Abstract
Evidence suggests that low 25-OH vitamin D 25(OH)D concentrations may increase the risk of several cardiovascular diseases such as hypertension, peripheral vascular disease, diabetes mellitus, obesity, myocardial infarction, heart failure and cardiovascular mortality. Recent studies suggested a possible relationship between vitamin D deficiency and increased carotid intima-media wall thickness and vascular calcification. We hypothesized that low 25(OH)D may be associated with coronary atherosclerosis and coronary plaque burden and composition, and investigated the relationship between serum vitamin D levels and coronary atherosclerosis, plaque burden or structure, in young adult patients by using dual-source 128x2 slice coronary computed tomography angiography (CCTA). We included 98 patients with coronary atherosclerosis and 110, age and gender matched, subjects with normal findings on CCTA examinations. Patients with subclinical atherosclerosis had significantly higher serum total cholesterol, triglycerides, hs-CRP, uric acid, HbA1c and creatinine levels and lower serum 25(OH)D levels in comparison with controls. There was no significant correlation between 25(OH)D and plaque morphology. There was also a positive relationship between 25(OH)D and plaque burden of coronary atherosclerosis. In multivariate analysis, coronary atherosclerosis was associated high hs-CRP (adjusted OR: 2.832), uric acid (adjusted OR: 3.671) and low 25(OH)D (adjusted OR: 0.689). Low levels of 25(OH)D were associated with coronary atherosclerosis and plaque burden, but there was no significant correlation between 25(OH)D and plaque morphology.
KEY WORDS: vitamin D, coronary atherosclerosis, uric acid, hs-CRP
INTRODUCTION
25-OH vitamin D (25(OH)D) is now recognized to have several biological effects in addition to calcium and bone homeostasis. Evidence suggests that low 25(OH)D concentrations may increase the risk of hypertension, peripheral vascular disease, diabetes mellitus, obesity, myocardial infarction, heart failure and cardiac mortality [1-3]. Recent studies showed that 25(OH)D might also play a role in the pathogenesis of cardiovascular disease through a shared association with atherosclerotic plaque formation and progression [4,5]. Low 25(OH)D levels have been found to be associated with endothelial dysfunction, inflammation, increased vascular stiffness and high coronary artery calcium scores [5]. In addition, 25(OH)D deficiency affects vascular functions by aggravating atherosclerosis, while treatment with 25(OH)D may have protective effects on atherosclerosis [6,7]. Low 25(OH)D levels have been found to be associated with early signs of atherosclerosis such as increased carotid intima-media wall thickness [8]. Clinical studies also suggest a possible relationship between 25(OH)D deficiency and vascular calcification [9] which is a major determinant of morbidity and mortality of the affected patients [10]. Although the incidence of cardiovascular disease events is low in individuals younger than 45 years old, subclinical atherosclerosis often begins in childhood or young adulthood. Early detection of coronary artery disease (CAD) in young patients is necessary because young patients with myocardial infarction should have more favorable early and late prognosis than their older counterparts. To our knowledge, there is no published data on the relationship between serum 25(OH)D levels and CAD detected by coronary computed tomography angiography (CCTA) in young adult population. We hypothesized that low 25(OH)D may be associated with subclinical coronary atherosclerosis and coronary plaque burden and composition in young adults. In this study, we aimed to determine the associations between 25(OH)D levels and CAD by using dual-source 128x2 slice CCTA in young adult subjects.
MATERIALS AND METHODS
Study population
The study was a single center, crossover, observational and was conducted in tertiary healthcare level (heart center) settings. A total of 208 patients, age under 45 years, were subjected to CCTA between September and May 2014. Of those, 98 patients (study group) diagnosed with coronary atherosclerosis on CCTA, and 110 age- and gender-matched subjects with normal CCTA (control group), were included. The indications for CCTA included: intermediate or high risk by Framingham score, asymptomatic, contraindications to exercise stress test, uninterpretable stress test results, suspected coronary anomalies or congenital heart disease for the exclusion of significant CAD before non-coronary cardiac surgery. The exclusion criteria were: prior diagnosis of CAD or severe valve disease, liver or kidney failure, diseases related to bone metabolism, primary or secondary hyperparathyroidism, use of drugs affecting calcium metabolism, history of malignancy or osteoporosis.
Demographic and clinical properties were recorded, and blood samples were obtained for biochemical and 25(OH)D analyzes. Written informed consent was obtained from all study participants and the local ethics committee approved the study protocol.
Coronary CT angiography
CCTA angiograms were obtained using the dual-source CT system (Definition Flash, Siemens Medical Solutions, Forchheim, Germany) with 280 milliseconds of rotation time, 2×28 slices, a pitch of 3.4 and triggering at 60% of the R-R interval. The tube current for the protocol was set at 180-300 mAs. Slice collimation of 0.6 mm was achieved. A dose of 80-100 ml of nonionic contrast media (Iomeron 400 mg/mL, Bracco, Milan, Italy) was administered at a rate of 5 mL/s with a dual-head power injector attached to an 18-gauge needle positioned in an ante-cubital vein. The bolus tracking technique was used, and images were obtained during a single breath-hold of 6 seconds.
Image analysis
Two experienced radiologists blinded to clinical information evaluated all scans with a 3-dimensional workstation Syngo.via (Siemens Healthcare, Forchheim, Germany). Consensus interpretation was performed to obtain a final CCTA diagnosis. The characteristics of the stenoses, plaques and the number of plaques to per-segment were analyzed according to the modified American Heart Association classification [11]. A stenosis of more than 50% and 75% was defined as significant or severe, respectively. Plaques were defined as structures that were 1 mm2 and within or adjacent to the vessel lumen and that could be clearly distinguished from the lumen and the surrounding pericardial tissue. Plaques with more than 50% of the plaque area occupied by calcifications (density C130 HU in native scans) were classified as calcified. Plaques with 50% calcium were classified as mixed, and plaques without any calcification were classified as non-calcified lesions [12]. Coronary atherosclerosis was defined as the presence of any plaques on a CCTA.
Laboratory measurements
Blood samples were collected in the morning after an overnight fasting. Levels of total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG) in serum were measured using an Abbott Aeroset autoanalyser with original kits (Abbott Laboratories, Abbott Park, Illinois, U.S.A). Low-density lipoprotein cholesterol (LDL-C) levels were calculated using Friedewald equation. A radioimmunoassay procedure was used to measure 25(OH)D (DiaSorin, Stillwater, MN). The intra- and inter-assay coefficients of variations (CVs) were 3% and 3.3% for the 25(OH)D. High-sensitivity C-reactive protein (hs-CRP) level was determined by immunoturbidimetric method (Abbott Aeroset 1600, Abbott reagents, Germany). Serum uric acid levels were measured using an enzymatic colorimetric test on a Roche Hitachi 911 Chemistry Analyzer (Roche Diagnostics, Germany)
Statistical analysis
All analyzes were performed using SPSS V 16.0 for Windows (version 16.0, SPSS, Chicago, Illinois). All data are presented as mean ± standard deviation unless otherwise stated. Comparison of parametric values between the two groups was performed using independent samples t test. Comparisons of nonparametric values between the two groups were performed using Mann-Whitney U test. Categorical variables were compared using the chi-square test. Spearman correlation coefficient was computed to examine the association between two continuous variables. The Kruskal-Wallis test was used to compare 25(OH)D levels in groups according to three subgroups based on the plaque characteristics. Univariate logistic regression models were firstly performed to evaluate the crude association between the presence of coronary atherosclerosis and each of the factors including age, sex, serum total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, hs-CRP, creatinine, cigarette smoking, diabetes, hypertension, body mass index, uric acid and 25(OH)D, individually. Those factors that were significant at the p≤0.10 level in the univariate models were put into the multiple logistic regression models to identify the factors that were independently associated with the presence of coronary atherosclerosis. A receiver operating characteristic (ROC) curve was constructed to determine the predictive value of vitamin D on coronary atherosclerosis. All statistical tests were two-sided, and p<0.05 was considered statistically significant.
RESULTS
Demographic and clinical characteristics of the study participants are presented in Table 1. Age, gender, body mass index (BMI), presence of hypertension and LDL cholesterol levels was not different between two groups. The study group had a higher prevalence of diabetes, hypertension, and smoking and higher levels of serum creatinine, total cholesterol, triglycerides, hemoglobin A1c (HbA1c), uric acid and hs-CRP levels than control group (Table 1). The study group had lower 25(OH)D, and HDL cholesterol levels compared to control group (Table 1). In all subjects, 25(OH)D was negatively correlated to hs-CRP (r= -0.344; P<0.001). There was a negative correlation between 25(OH)D and the number of plaques (r=-0.346; p< 0.001). The cohort was also divided into three groups according to the plaque characteristics. Serum 25(OH)D levels were not different between calcified, mixed and non-calcified plaque groups (17.38±5.6 vs. 17.64±5.2 vs. 17.7±4.3 ng/ml, p= 0.75) (Table 2). Subjects with subclinical coronary atherosclerosis had lower 25(OH)D and HDL cholesterol levels (Table 1). In all subjects, the 25(OH)D was negatively correlated with hs-CRP (r= -0.344; p<0.001). There was no significant correlation between 25(OH)D and plaque morphology (Table 2). The cohort was also divided into 3 groups according to the plaque characteristics. Univariate logistic regression analysis revealed following risk factors associated with the presence of coronary atherosclerosis: diabetes, smoking, total cholesterol, LDL cholesterol, HDL cholesterol, triglyceride, uric acid and (25(OH)D) (Table 3). In multivariate logistic regression analysis, after adjusting for all covariates, coronary atherosclerosis was associated with diabetes (adjusted OR: 5.235, 95% CI: 1.032-26.555), smoking (adjusted OR: 13.347, 95% CI: 3.316-53.718), hs-CRP (adjusted OR: 2.832, 95% CI: 1.095-7.829), HDL cholesterol (adjusted OR: 0.874, 95% CI: 0.798-0.958), 25(OH)D (adjusted OR: 0.689, 95% CI: 0.595-0.797), and uric acid (adjusted OR: 3.671, 95% CI: 1.974-6.827) (Table 4). The ROC curve analysis was performed to detect the best cutoff value of (25(OH)D) in the prediction of coronary atherosclerosis. The 25(OH)D value of < 20ng/mL yielded an area under curve value of 0.810. Furthermore, 25(OH)D value < 20ng/mL demonstrated a 77% sensitivity and 73% specificity for the prediction of coronary atherosclerosis (AUC: 810, p<0.001) (Figure 1).
TABLE 1.
Comparisons of clinical and laboratory characteristics of participants
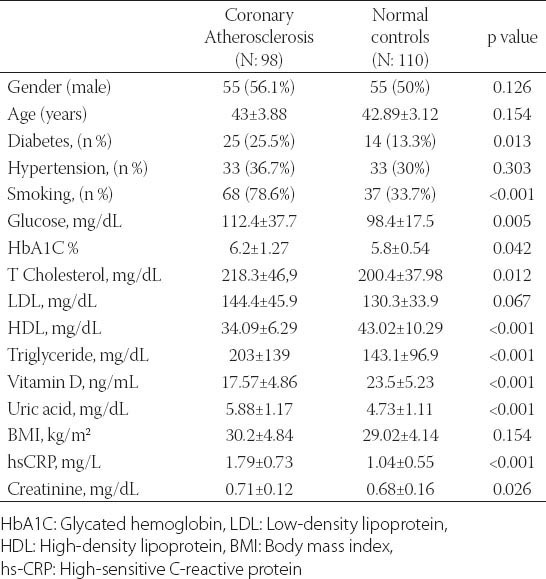
TABLE 2.
Relationships between Vitamin D, plaque morphology and inflammation
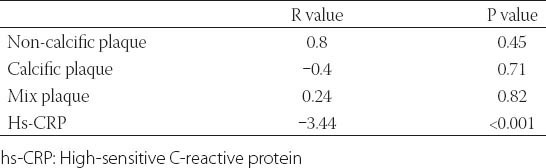
TABLE 3.
Univariate analysis of presence of subclinical atherosclerosis
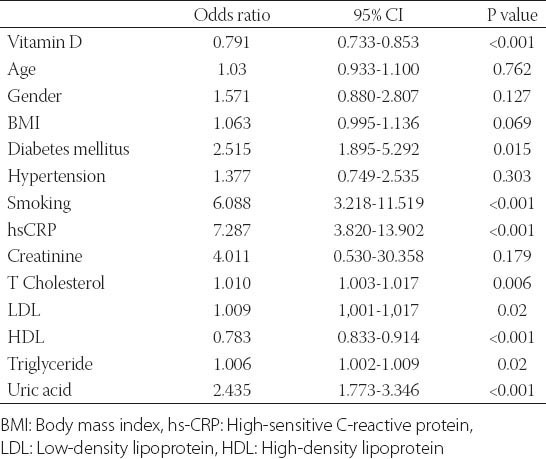
TABLE 4.
Multivariate analysis of presence of subclinical atherosclerosis
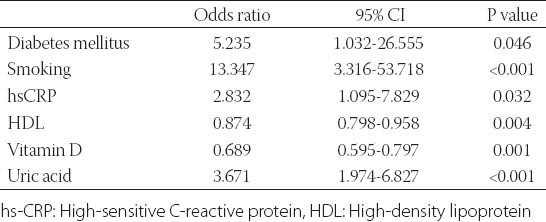
FIGURE 1.
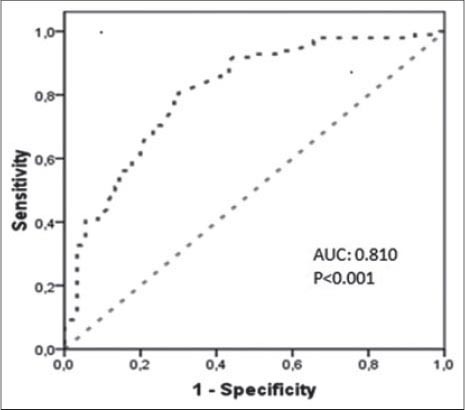
ROC Curve of Vitamin D for Prediction of Subclinical Coronary Atherosclerosis.
DISCUSSION
We found a significant inverse association between serum 25(OH)D levels and presence and severity of coronary atherosclerosis in young adults, but no association with plaque composition. The high prevalence of 25(OH)D deficiency in our cohort beginning at an early age may play a major role in the pathogenesis of coronary atherosclerosis. The results of previous studies evaluating an association between 25(OH)D and subclinical atherosclerosis assessed by carotid intima-media wall thickness (IMT) have been inconsistent. In a population-based cohort of the elderly, a significant inverse stepwise association has been found between serum 25(OH)D levels and internal carotid IMT [8]. However, in the Multi-Ethnic Study of Atherosclerosis [13] serum levels of 25(OH)D were not significantly associated with carotid IMT or plaque. The results of the present study are consistent with the previous studies suggesting the role of (25(OH)D) deficiency in the development and progression of atherosclerotic cardiovascular disease in different populations. Recently, Akin et al. [4] reported that (25(OH)D) insufficiency was associated with the severity of coronary artery stenosis. The relation between (25(OH)D) and coronary artery stenosis has been demonstrated previously by CCTA in a particular data set: asymptomatic African American long-term cocaine users [5]. In the Korean longitudinal study on health and aging, a significant relationship between (25(OH)D) status and coronary artery stenosis was found in a community-based elderly cohort [14]. As different from these studies, our patient population was consisted of young adults and additionally the relation between 25(OH)D and the severity of coronary atherosclerosis and plaque morphology was also investigated. There are some mechanisms proposed to explain the relation between 25(OH)D deficiency and coronary atherosclerosis. 25(OH)D receptor has been found in most tissues and cells, which includes vascular smooth muscle cells [15], macrophages [16], cardiomyocytes [17], endothelium [18], and lymphocytes [19]. 25(OH)D stimulates the production of prostacyclin by vascular smooth muscle cells, which prevents thrombus formation, cell adhesion, and smooth muscle cell proliferation [20]. Vascular smooth muscle cells and endothelial cells express receptors for 25(OH)D and could convert 25- hydroxyvitamin D3, the major circulating metabolite of vitamin D3, to 1,25-dihydroxyvitamin D3 (1,25(OH)2D) [21]. In addition, Al Mheid et al. [22] reported that 25(OH)D insufficiency was associated with increased arterial stiffness and endothelial dysfunction in a community-based asymptomatic population. Another mechanism may be pancreatic β-cell dysfunction, peripheral tissue resistance to insulin predisposing to diabetes [23], and activation of the renin-angiotensin system predisposing to hypertension [24]. In addition, Oh et al. [25]. Reported that active 25(OH)D suppresses foam-cell formation by reducing macrophage cholesterol uptake in diabetics. A final mechanism explaining the effects of 25(OH)D and its metabolites on atherosclerosis may be their anti-inflammatory actions. The inflammatory process plays a major role at all stages of atherosclerosis from initiation through progression and in the thrombotic complications of this disease [26]. The association of 25(OH)D with inflammatory markers is controversial. The Ludwigshafen Risk and Cardiovascular Health study [27] reported an increased inflammatory process in patients with low 25(OH)D status. By contrast, a recent analysis from the Framingham Offspring Study found that 25(OH)D deficiency was not associated with systemic inflammatory markers [28]. In our cohort, we found a significant inverse association between serum 25(OH)D and hs-CRP levels. Studies have shown that atherosclerosis begins at a very early stage in life [29], and the prevalence of subclinical coronary atherosclerosis in asymptomatic young adults is remarkable. The prevalence of subclinical atherosclerosis in young people was found to be 9.4% by KN Jin et al. [30], which is a similar result to those of other studies [31, 32]. Early detection of subclinical coronary atherosclerosis may be beneficial for reducing cardiovascular morbidity and mortality in this population. Noninvasive imaging modalities have been widely used to investigate coronary atherosclerotic lesions. CCTA offers additional information on coronary artery calcification score (CACS), with regard to the degree of stenosis and detailed plaque composition with high diagnostic accuracy. In the present study, we also found a significant inverse association between serum uric acid (SUA) levels and subclinical atherosclerosis in young patients. A growing body of evidence from experimental, clinical, and epidemiological studies has suggested a possible association between elevated levels of uric acid and several indices of vascular function including the development and progression of atherosclerotic cardiovascular disease [33]. Consistent with our results, Kaya et al. [34] demonstrated that SUA was significantly associated with the severity and morphology of coronary atherosclerosis detected by multidetector computed tomography. However, in a recent report, no association was found between SUA and brachial flow-mediated dilatation or the presence of carotid plaque in young adults [35].
The present study has several limitations. First, the sample size in our study was relatively small. Serum 25(OH)D levels vary with geography, seasonality, latitude, and altitude presumably as a result of sunlight exposure. Although 25(OH)D has a relatively long circulating half-life approximately 3 weeks and is considered a good biomarker, serum vitamin D levels may change throughout days and seasons of the year. Since coronary atherosclerosis develops and progresses over the years, a single measurement of vitamin D may not reflect lifetime status. However, in our study population, there was no difference in terms of distribution by months between control and study groups. In addition, this was a cross-sectional study, therefore, reversed causation bias cannot be excluded, e.g. that low 25(OH)D levels are the result and not the cause of the disease. Despite these limitations, our study has strength as well since it is the first study demonstrating the link between 25(OH)D and coronary atherosclerosis and plaque burden with CCTA, being current standard, in young adults.
CONCLUSION
Our study suggests an inverse association between levels of serum 25(OH)D and the presence and the severity of subclinical coronary atherosclerosis determined by CCTA in young adults, independent of other traditional cardiovascular disease risk factors. 25(OH)D may play a role in the development of subclinical coronary atherosclerosis in young patients.
DECLARATION OF INTERESTS
The authors declare no conflict of interest.
REFERENCES
- [1].Melamed ML, Muntner P, Michos ED, Uribarri J, Weber C, Sharma J, et al. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol. 2008;28:1179–1185. doi: 10.1161/ATVBAHA.108.165886. http://dx.doi.org/10.1161/ATVBAHA.108.165886 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Siasos G, Tousoulis D, Oikonomou E, Maniatis K, Kioufis S, Kokkou E, et al. Vitamin D serum levels are associated with cardiovascular outcome in coronary artery disease. Int J Cardiol. 2013;168(4):4445–7. doi: 10.1016/j.ijcard.2013.06.151. http://dx.doi.org/10.1016/j.ijcard.2013.06.151 . [DOI] [PubMed] [Google Scholar]
- [3].Schierbeck LL, Jensen TS, Bang U, Jensen G, Køber L, Jensen JE. Parathyroid hormone and vitamin D-markers for cardiovascular and all cause mortality in heart failure. Eur J Heart Fail. 2011;13:626–632. doi: 10.1093/eurjhf/hfr016. http://dx.doi.org/10.1093/eurjhf/hfr016 . [DOI] [PubMed] [Google Scholar]
- [4].Akin F, Ayça B, Köse N, Duran M, Sari M, Uysal OK, et al. Serum vitamin D levels are independently associated with severity of coronary artery disease. J Investig Med. 2012;60(6):869–73. doi: 10.2310/JIM.0b013e31825457cb. [DOI] [PubMed] [Google Scholar]
- [5].Lai H, Fishman EK, Gerstenblith G, Brinker JA, Tong W, Bhatia S, et al. Vitamin D deficiency is associated with significant coronary stenoses in asymptomatic African American chronic cocaine users. Int J Cardiol. 2012;158(2):211–6. doi: 10.1016/j.ijcard.2011.01.032. http://dx.doi.org/10.1016/j.ijcard.2011.01.032 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yiu YF, Yiu KH, Siu CW, Chan YH, Li SW, Wong LY, et al. Randomized controlled trial of vitamin D supplement on endothelial function in patients with type 2 diabetes. Atherosclerosis. 2013;227(1):140–6. doi: 10.1016/j.atherosclerosis.2012.12.013. http://dx.doi.org/10.1016/j.atherosclerosis.2012.12.013 . [DOI] [PubMed] [Google Scholar]
- [7].Tarcin O, Yavuz DG, Ozben B, Telli A, Ogunc AV, Yuksel M, et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab. 2009;94:4023–4030. doi: 10.1210/jc.2008-1212. http://dx.doi.org/10.1210/jc.2008-1212 . [DOI] [PubMed] [Google Scholar]
- [8].Reis JP, von Mühlen D, Michos ED, Miller ER, Appel LJ, Araneta MR, et al. Serum vitamin D, parathyroid hormone levels, and carotid atherosclerosis. Atherosclerosis. 2009;207:585–590. doi: 10.1016/j.atherosclerosis.2009.05.030. http://dx.doi.org/10.1016/j.atherosclerosis.2009.05.030 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Watson KE, Abrolat ML, Malone LL, Hoeg JM, Doherty T, Detrano R, et al. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96:1755–1760. doi: 10.1161/01.cir.96.6.1755. http://dx.doi.org/10.1161/01.CIR.96.6.1755 . [DOI] [PubMed] [Google Scholar]
- [10].Lee MJ, Shin DH, Kim SJ, Oh HJ, Yoo DE, Ko KI, et al. Progression of aortic arch calcification over 1 year is an independent predictor of mortality in incident peritoneal dialysis patients. PLoS One. 2012;7(11):e48793. doi: 10.1371/journal.pone.0048793. doi: 10.1371/journal.pone.0048793. Epub 2012 Nov 7. PMID: 23144974. http://dx.doi.org/10.1371/journal.pone.0048793 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc committee for grading of coronary artery disease, council on cardiovascular surgery, American heart association. Circulation. 1975;51(4):5–40. doi: 10.1161/01.cir.51.4.5. http://dx.doi.org/10.1161/01.CIR.51.4.5 . [DOI] [PubMed] [Google Scholar]
- [12].Leber AW, Becker A, Knez A, von Ziegler F, Sirol M, Nikolaou K, et al. Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: a comparative study using intravascular ultrasound. J Am Coll Cardiol. 2006;47(3):672–677. doi: 10.1016/j.jacc.2005.10.058. http://dx.doi.org/10.1016/j.jacc.2005.10.058 . [DOI] [PubMed] [Google Scholar]
- [13].Blondon M, Sachs M, Hoofnagle AN, Ix JH, Michos ED, Korcarz C, et al. 25-Hydroxyvitamin D and Parathyroid Hormone Are Not Associated With Carotid Intima-Media Thickness or Plaque in the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2013;33(11):2639–45. doi: 10.1161/ATVBAHA.113.301781. http://dx.doi.org/10.1161/ATVBAHA.113.301781 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lim S, Shin H, Kim MJ, Ahn HY, Kang SM, Yoon JW, et al. Vitamin D inadequacy is associated with significant coronary artery stenosis in a community-based elderly cohort: the Korean Longitudinal Study on Health and Aging. J Clin Endocrinol Metab. 2012;97(1):169–78. doi: 10.1210/jc.2011-1580. http://dx.doi.org/10.1210/jc.2011-1580 . [DOI] [PubMed] [Google Scholar]
- [15].Carthy EP, Yamashita W, Hsu A, Ooi BS. 1,25-Dihydroxyvitamin D3 and rat vascular smooth muscle cell growth. Hypertension. 1989;13:954–959. doi: 10.1161/01.hyp.13.6.954. http://dx.doi.org/10.1161/01.HYP.13.6.954 . [DOI] [PubMed] [Google Scholar]
- [16].Shioi A, Katagi M, Okuno Y, Mori K, Jono S, Koyama H, Nishizawa Y. Induction of bone-type alkaline phosphatase in human vascular smooth muscle cells: roles of tumor necrosis factor-alpha and oncostatin M derived from macrophages. Circ Res. 2002;91(1):9–16. doi: 10.1161/01.res.0000026421.61398.f2. http://dx.doi.org/10.1161/01.RES.0000026421.61398.F2 . [DOI] [PubMed] [Google Scholar]
- [17].Nibbelink KA, Tishkoff DX, Hershey SD, Rahman A, Simpson RU. 1,25(OH)2-vitamin D3 actions on cell proliferation, size, gene expression, and receptor localization, in the HL-1 cardiac myocyte. J Steroid Biochem Mol Biol. 2007;103:533–537. doi: 10.1016/j.jsbmb.2006.12.099. http://dx.doi.org/10.1016/j.jsbmb.2006.12.099 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Merke J, Milde P, Lewicka S, Hügel U, Klaus G, Mangelsdorf DJ, et al. Identification and regulation of 1,25-dihydroxyvitamin D3 receptor activity and biosynthesis of 1,25-dihydroxyvitamin D3. Studies in cultured bovine aortic endothelial cells and human dermal capillaries. J Clin Invest. 1989;83:1903–1915. doi: 10.1172/JCI114097. http://dx.doi.org/10.1172/JCI114097 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rigby WF, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1,25 dihydroxyvitamin D3 (calcitriol) J Clin Invest. 1984;74:1451–1455. doi: 10.1172/JCI111557. http://dx.doi.org/10.1172/JCI111557 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wakasugi M, Noguchi T, Inoue M, Kazama Y, Tawata M, Kanemaru Y, et al. Vitamin D3 stimulates the production of prostacyclin by vascular smooth muscle cells. Prostaglandins. 1991;42:127–136. doi: 10.1016/0090-6980(91)90072-n. http://dx.doi.org/10.1016/0090- 6980(91)90072-N . [DOI] [PubMed] [Google Scholar]
- [21].Zehnder D, Bland R, Chana RS, Wheeler DC, Howie AJ, Williams MC, et al. Synthesis of 1,25-dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol. 2002;13:621–629. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- [22].Al Mheid I, Patel R, Murrow J, Morris A, Rahman A, Fike L, et al. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. J Am Coll Cardiol. 2011;58:186–192. doi: 10.1016/j.jacc.2011.02.051. http://dx.doi.org/10.1016/j.jacc.2011.02.051 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pittas AG, Lau J, Hu FB, Dowson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. http://dx.doi.org/10.1210/jc.2007-0298 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. http://dx.doi.org/10.1172/JCI0215219 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Oh J, Weng S, Felton SK, Bhandare S, Riek A, Butler B, et al. 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120:687–698. doi: 10.1161/CIRCULATIONAHA.109.856070. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.856070 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. http://dx.doi.org/10.1038/nature01323 . [DOI] [PubMed] [Google Scholar]
- [27].Murr C, Pilz S, Grammer TB, Kleber ME, Meinitzer A, Boehm BO, et al. Vitamin D deficiency parallels inflammation and immune activation, the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin Chem Lab Med. 2012;50(12):2205–12. doi: 10.1515/cclm-2012-0157. http://dx.doi.org/10.1515/cclm- 2012-0157 . [DOI] [PubMed] [Google Scholar]
- [28].Shea MK, Booth SL, Massaro JM, Jacques PF, D’Agostino RB, Sr, Dawson-Hughes B, et al. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol. 2008;167:313–320. doi: 10.1093/aje/kwm306. http://dx.doi.org/10.1093/aje/kwm306 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tuzcu EM, Kapadia SR, Tutar E, Ziada KM, Hobbs RE, McCarthy PM, et al. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation. 2001;103(22):2705–10. doi: 10.1161/01.cir.103.22.2705. http://dx.doi.org/10.1161/01.CIR.103.22.2705 . [DOI] [PubMed] [Google Scholar]
- [30].Kwang Nam Jin, Eun Ju Chun, Chang-Hoon Lee, Jeong A. Kim, Min Su Lee, et al. Subclinical coronary atherosclerosis in young adults: prevalence, characteristics, predictors with coronary computed tomography angiography. Int J Cardiovasc Imaging. 2012;28:93–100. doi: 10.1007/s10554-012-0143-0. http://dx.doi.org/10.1007/s10554-012-0143-0 . [DOI] [PubMed] [Google Scholar]
- [31].Berry JD, Liu K, Folsom AR, Lewis CE, Carr JJ, Polak JF, et al. Prevalence and progression of subclinical atherosclerosis in younger adults with low short-term but high lifetime estimated risk for cardiovascular disease: the coronary artery risk development in young adults study and multi-ethnic study of atherosclerosis. Circulation. 2009;119:382–9. doi: 10.1161/CIRCULATIONAHA.108.800235. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.800235 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Celik O, Cakmak HA, Satilmis S, Gungor B, Akin F, Ozturk D, et al. The relationship between gamma-glutamyl transferase levels and coronary plaque burdens and plaque structures in young adults with coronary atherosclerosis. Clin Cardiol. 2014;37(9):552–557. doi: 10.1002/clc.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kanbay M, Segal M, Afsar B, Kang DH, Rodriguez-Iturbe B, Johnson RJ. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart. 2013;99(11):759–66. doi: 10.1136/heartjnl-2012-302535. http://dx.doi.org/10.1136/heartjnl- 2012-302535 . [DOI] [PubMed] [Google Scholar]
- [34].Kaya EB, Yorgun H, Canpolat U, Hazırolan T, Sunman H, Ülgen A, et al. Serum uric acid levels predict the severity and morphology of coronary atherosclerosis detected by multidetector computed tomography. Atherosclerosis. 2010;213(1):178–83. doi: 10.1016/j.atherosclerosis.2010.08.077. http://dx.doi.org/10.1016/j.atherosclerosis.2010.08.077 . [DOI] [PubMed] [Google Scholar]
- [35].Oikonen M, Wendelin-Saarenhovi M, Lyytikäinen LP, Siitonen N, Loo BM, Jula A, et al. Associations between serum uric acid and markers of subclinical atherosclerosis in young adults. The cardiovascular risk in Young Finns study. Atherosclerosis. 2012;223(2):497–503. doi: 10.1016/j.atherosclerosis.2012.05.036. http://dx.doi.org/10.1016/j.atherosclerosis.2012.05.036 . [DOI] [PubMed] [Google Scholar]


