Abstract
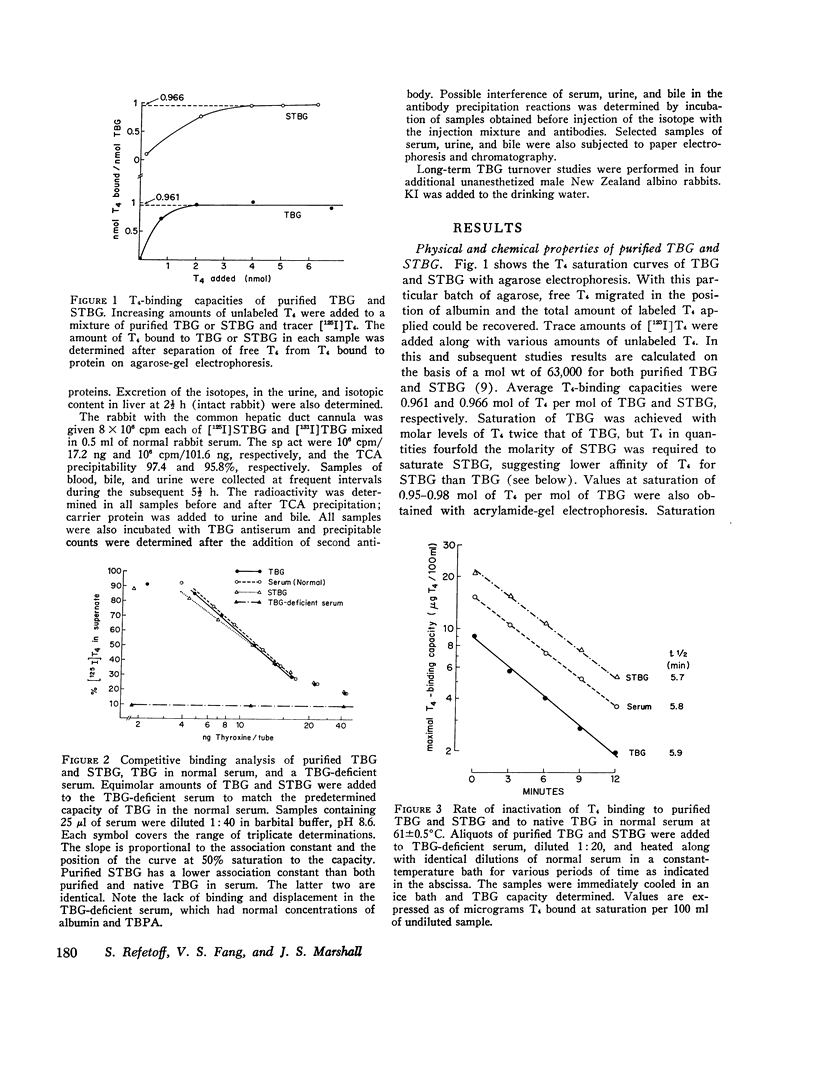
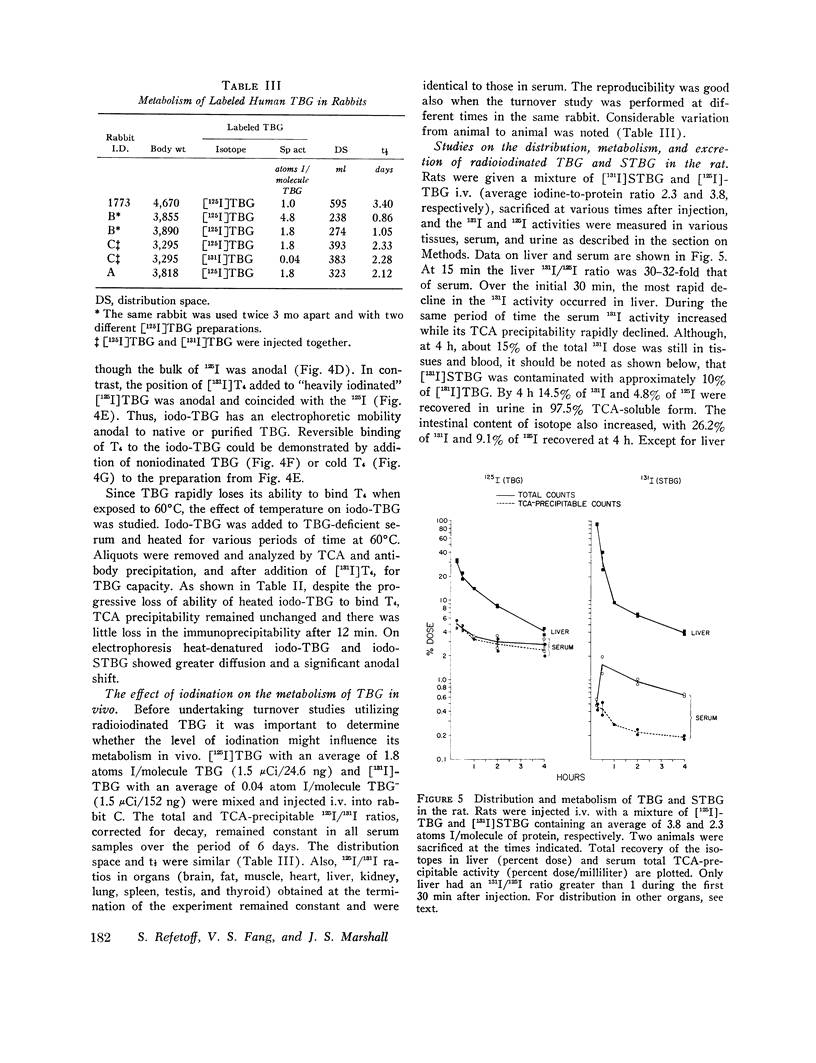
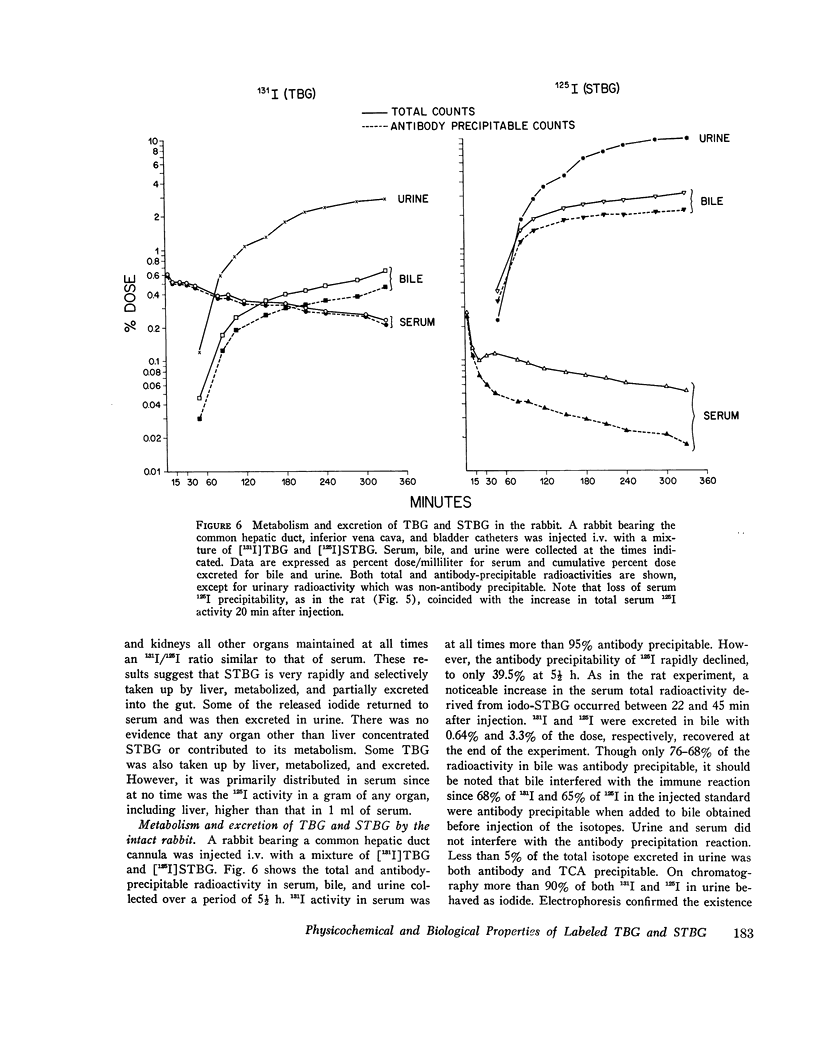
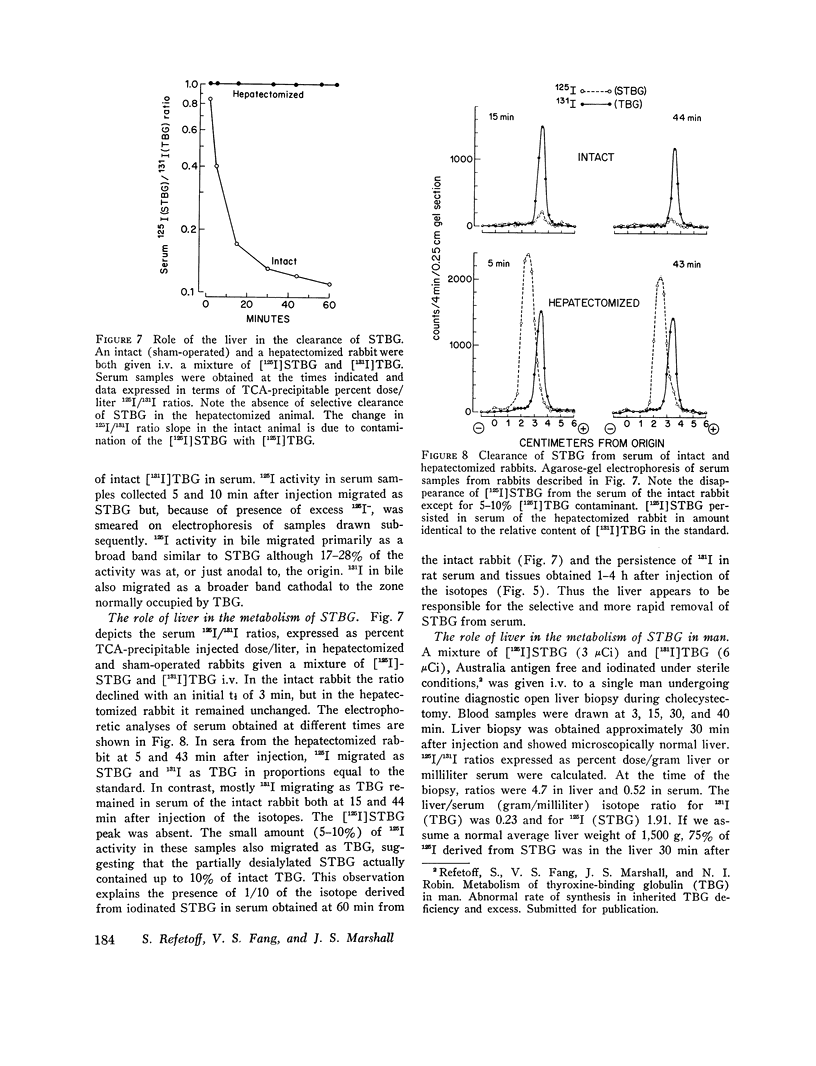
Thyroxine-binding globulin (TBG) and partially desialylated or slow TBG (STBG) were purified from human serum by affinity chromatography. Purified TBG was identical to TBG present in serum by the criteria of electrophoretic mobility, affinity for thyroxine (T4), and heat-inactivation response. Purified STBG had slower electrophoretic mobility and lower affinity for T4. Both bound T4 in an equimolar ratio, were immunoprecipitable, and had similar inactivation t1/2 at 61 degrees C. TBG and STBG were iodinated by the chloramine-T-catalyzed reaction. An average of from 0.02 to 6 atoms I could be incorporated per molecule of the protein by adjusting the conditions of the reaction (time, protein and iodide concentrations). 125-I, 131-I, and 127-I were used. Iodination increased the anodal mobility of TBG but did not affect the reversible T4-binding, precipitation by antiserum, or the heat-inactivation properties. "Heavily" and "lightly" iodinated TBG had identical disappearance half-times from serum in the rabbit. 15 min after the intravenous administration of [131-I]-STBG and [125-I]TBG mixture to rats, more than 90% of the injected 131-I dose was in the liver, and the liver 131-I/125-I ratio was 32-fold that of serum. Selective uptake of STBG by the liver was also observed in the rabbit and in man. The serum [125-I]STBG/[131-I]TBG ratio declined from 1 to 0.2 in 10 min in the intact rabbit but remained unchanged for 1 h in the acutely hepatectomized animal. In the rabbit, t 1/2 was approximately 3 min for STBG and 0.8-3.4 days for TBG. The radioiodine derived from the iodinated proteins is partly excreted in bile but the bulk was precipitable with specific antibodies. Some isotope in the form of iodide appeared in blood and was excreted in the urine. Since radioiodinated TBG and STBG preserve their biologic and immunologic properties they are useful as tracer materials for metabolic studies. In rat, rabbit, and man STBG is rapidly cleared from serum by the liver. Conversion of TBG to STBG may be the limiting step in the regulation of TBG metabolism.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLUMBERG B. S., ROBBINS J. Thyroxine-serum protein complexes: single dimension gel and paper electrophoresis studies. Endocrinology. 1960 Sep;67:368–378. doi: 10.1210/endo-67-3-368. [DOI] [PubMed] [Google Scholar]
- Bellabarba D., Peterson R. E., Sterling K. An improved method for chromatography of iodothyronines. J Clin Endocrinol Metab. 1968 Feb;28(2):305–307. doi: 10.1210/jcem-28-2-305. [DOI] [PubMed] [Google Scholar]
- DOWLING J. T., FREINKEL N., INGBAR S. H. The influence of extracellular thyroxine-binding protein upon the accumulation of thyroxine by tissue slices. J Clin Invest. 1957 Jan;36(1 Pt 1):25–37. doi: 10.1172/JCI103406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELZINGA K. E., CARR E. A., Jr, BEIERWALTES W. H. Adaptation of the standard Durrum-type cell for reverse-flow paper electrophoresis. Am J Clin Pathol. 1961 Aug;36:125–131. doi: 10.1093/ajcp/36.2.125. [DOI] [PubMed] [Google Scholar]
- Eylar E. H. On the biological role of glycoproteins. J Theor Biol. 1966 Jan;10(1):89–113. doi: 10.1016/0022-5193(66)90179-2. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio N. A., Jr, Tabachnick M. Thyroxine-protein interactions. V. Isolation and characterization of a thyroxine-binding globulin from human plasma. J Biol Chem. 1968 May 10;243(9):2247–2259. [PubMed] [Google Scholar]
- Green A. M., Marshall J. S., Pensky J., Stanbury J. B. Studies on human thyroxine-binding globulin. VII. The effect of environmental changes on the fluorescence of I,8-anilinonaphthalene sulfonic acid bound to thyroxine-binding globulin. Biochim Biophys Acta. 1972 Sep 29;278(2):305–315. [PubMed] [Google Scholar]
- Green A. M., Marshall J. S., Pensky J., Stanbury J. B. Studies on thyroxine-binding globulin. IV. The interaction of thyroxine with thyroxine-binding globulin. Biochim Biophys Acta. 1972 Aug 31;278(1):117–124. doi: 10.1016/0005-2795(72)90112-2. [DOI] [PubMed] [Google Scholar]
- Green A. M., Marshall J. S., Pensky J., Stanbury J. B. Thyroxine-binding globulin: characterization of the binding site with a fluorescent dye as a probe. Science. 1972 Mar 24;175(4028):1378–1380. doi: 10.1126/science.175.4028.1378. [DOI] [PubMed] [Google Scholar]
- Laurell C. B., Niléhn J. E. A new type of inherited serum albumin anomaly. J Clin Invest. 1966 Dec;45(12):1935–1945. doi: 10.1172/JCI105498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. S., Pensky J., Green A. M. Studies on human thyroxine-binding globulin. VI. The nature of slow thyroxine-binding globulin. J Clin Invest. 1972 Dec;51(12):3173–3181. doi: 10.1172/JCI107144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. S., Pensky J. Studies on human thyroxine-binding globulin (TBG). I. Purification of TBG and immunologic studies on the relationship between TBG from normal persons and those with TBG "deficiency". J Clin Invest. 1969 Mar;48(3):508–515. doi: 10.1172/JCI106008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. S., Pensky J. Studies on thyroxine-binding globulin (TBG). 3. Some physical characteristics of TBG and its interaction with thyroxine. Arch Biochem Biophys. 1971 Sep;146(1):76–83. doi: 10.1016/s0003-9861(71)80043-7. [DOI] [PubMed] [Google Scholar]
- Morell A. G., Gregoriadis G., Scheinberg I. H., Hickman J., Ashwell G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem. 1971 Mar 10;246(5):1461–1467. [PubMed] [Google Scholar]
- Oppenheimer J. H. Role of plasma proteins in the binding, distribution and metabolism of the thyroid hormones. N Engl J Med. 1968 May 23;278(21):1153–1162. doi: 10.1056/NEJM196805232782107. [DOI] [PubMed] [Google Scholar]
- Pensky J., Marshall J. S. Studies on thyroxine-binding globulin (TBG). II. Separation from human serum by affinity chromatography. Arch Biochem Biophys. 1969 Dec;135(1):304–310. doi: 10.1016/0003-9861(69)90544-x. [DOI] [PubMed] [Google Scholar]
- Premachandra B. N., Perlstein I. B., Blumenthal H. T. Studies on obesity. II. Slow-moving thyroxine binding globulin in the sera of normal and obese subjects. J Clin Endocrinol Metab. 1970 Jun;30(6):752–762. doi: 10.1210/jcem-30-6-752. [DOI] [PubMed] [Google Scholar]
- ROBBINS J. Reverse-flow zone electrophoresis; a method for determining the thyroxine-binding capacity of serum protein. Arch Biochem Biophys. 1956 Aug;63(2):461–469. doi: 10.1016/0003-9861(56)90062-5. [DOI] [PubMed] [Google Scholar]
- Refetoff S., Hagen S. R., Selenkow H. A. Estimation of the T 4 binding capacity of serum TBG and TBPA by a single T 4 load ion exchange resin method. J Nucl Med. 1972 Jan;13(1):2–12. [PubMed] [Google Scholar]
- Refetoff S., Matalon R., Bigazzi M. Metabolism of L-thyroxine (T4) and L-triiodothyronine (T3) by human fibroblasts in tissue culture: evidence for cellular binding proteins and conversion of T4 to T3. Endocrinology. 1972 Oct;91(4):934–947. doi: 10.1210/endo-91-4-934. [DOI] [PubMed] [Google Scholar]
- Refetoff S., Robin N. I., Alper C. A. Study of four new kindreds with inherited thyroxine-binding globulin abnormalities. Possible mutations of a single gene locus. J Clin Invest. 1972 Apr;51(4):848–867. doi: 10.1172/JCI106880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refetoff S., Robin N. I., Fang V. S. Parameters of thyroid function in serum of 16 selected vertebrate species: a study of PBI, serum T4, free T4, and the pattern of T4 and T3 binding to serum proteins. Endocrinology. 1970 Apr;86(4):793–805. doi: 10.1210/endo-86-4-793. [DOI] [PubMed] [Google Scholar]
- Refetoff S., Selenkow H. A. Familial thyroxine-binding globulin deficiency in a patient with Turner's syndrome (XO). Genetic study of a kindred. N Engl J Med. 1968 May 16;278(20):1081–1087. doi: 10.1056/NEJM196805162782002. [DOI] [PubMed] [Google Scholar]
- Robbins J. Inherited variations in thyroxine transport. Mt Sinai J Med. 1973 May-Jun;40(3):511–519. [PubMed] [Google Scholar]
- Rosenberg L. L., Nataf B. M., Cavalieri R. R. Iodine exchange reactions of iodoamino acids and thyroglobulin in in vitro iodinating systems with peroxidases. Endocrinology. 1973 Nov;93(5):1066–1076. doi: 10.1210/endo-93-5-1066. [DOI] [PubMed] [Google Scholar]
- SCHULTZE H. E. Influence of bound sialic acid on electrophoretic mobility of human serum proteins. Arch Biochem Biophys. 1962 Sep;Suppl 1:290–294. [PubMed] [Google Scholar]
- Scherberg N. H., Refetoff S. Hybridization of RNA labelled with 125 I to high specific activity. Nat New Biol. 1973 Apr 4;242(118):142–145. doi: 10.1038/newbio242142a0. [DOI] [PubMed] [Google Scholar]
- Sterling K., Hamada S., Takemura Y., Brenner M. A., Newman E. S., Inada M. Preparation and properties of thyroxine-binding alpha globulin (TBG). J Clin Invest. 1971 Aug;50(8):1758–1771. doi: 10.1172/JCI106665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura Y., Hocman G., Sterling K. Thermal stability of serum thyroxine-binding proteins. J Clin Endocrinol Metab. 1971 Feb;32(2):222–224. doi: 10.1210/jcem-32-2-222. [DOI] [PubMed] [Google Scholar]
- Thorson S. C., Tauxe W. N., Taswell H. F. Evidence for the existence of two thyroxine-binding globulin moieties: correlation between paper and starch-gel electrophoretic patterns utilizing thyroxine-binding globulin-deficient sera. J Clin Endocrinol Metab. 1966 Feb;26(2):181–188. doi: 10.1210/jcem-26-2-181. [DOI] [PubMed] [Google Scholar]
- Tsuruhara T., Van Hall E. V., Dufau M. L., Catt K. J. Ovarian binding of intact and desialytated hcg in vivo and in vitro. Endocrinology. 1972 Aug;91(2):463–469. doi: 10.1210/endo-91-2-463. [DOI] [PubMed] [Google Scholar]
- Van Den Hamer C. J., Morell A. G., Scheinberg I. H., Hickman J., Ashwell G. Physical and chemical studies on ceruloplasmin. IX. The role of galactosyl residues in the clearance of ceruloplasmin from the circulation. J Biol Chem. 1970 Sep 10;245(17):4397–4402. [PubMed] [Google Scholar]
- Winterburn P. J., Phelps C. F. The significance of glycosylated proteins. Nature. 1972 Mar 24;236(5343):147–151. doi: 10.1038/236147a0. [DOI] [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. Labeling of proteins--problems and practices. Trans N Y Acad Sci. 1966 Jun;28(8):1033–1044. doi: 10.1111/j.2164-0947.1966.tb02406.x. [DOI] [PubMed] [Google Scholar]


