Abstract
A range of factors are believed to exert a negative influence on opinions of physicians about generic drugs. The aim of this study was to survey the opinions of primary care doctors on generics, and determine the factors which may affect them. A questionnaire comprising thirty eight questions was distributed among primary care doctors working in seventy out-patient clinics of the Lodzkie province, Poland, during the period of January 1, 2010 – December 31, 2010. A total of 170 of 183 participants completed the survey (average age 48.5; 70.0% women): a 92.9% response rate. While 38.8% of physicians claimed that generics were worse than brand name drugs, 54.1% considered them to be better. However, 36.5% of the doctors did not choose generics for their own use. Two key opinions were identified among the responses concerning the effectiveness of generic drugs: use of generic drugs by the physician (p<0.001), and their opinion that pharmacists do inform patients about generic drugs (p<0.05). Although existing evidence confirms that generic and brand name drugs are equally effective, many physicians doubt this, which prevents them from being used as cost effective drug therapy. In order to increase healthcare savings through the use of generics, these factors should be addressed: for example, convincing a physician to adopt generics for personal use may be an efficient way to support more cost effective treatment of his patients.
KEY WORDS: Drug therapy, generic drugs, drug substitution, health care surveys, general practice/family medicine
INTRODUCTION
Generic drugs are medicines which are identical, or are bioequivalent to brand name drugs in dosage, form, safety, strength, route of administration, quality, performance characteristics and intended use [1]. Instead of clinical trials, bioequivalence studies are obligatory for generic drugs to be registered as a new product and launched onto the market. Bioequivalence studies are mainly based on two pharmacokinetic parameters: AUC, or area under the plasma concentration curve from administration to last observed concentration, and Cmax, or maximal plasma concentration. For the generic drug to be regarded as bioequivalent, and hence to be accepted for sale on the US or EU markets, its AUC and Cmax values must be within an acceptance range of 0.80 – 1.25 of those of the reference product [2,3]. In Poland, prior to being introduced to the market, medications have to be approved by the office of medicinal products, medical devices and biocidal products [4].
The prices of generic drug are set by the producers; however, if they are included on the reimbursement lists of the Polish Ministry of Health, the prices are fixed and identical in every pharmacy and for every patient [5]. As there is no need for expensive preclinical and clinical trials, generic drugs are much cheaper than their brand-name counterparts, and hence, much more affordable for the average patient.
Worldwide expenditure on medicines in 2010 reached approximately $856 billion, of which 27% accounted for generics [6], a figure which is expected to increase up to 39% in 2015 [6]. Generic drug use saved the healthcare systems and consumers over $192 billion worldwide in 2011 alone [7]. Although generics are regarded as safe with regard to their bioequivalence [7,8], studies in the USA [9,10], Australia [11], Spain [12] and Greece [13] indicate that they are mistrusted by physicians. A literature review reveals that despite Poland being the largest European market for generics in 2010, and one where generics represent 40% of total drug sales in terms of value [14], no questionnaire-based study of physicians’ opinions on generic drugs has been conducted. Hence the aim of the study is to evaluate the opinions of physicians on generic drugs and secondly, to identify the factors affecting their opinions. Primary care physicians, having the highest number of consultations with their patients yearly [15] and thus having the most opportunities to prescribe generics, were chosen as a target group for this study.
MATERIALS AND METHODS
Study design
A cross sectional study was performed. A thirty-eight item questionnaire was designed, covering a wide spectrum of issues related to the perception of generics and their use: the effectiveness of generic drugs, the brand name or generic drugs the physician would personally use, the frequency of informing patients about generics, and the knowledge of generic brands and generic substitution: i.e., the exchange of prescribed drug with its cheaper equivalent at the pharmacy, which is allowed by Polish law. In most questions, the participants were allowed to choose only one answer unless stated otherwise. If the physician chose more than one answer, this question was counted as “no answer”. The study protocol was accepted by Ethics committee of the Medical University of Lodz (No. RNN/616/08/KB).
The questionnaire was distributed among all family physicians working in seventy primary care clinics randomly chosen from the list available on the National Health Fund (Narodowy Fundusz Zdrowia) website [16] within the Lodzkie province, Poland. The total population of the province in 2012 was 2.5 million, i.e. approximately 6.5% of Poland’s population, 52% of whom were women. The province employs around 8% of the approximately 140,000 physicians working in Poland, of whom 6,509 were family medicine specialists. In 2012 there were 1523 outpatient clinics in Lodzkie province, representing 8% of all Polish outpatient departments, and over 18 million visits took place that year - 7% of visits made in the country as a whole [17].
Selection of study subjects
The inclusion criteria were as follows: active employment as a physician in a primary care clinic in the Lodzkie province, no previous participation in the study, and oral agreement to fill in the survey.
The questionnaire was directly distributed by one of the authors (PL) in the period of January 1, 2010 – December 31, 2010. A total number of 183 physicians received the questionnaire. Completing the survey was voluntary and anonymous. The questionnaire was first piloted with 15 family physicians of the 183 respondents in order to identify mistakes, correct them and ensure that questions were well perceived by physicians. The only mistakes found were spelling errors and the numbering of questions, which were corrected. The answers given in the pilot study were included in the final results.
Statistical Analysis
Statistica (v.10.0.1011.7, StatSoft, Inc. 2011, Tulsa, OK, USA) software was used for statistical analysis. The Chi2 test and Fisher’s exact test were used for correlation analysis. The factors which returned a correlation with a positive opinion on generic drugs were used for multivariate analysis by logistic regression. The multivariate analysis was used to determine factors that predict the positive opinions of primary care physicians about generic drugs. Odds ratios and 95% confidence intervals were derived.
RESULTS
One hundred and seventy primary care physicians, i.e., 92.9% of the 183 addressed, agreed to take part in the study and completed the paper questionnaire: thirteen physicians did not agree to participate. Not every participant filled in the full survey (the number of participants is marked if less than n=170).
Demographic characteristic of participants
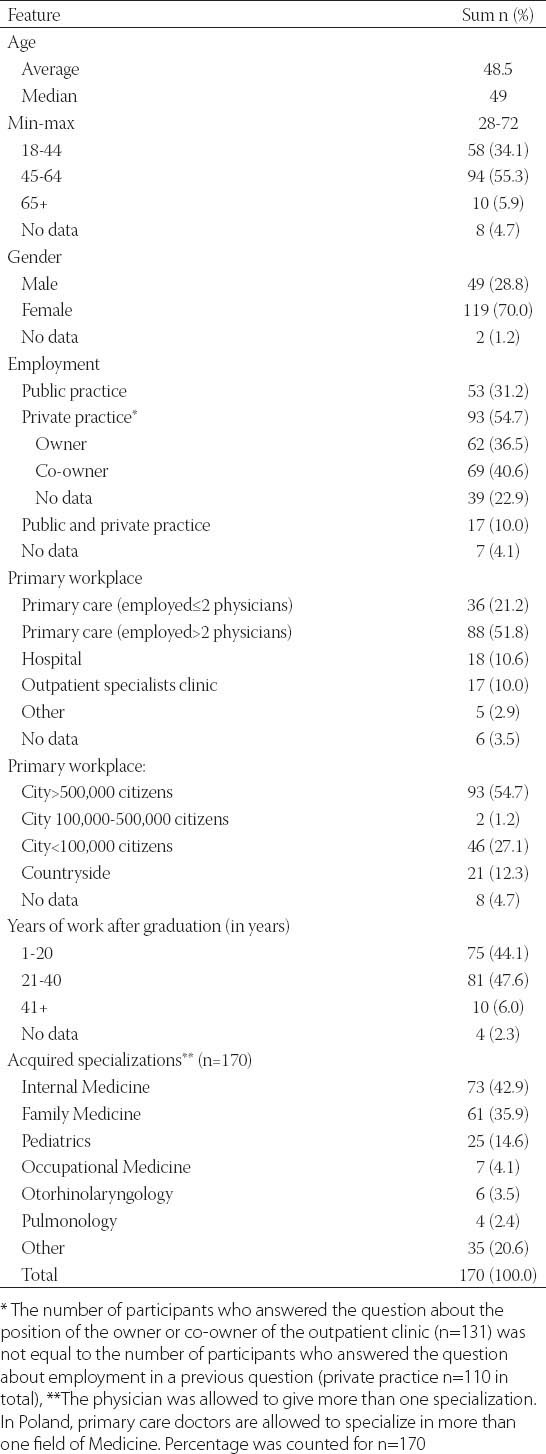
The demographic characteristics of the survey participants are presented in Table 1.
TABLE 1.
Demographic characteristics of survey participants.
General knowledge of generics
Almost all (96.2%) physicians knew the term “generic drug”, but only 84.1% chose a correct synonym. From the range of generic drugs prescribed in Poland, the three most commonly given by the respondents were Diuresin SR (indapamide; 11.0%), Amlopin (amlodipine; 7.6%) and Amoksiklav (amoxicillin and clavulanic acid; 7.0%). The most commonly reported sources of information about generic drugs were pharmaceutical company representatives (82.3%), the medical press (73.5%) and medical conferences (52.3%). While 57.1% of participants were familiar with the term “generic substitution”, 41.2% were not. Almost 6% of physicians incorrectly claimed that it was not possible to change a drug to its cheaper equivalent according to Polish law.
Knowledge of the price of generics
Almost every doctor (94.1%) confirmed that financial constraints may prevent patients from buying medications, but only 37.1% knew the prices of drugs well, with 60.5% able to give a rough estimate. Nonetheless, 73.0% of respondents were considering prescribing generics and 71.1% regularly informed patients of this possibility. The respondents who considered generics when prescribing a drug tended to report doing so either often or always (in 50-100% of cases; p<0.001) and were more ready to inform patients about generic substitution (p<0.001). These physicians were also more likely to ask patients if they could afford the prescribed drugs (p<0.001), most of whom doing so in 50-100% of cases (p<0.01). No correlation was found between the physician considering the use of generics and their knowledge of cost of the drug (p>0.05). Most physicians (68.2%) believed that pharmacists inform patients about the possibility of buying cheaper equivalents of prescribed drugs.
General opinion about generic drugs
While approximately two-fifths (38.8%, n=170) of the participants claimed that generics were worse than brand name drugs, 54.1% were more positive: 53.5% perceived them as equally effective and 0.6% as better than brand name drugs. The most common negative opinions provided by the survey respondents concerned poorer drug parameters such as taste, pharmacokinetic and physical factors (34.8%), poorer effectiveness (28.8%) and a greater number of adverse effects (12.1%).
The respondents indicated that the prescription (34.1%) and other information given by the pharmacist (32.9%) had the greatest influence on the decision by the patient to choose generic or brand-name drugs. Sixty percent of physicians reported choosing cheaper equivalents of brand-name drugs when buying prescription drugs for themselves and were more willing to consider generics when prescribing drugs for patients (p<0.005).
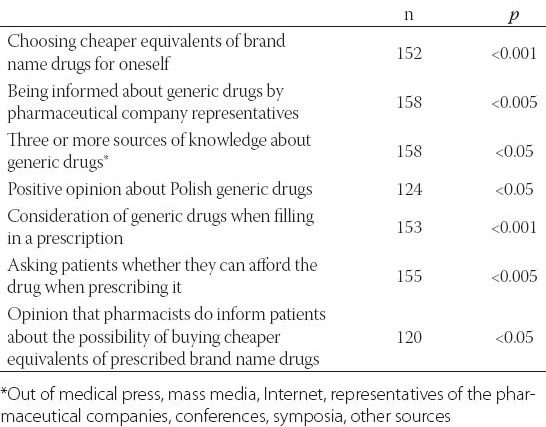
Logistic regression indicated that the respondents’ own choice of cheaper equivalent of brand name drug (p<0.001, 95% CI: 2.50-13.80, OR=5.87) and their opinion that pharmacists do inform patients about the possibility of buying cheaper equivalents of prescribed brand name drugs (p<0.05, 95% CI: 0.03-0.84, OR=0.16) had a significant effect on the respondents’ positive perception of the effectiveness of generic drugs in the surveyed sample (n=117). However, the awareness of the possibility to change a drug for its cheaper equivalent in a pharmacy, self-assessment of knowledge of generic drugs, familiarity with the term “generic drug”, the opinion that patients may not buy their drugs due to financial constraints or age of the respondent had no significant influence on the perception of generics. For details, see Table 2.
TABLE 2.
Factors associated with opinion of physicians of generic drugs having equal or better effectiveness then brand-name drugs in correlation analysis using chi2 test. “Hard to say” or no answers were omitted.
DISCUSSION
The findings of this study suggest that primary care doctors doubt the equal effectiveness of generic and brand name drugs. Moreover, they indicate that two key factors may influence respondent opinions regarding the effectiveness of generic drugs: the readiness of the respondents to use generic drugs personally, and doubts whether patients are fully informed in the pharmacy about the possibility of buying generic drugs.
The doubts of the primary care physicians regarding the effectiveness of generic drugs given in the present study are consistent with those revealed by other studies conducted on physicians [10,13,18] or medical students [19]. Although many physicians indicate a negative opinion of the effectiveness of generic medications, studies on narrow therapeutic index drugs like antiepileptics [20] or anticoagulants [21], systematic reviews on cardiovascular medications [22] and the long history of their mass use in numerous countries, including Poland, suggest that this perspective is baseless, and hence, the negative opinions of doctors are surprising.
The findings of the present study confirm those of Shrank [10], who found that pharmaceutical company representatives (75%) and medical journals (42%) were the most common sources of information about generic drugs for the surveyed physicians. Although physicians are known to be aware that the information provided by pharmaceutical representatives may be biased [23], it has been shown that such information may nevertheless influence the perception of a drug by the physician [24], as well as increase both the rationality and cost of prescription [25]. The negative opinion of physicians concerning cheaper equivalents may be an important barrier to the use of cheaper drugs, which was given as one of the key causes of the considerably smaller sale of generics observed in the Republic of Ireland compared to England in 1993 (around 50%) [26]. As many as 34.8% of respondents in the present study believed that the perceived poorer effectiveness of generic drugs was due to their pharmacokinetic and physical parameters. As approval is only awarded to generics after being proven equivalent to brand-name products by a series of bioequivalence tests, this negative opinion appears to be based on a lack of awareness of the registration procedure and associated regulations, rather than the product quality of the generic itself.
In Poland, the physician may prescribe a medication using the name of an original brand, a generic brand or the international non-proprietary name of a drug [27]. Community pharmacies may exchange the prescribed drug with a cheaper one. This procedure, which has existed in Poland for many years, is called “generic substitution”. Our present findings reveal a correlation between the information about generic substitution given to patients and their consideration of generics. Physicians who considered generics when prescribing a brand-name drug were more likely to inform patients that it may be exchanged for a cheaper generic, which illustrates how the behavior of the physician may influence the patient to choose a cheaper drug at pharmacy level, making generics more accessible.
The National Health Fund (NFZ) provides a nationwide public insurance program which, with a few exceptions, covers every Polish citizen. The costs of most crucial drugs are reimbursed by the NFZ, and as different products with the same active compound but a different price receive the same level of reimbursement, promoting generic medicines decreases patient expenditure on drugs. The factors associated with the reluctance to use generics identified by the present study, if confirmed on a representative group of physicians, should be addressed in order to increase generic usage. Persuading physicians to choose generics for personal use, to seek information about generics from more varied sources, to consider cheaper equivalents when prescribing medications for patients and to ask patients if they can afford prescribed medications are the most important actions which may help decrease the cost of healthcare both for the patient and the national healthcare system as a whole.
Contrary to Shrank et al. [10], no correlation was found in the present survey between physician age and opinion on the efficacy of generics, or physician age and preference for generics. One study highlights the positive opinions held by physicians about generic drugs [12]. The significant discrepancies suggest that these opinions have a multifactorial background, which may be linked to the practitioners, the patients and the drugs themselves [28].
An obvious limitation of this study is the fact that the survey was conducted among primary care physicians in the Lodzkie province only, an area of 2.5 million inhabitants, and is not representative of the entire group of Polish primary care physicians. The number of primary care doctors surveyed, one hundred and seventy, was smaller than those included in some other studies [10,13]. However, contrary to the studies based on postal [9,12,13] or web-based surveys [10], the three key advantages of this study were, firstly, that the population was homogenous and was limited to primary care doctors only, secondly, that target physicians completed the survey themselves, and thirdly, that the statistical analysis returned highly significant results (p<0.001), suggesting that this strong correlation between variables could be observed also in a larger population.
This is the first study to indicate that the perception of generics by physicians is related to both their own usage of generic drugs, and their perception of information given by pharmacists. Further representative studies are needed to confirm these results. However, action is required in these areas to improve the perception of the effectiveness of generic drugs among primary care physicians in Poland.
CONCLUSIONS
Two factors affecting the perception of generic drugs by physicians have been identified in the studied sample: the choice of cheaper equivalents of brand name drugs for personal use and the opinion that pharmacists do inform patients about the possibility of buying cheaper equivalents of prescribed brand name drugs.
ACKNOWLEDGMENTS
This research was financially supported by Medical University of Lodz grant No. 502-03/6-029-03/502-64-069.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- [1].U.S. Food and, Drug Administration. [Accessed: October 5 2014]. Available from: http://www.fda.gov/drugs/resourcesforyou/consumers/buyingusingmedicinesafely/understandinggenericdrugs/default.htm .
- [2].Note for guidance on the investigation of bioavailability and bioequivalence. The European Agency for the Evaluation of Medicinal Products. London 14.12.2000, CPMP/EWP/QWP/1401/98. [Accessed: October 5 2014]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003519.pdf .
- [3].U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) March; 2003. [Accessed October 5 2014]. Guidance for Industry. Bioavailability and Bioequivalence Studies for Orally Administered Drug Products —General Considerations. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070124.pdf . [Google Scholar]
- [4].The Office of Medicinal Products, Medical Devices and Biocidal Products. [Accessed October 5 2014]. Available at: http://en.urpl.gov.pl .
- [5].Ustawa z dn. 12 maja 2011 o refundacji leków, środków spożywczych specjalnego przeznaczenia żywieniowego oraz wyrobów medycznych. [Act of May 12, 2011 on drugs reimbursement, food products of special nutritional use and medical products] [Google Scholar]
- [6].The Global Use of Medicines: Outlook Through 2015. Report by the IMS Institute for Healthcare Informatics. [Accessed: October 5 2014]. Available from: http://www.imshealth.com/deployedfiles/ims/Global/Content/Insights/IMS%20Institute%20for%20Healthcare%20Informatics/Global_Use_of_Medicines_Report.pdf .
- [7].Zore M, Harris A, Tobe LA, Siesky B, Januleviciene I, Behzadi J, et al. Generic medications in ophthalmology. Br J Ophthalmol. 2013;97(3):253–7. doi: 10.1136/bjophthalmol-2012-302245. http://dx.doi.org/10.1136/bjophthalmol- 2012-302245 . [DOI] [PubMed] [Google Scholar]
- [8].Lewek P, Kardas P. Generic drugs: the benefits and risks of making the switch. J FamPract. 2010;59:634–40. http://dx.doi.org/10.1016/j.seizure.2005.12.010 . [PubMed] [Google Scholar]
- [9].Banahan BF, 3rd, Kolassa EM. A physician survey on generic drugs and substitution of critical dose medications. Arch Intern Med. 1997;157(18):2080–2088. http://dx.doi.org/10.1001/archinte.1997.00440390066010 . [PubMed] [Google Scholar]
- [10].Shrank WH, Liberman JN, Fischer MA, Girdish C, Brennan TA, Choudhry NK. Physician perceptions about generic drugs. Ann Pharmacother. 2011;45(1):31–8. doi: 10.1345/aph.1P389. http://dx.doi.org/10.1345/aph.1P389 . [DOI] [PubMed] [Google Scholar]
- [11].Hassali MA, Kong DCM, Stewart K. 2006. Generic medicines: perceptions of general practitioners in Melbourne, Australia. Journal of Generic Medicines. 2006;3(3):214–225. http://dx.doi.org/10.1057/palgrave.jgm.4940122 . [Google Scholar]
- [12].García AJ, Martos F, Leiva F, Sánchez de la Cuesta F. [Genéricos: ¿buenos o malos? Conocimientos y actitudes de los médicos ante los medicamentosgenéricos] Gac Sanit. 2003;17:144–9. doi: 10.1016/s0213-9111(03)71712-9. http://dx.doi.org/10.1016/S0213-9111(03)71712-9 . [DOI] [PubMed] [Google Scholar]
- [13].Tsiantou V, Zavras D, Kousoulakou H, Geitona M, Kyriopoulos J. Generic medicines: Greek physicians’ perceptions and prescribing practices. J Clin Pharm Ther. 2009;34:547–54. doi: 10.1111/j.1365-2710.2009.01037.x. http://dx.doi.org/10.1111/j.1365-2710.2009.01037.x . [DOI] [PubMed] [Google Scholar]
- [14].European Generic medicines Association. [Accessed Aug 7 2013]. Available from: http://www.egagenerics.com/images/factsheet/IMS_deck_for_website.pdf .
- [15].Warsaw: Central Statistical Office; 2012. Statistical Yearbook of Republic of Poland. [Google Scholar]
- [16].Narodowy Fundusz Zdrowia. [Accessed August 7 2013]. Available from: http://www.nfz-lodz.pl/index.php/dlapacjentow/gdzie-sie-leczyc .
- [17].Central Statistical Office of Poland. [Accessed October 5 2014]. Available from: http://stat.gov.pl .
- [18].Heikkilä R, Mäntyselkä P, Hartikainen-Herranen K, Ahonen R. Customers ‘and physicians’ opinions of and experiences with generic substitution during the first year in Finland. Health Policy. 2007;82(3):366–74. doi: 10.1016/j.healthpol.2006.10.006. http://dx.doi.org/10.1016/j.healthpol.2006.10.006 . [DOI] [PubMed] [Google Scholar]
- [19].Hassali MA, Stewart K, Kong DC. A national survey on knowledge and perceptions of senior medical students in Australia about generic medicines. Med J Aust. 2008;21(188) 2:123–4. doi: 10.5694/j.1326-5377.2008.tb01543.x. [DOI] [PubMed] [Google Scholar]
- [20].Kesselheim AS, Stedman MR, Bubrick EJ, Gagne JJ, Misono AS, Lee JL, et al. Seizure outcomes following the use of generic versus brand-name antiepileptic drugs: a systematic review and meta-analysis. Drugs. 2010;70(5):605–21. doi: 10.2165/10898530-000000000-00000. http://dx.doi.org/10.2165/10898530-000000000-00000 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dentali F, Donadini MP, Clark N, Crowther MA, Garcia D, Hylek E, et al. Warfarin associated research projects and other endeavors (WARPED) consortium. Brand name versus generic warfarin: a systematic review of the literature. Pharmacotherapy. 2011;31(4):386–93. doi: 10.1592/phco.31.4.386. http://dx.doi.org/10.1592/phco.31.4.386 . [DOI] [PubMed] [Google Scholar]
- [22].Kesselheim AS, Misono AS, Lee JL, Stedman MR, Brookhart MA, Choudhry NK, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA. 2008;300(21):2514–26. doi: 10.1001/jama.2008.758. http://dx.doi.org/10.1001/jama.2008.758 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hodges B. Interactions with the pharmaceutical industry: experiences and attitudes of psychiatry residents, interns and clerks. CMAJ. 1995;153(5):553–9. [PMC free article] [PubMed] [Google Scholar]
- [24].Peay MY, Peay ER. The role of commercial sources in the adoption of a new drug. SocSciMed. 1988;26(12):1183–9. doi: 10.1016/0277-9536(88)90149-9. http://dx.doi.org/10.1016/0277- 9536(88)90149-9 . [DOI] [PubMed] [Google Scholar]
- [25].Wazana A. Physicians and the pharmaceutical industry: is a gift ever just a gift? JAMA. 2000;283(3):373–80. doi: 10.1001/jama.283.3.373. http://dx.doi.org/10.1001/jama.283.3.373 . [DOI] [PubMed] [Google Scholar]
- [26].McGettigan P, McManus J, O’Shea B, Chan R, Feely J. Low rate of generic prescribing in the Republic of Ireland compared to England and Northern Ireland: prescribers’ concerns. Ir Med J. 1997;90(4):146–7. [PubMed] [Google Scholar]
- [27].Rozporządzenie Ministra Zdrowia z dn. 8.03.2012 w sprawie recept lekarskich. [Regulation of the Minister of Health of March 8, 2012 on medical prescriptions] [Google Scholar]
- [28].Lagarce L, Lusson-Brisset C, Bruhat C, Diquet B, Lainé-Cessac P. How practitioners view generic drugs: an opinion study from general practitioners in Maine-et-Loire (France) Therapie. 2005;60:67–74. doi: 10.2515/therapie:2005009. http://dx.doi.org/10.2515/therapie:2005009 . [DOI] [PubMed] [Google Scholar]


