Abstract
Sofosbuvir (SOF) in combination with ribavirin (RBV) for 12 or 24 weeks is the current standard of care for patients infected with hepatitis C virus (HCV) genotypes 2 and 3, respectively. However, in clinical trials treatment-experienced patients, particularly those with cirrhosis, had suboptimal sustained virological response (SVR) rates. We assessed the efficacy and safety of sofosbuvir plus peginterferon and ribavirin (SOF+Peg-IFN+RBV) administered for 12 weeks to treatment-experienced patients with HCV genotypes 2 and 3, with and without cirrhosis. We enrolled 47 patients in this open-label, nonrandomized, uncontrolled phase 2 study. The primary endpoint was the proportion of patients with SVR at 12 weeks after cessation of study treatment (SVR12). The overall rate of SVR12 was 89% (95% confidence interval [CI]: 77-97). Rates of SVR12 were higher in patients with genotype 2 than in those with genotype 3, 96% (95% CI: 78-100) and 83% (95% CI: 62-95), respectively. Rates of SVR12 were similar in patients with and without cirrhosis: for genotype 2, 93% of patients with cirrhosis and 100% of patients without cirrhosis achieved SVR12, and for genotype 3, the SVR12 rate was 83% in patients both with and without cirrhosis. One patient discontinued study treatment because of an adverse event and four patients experienced serious adverse events. The most common adverse events were influenza-like illness, fatigue, anemia, and neutropenia. Conclusion: In treatment-experienced patients with HCV genotypes 2 and 3, 12-week administration of SOF+Peg-IFN+RBV provided high SVR rates, irrespective of cirrhosis status. No safety concerns were identified. (Hepatology 2015;61:769–775)
An estimated 180 million people worldwide are chronically infected with the hepatitis C virus (HCV).1 Up to 20% of these patients will develop HCV-related complications, including cirrhosis, end-stage liver disease, and hepatocellular carcinoma.2 Infection with HCV genotypes 2 and 3 accounts for ∼30% of all chronic HCV cases worldwide.3,4 Although HCV genotypes 2 and 3 have been grouped together in clinical studies and treatment guidelines,1 there are important clinical differences between them. Patients with genotype 3 HCV have a higher incidence of hepatic steatosis and a more rapid progression of fibrosis, as well as a higher risk of development of hepatocellular carcinoma than patients with genotype 2 HCV.5,6 Moreover, patients with genotype 3 HCV infection have a lower rate of response to HCV treatment than those with genotype 2 HCV.7
When this study was initiated there were no approved treatment options for patients with HCV genotypes 2 and 3 who did not achieve sustained virologic response (SVR) after a 24-week regimen of peginterferon-ribavirin (Peg-IFN+RBV) or for those patients in whom interferon therapy was contraindicated.8,9 Sofosbuvir (SOF), a first-in-class nucleotide analog HCV NS5B polymerase inhibitor, has recently been approved in the United States and Europe. The approved regimen for treatment-experienced patients with HCV genotype 2 or 3 was based on data from the FUSION10 and VALENCE11 studies. For patients with HCV genotype 2, SOF+RBV for 12 weeks is now approved. Rates of SVR for 12 weeks after completion of treatment (SVR12) ranged from 94-96% in patients without cirrhosis and from 60-82% in those with cirrhosis. Extending the treatment duration to 16 weeks, as was tested in the FUSION study, did not markedly improve SVR12 rates. For patients with HCV genotype 3, the approved regimen is 24 weeks of SOF+RBV, which was found to result in higher SVR12 rates than 12 or 16 weeks of treatment. SVR12 rates were 87% for treatment-experienced patients without cirrhosis and 62% for those with cirrhosis.
In previous studies, SOF+Peg-IFN+RBV was well tolerated and highly efficacious, with SVR rates of 92-100%, when administered for 12 weeks to treatment-naïve, noncirrhosis patients with genotypes 2 and 3.12,13 Based on these data, we hypothesized that 12 weeks of treatment with SOF+Peg-IFN+RBV might improve response rates in treatment-experienced patients with cirrhosis and HCV genotypes 2 and 3 without compromising safety. The purpose of our study was to assess the efficacy, safety, and tolerability of SOF+Peg-IFN+RBV in treatment-experienced patients with HCV genotype 2 and 3, with and without cirrhosis.
Materials and Methods
Study Population
We enrolled men and nonpregnant women ≥18 years of age with a body mass index ≥18 kg/m2. Patients with HCV genotype 2 or 3 infection and HCV RNA levels ≥104 IU/mL at screening were eligible. Patients were HCV treatment-experienced, defined as patients who experienced virologic failure after receiving a previous course of interferon and RBV; all but one patient had previously received Peg-IFN+RBV. The enrollment of ∼50% of patients with compensated cirrhosis was permitted. The presence of cirrhosis was established by liver biopsy or by a FibroTest score of >0.75 and an aspartate aminotransferase (AST): platelet ratio index of >2. To be eligible, patients were required to have platelet levels at baseline ≥90,000/μL, or ≥75,000/μL for patients with cirrhosis. Patients were excluded from the study if they had previously taken direct-acting antivirals targeting the HCV NS5B polymerase, had chronic liver disease of a non-HCV etiology, or were coinfected with the human immunodeficiency virus or hepatitis B viruses.
Written consent was obtained from all patients prior to screening. This study was approved by the Institutional Review Board and was conducted in compliance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Study Design
This was an open-label, nonrandomized, uncontrolled phase 2 study conducted at a single site in San Antonio, Texas, from February to December 2013. All patients received sofosbuvir (SOF; Gilead Sciences, Foster City, CA) in combination with peg-IFN-alpha (Pegasys, Roche Laboratories, Nutley, NJ) and RBV (Ribasphere, Kadmon, New York, NY) for 12 weeks. Sofosbuvir 400 mg was administered orally once-daily in the morning, peginterferon 180 μg was administered as a weekly subcutaneous injection, and ribavirin was administered orally twice-daily in the morning and evening with food (doses were determined according to body weight: 1,000 mg daily in patients with a body weight of <75 kg and 1,200 mg daily in patients with a body weight of ≥75 kg). The sponsor collected the data, monitored study conduct, and performed the statistical analyses. An internal data monitoring committee reviewed the progress of the study.
Study Assessments: Efficacy Analysis
Plasma samples for HCV viral sequencing were collected at baseline and at every visit thereafter. Due to changes in the central laboratory, two assays were used to quantify HCV RNA, the COBAS TaqMan HCV Test, V2.0 for use with the High Pure System (Roche Molecular Systems, West Sussex, UK) with a lower limit of quantification (LLOQ) of 25 IU/mL and the COBAS AmpliPrep/COBAS TaqMan HCV Test v. 2.0 with an LLOQ of 15 IU/mL. All posttreatment results used the latter assay.
The IL28B genotype was determined by amplification and sequencing of the rs12979860 single-nucleotide polymorphism.
Viral relapse was defined as HCV RNA >LLOQ at posttreatment weeks 4, 12, and 24, in patients who had HCV RNA <LLOQ at the end of treatment. Viral breakthrough was defined as a patient having HCV RNA ≥LLOQ during treatment after having previously had HCV RNA <LLOQ while on treatment. If relapse or breakthrough was observed during the study, deep sequencing on the HCV NS5B region was performed.
For all subjects with virologic failure, amplification and deep sequencing for the HCV NS5B region was performed at baseline and at the first virologic failure timepoint if HCV RNA levels were ≥1,000 IU/mL. The resulting sequences were compared to detect resistance-associated variants that emerged during treatment. We report variants that were present in more than 1% of the sequence reads.
Safety Analysis
Safety assessments included monitoring of patients for adverse events, review of concomitant medications, clinical laboratory analyses, vital signs, and physical examinations. Follow-up visits took place 4, 12, and 24 weeks after patients received their last dose of study treatment.
Statistical Analysis
The primary endpoint was to determine the proportion of patients with SVR12. No sample size calculations or formal hypothesis-testing were performed. Point estimates and two-sided 95% exact confidence intervals (CIs; based on the Clopper-Pearson method) were provided for SVR12 rates for subgroups. Exploratory multivariate logistic-regression analyses characterizing the relationship between SVR12 and various prespecified demographic and baseline clinical characteristics were performed for each genotype. Data are presented for patients who received at least one dose of study treatment.
Results
Study Population Disposition and Demographics
A total of 56 patients with chronic HCV were screened for the study; 47 were enrolled and 44 patients completed study treatment. Three patients discontinued the study treatment, one patient was lost to follow-up, one patient was not adherent to the protocol, and one patient withdrew due to an adverse event. Forty-five patients attended the week 4 and 12 posttreatment visits, and 43 returned for the week 24 posttreatment visit (Fig. 1). Twenty-three (49%) patients had HCV genotype 2 and 24 (51%) patients had HCV genotype 3 (Table1). Most patients were white (96%), male (68%), and had cirrhosis (55%). Of the 26 patients with cirrhosis, 25 had the presence of cirrhosis confirmed by biopsy. There were 36% patients with the CC IL28B genotype. At baseline, mean HCV RNA levels were 6.2 log10 IU/mL and mean alanine transaminase (ALT) was 109 U/L across genotypes 2 and 3. Overall, 85% of patients had experienced a relapse/breakthrough after previous treatment with an interferon-based regimen and 15% were nonresponders.
Figure 1.
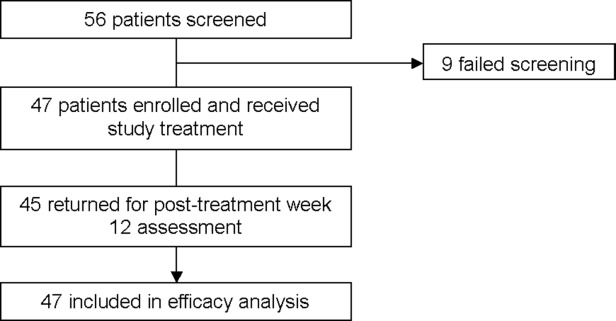
CONSORT diagram of patient disposition.
Table 1.
Demographic and Clinical Characteristics of the Patients at Baseline
| Patient Characteristics | Genotype 2 (N = 23) | Genotype 3 (N = 24) | Total (N = 47) |
|---|---|---|---|
| Age in years, mean (range) | 58 (46-72) | 54 (39-64) | 56 (39-72) |
| Male sex, n (%) | 14 (61) | 18 (75) | 32 (68) |
| Body mass index in kg/m2, mean (SD) | 32 (21-45) | 31 (21-53) | 31 (21-53) |
| Race, n (%) | |||
| White | 22 (96) | 23 (96) | 45 (96) |
| Black/African American | 0 (0) | 1 (4) | 1 (2) |
| Asian | 1 (4) | 0 | 1 (2) |
| Ethnicity, n (%) | |||
| Hispanic/Latino | 11 (48) | 10 (42) | 21 (45) |
| Non-Hispanic/Latino | 12 (52) | 14 (58) | 26 (55) |
| Disease characteristic | |||
| Cirrhosis, n (%) | |||
| No | 9 (39) | 12 (50) | 21 (45) |
| Yes | 14 (61) | 12 (50) | 26 (55) |
| IL28B, n (%) | |||
| CC | 10 (43) | 7 (29) | 17 (36) |
| CT | 10 (44) | 15 (63) | 25 (53) |
| TT | 3 (13) | 2 (8) | 5 (11) |
| Baseline HCV RNA (log10 IU/mL), Mean (SD) | 6.4 (0.7) | 6.0 (0.6) | 6.2 (0.7) |
| Baseline alanine aminotransferase (U/L), Mean (SD) | 91 (54) | 126 (86) | 109 (74) |
| Previous treatment, n (%) | |||
| No response | 2 (9) | 5 (21) | 7 (15) |
| Relapse or breakthrough infection | 21 (91) | 19 (79) | 40 (85) |
BMI, body mass index; SD, standard deviation.
On-treatment and SVR
Treatment with SOF+Peg-IFN+RBV resulted in rapid suppression of HCV RNA. By week 4, 96% of patients had HCV RNA <LLOQ, 100% of patients with genotype 2 HCV and 91% with genotype 3 HCV. By week 8, all 45 patients receiving treatment had HCV RNA <LLOQ (Table2).
Table 2.
Response During and After Treatment
| Response | Genotype 2 (N = 23) | Genotype 3 (N = 24) | Total (N = 47) |
|---|---|---|---|
| HCV RNA < LLOQ during treatment, n/N (%) | |||
| At baseline | 0/23 | 0/24 | 0/47 |
| Week 1 | 10/23 (43) | 8/24 (33) | 18/47 (38) |
| Week 2 | 18/23 (78) | 16/23 (70) | 34/46 (74) |
| Week 4 | 22/22 (100) | 21/23 (91) | 43/45 (96) |
| Week 6 | 22/22 (100) | 22/23 (96) | 44/45 (98) |
| Week 8 | 22/22 (100) | 23/23 (100) | 45/45 (100) |
| Week 12 | 22/22 (100) | 22/22 (100) | 44/44 (100) |
| HCV RNA < LLOQ after end of treatment, n/N (%) | |||
| SVR4 | 22/23 (96) | 21/24 (88) | 43/47 (92) |
| 95% CI | [78-100] | [68-97] | [80-98] |
| SVR12 | 22/23 (96) | 20/24 (83) | 42/47 (89) |
| 95% CI | [78-100] | [63-95] | [77-97] |
| SVR 24 | 22/23 (96) | 20/24 (83) | 42/47 (89) |
| 95% CI | [78-100] | [63-95] | [77-97] |
| Overall virologic failure, n/N (%) | 0/23 | 2/24 (8) | 2/47 (4) |
| Relapse | 0/22 | 2/23 (9) | 2/45 (4) |
| Study drug completer | 0/22 | 2/22 (9) | 2/44 (5) |
| Study drug noncompleter | 0/0 | 0/1 | 0/1 |
| On-treatment virologic failure | 0/23 | 0/24 | 0/47 |
| Other | 1/23 (4) | 2/24 (8) | 3/47 (6) |
SVR, sustained virologic response; Other, subjects who did not achieve SVR12 and did not meet virologic failure criteria.
Overall, the proportion of patients with SVR12 was 89% (95% CI: 77-97). The rates of SVR12 were higher in patients with genotype 2 than in those with genotype 3, 96% (95% CI: 78-100) and 83% (95% CI: 63-95), respectively (Table3). Five patients did not achieve SVR12: one with genotype 2 HCV (4% of the subgroup) and four with genotype 3 HCV (17% of the subgroup). The patient with genotype 2 HCV who did not achieve SVR12 discontinued treatment after 15 days without achieving HCV RNA <LLOQ. Among the four patients with genotype 3 who did not achieve SVR12, two had observed virologic relapse after treatment (9%) and two were lost to follow-up. Both patients who relapsed were white males with genotype 3a HCV and both had CT alleles of the IL28B gene. One of the relapsers, a 56-year-old man with cirrhosis, relapsed between posttreatment weeks 4 and 12. The other, a 61-year-old man without cirrhosis, relapsed by posttreatment week 4. No on-treatment breakthrough was observed in any patient, and there were no virologic failures among patients with genotype 2 HCV. There was no virologic relapse after posttreatment week 12; all patients with SVR12 also achieved SVR24.
Table 3.
Subgroup Analysis of SVR12 Rates and Virologic Failure
| Genotype 2(N = 23) | Genotype 3(N = 24) | Total (N = 47) | |
|---|---|---|---|
| Overall SVR12 n/N (%) | 22/23 (96) | 20/24 (83) | 42/47 (89) |
| 95% CI | [78-100] | [63-95] | [77-97] |
| Age at baseline (years) | |||
| <50 | 2/2 (100) | 8/8 (100) | 10/10 (100) |
| 95% CI | [16-100] | [63-100] | [69-100] |
| ≥50 | 20/21 (95) | 12/16 (75) | 32/37 (87) |
| 95% CI | [76-100] | [48-93] | [71-96] |
| Sex | |||
| Male | 14/14 (100) | 14/18 (78) | 28/32 (88) |
| 95% CI | [77-100] | [52-94] | [71-97] |
| Female | 8/9 (89) | 6/6 (100) | 14/15 (93) |
| 95% CI | [52-100] | [54-100] | [68-100] |
| Race | |||
| Black | 0/0 | 1/1 (100) | 1/1 (100) |
| 95% CI (%) | [3-100] | [3-100] | |
| Non-Black | 22/23 (96) | 19/23 (83) | 41/46 (89) |
| 95% CI | [78-100] | [61-95] | [76-96] |
| Ethnicity | |||
| Hispanic or Latino | 10/11 (91) | 9/10 (90) | 19/21 (91) |
| 95% CI | [59-100] | [56-100] | [70-99] |
| Not Hispanic or Latino | 12/12 (100) | 11/14 (79) | 23/26 (89) |
| 95% CI | [74-100] | [49-95] | [70-98] |
| Cirrhosis | |||
| No | 9/9 (100) | 10/12 (83) | 19/21 (91) |
| 95% CI | [66-100] | [52-98] | [70-99] |
| Yes | 13/14 (93) | 10/12 (83) | 23/26 (89) |
| 95% CI | [66-100] | [52-98] | [70-98] |
| Baseline HCV RNA | |||
| <6 log10 IU/mL | 4/4 (100) | 11/13 (85) | 15/17 (88) |
| 95% CI | [40-100] | [55-98] | [64-99] |
| ≥6 log10 IU/mL | 18/19 (95) | 9/11 (82) | 27/30 (90) |
| 95% CI | [74-100] | [48-98] | [74-98] |
A subgroup analysis was performed to evaluate the effects of age and the presence or absence of cirrhosis on SVR12. Due to the small sizes of the subgroups and the high overall response rate, no difference in response by subgroup, including the presence of cirrhosis, could be detected.
Viral Resistance Testing
The NS5B region was deep-sequenced at baseline and at the time of virologic failure in samples collected from the two patients with genotype 3 HCV infection who experienced viral relapse. Low levels of F289L (1.11%), a variant associated with resistance to nucleotide inhibitors, was detected at baseline in one patient, but was not detected at the time of virologic failure. L159F, an SOF treatment-emergent variant, was detected in the same subject at posttreatment week 4 (>99%). The S282T substitution was not detected and no changes in susceptibilities to SOF or RBV were detected in any patient.
Safety
Overall, 45 patients (96%) reported at least one adverse event and 91% of patients experienced at least one adverse event that was considered by the physician to be related to study treatment. No deaths occurred. Three patients discontinued RBV due to ribavirin-related anemia and one patient discontinued all therapy due to pain. Four patients (9%) had five serious adverse events: of cholecystitis, sepsis, anemia, decompensated cirrhosis, and hemorrhaging from esophageal varices. Three of the serious adverse events were considered by the physician to be related to either Peg-IFN or RBV (sepsis, anemia, decompensated cirrhosis) and none was considered to be related to SOF. The most frequently reported adverse events (in ≥20% of patients) were influenza-like illness, fatigue, anemia, and neutropenia (Table4); most adverse events were rated by the physician as mild or moderate in severity.
Table 4.
Overall Adverse Events, Discontinuations, and Hematologic and Chemistry Abnormalities
| Event, n (%) | Genotype 2 or 3(N = 47) |
|---|---|
| Any adverse event | 45 (96) |
| Discontinuation of any study drug owing to an adverse event | 4 (9) |
| Serious adverse event | 4 (9) |
| Common adverse events* | |
| Influenza-like illness | 26 (55) |
| Fatigue | 15 (32) |
| Anemia | 14 (30) |
| Neutropenia | 11 (23) |
| Nausea | 8 (17) |
| Headache | 7 (15) |
| Rash | 7 (15) |
| Thrombocytopenia | 7 (15) |
| Insomnia | 6 (13) |
| Diarrhoea | 5 (11) |
| Hypertension | 5 (11) |
| Grade 3 and 4 laboratory abnormalities | |
| Decreased hemoglobin level | 13 (28) |
| Decreased lymphocyte count <499/ mm3 | 2 (4) |
| Decreased neutrophil count <750/ mm3 | 13 (28) |
| Platelet count <50,000/ mm3 | 7 (15) |
| White-cell count <1,000/ mm3 | 3 (7) |
Adverse events reported in at least 10% of patients in any study group.
The most common hematologic abnormalities observed were decreased concentrations of hemoglobin and decreased counts of neutrophils and platelets. Declines in hemoglobin concentrations to below 10 g/dL were observed in 13 patients, with four patients reporting a decline in hemoglobin to below 8.5 g/dL. Neutrophil counts decreased rapidly during the first week of treatment, with absolute neutrophil counts below 750/mm3 in 13 patients, of whom three were below 500/mm3. Posttherapy neutrophil counts returned to baseline values. These results are consistent with the known effects of Peg-IFN+RBV treatment.14 Grade 3 elevations in bilirubin were seen in four patients, of whom three had cirrhosis. The changes in total bilirubin levels were consistent with ribavirin-induced hemolysis. No notable findings related to vital signs (systolic and diastolic blood pressure and pulse) were observed during the study and no patient had a clinically significant ECG abnormality.
Discussion
This phase 2 study was the first to evaluate the efficacy, safety, and tolerability of 12-week administration of SOF+Peg-IFN+RBV in treatment-experienced patients with genotype 2 and 3 HCV infection, with and without cirrhosis. This study showed high SVR rates in patients who have historically exhibited suboptimal response rates to HCV treatment and for whom there are few treatment options available.
The 96% SVR12 rate in treatment-experienced patients with HCV genotype 2 after 12 weeks of treatment with SOF+Peg-IFN+RBV is similar to rates previously reported in treatment-experienced noncirrhosis patients who received an IFN-free regimen consisting of SOF+RBV for the same duration (SVR of 94-96%).10,11 Importantly, there were no genotype 2 patients who experienced virologic failure, including the two genotype 2 patients with prior virologic nonresponse to Peg-IFN+RBV therapy. The only patient who did not achieve SVR was one who discontinued treatment prior to complete viral suppression. In our study, patients infected with genotype 2 HCV with cirrhosis had an SVR rate of 93%, while previous studies reported rates of 60-78% in patients who received SOF+RBV for 12 to 16 weeks.10,11 The higher SVR12 rates we observed in patients with HCV genotype 2 and cirrhosis may therefore represent improved efficacy in those with cirrhosis, compared with SOF+RBV for 12 or 16 weeks. The regimen of SOF+Peg-IFN+RBV appears to provide an effective treatment option for treatment-experienced HCV genotype 2 patients with cirrhosis who are able and willing to receive IFN. Given that, by definition, treatment-experienced patients have already received a course of Peg-IFN, this would be expected to be a large proportion of this subpopulation.
Although a lower SVR12 rate (83%) was seen in genotype 3 patients as compared to genotype 2 patients, this numeric difference was attributable to a higher rate of patients with nonvirologic treatment failure (e.g., early treatment discontinuation, loss to follow-up). Virologic relapse occurred in only two (8%) patients, one with cirrhosis and one without cirrhosis. Given the small sample size and low number of relapsers, no clear baseline predictors of treatment failure were evident. It is not possible to assess the impact of prior response to therapy on treatment outcome; one patient who relapsed after SOF+Peg-IFN+RBV was a prior nonresponder to Peg-IFN+RBV and the other was a prior relapser. Whether comparing rates of SVR or relapse, this regimen demonstrated better efficacy in treatment-experienced genotype 3 patients with cirrhosis than SOF+RBV therapy for up to 24 weeks, which had a 62% SVR12 rate in a phase 3 trial.11 In the same trial, treatment-experienced patients without cirrhosis demonstrated an 87% SVR rate following 24 weeks of SOF+RBV, which is similar to the SVR rate of 83% observed in this trial in this subgroup. Thus, 12 weeks of SOF+Peg-IFN+RBV is an important treatment option for treatment-experienced genotype 3 patients who can take IFN, particularly for those with cirrhosis. However, the benefit of SOF+Peg-IFN+RBV must be weighed against the toxicities associated with interferon-based treatment, which are exacerbated in patients with cirrhosis.
This study confirms previous reports regarding the high barrier to resistance observed with sofosbuvir-containing regimens. Rapid virologic suppression occurred, with all but two genotype 3 HCV-infected patients achieving HCV RNA <LLOQ by week 4, and there were no virologic breakthroughs during treatment. Overall, virologic relapse was observed in two patients, both infected with genotype 3 HCV. Resistance analysis was performed for the two patients with genotype 3 HCV who experienced viral relapse. No S282T, the signature resistance mutation for sofosbuvir, was detected at the time of relapse using deep sequencing with a level of detection of 1%. The L159F treatment-emergent variant15 was detected in one patient at virologic relapse at posttreatment week 4. This substitution has been previously described at the time of virologic failure in sofosbuvir-treated patients but does not confer an in vitro shift in susceptibility to sofosbuvir. The clinical significance of this substitution and its persistence over time are unknown.
Treatment for 12 weeks with SOF+Peg-IFN+RBV was generally well tolerated in this treatment-experienced population, in which 55% of patients had compensated cirrhosis. Consistent with data from an earlier phase 3 trial of SOF+Peg-IFN+RBV in genotype 1 HCV-infected patients, one patient (2%) discontinued all treatment due to adverse events.7 This patient stopped treatment on day 2 of treatment due to generalized body pain. The low discontinuation rates suggest that the shortened 12-week course of interferon produces fewer early discontinuations compared to historic regimens of 24-week duration.1,16–18 Expected RBV-associated hematologic effects were successfully managed through RBV dose reductions or interruptions. In addition, the most frequently reported adverse events were influenza-like illness, fatigue, and anemia. These events are consistent with the historical safety profile of Peg-IFN+RBV; no additional safety signal was associated with the addition of SOF. These data further underscore that the 12-week duration of treatment, even in patients with compensated cirrhosis, facilitates treatment completion despite the expected untoward effects of both Peg-IFN and RBV.
In conclusion, in treatment-experienced patients with HCV genotypes 2 and 3, including those with compensated cirrhosis, 12-week administration of SOF+Peg-IFN+RBV provided high SVR rates. In addition, SOF+Peg-IFN+RBV was generally well tolerated, with low discontinuation rates and a profile of adverse events consistent with Peg-IFN+RBV. As discussed in guidance documents issued by the American Association for the Study of Liver Diseases (AASLD)19 and the European Association for the Study of the Liver,20 this regimen represents an important option for treatment-experienced patients who are able to tolerate 12 weeks of peg-IFN therapy, particularly those with compensated cirrhosis.
Acknowledgments
The authors thank the patients and staff who participated in the study. Laila Guzadhur, Ph.D., and Severina Moreira, Ph.D., from Niche Science and Technology (Richmond-Upon-Thames, London, United Kingdom) provided writing and editorial support during the development of the article; these services were paid for by Gilead Sciences.
Glossary
- AASLD
American Association for the Study of Liver Diseases
- ALT
alanine transaminase
- AST
aspartate aminotransferase
- HCV
hepatitis C virus
- IFN
interferon
- LLOQ
lower limit of quantification
- Peg-IFN
peginterferon
- RBV
ribavirin
- SOF
sofosbuvir
- SVR12
sustained virologic response at 12 weeks after cessation of study treatment
Footnotes
Author names in bold designate shared co-first authorship.
References
- Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naggie S, Patel K, McHutchison J. Hepatitis C virus directly acting antivirals: current developments with NS3/4A HCV serine protease inhibitors. J Antimicrob Chemother. 2010;65:2063–2069. doi: 10.1093/jac/dkq284. [DOI] [PubMed] [Google Scholar]
- Tapper EB, Afdhal NH. Is 3 the new 1: perspectives on virology, natural history and treatment for hepatitis C genotype 3. J Viral Hepat. 2013;20:669–677. doi: 10.1111/jvh.12168. [DOI] [PubMed] [Google Scholar]
- Gower E, Estes CC, Blach S, Razavi-Shearer K. Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus. J Hepatol. 2014 doi: 10.1016/j.jhep.2014.07.027. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Kanwal F, Kramer JR, Ilyas J, Duan Z, El-Serag HB. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. Veterans with HCV. Hepatology. 2014;60:98–105. doi: 10.1002/hep.27095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens N, Negro F. Is genotype 3 of the hepatitis C virus the new villain? Hepatology. 2014;59:2403–2412. doi: 10.1002/hep.26905. [DOI] [PubMed] [Google Scholar]
- Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013a;368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- Assis DN, Lim JK. New pharmacotherapy for hepatitis C. Clin Pharmacol Ther. 2012;92:294–305. doi: 10.1038/clpt.2012.103. [DOI] [PubMed] [Google Scholar]
- Wyles DL. Beyond telaprevir and boceprevir: resistance and new agents for hepatitis C virus infection. Top Antivir Med. 2012;20:139–145. [PMC free article] [PubMed] [Google Scholar]
- Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867–1877. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370:1993–2001. doi: 10.1056/NEJMoa1316145. [DOI] [PubMed] [Google Scholar]
- Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Symonds WT, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med. 2013;368:34–44. doi: 10.1056/NEJMoa1208953. [DOI] [PubMed] [Google Scholar]
- Lawitz E, Lalezari JP, Hassanein T, Kowdley KV, Poordad FF, Sheikh AM. Sofosbuvir in combination with peginterferon alfa-2a and ribavirin for non-cirrhotic, treatment-naive patients with genotypes 1, 2, and 3 hepatitis C infection: a randomised, double-blind, phase 2 trial. Lancet Infect Dis. 2013b;13:401–408. doi: 10.1016/S1473-3099(13)70033-1. [DOI] [PubMed] [Google Scholar]
- Papastergiou V, Stampori M, Lisgos P, Pselas C, Prodromidou K, Karatapanis S. Durability of a sustained virological response, late clinical sequelae, and long-term changes in aspartate aminotransferase to the platelet ratio index after successful treatment with peginterferon/ribavirin for chronic hepatitis C: a prospective study. Eur J Gastroenterol Hepatol. 2013;25:798–805. doi: 10.1097/MEG.0b013e32835eb8bf. [DOI] [PubMed] [Google Scholar]
- Svarovskaia ES, Dvory-Sobol H, Parkin N, Hebner C, Gontcharova V, et al. Infrequent development of resistance in genotype 1 through 6 HCV-infected subjects treated with sofosbuvir in phase 2 and 3 clinical trials. Clin Infect Dis. 2014 doi: 10.1093/cid/ciu697. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman ML, Suter F, Bacon BR, Nelson D, Harley H, Solá R, et al. Peginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2007;357:124–134. doi: 10.1056/NEJMoa066403. [DOI] [PubMed] [Google Scholar]
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- Poynard T, Colombo M, Bruix J, Schiff E, Terg R, Flamm S, et al. Peginterferon alfa-2b and ribavirin: effective in patients with hepatitis C who failed interferon alfa/ribavirin therapy. Gastroenterology. 2009;136:1618–1628. doi: 10.1053/j.gastro.2009.01.039. [DOI] [PubMed] [Google Scholar]
- American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. Available at. http://www.hcvguidelines.org/ [DOI] [PMC free article] [PubMed]
- European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392–420. doi: 10.1016/j.jhep.2013.11.003. [DOI] [PubMed] [Google Scholar]


