Abstract
Background
Cervical total disk replacement (TDR) is intended to address pain and preserve motion between vertebral bodies in patients with symptomatic cervical disk disease. Two-year follow-up for the ProDisc-C (Synthes USA Products, LLC, West Chester, Pennsylvania) TDR clinical trial showed non-inferiority versus anterior cervical discectomy and fusion (ACDF), showing superiority in many clinical outcomes. We present the 4-year interim follow-up results.
Methods
Patients were randomized (1:1) to ProDisc-C (PDC-R) or ACDF. Patients were assessed preoperatively, and postoperatively at 6 weeks and 3, 6, 12, 18, 24, 36, and 48 months. After the randomized portion, continued access (CA) patients also underwent ProDisc-C implantation, with follow-up visits up to 24 months. Evaluations included Neck Disability Index (NDI), Visual Analog Scale (VAS) for pain/satisfaction, and radiographic and physical/neurologic examinations.
Results
Randomized patients (103 PDC-R and 106 ACDF) and 136 CA patients were treated at 13 sites. VAS pain and NDI score improvements from baseline were significant for all patients (P < .0001) but did not differ among groups. VAS satisfaction was higher at all time points for PDC-R versus ACDF patients (P = .0499 at 48 months). The percentage of patients who responded yes to surgery again was 85.6% at 24 months and 88.9% at 48 months in the PDC-R group, 80.9% at 24 months and 81.0% at 48 months in the ACDF group, and 86.3% at 24 months in the CA group. Five PDC-R patients (48 months) and no CA patients (24 months) had index-level bridging bone. By 48 months, approximately 4-fold more ACDF patients required secondary surgery (3 of 103 PDC-R patients [2.9%] vs 12 of 106 ACDF patients [11.3%], P = .0292). Of these, 6 ACDF patients (5.6%) required procedures at adjacent levels. Three CA patients required secondary procedures (24 months).
Conclusions
Our 4-year data support that ProDisc-C TDR and ACDF are viable surgical options for symptomatic cervical disk disease. Although ACDF patients may be at higher risk for additional surgical intervention, patients in both groups show good clinical results at longer-term follow-up.
Keywords: Cervical arthroplasty, Symptomatic cervical disc disease, Total disc replacement
Since 2007, 3 cervical total disk replacement (TDR) prostheses have been approved by the Food and Drug Administration (FDA) for marketing in the United States. As part of their application to the FDA, these devices underwent an Investigational Device Exemption clinical trial and 2-year follow-up results were presented.1–3 All 3 studies showed non-inferiority of the investigational device compared with anterior cervical discectomy and fusion (ACDF). The ProDisc-C (Synthes USA Products, LLC, West Chester, Pennsylvania) TDR was also able to prove superiority in many clinical outcomes in comparison to ACDF.1 However, there was still a significant concern in the medical community regarding performance over the longer term and whether these areas of superiority compared with ACDF would bear out over time.
Widely accepted as one of the most successful spine procedures performed today, ACDF was first described by Smith and Robinson4 in 1958 and remained largely unchanged until the 1990s, when anterior cervical plates were introduced. However, there has been increased reporting of adjacent segment degeneration ranging from 2.9% to 6.9% per year as a consequence of ACDF5–10 despite reports of high fusion rates.5, 11–13 It has been shown in biomechanical in vitro studies that fusion causes increased stress or motion at the adjacent levels,14–16 and it has been hypothesized that this may contribute to adjacent segment breakdown by changing segmental motion and increasing strains in the intervertebral disk adjacent to fusion.16 Long-term data will show whether substituting arthroplasty for fusion will reduce the incidence of adjacent segment degeneration.
The purpose of this report is to present the 4-year follow-up results of the ProDisc-C TDR clinical trial.
Materials and methods
Complete methodology of this prospective, randomized, controlled, multicenter trial was previously described by Murrey et al.1 In brief, patients with symptomatic cervical disk disease who were unresponsive to nonoperative treatments for at least 6 weeks, met the inclusion/exclusion criteria, and had signed informed consent forms were randomized in a 1:1 ratio to ProDisc-C (PDC-R) or ACDF. Patients remained blinded to randomization until immediately after surgery. After enrollment of the randomized portion of the clinical trial was completed, the FDA allowed the study investigators to continue to implant the investigational device in patients who met the original study criteria until study approval. These patients were termed continued access (CA).
Patients were evaluated preoperatively and postoperatively at 6 weeks and 3, 6, 12, 18, 24, 36, and 48 months. CA patients had only reached the 24-month follow-up time point at the time of this report. Patient self-assessments included Neck Disability Index (NDI) questionnaire, Short Form 36 (SF-36), neck and arm pain intensity on a 100-mm visual analog scale (VAS), and VAS for patient satisfaction. Physical and neurologic examinations included root tension, reflexes, muscle strength, and sensory deficits. Radio-graphic evaluation consisted of anteroposterior and lateral standing, flexion, and extension films. Radiographic analysis of range of motion (ROM) was measured by an independent third party (Medical Metrics Inc., Houston, Texas).
For between-treatment group comparisons of continuous measurements such as NDI, VAS for pain, and SF-36 scores, the Wilcoxon rank sum test was used. To compare the mean improvement from baseline within the treatment groups for the patient self-assessment data at each follow-up visit, paired t tests were performed. The Fisher's exact test was used to compare success rates between treatment groups such as neurologic success and the percentage of patients indicating that they would have the surgery again.
Results
From August 2003 to October 2004, 209 randomized patients (103 PDC-R and 106 ACDF) had surgery at 13 investigational sites across the United States and are continuing to be followed up for 7 years. After closure of randomized enrollment in 2004, an additional series of 136 CA patients had ProDisc-C TDR surgery from March 2005 to January 2008. Follow-up rates at 24 months were 98.0% for PDC-R, 94.8% for ACDF, and 77.4% for CA. At the time of this publication, the follow-up rates for PDC-R and ACDF were 63.0% and 46.2%, respectively, at 48 months. Overall patient demographics showed no difference between randomized and CA cohorts of ProDisc-C-treated or ACDF-treated patients (Table 1). Operative time (PDC-R, 107.4 minutes; ACDF, 98.7 minutes; and CA, 108.8 minutes) and estimated blood loss (PDC-R, 83.5 mL; ACDF, 63.5 mL; and CA, 84.2 mL) were statistically lower for ACDF compared with PDC-R patients (P = .0063 and P = .0094, respectively). There was no difference between PDC-R or ACDF patients and CA patients in operative time or blood loss.
Table 1.
Patient demographics and intraoperative data.
| Variable | ACDF (n = 106) | ProDisc-C (PDC-R): (n = 103) | ProDisc-C (CA): (n = 136) | P value* for PDC-R vs ACDF |
|---|---|---|---|---|
| Patient demographics | ||||
| Gender [n (%)] | .89 | |||
| Male | 57 (53.8%) | 57 (55.3%) | 58 (42.7%) | |
| Female | 49 (46.2%) | 46 (44.7%) | 78 (57.4%) | |
| Age (years) | .20 | |||
| n | 106 | 103 | 134 | |
| Mean (SD) | 43.5 (7.2) | 42.1 (8.4) | 43.5 (8.0) | |
| Race [n (%)] | .10 | |||
| White | 88 (85.4%) | 97 (91.5%) | 125 (92.6%) | |
| African American | 4 (3.9%) | 1 (0.9%) | 0 (0%) | |
| Hispanic | 3 (2.9%) | 5 (4.7%) | 1 (0.7%) | |
| Asian | 5 (4.9%) | 0 (0%) | 5 (3.7%) | |
| Other | 3 (2.9%) | 3 (2.8%) | 4 (3.0%) | |
| Body mass index | .09 | |||
| n | 106 | 103 | 135 | |
| Mean (SD) (kg/m2) | 27.3 (5.5) | 26.4 (5.3) | 26.7 (5.1) | |
| Smoking status | .88 | |||
| Former | 20 (22.5%) | 18 (20.0%) | 30 (24.2%) | |
| Current | 37 (34.9%) | 34 (33.0%) | 27 (19.9%) | |
| Intraoperative data | ||||
| Implant level | ||||
| C3-C4 | 1 (0.09%) | 3 (2.9%) | 4 (2.9%) | .48 |
| C4-C5 | 6 (5.7%) | 10 (9.7%) | 14 (10.3%) | |
| C5-C6 | 61 (57.5%) | 58 (56.3%) | 82 (60.3%) | |
| C6-C7 | 38 (35.8%) | 32 (31.1%) | 36 (26.5%) | |
| Intraoperative time | .0063 | |||
| n | 106 | 103 | 135 | |
| Mean (SD) (minutes) | 98.7 (47.0) | 107.4 (35.6) | 108.8 (48.6) | |
| Estimated blood loss | .009 | |||
| N | 105 | 103 | 135 | |
| Mean (SD) (mL) | 63.5 (50.4) | 83.5 (64.9) | 84.2 (84.6) |
SD = standard deviation.
Continuous variables were analyzed by the Wilcoxon rank sum test; categorical variables were analyzed using Fisher's exact test.
Neurologic success
Neurologic success was defined as maintenance or improvement in each of the neurologic evaluations including sensory, motor, and reflex functions. At 24 months, the neurologic success rate was not different among the 3 groups (PDC-R, 90.9%; ACDF, 88.0%; and CA, 94.3%). Both the PDC-R and ACDF groups were able to maintain neurologic success levels from 24 to 48 months, because 24-and 48-month follow-up values were not statistically different. At 48 months, the overall neurologic success rate trended toward significance for PDC-R patients (88.9%) compared with ACDF patients (74.4%) (P = .0665).
Neck Disability Index
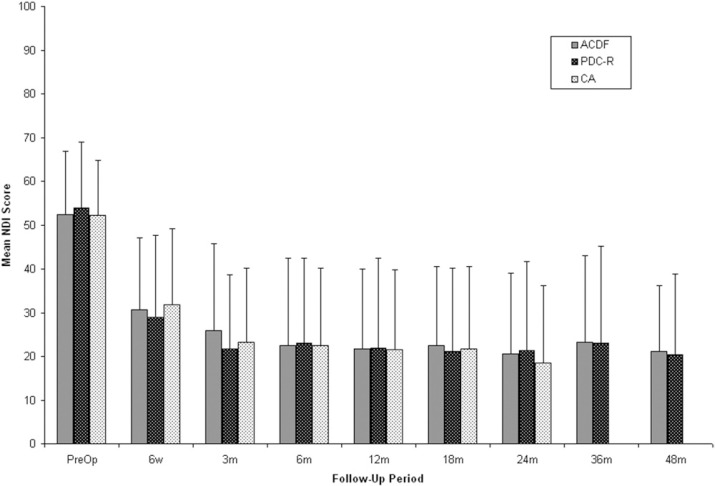
Preoperative NDI scores were not different between groups (PDC-R, 53.9 ± 15.1; ACDF, 52.2 ± 14.5; andCA, 52.1 ± 12.7). Regardless of treatment, all patients showed statistically significant improvement in NDI scores at all follow-up periods compared with baseline (P < .0001) (Fig. 1). At 24 months, there was no significant difference seen between groups. The mean NDI score was 21.4 ± 20.3 for PDC-R patients, 20.6 ± 18.4 for ACDF patients, and 18.5 ± 17.7 for CA patients. These scores represent a 60.3%, 60.6%, and 64.5% improvement from baseline, respectively. At 48 months, the mean NDI score was 20.3 ± 18.6 for PDC-R patients and 21.2 ± 14.9 for ACDF patients, a 62.3% and 59.5% improvement from baseline, respectively.
Fig. 1.
Mean Neck Disability Index (NDI) scores for anterior cervical discectomy and fusion (ACDF), ProDisc-C randomized (PDC-R), and ProDisc-C continued access (CA) patients over time. CA patients were followed out to 24 months. Error bars represent standard deviation.
VAS for neck and arm pain
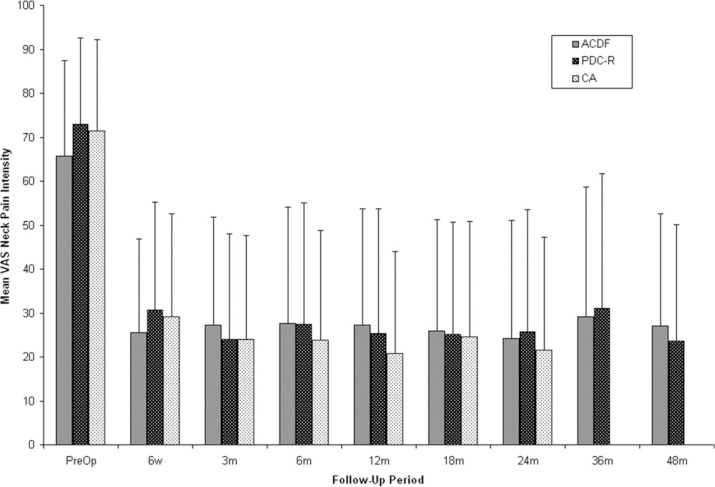
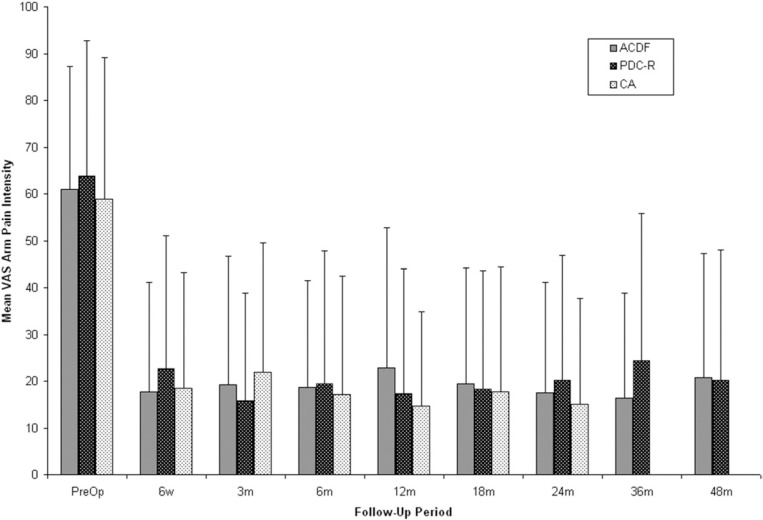
VAS neck and arm pain intensity assessments indicated statistically significant improvement from preoperative scores regardless of treatment (P < .0001) (Figs. 2 and 3). At 24 months, mean VAS neck pain intensity scores were reduced by 47.3 mm in the PDC-R group, 41.5 mm in the ACDF group, and 49.8 mm in the CA group. At 48 months, the ACDF group showed only a 38.7 mm reduction in mean VAS score from preoperative levels compared with 49.3 mm in the PDC-R group, although this difference was not statistically significant. At 24 months, a reduction of 43.7 mm was observed in mean VAS arm pain intensity scores in the PDC-R group, 43.4 mm in the ACDF group, and 43.8 mm in the CA group. At 48 months, the PDC-R group maintained a 43.8-mm reduction in mean VAS score, whereas ACDF patients showed a 40.2-mm reduction in mean VAS score compared with preoperative score.
Fig. 2.
Mean Visual Analog Scale (VAS) neck pain intensity scores for anterior cervical discectomy and fusion (ACDF), ProDisc-C randomized (PDC-R), and ProDisc-C continued access (CA) patients over time. CA patients were followed out to 24 months. Error bars represent standard deviation.
Fig. 3.
Mean Visual Analog Scale (VAS) arm pain intensity scores for anterior cervical discectomy and fusion (ACDF), ProDisc-C randomized (PDC-R), and ProDisc-C continued access (CA) patients over time. CA patients were followed out to 24 months. Error bars represent standard deviation.
Short Form 36
Regardless of treatment and at all time points, there was a statistically significant improvement in SF-36 scores from baseline (P < .0016). At 24 months, SF-36 physical component scores improved in 83.8% of PDC-R patients, 84.4% of ACDF patients, and 84.6% of CA patients. At 48 months, 87.1% of PDC-R patients and 83.3% of ACDF patients showed improvement. At 24 months, SF-36 mental component scores improved in 75.8% of PDC-R patients, 80.0% of ACDF patients, and 76.0% of CA patients. At 48 months, improvement was seen in 80.6% of patients in the PDC-R group whereas only 73.8% of ACDF patients showed improvement.
Patient satisfaction and surgery again
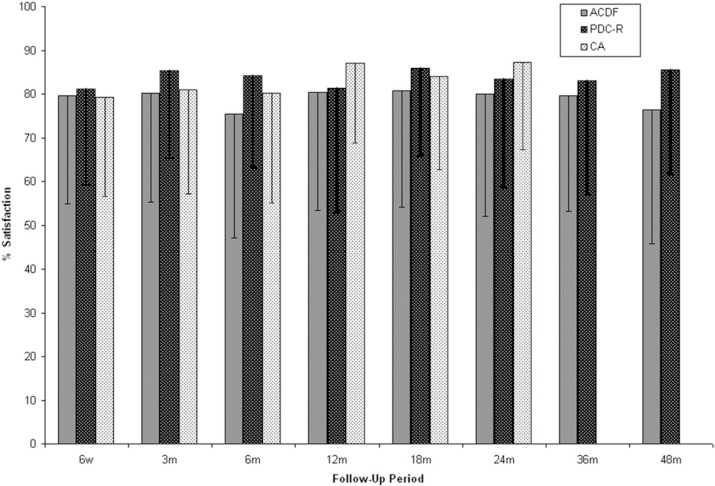
VAS patient satisfaction (Fig. 4) was higher at all time points for PDC-R patients compared with the ACDF group. At 24 months, the mean satisfaction score was 83.4 ± 24.8 mm for PDC-R patients, 80.0 ± 28.0 mm for ACDF patients, and 87.3 ± 20.0 mm for CA patients. At 48 months, there was a statistically significant difference in the mean satisfaction score in PDC-R patients (85.5 ± 23.7 mm) compared with ACDF patients (76.4 ± 30.6 mm) (P = .0499). The percentage of patients considered very to completely satisfied (60-100 mm) was 86.3% in the PDC-R group, 83.0% in the ACDF group, and 90.5% in the CA group. At 48 months, the percentage of patients considered very to completely satisfied (60–100 mm) stayed consistent for PDC-R patients (85.7%) while dropping in the ACDF group (76.2%).
Fig. 4.
Mean Visual Analog Scale (VAS) patient satisfaction scores for anterior cervical discectomy and fusion (ACDF), ProDisc-C randomized (PDC-R), and ProDisc-C continued access (CA) patients over time. CA patients were followed out to 24 months. Error bars represent standard deviation.
Patients were asked whether they would have the same surgical treatment again. At 24 months, 85.6% of PDC-R patients, 80.9% of ACDF patients, and 86.3% of CA patients responded yes, they would have the same surgery again. At 48 months, 88.9% of PDC-R and 81.0% of ACDF patients responded yes.
Radiography
At 24 months, flexion-extension ROM at the index level averaged 9.38° ± 5.97° for PDC-R patients and 9.50° ± 5.15°for CA patients. At 48 months, flexion-extension ROM was maintained in PDC-R patients (9.12° ± 6.06°). By 24 months, 3 cases of bridging bone were identified at the index level in the PDC-R group. Between 24 and 48 months, an additional 2 cases of bridging bone were identified in the PDC-R group. No cases of bridging bone were observed in the CA group at up to 24 months.
At 24 months, 91.2% of the ACDF patients had <2° ROM; by 48 months, 95.5% of the ACDF group had <2° ROM
Secondary surgical procedures were defined as any reoperation, revision, supplemental fixation, or removal of the implant. By 24 months, of the patients originally enrolled in the study, 2 (1.9%) in the PDC-R group and 9 (8.5%) in the ACDF group required a secondary surgical procedure. From 24 to 48 months, 1 additional PDC-R patient and 3 additional ACDF patients required secondary surgery. By 48 months, 2.9% of PDC-R patients and 11.3% of ACDF patients had required a secondary surgical procedure—an approximately 4-fold difference (P = .0292).
The 3 PDC-R patients reported ongoing pain and were converted to fusion. One patient reported worsening pain; the TDR was removed and the level converted to a fusion. The second case involved removal of the TDR and revision to a 2-level ACDF because of return of nonspecific neck pain. The third patient underwent a posterior decompression with supplemental fixation at C6-T1; the TDR was left intact.
Of the 12 secondary surgeries that occurred in the ACDF group, 6 included an adjacent level in addition to the index level. The primary reason for revision at the index level was pseudarthrosis; 1 patient underwent revision because of dysphagia associated with plate liftoff, and 1 patient underwent posterior decompression for foraminal stenosis. Of the 6 patients (5.6%) who required procedures at the adjacent level, an ACDF was performed at 1 adjacent level in 3 patients and at both adjacent levels in 3 patients.
In the CA group, 3 patients required a secondary surgical procedure by the 24-month follow-up time point. In 1 case the implant was repositioned 4 days after surgery. Increasing radiculopathy developed in 1 patient due to hypermobility at the index level, and this patient underwent revision to anterior fusion. One patient reported increasing trapezial and arm pain; the implant was removed, and the index and adjacent levels were converted to anterior fusion.
Discussion
Although a hypothesized benefit of TDR surgery is the reduction of adjacent segment disease alone, this prospective study shows a reduction in the overall rate of secondary surgical interventions at both the index and adjacent levels. A 4-fold increase in reoperation rates at the index and adjacent levels for ACDF-treated patients compared with PDC-R-treated patients was shown in these longer-term results. The reoperations in the ACDF group were mainly for pseudarthrosis at the index level or the development of symptomatic degeneration at an adjacent level. By 48 months, 5.6% of ACDF patients required further surgery at an adjacent level. This rate was higher than the 1.9% rate observed in the PDC-R group. Though preliminary, these results indicate that TDR does slow the rate of adjacent-level disease.
The rate of reoperation for symptomatic adjacent segment disease after cervical fusion was 1.4% per year in this study, slightly lower than the 1.5% to 4% rate reported in the literature.7 However, it must be noted that the patients enrolled in this study were limited to single-level disease, whereas the large patient series reported by Hilibrand et al6 and Goffin et al17 include a substantial number of multilevel procedures. Many clinical series have established that patients with multilevel cervical fusions have a higher incidence of adjacent-level disease progression when compared with single-level fusions.
In the PDC-R group, radiographic evaluation showed 5 cases of bridging bone. Three of these cases were observed at 12 to 18 months follow-up and two were noted at the 48-month follow-up. This radiographic finding was asymptomatic in these 5 patients. Extensive analysis has shown that in none of these cases was there any correlation between the development of bridging bone and an effect on clinical outcomes compared with the remainder of the PDC-R group. There were no cases of bridging bone reported in the CA group. This difference is likely because of greater attention to soft-tissue handling, endplate preparation, controlling bleeding, and use of bone wax. Nonsteroidal anti-inflammatory drugs were not required in this study, and most investigators did not administer them.
With any new technology, there are always the questions of how steep the learning curve is and how experience affects patient outcomes. In this study the clinical outcomes of the randomized and CA ProDisc-C patients observed up to 24 months were comparable. Given the relatively straightforward surgical technique, it appears that a learning curve does not play a factor and patient outcomes are not affected.
This report is limited by the lower patient accountability at 48 months compared with 24 months. This is a continuing study, and follow-up is ongoing. Nevertheless, these preliminary data at 4 years show that both TDR and ACDF are viable surgical options for patients with symptomatic cervical disk disease. Although it appears that ACDF patients are at higher risk for having an additional surgical intervention at the index level or an adjacent level, patients in both groups continue to show good clinical results at longer-term follow-up.
Acknowledgement
The authors thank Johanna Hantel, Synthes USA Products, LLC, for her editorial collaboration, and Jeff Stein, PhD, Synthes USA Products, LLC, for his assistance with the statistical analysis.
References
- 1.Murrey D, Janssen M, Delamarter R, et al. Results of the prospective, randomized, controlled multicenter Food and Drug Administration investigational device exemption study of the ProDisc-C total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomatic cervical disc disease. Spine J. 2009;9:275–86. doi: 10.1016/j.spinee.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Mummaneni PV, Burkus JK, Haid RW, Traynelis VC, Zdeblick TA. Clinical and radiographic analysis of cervical disc arthroplasty com pared with allograft fusion: a randomized controlled clinical trial. J Neurosurg Spine. 2007;6:198–209. doi: 10.3171/spi.2007.6.3.198. [DOI] [PubMed] [Google Scholar]
- 3.Sasso RC, Smucker JD, Hacker RJ, Heller JG. Artificial disc versus fusion: a prospective, randomized study with 2-year follow-up on 99 patients. Spine. 2007;32:2933–40. doi: 10.1097/BRS.0b013e31815d0034. [DOI] [PubMed] [Google Scholar]
- 4.Smith GW, Robinson RA. The treatment of certain cervical spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am. 1958;40:607–24. [PubMed] [Google Scholar]
- 5.Yue WM, Brodner W, Highland TR. Long-term results after anterior cervical discectomy and fusion with allograft and plating: a 5- to 11-year radiologic and clinical follow-up study. Spine. 2005;30:2138–44. doi: 10.1097/01.brs.0000180479.63092.17. [DOI] [PubMed] [Google Scholar]
- 6.Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519–28. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Hilibrand AS, Robbins BA. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J. 2004;4:S190–4. doi: 10.1016/j.spinee.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Gore DR, Sepic SB. Anterior cervical fusion for degenerated or protruded discs: a review of one hundred forty-six patients. Spine. 1984;9:667–71. doi: 10.1097/00007632-198410000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Robertson JT, Papadopoulos SM, Traynelis VC. Assessment of adjacent-segment disease in patients treated with cervical fusion or arthroplasty: a prospective 2-year study. J Neurosurg Spine. 2005;3:417–23. doi: 10.3171/spi.2005.3.6.0417. [DOI] [PubMed] [Google Scholar]
- 10.Ishihara H, Kanamori M, Kawaguchi Y, Nakamura H, Kimura T. Adjacent segment disease after anterior cervical interbody fusion. Spine J. 2004;4:624–8. doi: 10.1016/j.spinee.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Bohlman HH, Emery SE, Goodfellow DB, Jones PK. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy: long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am. 1993;75:1298–307. doi: 10.2106/00004623-199309000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser MG, Haid RW, Jr, Subach BR, Barnes B, Rodts GE., Jr Anterior cervical plating enhances arthrodesis after discectomy and fusion with cortical allograft. Neurosurgery. 2002;50:229–38. doi: 10.1097/00006123-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Fraser JF, Hartl R. Anterior approaches to fusion of the cervical spine: a metaanalysis of fusion rates. J Neurosurg Spine. 2007;6:298–303. doi: 10.3171/spi.2007.6.4.2. [DOI] [PubMed] [Google Scholar]
- 14.Puttlitz CM, Rousseau MA, Xu Z, Hu S, Tay BK, Lotz JC. Interver-tebral disc replacement maintains cervical spine kinetics. Spine. 2004;29:2809–14. doi: 10.1097/01.brs.0000147739.42354.a9. [DOI] [PubMed] [Google Scholar]
- 15.DiAngelo DJ, Foley KT, Morrow BR, et al. In vitro biomechanics of cervical disc arthroplasty with the ProDisc-C total disc implant. Neu-rosurg Focus. 2004;17:E7. [PubMed] [Google Scholar]
- 16.Matsunaga S, Kabayama S, Yamamoto T, Yone K, Sakou T, Nakan-ishi K. Strain on intervertebral discs after anterior cervical decompression and fusion. Spine. 1999;24:670–5. doi: 10.1097/00007632-199904010-00011. [DOI] [PubMed] [Google Scholar]
- 17.Goffin J, Geusens E, Vantomme N, et al. Long-term follow-up after interbody fusion of the cervical spine. J Spinal Disord Tech. 2004;17:79–85. doi: 10.1097/00024720-200404000-00001. [DOI] [PubMed] [Google Scholar]