Abstract
Diabetic cardiomyopathy is a prominent cause of heart failure in patients with diabetes mellitus. Currently, there is no specific treatment for diabetic cardiomyopathy. This study aimed to investigate the effect and underlying mechanisms of Zinc (Zn) supplementation in the protection against diabetic cardiomyopathy in a rat model of type 2 diabetes mellitus (T2DM). T2DM-like lesions in male Wistar rats were induced by introducing the high-fat diet and by administration of streptozocin (STZ). After STZ induction, animals with fasting plasma glucose level ≥16.7 mM were considered as diabetic, and randomly assigned to the group receiving physiological saline (control) or ZnSO4 for 56 days. On days 0, 7, 28 and 56 of treatment, animals were weighed, and their blood samples were analyzed. On day 56, hemodynamic assessment was performed right before the sacrifice of animals. Cardiac tissue specimens were collected and subjected to pathologic assessment, metallothionein (MT) concentration measurement and Western blot analysis of microtubule-associated protein light chain 3 (LC3), the marker of autophagy, and glucose-regulated protein-78 (GRP78), an oxidative stress marker. High-fat diet feeding followed by STZ administration resulted in weight loss, hyperglycemia, polydipsia, polyphagia, hemodynamic anomalies and a significant increase in the myocardial content of LC3 and GRP78 proteins, but not in MT protein. Zn supplementation effectively attenuated all these aberrations induced by high-fat diet and STZ. These findings suggest that Zn might be a protective factor in diabetic cardiomyopathy, acting in two ways: at least partially, through inhibiting autophagy and by endoplasmic reticulum stress.
KEY WORDS: Diabetic cardiomyopathy, zinc, autophagy, metallothionein, oxidative stress, rats
INTRODUCTION
Diabetes mellitus (DM) is one of the most common non-communicable diseases, and its prevalence has been increasing rapidly in both developed and developing countries [1]. However, the burden of DM is particularly prominent in China. Based on the latest information published on the website of the International Diabetes Federation, China has overtaken India and become the leading country in terms of diabetes prevalence. The total number of diagnosed patients with diabetes was 92.4 million in 2010 and is projected to reach half a billion in 2030 [2].
Cardiovascular complications, including diabetic cardiomyopathy, are the major causes of morbidity and mortality in diabetic patients. Defined as the presence of functional and structural abnormalities in the absence of coronary artery disease, hypertension and significant valvular disease [3, 4], diabetic cardiomyopathy manifests initially as asymptomatic diastolic dysfunction that eventually progresses to symptomatic heart failure. Early echocardiography-based studies showed that 30% of type 2 diabetic patients had diastolic dysfunction [5, 6]. Recent studies using more rigorous and more sensitive Doppler methods showed that over 50% of type 2 diabetic patients had mild and early diastolic dysfunction [7,8]. The pathophysiology of diabetic cardiomyopathy is not completely understood, and it appears to be multifactorial involving hyperglycemia, hyperinsulinemia, abnormal fatty acid metabolism and oxidative stress, cardiac autonomic neuropathy and the local renin-angiotensin-aldosterone system overactivation [9].
Currently, there is no specific and efficient treatment available for diabetic cardiomyopathy. Glycemic control, through supportive measures such as diet modifications, is the cornerstone of diabetic cardiomyopathy management [10, 11]. Zinc (Zn) is one of the most important essential trace elements playing indispensable role in human health and disease [12]. It has been documented that Zn level in blood is significantly lower in patients with type 1 diabetes (64.2±12.6 µg/dL) or type 2 diabetes (68.9±11.9 µg/dL) than in healthy subjects (83.4±12.5 µg/dL) [13]. Therefore, Zn supplementation might help patients with diabetes to achieve an effective and sustainable glycemic control [13-15]. In addition to glycemic control, other mechanisms have also been postulated to explain the benefits of Zn supplementation for patients with diabetic cardiomyopathy. Nevertheless, the current understanding of mechanisms underlying Zn-mediated cardioprotection in patients with diabetes is still obscure. We hypothesize that Zn may exert its cardioprotective effect via Akt activation, upregulation of metallothionein expression, and inhibition of autophagy. This study aimed to test this hypothesis in a rat model of diabetic cardiomyopathy.
MATERIALS AND METHODS
Ethical statement
All experiments on animals were performed in accordance with the ethical guidelines for the Care and Use of Laboratory Animals of the United States National Institutes of Health. The study protocol, including procedures for animal handling and husbandry, was reviewed and approved by the Animal Care and Use Committee of Jilin University.
Animals and groups
Male Wistar rats, weighing between 180 and 220 g were purchased from the Experimental Animal Center of the College of Basic Sciences of Jilin University in Changchun, China. After adaption to the new environment for seven days, animals were randomly allocated into three groups: “healthy controls”, “diabetes”, and “diabetes + Zn supplementation”. Based on the calculations done using a free online software, Raosoft® Sample Size Calculator, a sample size of 8 was used to ensure a desired analysis power of 0.9 for 2-sided tests at α=0.05. However, bearing in mind the possibility of animal illness or death during the experiment, more animals were included, so that each group was assigned a total of 13 animals.
For induction of a human type 2 diabetes-like condition, animals in the “diabetes” and “diabetes + Zn supplementation” groups were fed with a high-fat diet for 35 days, followed by a single intraperitoneal injection (40 mg/kg) of streptozocin (STZ). The level of fasting plasma glucose (FPG) was measured on days 0, 3 and 56 after STZ treatment. Animals with an FPG level ≥16.7 mM after STZ treatment were considered as diabetic. While animals in the “diabetes” group had been given physiological saline, those in the “diabetes + Zn supplementation” group had been receiving Zn at 15 mg/kg/day in a form of gastric ZnSO4 solution during 56 days. Animals in the control group were left untreated and fed with a regular diet during the entire period of the experiment.
Invasive hemodynamic assessment
Invasive hemodynamic assessment was performed right before sacrifice of the animals on post-treatment day 56. A thoracotomy procedure was performed under anesthesia given by intraperitoneal injection of pentobarbital (30 mg/kg). The right carotid artery cannulation was performed by a plastic catheter (1 mm in diameter). Hemodynamic variables, including heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), left ventricular pressure (LVP), left ventricular end-diastolic dimension (LVEDD), maximum rates of systolic (dP/dt) and diastolic (-dP/dt) pressure alterations, were evaluated as previously described [16].
Pathologic assessment
Animals were sacrificed after hemodynamic assessment had been done on the 56th day of Zn supplementation. Hearts were immediately collected. For light microscopy, specimens were fixed in 10% formaldehyde solution (pH 7.0), embedded in paraffin, sectioned at 4-µm, and stained with hematoxylin and eosin (HE). For transmission electron microscopy, specimens were first immersed in 2.5% glutaraldehyde for 4 hours and then in 1% osmium tetroxide for 2 hours at 4ºC. Following dehydration through a graded ethanol series, specimens were embedded in Epon 812 resin. Ultrathin sections (1 µm) were prepared, stained with uranyl acetate and lead citrate and examined under a transmission electron microscope (Hitachi H8000, Tokyo, Japan).
Myocardial MT concentration
Myocardial tissue specimens were collected immediately after sacrifice of the animals at the end of the experiment and homogenized in four volumes of 10 mmol/L Tris-HCl buffer (pH 7.4). After centrifugation at 10,000 ×g min at 4°C for 10, supernatant was collected and heated for 2 min in a boiling water bath. Heat-precipitated proteins were removed following centrifugation at 10,000 ×g for 2 min. An aliquot of the 200µL heat-denatured tissue supernatant was placed in a 1.5-mL microcentrifuge tube. An amount of 200 µl of radioactive cadmium (2.0 mg 109CdCl2/mL with radioactivity of 1.0 µCi/mL) was added. The mixture was incubated at 25°C for 10 min. Free cadmium was removed through a repeated procedure of bovine hemoglobin binding. The radioactivity of the cadmium-labeled MT was measured on a gamma counter (Perkin Elmer 1470, Downers Grove, IL, USA), based on which the protein concentration of MT was calculated as micromoles of cadmium per gram of heat stable protein.
Western blot
Total protein was extracted with a lysis buffer containing 500 mM HEPES-KOH, 250 mM NaCl, 1% NP-40 (Sigma, USA), 1 mM PMSF (Sigma, USA) and 1 µg/mL Aprotinin (Amresco, USA). The concentration of protein was determined with BCA Protein Assay Kit (Pierce Biotechnology, USA) according to the manufacturer’s instructions. Proteins were resolved by 12% or 8% (w/v) SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The membranes were incubated overnight at 4° with primary antibodies. After washing 4 times in Tris-buffered saline (10 mM Tris-HCl and 150 mM NaCl) containing 1% (v/v) Tween 20, the membranes were incubated with a horseradish peroxidase-conjugated primary antibody against rat microtubule-associated protein light chain 3 (LC-3) or glucose-regulated protein 78 (GRP78, Beyotime Institute of Biotechnology, Jiangsu, China) for 1 h at room temperature. Immunoreactive bands were visualized using an enhanced chemiluminescence detection kit from GE Healthcare (Shanghai) Co., Ltd. (Shanghai, China).
Statistical analysis
Data were expressed as mean ± standard deviation and analyzed by ANOVA and Wilcoxon rank sum test or Student t test. All analyzes were performed using the statistical software SPSS 12.0 (Chicago, IL, USA). Differences were considered significant when p<0.05.
RESULTS
Changes in body weight
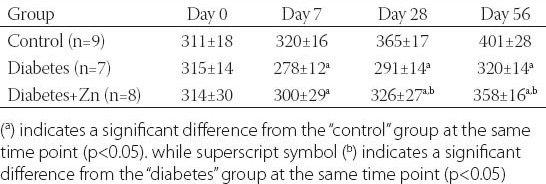
Body weight of animals allocated to three different groups was measured at different points of time, and its changes are presented in Table 1. On day 0 (i.e. before the experiment or at a baseline), there was no significant difference in body weight among three groups (p>0.05). Diabetes induction with STZ following high-fat diet feeding resulted in a body weight reduction that was significant on day 7 and sustained through experimental days 28 to 56 (p<0.05). Zn supplementation attenuated the high-fat diet- and STZ-induced weight loss slightly on day 7 (p>0.05) and significantly on days 28 and 56 (p<0.05).
TABLE 1.
Body weight (grams) of animals in three experimental groups
Changes in blood glucose
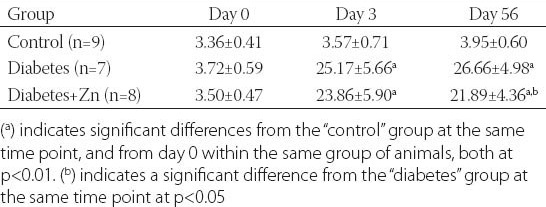
Blood glucose is an important indicator of the severity of diabetic lesions. In order to monitor changes in the FPG level over time, we measured the FPG level before and after STZ treatment (Table 2). Prior to STZ induction, the level of FPG was similar in all three groups of animals (p>0.05). While the FPG level had not changed significantly over time in control animals (p>0.05), it was dramatically increased in diabetic animals after STZ t\induction (p<0.01). Zn supplementation resulted in a significant decrease in the level of FPG on day 56 (p<0.05), but not on day 3 (p>0.05). Despite a significant decrease at later time points, the absolute level of FPG in diabetic animals after Zn treatment remained much higher than the baseline level in control animals (p<0.01).
TABLE 2.
Levels (mM) of FPG at different time points after STZ induction in the indicated groups of animals
Changes in hemodynamic variables
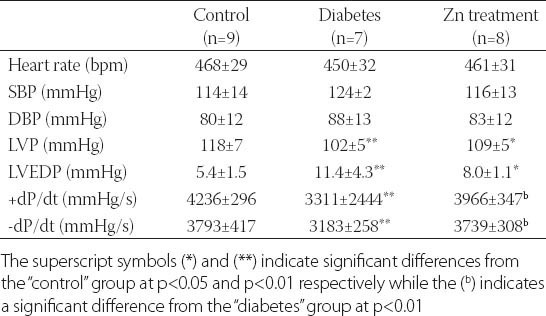
There were no significant differences in heart rate, SBP and DBP between different groups of animals (p>0.05), as shown in Table 3. High-fat diet and STZ significantly worsened the readings of LVP, LVEDP, +dp/dt and –dp/dt (p<0.01). Zn supplementation effectively attenuated the detrimental effects of high-fat diet and STZ on +dP/dt and –dP/dt (p<0.01), but not on LVP and LVEDP (p>0.05).
TABLE 3.
Hemodynamic variables in indicated groups of animals on the experimental day 56 of Zn supplementation
Histopathologic changes in the myocardium
Light and electron microscopic images of myocardial tissue sections are presented in Figure 1. HE staining showed regularly oriented cardiomyocytes with 1 or 2 centrally located oval nuclei and uniform cytoplasm in normal control animals, the finding that is in a sharp contrast to disarray with abnormalities such as hypertrophy, twisting, vacuolar degeneration, fatty degeneration, focal myocardial necrosis, and fibrosis in myocytes in animals with experimental diabetes. However, there is a significant reversion of these abnormalities in diabetic animals after Zn treatment. When examined with a scanning electron microscope, cardiomyocytes of healthy control animals displayed a latticework arrangement of myofibrils and dense mitochondria with clearly visible intercalated disc structures in between. In contrast, fat accumulation, focal necrosis, partial fragmentation and dissolution of myofibrils, distortion of mitochondrial structure, swelling hyperplasia, local vacuolation as well as increased intra-myocardial gaps were seen in cardiomyocytes of animals with experimental diabetes. Zn treatment significantly attenuated the detrimental effects of high-fat diet and STZ.
FIGURE 1.
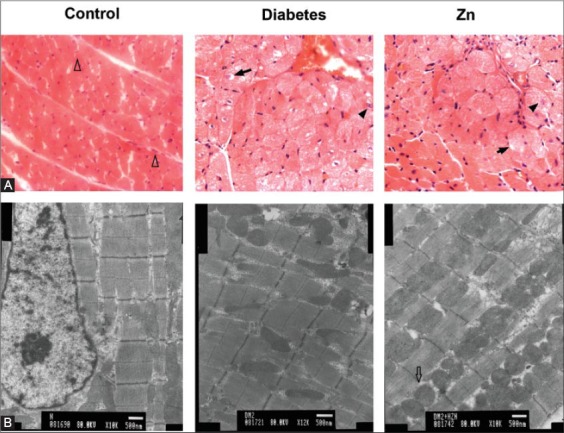
Light (A) and electron (B) microscopic images of representative myocardial tissue specimens in the indicated groups of rats on the day 56, showing normal myocardiocytes (empty arrow head), vacuolization (solid arrow), necrosis (solid arrow head) and dense mitochondria (empty arrow).
Changes in MT concentration
Myocardial concentration of MT was not different between the control and diabetes groups (p>0.05), but was significantly increased in animals with experimental diabetes after Zn treatment (p<0.01, Figure 2).
FIGURE 2.
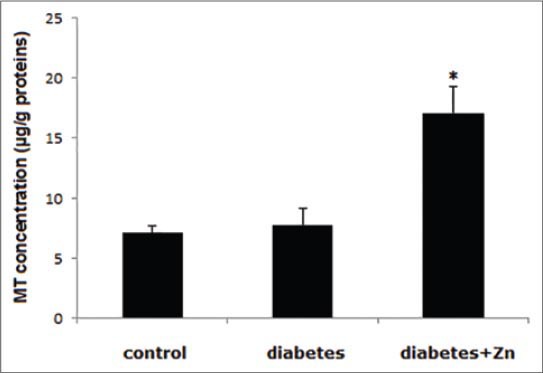
MT concentration in myocardial tissue in the indicated groups of rats on day 56 after treatment. Asterisk (*) indicates a significant difference at p=0.01.
Changes in LC3-II concentration
The basal level of LC3-II in myocardial tissue in control animals was low, as shown in Figure 3. Diabetes induction with a high-fat diet and STZ injection resulted in a significant increase in myocardial level of LC3-II (p<0.01). However, Zn treatment effectively attenuated the increase (p<0.05).
FIGURE 3.
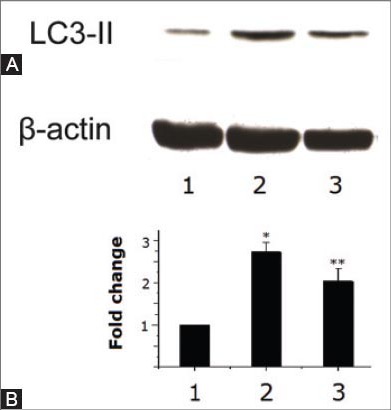
Western blotting analysis of LC3-II in cardiac specimens in control rats (1) and experimental diabetic rats without (2) and with (3) Zn treatment. (A) A representative Western blot. (B) A bar graph showing fold change in the average level of LC3-II. A single asterisk (*) indicates a significant difference from control rats at p<0.01 and double asterisks (**) indicate a significant difference from the diabetes group at p<0.05.
Changes in GRP78 concentration
Results of Western blotting analysis indicate that GRP78 protein was undetectable in myocardial tissue in healthy control animals as seen in Figure 4. High-fat diet and STZ induced a robust increase in myocardial GRP78 protein that was significantly attenuated by Zn treatment (p<0.05).
FIGURE 4.
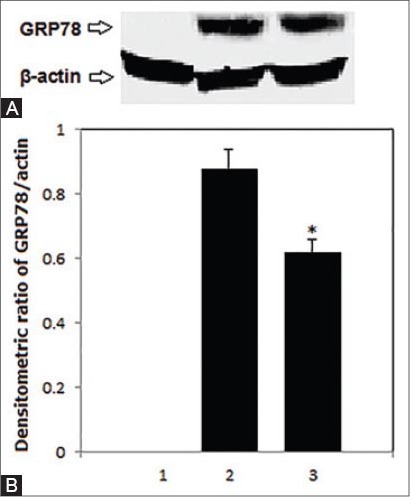
Western blotting analysis of GRP78 in cardiac specimens in control healthy rats (1) and those with experimental diabetes without (2) and with (3) Zn treatment. (A) A representative Western blot. (B) A bar graph showing average densitometric ratio of GRP78/β-actin. The single asterisk (*) indicates a significant difference from the control at p<0.01 while the double asterisks (**) indicate a significant difference between Zn supplementation and non-Zn supplementation in diabetic animals at p<0.05.
DISCUSSION
Diabetes mellitus is a metabolic disorder characterized by a chronically increased blood glucose level (i.e. hyperglycemia) either due to a deficiency in insulin secretion consequent to beta cell loss in the pancreas (type 1) or due to loss of insulin sensitivity in target organs in the presence of normal insulin secretion (type 2). Approximately 95% of patients with diabetes in developing countries have type 2 diabetes mellitus [17]. Several animal models of human type 2 diabetes, have been reported, but none of them are without limitations [17, 18]. In this study, we successfully induced development of human type 2 diabetes-like pathology with with a high-fat diet and a single intraperitoneal dose of STZ in male Wistar rats, thereby further validating the usefulness of this non-genetic model in the research of type 2 diabetes [19].
Type 2 diabetes is associated with many complications, among which cardiomyopathy occurs in 52-60% of well-controlled patients with diabetes [20] and is the primary cause of morbidity and mortality in this population [21]. Currently, no specific prevention and treatment modalities are available for diabetic cardiomyopathy. Although a low-dose aspirin regimen has been recommended for patients with high risk of cardiovascular complications, more recent data have shown that this regimen has little cardiovascular benefits in clinical settings [22]. Lifestyle modifications, control of glucose and lipid abnormalities, and treatment of hypertension and cardiovascular disease, if present, are among the major strategies of diabetic cardiomyopathy management [23]. Recently, dietary supplementation of trace elements, important in insulin-mimetic or antioxidant activity, has been recommended as a novel strategy to manage diabetes-associated cardiac complications [24]. Among these trace elements, Zn has drawn particular attention [12, 14, 15, 25]. Here, we attempted to validate the benefits of Zn further in the protection against diabetic cardiomyopathy and to study the underlying mechanisms in a rat model of high-fat diet feeding and STZ-induced type 2 diabetes.
In previous animal studies, body weight loss has been observed in diabetes over the course of the lesion progression [26, 27]. In consistency with these observations, we demonstrated significant weight loss in diabetic rats after induction with high-fat diet feeding and application of STZ, that is in a sharp contrast to the time-dependent weight gain over time in control animals. Our study also demonstrated Zn-mediated prevention of diabetes-associated weight loss (Table 1).
Hemodynamically, diabetic cardiomyopathy is characterized by impaired cardiac relaxation progressing to overt contractile failure. To evaluate the role of high-fat diet feeding and STZ administration in impairing diastolic function, and the impact of Zn supplementation in prevention or reversal of this impairment, we performed hemodynamic assessment. Our results showed that STZ administration following high-fat diet significantly worsened the readings of LVP, LVEDP, +dp/dt and –dp/dt, while Zn supplementation effectively attenuated the detrimental effects of high-fat diet and STZ on +dP/dt and –dP/dt but not on LVP and LVEDP (Table 3). These results indicate that normal diastolic function was impaired in rats following diabetes induction with high-fat diet and STZ and that Zn supplementation had limited effects against diastolic dysfunction in rats with diabetic cardiomyopathy. In a French study in a pig model of diabetic cardiomyopathy, also limited effects were observed for algal polysaccharides in improving diastolic function in diabetic cardiomyopathy [28].
Pathologically, diabetic cardiomyopathy is characterized by cellular structural insults and abnormalities including cardiomyocyte hypertrophy and apoptosis. Our light and electron microscopic assessments demonstrated hypertrophy, vacuolar degeneration, focal necrosis, fibrosis, myofibril disarray and partial fragmentation, distortion of mitochondrial structure, swelling hyperplasia, and local vacuolation in cardiomyocytes in rat with experimental diabetes and significant improvement of these abnormalities in diabetic rats after Zn treatment (Figure 1). These findings further confirmed the success in the induction of diabetic cardiomyopathy by high-fat feeding and STZ administration as well as the protective and therapeutic benefits of Zn supplementation.
MT is a cysteine-rich protein that binds predominantly to zinc and serves as an oxidoreductive metabolic zinc link, thus playing important roles in cellular zinc transport, energy production, and protection against oxidative stress [29]. Zn supplementation has been shown to induce MT synthesis in vivo in experimental animals and to reduce diabetes-related complications in both humans and animal models [30]. In this study, we demonstrated that MT protein content in myocardial tissue was robustly increased in rats with experimentally induced diabetes. This observation is in accordance with results from a previous mouse study where Zn supplementation significantly increased cardiac MT expression [25].
Autophagy is the process by which cells recycle cytoplasm and dispose of excess or defective organelles and plays crucial roles in both health and disease [31]. Given that diabetes is a metabolic disorder and that autophagy is a major homeostatic mechanism to eliminate damaged organelles, long-lived or aberrant proteins and superfluous portions of the cytoplasm, it is logical to conjecture that autophagy is playing indispensable parts in nutrient homeostasis and the development of diabetes [32]. It has been suggested that autophagy is a two-edged sword, playing opposing roles, in DM [33]. Using electron microscopy, Marchetti and Masini showed that there were significantly more dead pancreatic beta-cells in type 2 diabetes patients than in control subjects and approximately 50% of the dead beta-cells were characterized by massive vacuole overload in the cytoplasm (with no signs of nuclear morphological alterations) and were possibly autophagy-associated [34]. In contrast, Jung and Lee demonstrated beta-cell mass reduction, mitochondrial swelling and ex vivo insulin secretory function impairment in beta-cells, and hypoinsulinemia and hyperglycemia in beta-cell-specific autophagy-related 7 (Atg7) gene-null mice [35]. In this study, we demonstrated mitochondrial swelling and cytoplasmic vacuolation in cardiomyocytes in rats with experimentally induced diabetes by electron microscopy (Figure 1A). In addition, we also showed a significant increase and a significant decrease in myocardial content of LC3-II protein, the marker of autophagy, in experimental diabetes rats receiving normal saline and Zn treatments respectively (Figure 3). These observations suggest autophagy-associated structural and functional aberrations in experimentally induced DM.
Accumulating data point to an essential role for oxidative stress in the activation of autophagy [36]. The glucose-regulated protein 78 (GRP78), also known as BiP, is a marker of the endoplasmic reticulum (ER) stress [37]. In this study, myocardial GRP78 protein content showed a pattern of changes similar to that of changes in LC3-II protein content in different groups of animals, strongly suggesting an association between autophagy and oxidative stress.
CONCLUSION
In summary, using a rat model of diabetic cardiomyopathy experimentally induced by high fat diet and STZ administration, this study demonstrated that Zn supplementation was sufficient to protect against diabetic cardiomyopathy, at least partially, by reducing oxidative stress and autophagy in cardiac tissue.
ACKNOWLEDGMENTS
This work was supported by the Graduate Student Grant of Jilin University. We would like to express our gratitude to Dr. Ziming Yu for proofreading the manuscript.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- [1].Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since. 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. http://dx.doi.org/10.1016/S0140-6736(11)60679-X . [DOI] [PubMed] [Google Scholar]
- [2].New diabetes figures in China: IDF press statement. [Accessed 2014 July 20]. Available from: http://www.idf.org/press-releases/idf-press-statement-china-study .
- [3].Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.679597 . [DOI] [PubMed] [Google Scholar]
- [4].Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. http://dx.doi.org/10.1016/0002-9149(72)90595-4 . [DOI] [PubMed] [Google Scholar]
- [5].Nicolino A, Longobardi G, Furgi G, Rossi M, Zoccolillo N, Ferrara N, Rengo F. Left ventricular diastolic filling in diabetes mellitus with and without hypertension. Am J Hypertens. 1995;8:382–389. doi: 10.1016/0895-7061(95)00022-h. http://dx.doi.org/10.1016/0895-7061(95) 00022-H . [DOI] [PubMed] [Google Scholar]
- [6].Di Bonito P, Cuomo S, Moio N, Sibilio G, Sabatini D, Quattrin S, Capaldo B. Diastolic dysfunction in patients with non-insulin-dependent diabetes mellitus of short duration. Diabet Med. 1996;13:321–324. doi: 10.1002/(SICI)1096-9136(199604)13:4<321::AID-DIA3>3.0.CO;2-7. http://dx.doi.org/10.1002/(SICI)1096-9136(199604) 13: 4<321: AID-DIA3>3.0.CO;2-7 . [DOI] [PubMed] [Google Scholar]
- [7].Poirier P, Bogaty P, Garneau C, Marois L, Dumesnil JG. Diastolic dysfunction in normotensive men with well-controlled type 2 diabetes: importance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. Diabetes Care. 2001;24:5–10. doi: 10.2337/diacare.24.1.5. http://dx.doi.org/10.2337/diacare.24.1.5 . [DOI] [PubMed] [Google Scholar]
- [8].Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. http://dx.doi.org/10.1001/jama.289.2.194 . [DOI] [PubMed] [Google Scholar]
- [9].Acar E, Ural D, Bildirici U, Sahin T, Yilmaz I. Diabetic cardiomyopathy. Anadolu Kardiyol Derg. 2011;11:732–737. doi: 10.5152/akd.2011.196. [DOI] [PubMed] [Google Scholar]
- [10].Hayat SA, Patel B, Khattar RS, Malik RA. Diabetic cardiomyopathy: mechanisms, diagnosis and treatment. Clin Sci (Lond) 2004;107:539–557. doi: 10.1042/CS20040057. http://dx.doi.org/10.1042/CS20040057 . [DOI] [PubMed] [Google Scholar]
- [11].Voulgari C, Papadogiannis D, Tentolouris N. Diabetic cardiomyopathy: from the pathophysiology of the cardiac myocytes to current diagnosis and management strategies. Vasc Health Risk Manag. 2010;6:883–903. doi: 10.2147/VHRM.S11681. http://dx.doi.org/10.2147/VHRM.S11681 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME. Zinc and human health: an update. Arch Toxicol. 2012;86:521–534. doi: 10.1007/s00204-011-0775-1. http://dx.doi.org/10.1007/s00204-011-0775-1 . [DOI] [PubMed] [Google Scholar]
- [13].Al-Maroof RA, Al-Sharbatti SS. Serum zinc levels in diabetic patients and effect of zinc supplementation on glycemic control of type 2 diabetics. Saudi Med J. 2006;27:344–350. [PubMed] [Google Scholar]
- [14].Oh HM, Yoon JS. Glycemic control of type 2 diabetic patients after short-term zinc supplementation. Nutr Res Pract. 2008;2:283–288. doi: 10.4162/nrp.2008.2.4.283. http://dx.doi.org/10.4162/nrp.2008.2.4.283 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Capdor J, Foster M, Petocz P, Samman S. Zinc and glycemic control: a meta-analysis of randomised placebo controlled supplementation trials in humans. J Trace Elem Med Biol. 2013;27:137–142. doi: 10.1016/j.jtemb.2012.08.001. http://dx.doi.org/10.1016/j.jtemb.2012.08.001 . [DOI] [PubMed] [Google Scholar]
- [16].Azevedo PS, Polegato BF, Minicucci MF, Pio SM, Silva IA, Santos PP, et al. Early echocardiographic predictors of increased left ventricular end-diastolic pressure three months after myocardial infarction in rats. Med Sci Monit. 2012;18:BR253–258. doi: 10.12659/MSM.883202. http://dx.doi.org/10.12659/MSM.883202 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Islam MS, Wilson RD. Experimentally induced rodent models of type 2 diabetes. Methods Mol Biol. 2012;933:161–174. doi: 10.1007/978-1-62703-068-7_10. [DOI] [PubMed] [Google Scholar]
- [18].Akash MS, Rehman K, Chen S. Goto-Kakizaki rats: its suitability as non-obese diabetic animal model for spontaneous type 2 diabetes mellitus. Curr Diabetes Rev. 2013;9:387–396. doi: 10.2174/15733998113099990069. http://dx.doi.org/10.2174/15733998113099990069 . [DOI] [PubMed] [Google Scholar]
- [19].Bugger H, Abel ED. Rodent models of diabetic cardiomyopathy. Dis Model Mech. 2009;2:454–466. doi: 10.1242/dmm.001941. http://dx.doi.org/10.1242/dmm.001941 . [DOI] [PubMed] [Google Scholar]
- [20].Seferovic PM, Milinkovic I, Ristic AD, Seferovic Mitrovic JP, Lalic K, Jotic A, Kanjuh V, Lalic N, Maisch B. Diabetic cardiomyopathy: ongoing controversies in 2012. Herz. 2012;37:880–886. doi: 10.1007/s00059-012-3720-z. http://dx.doi.org/10.1007/s00059-012-3720-z . [DOI] [PubMed] [Google Scholar]
- [21].Farhangkhoee H, Khan ZA, Kaur H, Xin X, Chen S, Chakrabarti S. Vascular endothelial dysfunction in diabetic cardiomyopathy: pathogenesis and potential treatment targets. Pharmacol Ther. 2006;111:384–399. doi: 10.1016/j.pharmthera.2005.10.008. http://dx.doi.org/10.1016/j.pharmthera.2005.10.008 . [DOI] [PubMed] [Google Scholar]
- [22].Schmidt BM, Arora R. Primary prevention of cardiovascular complications in type II diabetes patients using aspirin: a complicated tale. Am J Ther. 2013;20:275–278. doi: 10.1097/MJT.0b013e3181afbf17. [DOI] [PubMed] [Google Scholar]
- [23].Pappachan JM, Varughese GI, Sriraman R, Arunagirinathan G. Diabetic cardiomyopathy: Pathophysiology, diagnostic evaluation and management. World J Diabetes. 2013;4:177–189. doi: 10.4239/wjd.v4.i5.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ozturk N, Olgar Y, Ozdemir S. Trace elements in diabetic cardiomyopathy: An electrophysiological overview. World J Diabetes. 2013;4:92–100. doi: 10.4239/wjd.v4.i4.92. http://dx.doi.org/10.4239/wjd.v4.i4.92 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang J, Song Y, Elsherif L, Song Z, Zhou G, Prabhu SD, et al. Cardiac metallothionein induction plays the major role in the prevention of diabetic cardiomyopathy by zinc supplementation. Circulation. 2006;113:544–554. doi: 10.1161/CIRCULATIONAHA.105.537894. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.537894 . [DOI] [PubMed] [Google Scholar]
- [26].Badole SL, Jangam GB, Chaudhari SM, Ghule AE, Zanwar AA. L-glutamine supplementation prevents the development of experimental diabetic cardiomyopathy in streptozotocin-nicotinamide induced diabetic rats. PLoS One. 2014;9:e92697. doi: 10.1371/journal.pone.0092697. http://dx.doi.org/10.1371/journal.pone.0092697 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Daniels A, Linz D, van Bilsen M, Rutten H, Sadowski T, Ruf S, et al. Long-term severe diabetes only leads to mild cardiac diastolic dysfunction in Zucker diabetic fatty rats. Eur J Heart Fail. 2012;14:193–201. doi: 10.1093/eurjhf/hfr166. http://dx.doi.org/10.1093/eurjhf/hfr166 . [DOI] [PubMed] [Google Scholar]
- [28].Vaugelade P, Hoebler C, Bernard F, Guillon F, Lahaye M, Duee PH, et al. Non-starch polysaccharides extracted from seaweed can modulate intestinal absorption of glucose and insulin response in the pig. Reprod Nutr Dev. 2000;40:33–47. doi: 10.1051/rnd:2000118. http://dx.doi.org/10.1051/rnd: 2000118 . [DOI] [PubMed] [Google Scholar]
- [29].Bell SG, Vallee BL. The metallothionein/thionein system: an oxidoreductive metabolic zinc link. Chembiochem. 2009;10:55–62. doi: 10.1002/cbic.200800511. http://dx.doi.org/10.1002/cbic.200800511 . [DOI] [PubMed] [Google Scholar]
- [30].Islam MS, Loots du T. Diabetes, metallothionein, and zinc interactions: a review. Biofactors. 2007;29:203–212. doi: 10.1002/biof.5520290404. http://dx.doi.org/10.1002/biof.5520290404 . [DOI] [PubMed] [Google Scholar]
- [31].Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. http://dx.doi.org/10.1126/science.1099993 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Quan W, Lee MS. Role of autophagy in the control of body metabolism. Endocrinol Metab (Seoul) 2013;28:6–11. doi: 10.3803/EnM.2013.28.1.6. http://dx.doi.org/10.3803/EnM.2013.28.1.6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yamamoto S, Kazama JJ, Fukagawa M. Autophagy: a two-edged sword in diabetes mellitus. Biochem J. 2013;456:e1–3. doi: 10.1042/BJ20131282. http://dx.doi.org/10.1042/BJ20131282 . [DOI] [PubMed] [Google Scholar]
- [34].Marchetti P, Masini M. Autophagy and the pancreatic beta-cell in human type 2 diabetes. Autophagy. 2009;5:1055–1056. doi: 10.4161/auto.5.7.9511. http://dx.doi.org/10.4161/auto.5.7.9511 . [DOI] [PubMed] [Google Scholar]
- [35].Jung HS, Lee MS. Role of autophagy in diabetes and mitochondria. Ann N Y Acad Sci. 2010;1201:79–83. doi: 10.1111/j.1749-6632.2010.05614.x. http://dx.doi.org/10.1111/j.1749-6632.2010.05614.x . [DOI] [PubMed] [Google Scholar]
- [36].Coto-Montes A, Boga JA, Rosales-Corral S, Fuentes-Broto L, Tan DX, Reiter RJ. Role of melatonin in the regulation of autophagy and mitophagy: a review. Mol Cell Endocrinol. 2012;361:12–23. doi: 10.1016/j.mce.2012.04.009. http://dx.doi.org/10.1016/j.mce.2012.04.009 . [DOI] [PubMed] [Google Scholar]
- [37].Haas IG. BiP (GRP78), an essential hsp70 resident protein in the endoplasmic reticulum. Experientia. 1994;50:1012–1020. doi: 10.1007/BF01923455. http://dx.doi.org/10.1007/BF01923455 . [DOI] [PubMed] [Google Scholar]


