Abstract
Trefoil factor family peptides (TFF1, TFF2, and TFF3) are predominantly found in mucous epithelia of various organs. However, they have also been reported in the nervous tissue, particularly mouse, rat, porcine, and human brain. The aim of this research was to determine the presence of TFF1 and TFF3 in the nervous system of developing mouse embryo. Mouse embryos, at the stages E15 to E17 were isolated, fixed in 4% paraformaldehyde and embedded in paraffin blocks. Sagittal 6µm sections were made, processed for immunohistochemistry, and incubated with anti-TFF1 or anti-TFF3 primary polyclonal rabbit antibodies. Labeled streptavidin-biotin method was used for TFF detection. TFF1 and 3 were found in the cytoplasm of ganglion cell somata, while TFF3 staining was also visible in the cytoplasm of neurons in different areas and nuclei of brain and medulla oblongata. Neurons in the gray matter of spinal cord were also TFF1 and TFF3 positive, and signal for both peptides was found in the choroid plexus. TFF peptides might be involved in the complex processes of nervous system development and differentiation and brain plasticity.
KEY WORDS: trefoil factor, nervous system, embryonic development, mouse, immunohistochemistry
INTRODUCTION
Research on trefoil factor family (TFF) peptides started in 1982, when pancreatic spasmolytic polypeptide, today known as TFF2, and sP2 peptide, today known as TFF1 were discovered [1]. A few years later, intestinal trefoil factor (now TFF3) was described. A three-looped, clover-like structural domain is a common characteristic of these peptides, and is important for their resistance to proteolytic degradation [2,3]. Members of this small family of peptides are predominantly found in mucous epithelia of the gastrointestinal system. However, they are also expressed in many other tissues, for example epithelia of respiratory tract, uterus, conjunctiva, thyroid gland, salivary glands, prostate, mammary gland, gallbladder, and thyroid gland [2–4]. Expression of these peptides was investigated in connection with different pathological conditions. It has been found that expression of TFF peptides is deregulated in numerous epithelial tumors [4–9] and chronic inflammatory diseases [10–13]. These peptides act through various mechanisms and participate in apoptosis, cell migration, and immune response, affect mucus viscosity and angiogenesis [4,7,11,14-16]. New properties and potential roles of TFF peptides are continually being discovered, making them a fruitful field for scientific research.
TFF1 and TFF3 are both found in the nervous tissue, however, to a much lower extent than in the mucous epithelia. Both peptides are expressed in different parts of adult rat, mouse, and human brain. Significant TFF1 expression was noticed in rat hippocampus, frontal cortex, and cerebellum [17], and weak, but uniform expression was detected in all regions of mouse brain [18]. In mouse and rat, astrocytes have been reported to synthesize TFF1 [17,19,20]. There are very limited data on the neural expression of TFF2, which is the least investigated of the three TFF peptides in the context of neural tissue. Until now, TFF2 was found in the ganglion cell layer of murine retina, which is of neural origin [21], whereas regarding TFF3, mouse brain shows significant expression of this peptide in hippocampus, temporal cortex and cerebellum [18]. TFF3 was also detected in human and rat hypothalamus, particularly in the supraoptic and paraventricular nuclei, co-localized with oxytocin, and accumulated together with oxytocin in the neural lobe of pituitary [22–24]. Ultrastructural examination of porcine pituitary gland revealed co-localization of TFF3 and oxytocin within the same vesicles, and was thus hypothesized that from there, TFF3 is released into the bloodstream to affect potential, undiscovered target receptors [25]. Injections of TFF3 monomer into rat amygdala exhibit a dose-dependent effect on animal behavior (anxiolytic effect at low dose or anxiogenic effect at high dose) [24]. Recent research identifies TFF3 as a neuropeptide that facilitates learning, object recognition and retention of memory [26]. Low TFF3 in cerebrospinal fluid is a predictive factor for brain atrophy, and its potential role in the pathogenesis of Alzheimer’s disease is suggested [27].
Since TFF1 and TFF3 are found in the adult brain of mice and rats, it is interesting to investigate their expression in mouse embryonic nervous tissue, and also to screen for their presence in the neurons of the peripheral nervous system both in adult animals and embryos. The aim of our study was to investigate the presence of TFF1 and TFF3 in nervous tissues of the mouse embryo. Expression of TFF2 was not investigated in this study, because TFF1 and TFF3 were of main interest in recent nervous system research in adult animals. However, this could be a subject of future research on TFF peptides.
MATERIALS AND METHODS
The use of the animals was reviewed and approved by the Croatian board for scientific animal experiments and approved by a local ethical committee. Twelve CD1 mouse embryos 15, 16 and 17 days old (Theiler stage 23, 24 and 25) were used in this study (five 15-day old, four 16-day old and three 17-day old embryos). Embryos of a specific age in our collection were obtained in the following manner: after placing the females and males in the same cage, vagina was checked for the vaginal plug every morning. The presence of the vaginal plug was considered the beginning of pregnancy (gestational day 0). On a particular day, depending on the age of embryos required, the females were sacrificed by cervical dislocation.
Embryos were isolated, fixed in 4% paraformaldehyde, and then embedded in paraffin. Prepared paraffin blocks were cut sagittally into 6 µm thick sections that were transferred onto adhesive slides, and sections from all embryos were carefully selected to show nervous tissues of interest. Slides were dried, deparaffinized and rehydrated. Epitope retrieval was performed by heating in the microwave for around five minutes, using 0.01 M citric acid (pH 6). Blocking of endogenous peroxidase was done using 0.3% hydrogen peroxide for 15 min. Non-specific protein blocking was made with SuperBlock® (Thermo Scientific, Rockford, USA) for 30 min. Affinity purified primary polyclonal rabbit anti-TFF1 and anti-TFF3 antibodies (proprietary, self-made) were applied to separate slides, which were incubated at 4° C until tomorrow morning. The antibodies were purified by immunoadsorption from the serum, validated by Western blotting and specificity was confirmed by immunostaining of the specific sections of the alimentary tract. All the antibodies were produced according to the specific peptide sequence. Antibody dilutions used were 1:250, 1:500 and 1:1000 for anti-TFF1, and 1:2500, 1:5000 and 1:10000 for anti-TFF3. Phosphate-buffered saline (PBS) (pH 7.4) was used as a negative control (primary antibody omitted), and for the dilution of antibodies as well. Best results were obtained using 1:500 antibody dilution for TFF1 peptide, and 1:5000 for TFF3 peptide. Sections of adult mouse stomach were used as a positive control for TFF1 staining, and adult mouse intestine for TFF3 staining. Next day, slides were rinsed with PBS+0.05% Tween (Sigma–Aldrich, St. Louis, MO, USA) four times for 5 minutes and after that biotinylated goat anti-rabbit secondary antibody (Dako, Glostrup, Denmark) was applied for 30 minutes at room temperature. After another four rounds of five-minute washes in PBS+0.05% Tween, Streptavidin-HRP (Dako, Glostrup, Denmark) was applied to the slides for 30 minutes at room temperature. Slides were further rinsed four times in PBS+0.05% Tween, after which final exposure to DAB (3,3’-diaminobenzidine) was performed (Sigma–Aldrich, St. Louis, MO, USA). Slides were then washed with PBS+0.05% Tween, counterstained with hematoxylin, dehydrated and mounted with Canada balsam.
The following nervous tissues were examined: brain, spinal cord, dorsal root ganglia and cerebral ganglia (if available). Tissues at different developmental stages were analyzed manually using light microscopy at magnifications 20×, 40×, 100×, and 400×, and photographed at 400×. Slides were monitored with Olympus® BX50 microscope, photographed using Olympus® C-5050 digital camera and QuickPHOTO PRO imaging software (Promicra s.r.o., Prague, Czech Republic). For image assembly, Paint.NET software was used (version 4.0.5., dotPDN LLC, Rick Brewster and contributors).
RESULTS
Sections revealed different parts of the nervous system of the embryos. Morphologically, no abnormalities were detected, and the nervous tissues were in line with the developmental stages of the embryos. Presence of TFF1 and TFF3 peptides was detected in different parts of embryonic nervous system at all examined stages, from day 15 to day 17. Brain, spinal medulla, spinal ganglia and in some cases cerebral ganglia were available for examination.
TFF1 peptide was found in the ganglion cell somata in the dorsal root ganglia and trigeminal ganglion. The signal was present in the cytoplasm of neurons, mostly sparing the nucleus (Figure 1A). Also, a mild to moderate TFF1 signal was detectable in the whole central nervous system, in the white matter, glial cells and neurons of the brain and spinal medulla (Figures 2A and 3A). This signal was diffusely present, consistently appearing on all sections. TFF3 peptide was present both in the cytoplasm and the nucleus of ganglion cells of trigeminal ganglion and dorsal root ganglia (Figure 1B). Positive staining for TFF3 peptide was also detected in the cytoplasm and nucleus of neurons occupying different nuclei of brain and medulla oblongata (Figure 2B). As for the spinal cord, neurons in the gray matter of spinal cord were also positive in the same manner (Figure 3B). At day 17, the staining for TFF3 peptide was more cytoplasmic than nuclear. Some gentle staining for TFF3 peptide was visible in the white matter of the embryonic central nervous system. Choroid plexus was both TFF1 peptide and TFF3 peptide positive (not shown in figures).
FIGURE 1.
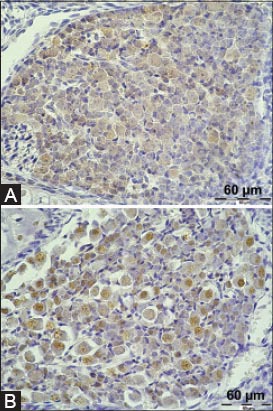
Presence of TFF1 and TFF3 in the dorsal root ganglion cells of mouse embryos. A TFF1 staining; B TFF3 staining. Magnification 400×.
FIGURE 2.
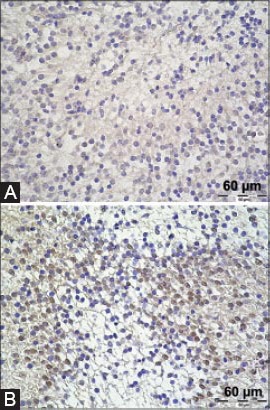
TFF1 and TFF3 signal in the brain of mouse embryos. A TFF1 staining, hippocampus; B TFF3 staining, olfactory bulb. Magnification 400×.
FIGURE 3.
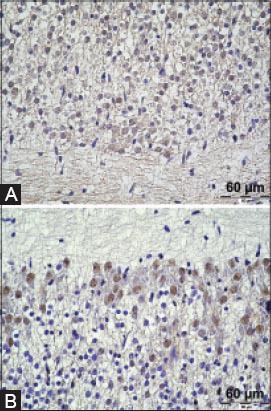
Positive staining for TFF1 and TFF3 in the neurons of embryonic spinal medulla. Cytoplasmic staining and a diffuse signal in the white matter for TFF1 and TFF3 localization in the nucleus and cytoplasm of the neurons in the grey matter. A TFF1 staining; B TFF3 staining. Magnification 400×
There were no differences in the staining pattern at the different stages of embryonic development. All negative controls showed no immunostaining, and all positive controls showed typical staining both for TFF1 and TFF3 peptide (not shown in figures).
DISCUSSION
The presence and localization of TFF1 and TFF3 peptides in the neural tissue at different stages of development was investigated using immunohistochemistry. Trefoil factors TFF1 and TFF3 were detected in mouse brain and spinal medulla at developmental stages E15-E17. At this point, we investigated the later stages of the embryonic development since they are morphologically and functionally closer to that of adult animals, and, therefore, more comparable to data currently available.
Although consistent with that of adult animals [18,20], detailed localization of TFF1 and TFF3 peptides in a developing brain was not determined in this study. However, we showed for the first time that TFF peptides can be located in the peripheral nervous tissue. Both examined molecules were found in ganglion cell somata: TFF1 peptide mostly in the cytoplasm and TFF3 peptide in the cytoplasm and the nucleus.
In our study, positive staining for TFF1 and TFF3 peptides was detected at similar developmental stages as the expression of TFF genes observed in developing mouse brain by other researchers (E16-E18) [18]. In adult nervous tissue, TFF1 gene was expressed at low level nearly uniformly in all brain regions, while TFF3 gene which was located in cerebral cortex and cerebellum before birth, was restricted to hippocampus, temporal cortex and cerebellum after birth [18]. Expression of TFF1 mRNA in the rat hippocampus reaches maximum around the birth [2].
The role of TFF peptides in the nervous system is still not fully understood. The presence of TFF3 peptide in porcine [25] and human [22] oxytocinergic neurons suggests their role as a neurotransmitter/modulator in various brain regions innervated by these neurons, like brain stem, spinal cord and pontine tegmentum [2], or as a neurohormone released into bloodstream and affecting potential target cells. Recent research has revealed various behavioral effects of TFF3 peptide. It affects anxiety bidirectionally in dose-dependent manner and improves learning and object recognition memory [24,26]. In our study, TFFs were found in different neuron somata, and (especially TFF1 peptide) diffusely in the white matter of the central nervous system. These results point to a possibility that TFFs may act as neuromodulators and/or neurotransmitters even during embryonic development.
Presence of TFF peptides in developing neural tissues raises questions regarding their possible role in the nervous tissue development. Since TFF peptides were associated with oxytocin secretion in neural tissue and mucin secretion in goblet cells [20], there is a possibility that TFFs may be markers for physiological maturation of specific tissues and cells. If this is the case, they might be used as an embryonic mark to pinpoint the start of an active physiological function. There is, however, another potential developmental role for TFF peptides. Research shows that they are also involved in cell proliferation and migration [15]. These processes are indispensable for the development of central nervous system. Since cell proliferation and migration are of particular importance in embryonic development, presence of TFFs in the developing nervous system goes well with similar roles of these peptides already demonstrated in adult tissues. For this reason, our results support the theory that TFFs might have an active role in nervous tissue development. There is also a possibility that TFF peptides are involved in development and plasticity of neural tissue through mechanisms responsible for their protective effects in epithelial tissues, for example, in the case of gastrointestinal mucosal protection and restitution [20]. Otto and Patel showed that all three TFFs were up-regulated in response to physical wounding of embryological gut (E17-18], but the expression showed differences in the adult [28]. Identification of novel rodent gene encoding for neural regeneration protein, which contains domain with homology to survival-promoting peptide (SPP) and TFF1 peptide, and exerts biological activities as neural migration, proliferation, differentiation, enhancement of neurite outgrowth and promotion of neuronal survival [29], supports proposed active role of TFF peptides in neural development and protective function.
Considering various effects of TFF peptides in cell migration, proliferation, apoptosis and angiogenesis, and the pattern of their expression in developing nervous system, it can be concluded that TFF peptides might be involved in complex processes of nervous system development and differentiation, and brain plasticity. These preliminary results suggest further research in order to elucidate these peptides’ features in the nervous tissues and embryonic development. Such research is important, since mechanisms of embryonic development can sometimes be linked to pathological processes leading to different debilitating diseases [30], and embryological research can suggest novel regenerative therapies [31]. Possible next steps would be to investigate TFF protein expression at the earlier stages of embryonic development, and also to investigate if TFF2 is expressed in adult and embryonic nervous system, both of which may be interesting for future research.
ACKNOWLEDGEMENTS
This study was supported by the Ministry of Science, Education and Sports of the Republic of Croatia through grant No. 219-0982914- 2179. The authors wish to thank Ms. Danica Matić for her valuable expertise in the histology laboratory.
DECLARATION OF INTERESTS
The authors hereby declare that they have no conflict of interests
REFERENCES
- [1].Blin N. Cytoprotective trefoil peptides abound in new functions. Cell Mol Life Sci. 2005;62:2907–9. doi: 10.1007/s00018-005-5477-5. http://dx.doi.org/10.1007/s00018-005-5477-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hoffmann W, Jagla W, Wiede A. Molecular medicine of TFF-peptides: from gut to brain. Histol Histopathol. 2001;16(1):319–34. doi: 10.14670/HH-16.319. [DOI] [PubMed] [Google Scholar]
- [3].Madsen J, Nielsen O, Tornoe I, Thim L, Holmskov U. Tissue Localization of Human Trefoil Factors 1, 2, and 3. J Histochem Cytochem. 2007;55:505–13. doi: 10.1369/jhc.6A7100.2007. http://dx.doi.org/10.1369/jhc.6A7100.2007 . [DOI] [PubMed] [Google Scholar]
- [4].Regalo G, Wright NA, Machado JC. Trefoil factors: from ulceration to neoplasia. Cell Mol Life Sci. 2005;62(24):2910–5. doi: 10.1007/s00018-005-5478-4. http://dx.doi.org/10.1007/s00018-005-5478-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ahmed ARH, Griffiths AB, Tilby MT, Westley BR, May FEB. TFF3 is a normal breast epithelial protein and is associated with differentiated phenotype in early breast cancer but predisposes to invasion and metastasis in advanced disease. Am J Pathol. 2012;180(3):904–16. doi: 10.1016/j.ajpath.2011.11.022. http://dx.doi.org/10.1016/j.ajpath.2011.11.022 . [DOI] [PubMed] [Google Scholar]
- [6].Chaiyarit P, Utrawichian A, Leelayuwat C, Vatanasapt P, Chanchareonsook N, Samson MH, et al. Investigation of trefoil factor expression in saliva and oral mucosal tissues of patients with oral squamous cell carcinoma. Clin Oral Investig. 2012;16(6):1549–56. doi: 10.1007/s00784-011-0667-z. http://dx.doi.org/10.1007/s00784-011-0667-z . [DOI] [PubMed] [Google Scholar]
- [7].Dhar DK, Wang TC, Tabara H, Tonomoto Y, Maruyama R, Tachibana M, et al. Expression of trefoil factor family members correlates with patient prognosis and neoangiogenesis. Clin Cancer Res. 2005;11(18):6472–8. doi: 10.1158/1078-0432.CCR-05-0671. http://dx.doi.org/10.1158/1078-0432.CCR-05-0671 . [DOI] [PubMed] [Google Scholar]
- [8].Kannan N, Kang J, Kong X, Tang J, Perry JK, Mohankumar KM, et al. Trefoil factor 3 is oncogenic and mediates anti-estrogen resistance in human mammary carcinoma. Neoplasia. 2010;12(12):1041–53. doi: 10.1593/neo.10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kosriwong K, Menheniott TR, Giraud AS, Jearanaikoon P, Sripa B, Limpaiboon T. Trefoil factors: tumor progression markers and mitogens via EGFR/MAPK activation in cholangiocarcinoma. World J Gastroenterol. 2011;17(12):1631–41. doi: 10.3748/wjg.v17.i12.1631. http://dx.doi.org/10.3748/wjg.v17.i12.1631 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chaiyarit P, Chayasadom A, Wara-Aswapati N, Hormdee D, Sittisomwong S, Nakaresisoon S, et al. Trefoil factors in saliva and gingival tissues of patients with chronic periodontitis. J Periodontol. 2012;83(9):1129–38. doi: 10.1902/jop.2011.110431. http://dx.doi.org/10.1902/jop.2011.110431 . [DOI] [PubMed] [Google Scholar]
- [11].Rösler S, Haase T, Claassen H, Schulze U, Schicht M, Riemann D, et al. Trefoil factor 3 is induced during degenerative and inflammatory joint disease, activates matrix metalloproteinases, and enhances apoptosis of articular cartilage chondrocytes. Arthritis Rheum. 2010;62(3):815–25. doi: 10.1002/art.27295. http://dx.doi.org/10.1002/art.27295 . [DOI] [PubMed] [Google Scholar]
- [12].Wills-Karp M, Rani R, Dienger K, Lewkowich I, Fox JG, Perkins C, et al. Trefoil factor 2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection. J Exp Med. 2012;209(3):607–22. doi: 10.1084/jem.20110079. http://dx.doi.org/10.1084/jem.20110079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wright NA, Poulsom R, Stamp G, Van Norden S, Sarraf C, Elia G, et al. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Scand J Gastroenterol Suppl. 1992;193:76–82. doi: 10.3109/00365529209096010. http://dx.doi.org/10.3109/00365529209096010 . [DOI] [PubMed] [Google Scholar]
- [14].Baus-Loncar M, Kayademir T, Takaishi S, Wang T. Trefoil factor family 2 deficiency and immune response. Cell Mol Life Sci. 2005;62(24):2947–55. doi: 10.1007/s00018-005-5483-7. http://dx.doi.org/10.1007/s00018-005-5483-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hoffmann W. Trefoil factors TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell Mol Life Sci. 2005;62(24):2932–8. doi: 10.1007/s00018-005-5481-9. http://dx.doi.org/10.1007/s00018-005-5481-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Thim L, Madsen F, Poulsen SS. Effect of trefoil factors on the viscoelastic properties of mucus gels. Eur J Clin Invest. 2002;32(7):519–27. doi: 10.1046/j.1365-2362.2002.01014.x. http://dx.doi.org/10.1046/j.1365-2362.2002.01014.x . [DOI] [PubMed] [Google Scholar]
- [17].Hirota M, Awatsuji H, Sugihara Y, Miyashita S, Furukawa Y, Hayashi K. Expression of pS2 gene in rat brain. Biochem Mol Biol Int. 1995;35(5):1079–84. [PubMed] [Google Scholar]
- [18].Hinz M, Schwegler H, Chwieralski CE, Laube G, Linke R, Pohle W, et al. Trefoil factor family (TFF) expression in the mouse brain and pituitary: changes in the developing cerebellum. Peptides. 2004;25(5):827–32. doi: 10.1016/j.peptides.2004.01.020. http://dx.doi.org/10.1016/j.peptides.2004.01.020 . [DOI] [PubMed] [Google Scholar]
- [19].Hirota M, Awatsuji H, Furukawa Y, Hayashi K. Cytokine regulation of PS2 gene expression in mouse astrocytes. Biochem Mol Biol Int. 1994;33(3):515–20. [PubMed] [Google Scholar]
- [20].Hoffmann W, Jagla W. Cell type specific expression of secretory TFF peptides: colocalization with mucins and synthesis in the brain. Int Rev Cytol. 2002;213:147–81. doi: 10.1016/s0074-7696(02)13014-2. http://dx.doi.org/10.1016/S0074-7696(02)13014-2 . [DOI] [PubMed] [Google Scholar]
- [21].Paunel-Görgülü AN, Franke AG, Paulsen FP, Dünker N. Trefoil factor family peptide 2 acts pro-proliferative and pro-apoptotic in the murine retina. Histochem Cell Biol. 2011;135(5):461–73. doi: 10.1007/s00418-011-0810-6. http://dx.doi.org/10.1007/s00418-011-0810-6 . [DOI] [PubMed] [Google Scholar]
- [22].Jagla W, Wiede A, Dietzmann K, Rutkowski K, Hoffmann W. Co-localization of TFF3 peptide and oxytocin in the human hypothalamus. FASEB J. 2000;14(9):1126–31. doi: 10.1096/fasebj.14.9.1126. [DOI] [PubMed] [Google Scholar]
- [23].Probst JC, Skutella T, Müller-Schmid A, Jirikowski GF, Hoffmann W. Molecular and cellular analysis of rP1.B in the rat hypothalamus: in situ hybridization and immunohistochemistry of a new P-domain neuropeptide. Brain Res Mol Brain Res. 1995;33(2):269–76. doi: 10.1016/0169-328x(95)00137-h. http://dx.doi.org/10.1016/0169-328X(95)00137-H . [DOI] [PubMed] [Google Scholar]
- [24].Schwarzberg H, Kalbacher H, Hoffmann W. Differential behavioral effects of TFF peptides: injections of synthetic TFF3 into the rat amygdala. Pharmacol Biochem Behav. 1999;62(1):173–8. doi: 10.1016/s0091-3057(98)00137-3. http://dx.doi.org/10.1016/S0091-3057(98)00137-3 . [DOI] [PubMed] [Google Scholar]
- [25].Schwarz H, Jagla W, Wiede A, Hoffmann W. Ultrastructural co-localization of TFF3-peptide and oxytocin in the neural lobe of the porcine pituitary. Cell Tissue Res. 2001;305(3):411–6. doi: 10.1007/s004410100412. http://dx.doi.org/10.1007/s004410100412 . [DOI] [PubMed] [Google Scholar]
- [26].Shi H-S, Yin X, Song L, Guo Q-J, Luo X-H. Neuropeptide Trefoil factor 3 improves learning and retention of novel object recognition memory in mice. Behav Brain Res. 2012;227(1):265–9. doi: 10.1016/j.bbr.2011.10.051. http://dx.doi.org/10.1016/j.bbr.2011.10.051 . [DOI] [PubMed] [Google Scholar]
- [27].Paterson RW, Bartlett JW, Blennow K, Fox NC, Shaw LM, et al. Alzheimer's Disease Neuroimaging Initiative. Cerebrospinal fluid markers including trefoil factor 3 are associated with neurodegeneration in amyloid-positive individuals. Transl Psychiatry. 2014;4(7):e419. doi: 10.1038/tp.2014.58. http://dx.doi.org/10.1038/tp.2014.58 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Otto WR, Patel K. Trefoil factor family (TFF)-domain peptides in the mouse: embryonic gastrointestinal expression and wounding response. Anat Embryol (Berl) 1999;199(6):499–508. doi: 10.1007/s004290050247. http://dx.doi.org/10.1007/s004290050247 . [DOI] [PubMed] [Google Scholar]
- [29].Gorba T, Bradoo P, Antonic A, Marvin K, Liu D-X, Lobie PE, et al. Neural regeneration protein is a novel chemoattractive and neuronal survival-promoting factor. Exp Cell Res. 2006;312(16):3060–74. doi: 10.1016/j.yexcr.2006.06.020. http://dx.doi.org/10.1016/j.yexcr.2006.06.020 . [DOI] [PubMed] [Google Scholar]
- [30].Bijelić N, Belovari T, Baus Lončar M. Trefoil factor family protein 3 (TFF3) is present in cartilage during endochondral ossification in the developing mouse fetus. Acta Histochem. 2013;115(3):204–8. doi: 10.1016/j.acthis.2012.06.007. http://dx.doi.org/10.1016/j.acthis.2012.06.007 . [DOI] [PubMed] [Google Scholar]
- [31].Katusić A, Jurić-Lekić G, Jovanov-Milosević N, Vlahović M, Jezek D, Serman L, et al. Development of the fetal neural retina in vitro and in ectopic transplants in vivo. Coll Antropol. 2008;32(1):201–7. [PubMed] [Google Scholar]


