970 Jun 20;2(5711):706–707. doi: 10.1136/bmj.2.5711.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubalcava B., Rodbell M. The role of acidic phospholipids in glucagon action on rat liver adenylate cyclase. J Biol Chem. 1973 Jun 10;248(11):3831–3837. [PubMed] [Google Scholar]
- Sakurai H., Dobbs R., Unger R. H. Somatostatin-induced changes in insulin and glucagon secretion in normal and diabetic dogs. J Clin Invest. 1974 Dec;54(6):1395–1402. doi: 10.1172/JCI107886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Aguilar-Parada E., Müller W. A., Eisentraut A. M. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970 Apr;49(4):837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975 Jan 4;1(7897):14–16. doi: 10.1016/s0140-6736(75)92375-2. [DOI] [PubMed] [Google Scholar]
- Valverde I., Rigopoulou D., Marco J., Faloona G. R., Unger R. H. Characterization of glucagon-like immunoreactivity (GLI). Diabetes. 1970 Sep;19(9):614–623. doi: 10.2337/diab.19.9.614. [DOI] [PubMed] [Google Scholar]
- Vranic M., Pek S., Kawamori R. Increased "glucagon immunoreactivity" in plasma of totally depancreatized dogs. Diabetes. 1974 Nov;23(11):905–912. doi: 10.2337/diab.23.11.905. [DOI] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]