Abstract
The objective of this study was to examine the effect of calving difficulty or dystocia on the vitality of newborn calves and its association with blood pH, the apparent efficiency of immunoglobulin G (IgG) absorption (AEA), and weight gain. A total of 45 calving events (N = 48 calves) were monitored from the first sight of fetal membranes. All calves were assessed at the time of first attaining sternal recumbency (SR), at 2 and 24 h, and at 7 and 14 d of age. Measurements included time to SR, rectal temperature, respiration and heart rate, analysis of blood gases and other blood measures, suckling response, time to standing, passive transfer of IgG, and weight gain. Calves were separated from their dam 2 h after birth and fed a commercial colostrum replacer containing 180 g of IgG by esophageal tube feeder. Calves born following dystocia had lower venous blood pH and took longer to attain SR and attempt to stand than those born unassisted. Duration of calving interacted with the number of people required to extract the calf by pulling as a significant predictor of pH at SR. No association was found between pH at SR and AEA. However, reduced AEA was found in calves that were female and in calves that did not achieve SR within 15 min of birth. A longer calving duration, being born in July or August rather than June, and a shorter time spent standing in the first 2 d of life were significantly associated with reduced weight gain to 14 d. It was concluded that factors at calving impact the physiology, vitality, and subsequent weight gain of newborn calves.
Résumé
L’objectif de la présente étude était d’examiner les effets des difficultés au moment du vêlage ou dystocie sur la vitalité de veaux nouveaunés et l’association avec le pH sanguin, l’efficacité apparente d’absorption des immunoglobulines G (IgG) (EAA), et le gain de poids. Quarante-cinq vêlages (N = 48 veaux) furent surveillés à partir de la première visualisation des membranes foetales. Tous les veaux furent évalués au moment de la première fois qu’ils étaient en décubitus sternal (DS), à 2 et 24 h, et à 7 et 14 jours d’âge. Les données recueillies incluaient le délai pour atteindre le DS, la température rectale, les rythmes respiratoire et cardiaque, l’analyse des gaz sanguins et d’autres mesures sanguines, la réponse de tétée, le délai pour se tenir debout, le transfert passif d’IgG et le gain de poids. Les veaux furent séparés de leur mère 2 h après la naissance et nourris par tube oesophagien avec un substitut commercial du colostrum contenant 180 g d’IgG. Les veaux nés suivant une dystocie avaient un pH sanguin veineux plus bas et ont pris plus de temps pour atteindre le DS et tenter de se lever que ceux nés sans assistance. La durée du vêlage a interagit avec le nombre de personnes requis pour extraire le veau en tirant comme un prédicteur significatif du pH à DS. Aucune association ne fut trouvée entre le pH à DS et l’EAA. Toutefois, une EAA réduite fut notée chez les génisses et chez les veaux qui n’étaient pas en DS à l’intérieur d’un délai de 15 min suivant la naissance. Une durée plus longue du vêlage, une naissance en juillet ou août plutôt qu’en juin, et un temps plus court à se tenir debout pendant les deux premières journées de vie étaient associés significativement avec un gain de poids moindre après 14 j. Il a été conclu que des facteurs au moment du vêlage ont un impact sur la physiologie, la vitalité et le gain de poids à venir de veaux nouveau-nés.
(Traduit par Docteur Serge Messier)
Introduction
The percentage of dairy calves that require assistance at birth has recently increased (1). As a result of difficult births or dystocia, a considerable number of calves are either born dead or die within 48 h of birth. The most comprehensive study of calf loss in North America indicates that 15.9% of calves die before weaning and that 8.1% of these deaths result from events that occur during calving and in the initial 48 h after birth (2). Dystocia may interfere with the vitality of newborn calves. Vitality can be defined as the capacity to live and grow with physical and mental energy and strength (3). Low vitality may impact the physiology and behavior of newborn calves.
Acidosis in calves following dystocia or forced extraction may be due to the premature rupture of the umbilical vessels or irregular respiration after birth, which may lead to reduced vitality (4,5). If the umbilical cord ruptures prematurely, blood oxygenation from the placenta is terminated before the calf can regulate its respiration. In addition, if the newborn calf cannot maintain adequate ventilation for gas exchange, oxygen supply will diminish, which leads to the rapid development of asphyxia and respiratory acidosis (5,6). If the hypoxia is severe enough, tissues will derive energy from anaerobic glycolysis, resulting in the production of lactic acid, inducing a state of metabolic acidosis. Severe respiratory and metabolic acidosis resulting from hypoxia may compromise survival in the newborn calf (7,8). Schuijt and Taverne (9) found that calves born following severe dystocia were more acidotic, took longer to achieve a normal pH (> 7.2), and had a greater risk of mortality.
Suckling reflex and time to standing have been assessed as objective behavioral indicators of fetal stress in calves. Schuijt and Taverne (9) examined the time interval from birth to sternal recumbency (SR) as a measure of newborn calf vitality. It was determined that calves that were forcefully extracted [force of traction greater than the mean pulling power (± 300 kg) of 2 men] took significantly longer to achieve SR, had more severe acidosis, recovered more slowly from acidosis, had greater mortality, and exhibited evidence of trauma more frequently than calves that were born without assistance, were normally extracted [force less than the mean pulling power (± 160 kg) of 1 man], or were born by Caesarean section (9).
In other research, increased time to standing has been found to be associated with a reduced or delayed motivation to drink colostrum after birth. In calves with fetal distress, consumption of colostrum is reduced by 74% during the first 12 h after birth (10). Thus, failure of passive transfer in these calves may result from failing to suckle in a timely manner. In 1 study, acidosis (venous blood pH < 7.15) was associated with a 52% decrease in colostrum intake and a 35% decrease in serum immunoglobulin G (IgG) concentration compared to calves with venous blood pH > 7.25 (11). In other studies, the increased morbidity resulting from failure of passive transfer has been associated with reduced IgG absorption, rather than intake. Dystocia-induced hypoxia, hypercapnia, and respiratory acidosis have been associated with decreased absorption of IgG (12,13).
The objective of this study was to examine calving events and the resulting physiological and behavioral characteristics in newborn calves and to determine whether these characteristics were associated with newborn vitality, the passive transfer of colostral immunoglobulin, and weight gain.
Materials and methods
Calf enrollment and sampling
This project was conducted from May to September 2010 at the University of British Columbia’s Dairy Education and Research Centre in Agassiz, British Columbia. Forty-five cows and their calves (N = 48) were enrolled in the study. This experiment was conducted according to the standard operating procedures of the Centre and in accordance with guidelines set by the Canadian Council for Animal Care (14).
Near-term cows were housed in freestall pens and fed a diet containing 1.17% calcium (Ca) and a calculated dietary cation-anion difference (DCAD) of 2.3 mEq/100 g of dry matter (DM). Prior to calving, cows were moved to 1 of 4 maternity pens, which had a sand base and were deep-bedded with straw. Food and water were available at all times. Live and videotaped observations of the calving process were initiated at the first sight of fetal membranes, defined as the start of stage-II labor. If the observations did not begin before the amniotic sac ruptured, the calving was not enrolled. Enrolled calvings were observed by 1 or 2 persons and videotaped using 2 cameras mounted on opposite walls of the maternity pen area. Videotapes were used to confirm visual observations of the events during calving and afterwards. The presentation of the calf at the start of labor, the nature and duration of any intervention during the calving process (including the number of people required to extract the calf, if necessary), as well as the duration of the calving event beginning at stage-II labor, were observed and recorded. Calving difficulty was scored using a 3-point scale. An unassisted birth was classified as 1, an easily assisted birth as 2, and dystocia requiring substantial force to extract the calf as 3. The number of people required to apply traction to extract the calf was treated as a separate variable from calving score. Every attempt was made to allow the cow to calve unassisted (up to 2 h). If the cow or calf was considered to be in distress, assistance was provided immediately. After calving, all cows in their third lactation or greater were given Ca subcutaneously.
Newborn calves remained with the dam and the times when the calf first held its head up and attained SR were recorded. Sternal recumbency was defined as the calf resting on its sternum and holding its head up. If SR was not achieved within 15 min of birth, the calf was placed into SR manually. Achieving SR within 15 min was categorized as a dichotomous (yes or no) outcome. At SR, heart rate, respiratory rate, and rectal temperature were measured and blood samples collected. Blood oxygen saturation and suckling response were also measured.
Blood oxygen saturation was measured with a veterinary pulse oximeter (Edan VE-H100B; Apexx Veterinary Equipment, Englewood, Colorado, USA) fitted with a C-clip fixed to the calf’s upper lip. Suckling response was measured on the fingers using a 4-point scale (1 = no suckling response, 2 = weak suckling, 3 = moderate suckling, and 4 = strong suckling response). A second measure of suckling using a pressure manometer (VWR International 89094-728; Mississauga, Ontario) fitted with a Peach Teat (Skellerup, Christchurch, New Zealand) was also recorded. Minimum and maximum values of pressure were obtained from the manometer in units of centimeters of water (cm H2O). After assessments at SR, the calf was left with its dam until 2 h after birth but was closely observed to prevent suckling. During the first 2 h, the time when the calf first attempted to stand (defined as 2 feet placed firmly on the ground) and stood successfully (4 feet placed firmly on the ground) for 2.5 and 5 min was recorded.
At 2 h of age, another series of vitality assessments were taken, including heart and respiration rate, rectal temperature, oxygen saturation, and suckling response (using the finger score and manometer). Blood samples were also collected. The calf was then moved to a calf barn and placed in an individual pen, measuring 1.16 × 2.0 m and bedded with shavings. All calves were fed 180 g of IgG in a commercial colostrum product (Headstart; Saskatoon Colostrum, Saskatoon, Saskatchewan), reconstituted according to label directions to a 3-L feeding volume. Colostrum replacer was fed at 38°C using an esophageal tube feeder. After feeding, a HOBO Pendant G Data Logger (MicroDAQ; Contoocook, New Hampshire, USA) was attached to the right rear leg to record standing and lying behavior over a 14-d period. Depending on the timing of birth and colostrum feeding, calves were offered 3 L of whole milk by nipple bottle at the next feeding time (8 am or 4 pm).
At 24 h after colostrum feeding, vitality measurements were reassessed and blood samples collected. Body measurements were taken and recorded, including length, girth, hip width, height, and weight.
On days 1 to 5 of life, calves were housed individually and offered 3 L of a combination of bulk tank and pasteurized waste milk twice a day (8 am and 4 pm) by nipple bottle. The amount consumed was recorded. On day 5, calves were dehorned and on day 6 moved into group pens (4.9 × 7.3 m, with up to 10 calves/pen), where they could consume up to 12 L of whole milk/calf/day from an automated milk feeder (CF1000CS Combi; DeLaval, Tumba, Sweden). Hay and fresh water were supplied ad libitum. Calves were housed on shavings in the group pens. Daily observations for diarrhea and respiratory disease were recorded.
At 7 and 14 d of age, heart and respiration rate, rectal temperature, suckling response (finger score and manometer), and body weight were measured and recorded and blood samples collected. At 14 d of age, body size, including length, girth, hip width, height, and weight, was also measured and recorded and the HOBO Data Logger was removed.
Blood sampling
Blood was collected from the jugular vein using 20G 1-inch needles. Whole blood samples were harvested into collection tubes containing ethylenediamine tetra-acetic acid (EDTA) (BD Vacutainer, Mississauga, Ontario) for analysis of complete blood (cell) count (CBC) (SR, 2 h, 24 h, 7 d, and 14 d), as well as lactate and glucose (SR, 2 h, and 24 h). Blood smears were made and air dried.
Blood samples for analysis of serum were collected into glass, silicon-coated collection tubes without anticoagulant (BD Vacutainer). Clotted samples were centrifuged and the serum harvested and frozen at −20°C for analysis of creatine kinase (CK) (SR and 24 h) and IgG (2 h, 24h, 7 d, and 14 d). Samples for blood-gas analysis were drawn from the jugular vein at SR, 2, and 24 h into lithium heparin-coated syringes (Gas Lyte Arterial Blood Sampler; Vital Signs, Englewood, Colorado, USA) and injected within 5 min of collection into an IRMA TruPoint Blood Gas Analysis System (Nexus Dx, Edison, New Jersey, USA).
Laboratory analyses
IgG
Frozen sera were transported on ice to the Saskatoon Colostrum Co. Ltd. (Saskatoon, Saskatchewan) for IgG testing. Samples were tested for total IgG concentration (g/L) by radial immunodiffusion as described by Chelack et al (15) with minor modifications. Apparent efficiency of IgG absorption (AEA) was calculated using the formula:
Plasma volume was calculated by multiplying the 24-h calf body weight (kg) by 9.94%. The 24-h plasma IgG values were used and the IgG intake was 180 g.
Lactate and glucose
Lactate levels in whole blood were measured using a Lactate Pro hand-held meter (Arkray, Kyoto, Japan) according to the manufacturer’s instructions. Glucose levels in whole blood were measured using a Precision-Xtra hand-held meter (Abbott Diabetes Care, Alameda, California, USA) according to the manufacturer’s instructions.
Creatine kinase (CK)
Frozen serum was thawed and analyzed for CK within 1 mo of collection. Following manufacturer’s instructions, a 10-μL aliquot of undiluted serum was analyzed, in duplicate, using the Enzychrome Creatine Kinase Assay Kit (ECPK-100; BioAssay Systems, Hayward, California, USA).
Blood gases
Blood gases were analyzed using an IRMA TruPoint Blood Gas Analysis System (Nexus Dx) following instrument instructions. Parameters measured included blood pH, partial pressure of carbon dioxide (pCO2) and oxygen (pO2), hematocrit, sodium, potassium, and ionized calcium. Values were calculated for bicarbonate, total carbon dioxide, base excess of blood and extracellular fluid, oxygen saturation, and total hemoglobin.
Complete blood (cell) count (CBC)
Complete blood (cell) count data were generated for each calf at Little Mountain Veterinary Clinic (Chilliwack, British Columbia). This included enumeration of white blood cells (lymphocytes, monocytes, and granulocytes), red blood cells and platelets, hemoglobin, and mean corpuscular volume using a Heska HemaTrue Veterinary Hematology Analyzer (Loveland, Colorado, USA). Differential leukocyte counts were also done manually on blood smears from 2 h and 24 h for most calves at the Department of Veterinary Pathology, Western College of Veterinary Medicine, University of Saskatchewan.
Statistical analysis
Data were entered into Microsoft Office Excel (Microsoft, Redmond, Washington, USA) and exported into Stata/IC 10 (StataCorp, College Station, Texas, USA) for variable screening and statistical modeling. A causal diagram was constructed to determine the relationships that should be tested by the model. All variables were examined with descriptive statistics to determine the distribution of the data and identify missing values. Univariable linear regression models were constructed to determine associations of interest for continuous outcomes, including minutes to SR, rectal temperature, heart and respiration rate, blood-gas variables, suckling reflex, and 24-h weight. Univariable logistic regression was used for the dichotomous outcome of whether the calf achieved SR within 15 min, whereas univariable multinomial logistic regression was used to determine associations with calving score. In addition, univariable Cox proportional hazards models were made to determine associations with the time taken to achieve SR, to the calf’s first attempt to stand, to stand for 2.5 and 5 min after birth, due to the limited number of calves that achieved SR and successful standing in the 15 min and 2 h observation period, respectively.
Three separate multivariable linear regression models were constructed using the “reg” command in Stata/IC 10.1. Models were constructed to examine the effects of dystocia on blood pH at SR, as well as the physiological and behavioral characteristics of newborn calves that impact AEA and 14-d weight gain. Univariable linear regression was used to screen predictor variables for inclusion in each of the 3 multivariable models. Any predictor variables that were associated with the outcome using a liberal P-value (α ≤ 0.20) were considered for inclusion in the final model.
Spearman correlation coefficients were calculated between all main effects variables that were considered for inclusion in the final model to avoid issues associated with collinearity. Consequently, if the correlation coefficient between 2 variables had an absolute value greater than 0.75, the variable of most biological relevance or with the fewest missing observations was included in the multivariable model-building process.
Linearity was assessed graphically in all main effects variables with the outcome variable, using lowess smoothers. If a variable was non-linear, a quadratic term was constructed and tested in the model. If the quadratic term was significant (P < 0.05) in the model and the relationship was adequately modeled using a curve, the quadratic term was retained in the model. If not, the quadratic term was removed and log and square root transformations were assessed for linearity and significance. If none of the presented transformations appeared to improve linearity, the variable was categorized.
A manual stepwise model-building approach was used. Confounding was assessed throughout model building by backwards elimination and was defined as a > 25% change in the coefficients of significant variables with removal of the potential confounder.
Interaction terms were constructed between all biologically plausible variables in the model. Each interaction term was tested for significance by inclusion in the model with all main effects. Interaction terms that were found to be statistically significant (P < 0.05) through this process were added to the model. In the final multivariable model, variables were retained if they were statistically significant, acted as a confounder, or were part of a significant interaction term.
To satisfy the assumption of homoscedasticity, a Cook-Weisberg test was done using a non-significant test (P ≥ 0.05) as evidence for homoscedasticity. Homoscedasticity was also evaluated graphically by plotting the standardized residuals against the predicted values. A Shapiro-Wilk’s test was carried out on the standardized residuals, with a non-significant test (P ≥ 0.05) suggesting normality. Standardized residuals were examined graphically to identify observations with high leverage. Cook’s Distance, DFITS, and DFBETA values were examined graphically to identify observations with a relatively large influence on the model and/or coefficient estimates for specific variables. Outliers and influential observations were examined for recording errors and to see if their removal resulted in a change in interpretation of the model(s).
Results
Description of study population
A total of 48 neonatal Holstein calves born at the University of British Columbia’s Dairy Education and Research Centre was enrolled in this study from May to September, 2010. The study population consisted of 22 heifers, 25 bulls, and 1 freemartin calf and included 3 sets of twins. Of the 48 calves enrolled, 1 died between 24 and 48 h of birth and was not included in the statistical analyses of outcomes measured after 24 h. The freemartin calf was excluded from all analyses involving gender as an independent variable.
Twenty calves were born with a calving score of 1, 18 with a score of 2, and 10 with a score of 3. Descriptive statistics of calf gender, calving score, duration of calving, as well as 24 h IgG, AEA, and weight are presented in Table I. All 48 calves enrolled in the study achieved serum IgG levels indicative of successful passive transfer (≥ 10 g IgG/L) 24 h after colostrum feeding. The mean and standard deviation of the 24-h IgG concentrations and AEA for all calves were 17.1 ± 2.9 g/L and 40.5 ± 6.9%, respectively.
Table I.
Summary of the number of heifer, bull, and freemartin calves by calving score, duration of calving, 24-h IgG concentration, apparent efficiency of IgG absorption (AEA), and body weight at 24 h and 14 d (Mean ± SD)
| Sex | Calving score | Number of calves | Duration of stage-II labor (min) | 24-h IgG (mg/mL) | 24-h AEA (%) | 24-h weight (kg) | 14-d weight (kg) |
|---|---|---|---|---|---|---|---|
| Heifer | 1 | 12 | 61.4 ± 35.6 | 17.7 ± 4.13 | 38.4 ± 6.62 | 40.1 ± 5.66 | 52.9 ± 6.77 |
| 2 | 8 | 81.0 ± 32.6 | 16.8 ± 2.14 | 39.1 ± 4.49 | 42.4 ± 4.00 | 54.0 ± 2.66 | |
| 3 | 2 | 68.0 ± 48.1 | 13.4 ± 3.96 | 29.5 ± 9.08 | 39.8 ± 0.49 | 50.2 ± 0.85 | |
| Bull | 1 | 8 | 84.8 ± 71.2 | 17.3 ± 2.31 | 43.5 ± 5.59 | 45.7 ± 4.20 | 55.8 ± 5.04 |
| 2 | 10 | 65.6 ± 59.7 | 17.9 ± 1.93 | 43.6 ± 7.62 | 43.9 ± 5.41 | 56.2 ± 4.77 | |
| 3 | 9 | 50.6 ± 33.4 | 16.2 ± 2.32 | 42.6 ± 6.15 | 47.9 ± 5.27 | 60.0 ± 6.52 | |
| Free-martin | 2 | 1 | 13.0 | 17.1 | 30.41 | 32.2 | 42.0 |
Univariable statistics: Effects of dystocia
Dystocia was defined as a birth with a calving score of 3 (hard pull). In the current study, 20.8% (10/48) of calves were born following dystocia. There was a greater incidence of dystocia in primiparous dams than in multiparous dams over the study period. Fifty percent of calves (5/10) from primiparous dams were born following dystocia, compared to 13.2% (5/38) from multiparous dams. However, the association between parity and dystocia was not statistically significant [Relative Risk Ratio (RRR) = 5.67; 95% confidence interval (CI) = 0.99 to 32.43; P = 0.051].
The distribution of calving score by the gender of the calf is presented in Table I. Bull calves weighed 4.8 kg more than heifers at the 24-h sampling time (95% CI = 1.82 to 7.7; P = 0.002). Furthermore, compared to heifer calves, bull calves were at greater risk of being born following dystocia than an unassisted calving (RRR = 6.0; 95% CI = 1.00 to 35.91; P = 0.05).
The physiological effects of calving difficulty may include acid-base imbalance and elevated blood lactate concentrations. In the current study, 5 calves had blood pH of < 7.2 at SR, 1 of which was severely acidotic with a blood pH of 6.9. Calves born following dystocia had significantly lower venous blood pH at SR than those born unassisted (P = 0.03) (Table II). Low blood pH at SR was associated with a weaker suckling response 2 h after birth, as measured with the finger score (β = 4.55; 95% CI = 1.71 to 7.39; P = 0.002). The blood pH of all calves 24 h after colostrum feeding was normal (7.4 ± 0.04) (Table II). Furthermore, compared to an unassisted calving, calves born following dystocia had 2.93 mmol/L higher blood lactate concentration at SR (P = 0.02) (Table II).
Table II.
Summary of the time to sternal recumbency (SR), first attempt to stand, suckling response, blood pH, lactate, and creatine kinase (CK) by calving score (Mean ± SD). Univariable linear associations are presented between minutes to SR and first attempt to stand after birth, suckling score at 2 h, blood pH, lactate, and CK at SR with calving score
| Unassisted | Easy pull | Hard pull | |
|---|---|---|---|
| Number of calves | 20 | 18 | 10 |
| SR within 15 min | |||
| Yes (# of calves) | 18 | 16 | 7 |
| No (# of calves) | 2 | 2 | 3 |
| Minutes to SR | 5.17 ± 3.03 | 7.38 ± 2.68 | 8.29 ± 3.64 |
| Hazard ratio | Ref | 0.63 | 0.39 |
| P-value | — | 0.19 | 0.035 |
| 95% CI | — | 0.32 to 1.26 | 0.16 to 0.94 |
| First attempt to stand in 2 h | |||
| Yes (# of calves) | 20 | 17 | 8 |
| No (# of calves) | 0 | 1 | 2 |
| Minutes to first attempt to stand | 30.25 ± 25.01 | 39.24 ± 22.24 | 49.50 ± 39.23 |
| Hazard ratio | Ref | 0.63 | 0.40 |
| P-value | — | 0.17 | 0.033 |
| 95% CI | — | 0.32 to 1.22 | 0.17 to 0.93 |
| Average suckling scorea (finger test) | |||
| SR | 2.83 ± 0.84 | 2.84 ± 0.89 | 2.22 ± 0.83 |
| 2 h | 3.42 ± 0.86 | 3.08 ± 0.84 | 2.70 ± 0.82 |
| Coefficient | Ref | −0.33 | −0.72 |
| P-value | — | 0.19 | 0.02 |
| 95% CI | — | −0.84 to 0.17 | −1.32 to −0.12 |
| 24 h | 2.33 ± 0.79 | 2.13 ± 1.06 | 2.80 ± 0.92 |
| pH (blood-gas) | |||
| pH at SR | 7.28 ± 0.05 | 7.25 ± 0.07 | 7.21 ± 0.11 |
| Coefficient | Ref | −0.034 | −0.065 |
| P-value | — | 0.18 | 0.03 |
| 95% CI | — −0.084 to 0.017 | −0.12 to | −0.0066 |
| pH at 2 h | 7.33 ± 0.06 | 7.31 ± 0.05 | 7.31 ± 0.07 |
| pH at 24 h | 7.41 ± 0.03 | 7.40 ± 0.05 | 7.42 ± 0.04 |
| Blood lactate (mmol/L) | |||
| SR | 3.69 ± 2.19 | 5.28 ± 2.67 | 6.62 ± 4.68 |
| Coefficient | Ref | 1.59 | 2.93 |
| P-value | — | 0.12 | 0.016 |
| 95% CI | — | −0.42 to 3.60 | 0.57 to 5.29 |
| CK (IU/L) | |||
| CK at SR | 28.08 ± 12.26 | 38.86 ± 17.55 | 59.94 ± 22.88 |
| Coefficient | Refb | 10.78 | 31.86 |
| P-value | — | 0.055 | < 0.001 |
| 95% CI | — | −0.26 to 21.81 | 18.70 to 45.02 |
Suckling score: 1 — no response, 2 — weak, 3 — medium, 4 — strong.
Referent category.
Increased calving difficulty was also associated with higher CK concentration in the blood at SR. Calves born with a calving score of 3 had 31.86 IU/L higher CK concentration at SR than calves born unassisted (calving score of 1) (Table II). The number of people required to assist in extraction of the calf also had a positive association with CK concentration. For each additional person required to extract the calf, CK concentration at SR increased 9.16 IU/L (95% CI = 3.66 to 14.67; P = 0.002). Calves with higher CK concentrations took longer to achieve SR (β = 0.059; 95% CI = 0.0058 to 0.11; P = 0.031). In addition, CK at SR was negatively associated with pH at SR (β = −0.0013; 95% CI = −0.0024 to −0.00023; P = 0.02).
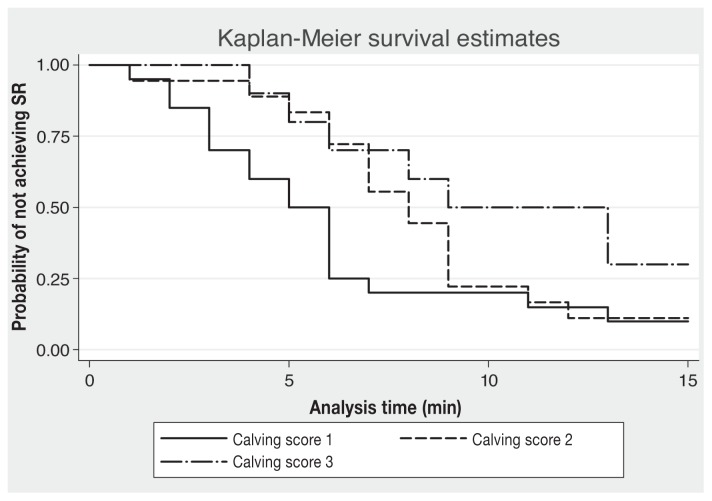
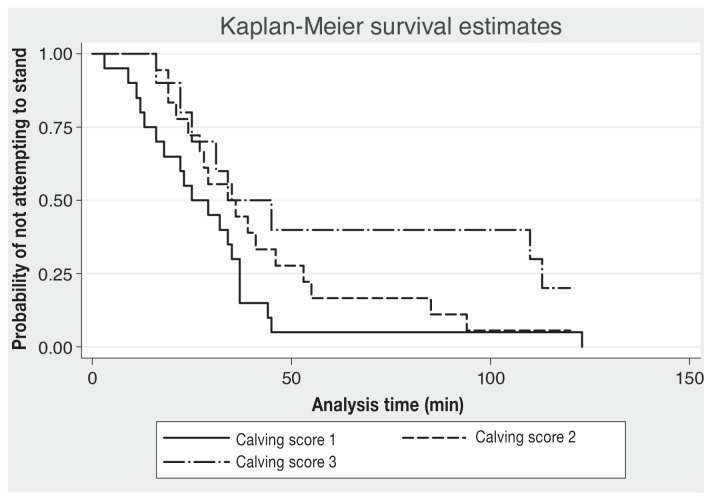
Behavioral differences in calves after a difficult calving included an increased time to achieve SR and first attempt to stand and reduced suckling response compared to calves born without difficulty. Calves born with dystocia took longer to attain SR (P = 0.04) (Figure 1) and to attempt to stand for the first time (P = 0.03) (Figure 2) than calves born with a calving score of 1 (an unassisted delivery) (Table II). There was no significant association between dystocia and time to standing for 2.5 min (Hazard Ratio = 0.45; 95% CI = 0.12 to 1.60; P = 0.22) or 5 min (Hazard Ratio = 0.40; 95% CI = 0.11 to 1.43; P = 0.16). Suckling response assessed by finger score was reduced with an increased calving score. Calves born following dystocia had a significantly weaker suckling response at 2 h than those born unassisted (P = 0.02) (Table II).
Figure 1.
Kaplan-Meier survival curves for the probability of not achieving sternal recumbency (SR) within 15 min of birth by calving score. Seven calves were treated as censored observations in the analysis: 2 calves from calving score 1, 2 from calving score 2, and 3 from calving score 3.
Figure 2.
Kaplan-Meier survival curves for the probability of not attempting to stand within 2 h of birth by calving score. Three calves were treated as censored observations in the analysis: 1 calf from calving score 2 and 2 calves from calving score 3.
No significant associations were found between calving score and heart rate, respiration rate, rectal temperature, suckling response measured by the manometer or pulse oximeter values, or with the various outcomes from the blood-gas analysis including pCO2, oxygen saturation, hematocrit, sodium, potassium, ionized calcium, bicarbonate, base excess, or total hemoglobin (data not shown).
Multivariable linear regression models
pH at SR
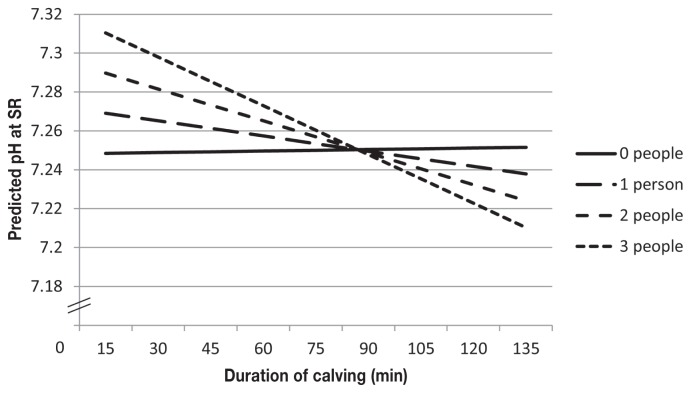
Factors associated with venous blood pH at birth were modeled using linear regression (Table III). There was a significant interaction between the number of people required to extract the calf and the duration of the calving event (P = 0.002). As the number of people pulling on the calf during delivery increased, the negative association between the duration of calving and pH at SR increased (Figure 3). Calves born following a calving score of 3 had lower blood pH than calves born following an unassisted calving (P = 0.01). Furthermore, calves with a higher respiration rate at SR had a lower blood pH (P = 0.002).
Table III.
Coefficient, 95% confidence interval, and significance level of the multivariable linear regression model for pH at sternal recumbency (SR) (n = 40)
| 95% Confidence interval | ||||
|---|---|---|---|---|
|
|
||||
| Parameter | Coefficient | Lower limit | Upper limit | P-value |
| Number of people pulling | 0.014 | −0.012 | 0.041 | 0.27 |
| Duration of stage II calving (min) | −0.000054 | −0.00048 | 0.00037 | 0.80 |
| Interaction between number of people pulling & duration of calving | −0.00045 | −0.00077 | −0.00013 | 0.007 |
| Calving score | ||||
| Unassisted | Refa | — | — | — |
| Easy pull | −0.029 | −0.097 | 0.039 | 0.39 |
| Hard pull | −0.13 | −0.23 | −0.033 | 0.011 |
| Respiration rate at SR | −0.0019 | −0.0031 | −0.00076 | 0.002 |
Referent category.
Figure 3.
Predicted values for pH at sternal recumbency (SR) as the duration of calving increases and interacts with the number of people pulling during calving. All other variables were set to median values.
One calf in this model was identified as an outlier due to low standardized residuals, low DFITS, high Cook’s Distance, as well as low DFBETA for 2 covariates in the model including respiration rate at SR and calving score 3. When this calf was removed from the model, calving score and respiration rate at SR were no longer significantly associated with pH at SR.
Apparent efficiency of IgG absorption (AEA)
Factors associated with reduced AEA included being a heifer rather than a bull calf (P = 0.014) and not achieving SR within 15 min after birth (P = 0.004) (Table IV).
Table IV.
Coefficient, 95% confidence interval and significance level of the multivariable linear regression model for apparent efficiency of IgG absorption (AEA) (n = 47)
| 95% Confidence interval | ||||
|---|---|---|---|---|
|
|
||||
| Parameter | Coefficient | Lower limit | Upper limit | P-value |
| Sex | ||||
| Bull | 4.99 | 1.44 | 8.53 | 0.007 |
| Heifer | Ref | — | — | — |
| SR within 15 min | ||||
| Yes | 6.3 | 1.35 | 11.28 | 0.014 |
| No | Ref | — | — | — |
Ref — referent category; SR — sternal recumbency.
14-day weight gain
A longer duration of calving was significantly associated with lower total weight gain during the 14-day study period (P = 0.015). Month of birth had a significant effect on weight gain. Calves born in July (P = 0.024) and August (P = 0.012) had a significantly lower weight gain by 14 d than calves born in June. In addition, calves that spent a shorter time standing in the first 2 d of life had significantly lower weight gain over the 14-day follow-up period (P < 0.001) (Table V).
Table V.
Coefficient, 95% confidence interval and significance level of the multivariable linear regression model for 14-d weight gain (kg) (n = 46)
| 95% Confidence interval | ||||
|---|---|---|---|---|
|
|
||||
| Parameter | Coefficient | Lower limit | Upper limit | P-value |
| Duration of stage-II calving (min) | −0.028 | −0.05 | −0.0057 | 0.015 |
| Month of birth | ||||
| May | −1.81 | −4.91 | 1.29 | 0.25 |
| June | Ref | — | — | — |
| July | −2.80 | −5.22 | −0.38 | 0.024 |
| August | −3.77 | −6.67 | −0.88 | 0.012 |
| September | −1.94 | −6.05 | 2.18 | 0.35 |
| Total min spent standing in first 2 d | 0.015 | 0.008 | 0.021 | < 0.001 |
Ref — referent category.
Discussion
The prevalence of dystocia in dairy cattle has increased as breeding programs have focused on production traits that have resulted in the size of calves increasing over time relative to their dams (1). The cumulative incidence of dystocia in the United States is similar to the 20.8% observed in the present study (1).
The most common cause of dystocia is high calf birth weight and a resulting disproportion of fetal-maternal size, especially in primiparous dams (16). Lombard et al (16) reported that in 7380 calvings, severe dystocia occurred in 18.9% of births in primiparous dams compared to 6.9% of births in multiparous dams. This finding is consistent with the current study, as there was a greater prevalence of dystocia in primiparous dams than in multiparous dams; 50% of primiparous dams delivered calves following dystocia compared to 13% of multiparous dams. This result probably did not reach statistical significance because only 10 of the 45 dams in this study were primiparous, resulting in limited statistical power.
Johanson and Berger (17) showed that calf birth weight was a better predictor of calving difficulty than the gender of the calf alone. It was determined that for every 1 kg increase in birth weight, there was a 13% increased probability of dystocia (17). In the current study, bull calves weighed an average of 4.8 kg more than heifers at 24 h. Thus, it is not surprising that gender was significantly associated with calving score. The risk of bull calves being born with a calving score of 3 rather than 1 was 6 times greater than heifer calves. When the 24-h calf weight was controlled for in this model, however, neither gender nor weight was significantly associated with calving score. The absence of an association between gender and calving score when weight was added to the model may be because heifer calves that were born following dystocia were lighter than heifers born in other assistance categories (Table I). The low sample size of calves born with dystocia may have also influenced this finding. Furthermore, it is possible that twin births had an effect on the association between weight and calving score. In most instances, the increased need for assistance in twin calves is due to malpositioning, not body size (18). Although twin calves are generally smaller at birth, they have an increased risk of dystocia. Thus, twins may bias the association between weight and calving score towards the null hypothesis. In the current study, 12.5% of calves were twins. Unexpectedly, however, none of these calves were malpositioned or born following dystocia. Therefore, it is unlikely that twins would have biased the association between weight and calving score in the current study.
During forced extraction of the calf, intense and prolonged uterine contractions and trauma may result in early umbilical cord rupture and an inability of the calf to regulate respiration, resulting in hypoxia (4,6). If newborn calves are severely hypoxic, tissues will derive energy from anaerobic glycolysis, resulting in the production of lactic acid and inducing metabolic acidosis (7,8). In previous studies, the duration of calving significantly affected the acid-base status in newborn calves, as did the method of assistance (19,20) and the duration of traction (4,21). The results of the current study suggest that the duration of calving, as well as the number of people required to assist during calving, are predictive of venous blood pH at SR and may be used as a proxy for fetal stress during calving.
Not surprisingly, calving score was also associated with pH at sternal recumbency (SR). Calves born following dystocia had lower venous blood pH. Furthermore, calves with higher respiratory rates following calving had lower blood pH. A higher respiratory rate may be needed in calves that experience respiratory acidosis in order to improve the oxygen-to-carbon dioxide ratio. However, neither of these associations remained significant after an outlier was removed from this model. This outlier calf was born with a calving score of 3 and had the lowest pH (6.9) and the highest respiratory rate (92 breaths/min) at SR. Thus, the associations between blood pH at SR and both calving score and respiratory rate may only be valid in extreme cases.
Boyd (12) found that serum CK concentration at birth was negatively correlated with blood pH. Exertion or damage to the cardiac or skeletal muscle can cause high serum CK, which can be the result of dystocia, birth trauma, asphyxia, hypoxia, hypercapnia, and respiratory acidosis (12). Thus, it is logical that CK levels would be highest in calves born during a difficult calving (12). In agreement, the current study found that calves born following dystocia had higher serum CK concentrations at SR than unassisted calves. Furthermore, calves with higher CK concentrations had lower venous blood pH at SR. Despite this, the highest CK levels in this study were still below the normal range for neonatal calves reported by Knowles et al (22).
In previous studies, time to SR was associated with dystocia and used as an objective behavioral indicator of stress and reduced vitality in newborn calves. Schuijt and Taverne (9) found that calves forcefully extracted took significantly longer to achieve SR than those born without assistance, normally extracted, or delivered by Caesarean section. In agreement, the current study found that calves born following dystocia took significantly longer to achieve SR than calves born unassisted. In contrast, Barrier et al (23) found that there was no difference in the time to achieve SR for assisted calves compared to calves born unassisted. However, there was a tendency for assisted calves to be less likely to stand and walk within the first 3 h of birth compared to calves born unassisted (23). Furthermore, Diesch et al (24) found that calves born with assistance took significantly longer to stand than calves born without assistance. Similar findings were seen in the current study, as calves born following dystocia took significantly longer to attempt to stand for the first time than calves born without assistance. Despite this, no significant relationship was found between calving score and time to successful standing for 2.5 or 5 min. However, 23 out of the 48 calves in the current study did not stand successfully by 2 h after birth, 30% of which were born following dystocia.
Dystocia causing reduced vitality, such as increased time to SR and standing, may have an effect on colostrum intake and passive transfer of IgG. Barrier et al (23) found that calves born with assistance spent more time lying on their flank and took longer to reach the udder after birth than calves that were unassisted. Calves that experience dystocia and are left with their dam may have reduced or delayed intake of colostrum from failure to suckle in a timely manner, which increases the risk of failure of passive transfer (25). The results of the current study indicate that calves that did not achieve SR within 15 min of birth had reduced AEA, compared to calves that did achieve SR within 15 min. Since all calves were fed the same quantity of colostral IgG at the same time after birth; however, reduced or delayed intake was not the cause of differences in AEA. It may be that calves that take longer to achieve SR have metabolic irregularities that reduce the absorption of IgG.
In some studies, failure of passive transfer has been associated with reduced IgG absorption, rather than intake. Dystocia-induced respiratory acidosis, hypoxia, and hypercapnia have been reported to be associated with decreased absorption of IgG (12,13). The current study found that dystocia was significantly associated with lower venous blood pH at SR. However, there was no relationship between blood pH (≤ 15 min after birth) and serum IgG or AEA. Drewry et al (25) observed that respiratory acidosis 1 h after birth had no association with serum IgG concentration. Drewry et al (25) used arterial blood samples to test for acidosis, whereas the current study, as well as those by Boyd (12) and Besser et al (13), used venous samples. Although there is a disagreement between studies on the effects of blood gases on passive transfer, it is unlikely that this disagreement relates to the differences in venous and arterial blood values. There are several studies reporting agreement between venous and arterial blood gas measurements. In humans, venous blood has been found to be a reliable substitute for arterial blood in the analysis of pH, bicarbonate, and pCO2 (26,27). As such, it was deemed appropriate to analyze blood gases using venous blood in this study. The procedure for obtaining arterial blood is more difficult, can be painful, and has the potential to cause complications if done repeatedly, as was required in this study (3 times within the first 24 h of life).
The use of suckling reflex to assess calf vitality and motivation to consume colostrum has produced conflicting results. Barrier et al (23) examined suckling behavior by observing position and time suckling at the udder after birth. Using these criteria, no association was seen between assistance at calving and successful suckling. It is uncertain, however, whether this method of assessment is strongly correlated with the actual strength of the suckling reflex. Alternatively, Vasseur et al (28) used a finger test to assess suckling reflex and found no association with the level of calving assistance or with colostrum intake or calf vigor. In the current study, there was no correlation between the finger test and manometer readings for suckling strength at SR, 2 h, or 24 h. The suckling reflex using the finger test was significantly associated with calving difficulty, whereas values from the manometer were not. Calves born following dystocia had weaker suckling responses, while calves with a calving score of 2 or an unassisted calving had stronger suckling responses based on the finger test. Thus, it is likely that the finger test provides a better assessment of suckling and is more directly correlated with the actual vigor of the calf. As this is the first study to use a manometer to assess suckling reflex, further research is needed to assess its accuracy.
In other research, newborn calf vitality was indicative of suckling behavior and colostrum intake. Schulz et al (29) found that the quantity of colostrum ingested was best predicted by a combination of birth weight, vigor during colostrum feeding, and vigor during the first hour of life. In the current study, it may be inferred that, since calves born following dystocia had a weak suckling response and took longer to achieve SR, their vitality and therefore their ability to consume colostrum would have been decreased. Since all calves were fed the same quantity of colostrum replacer by esophageal tube feeder; however, the success of passive transfer was not impacted by suckling reflex or calf vitality.
In the current study, bull calves had a higher AEA than heifers, although previous research has suggested the opposite (30). The association between gender and AEA may be due to metabolic state and calf vitality. Larger calves at birth are more affected by dystocia, resulting in greater risks of acidosis and lower calf vitality, which may reduce or delay IgG intake or absorption (10,12,13,29). However, since calves in the current study were fed a standard amount of IgG at the same time after birth, calf vitality would not influence the amount or timing of IgG intake. It is possible that gender is related to blood volume, which may also affect AEA. In other research in beef calves, Vann et al (31) reported no effect of calf gender on AEA. Thus, if the association between gender and AEA exists, it is unclear and further research is warranted.
Environmental temperatures may have an impact on serum Ig concentrations, as well as future health and performance. Olson et al (32) suggested that cold stress can impact the absorption of colostral Ig by delaying the onset and decreasing the rate of absorption. Other studies have shown that calves exposed to heat stress have lower serum IgG concentrations and a higher risk of mortality (33,34). Although no association was found in the current study between month of birth and AEA, calves born in July and August (the hottest months of the year) had lower weight gain up to 14 d of age. It is possible that heat stress led to reduced calf health and subsequent weight gain. In agreement, another study suggested that calves born in the summer months had lower weight gain in the first week of life (35).
Calving difficulty may also affect calf growth. In the current study, the duration of calving was negatively associated with weight gain up to 14 d. Each additional minute of calving time was associated with a 0.03 kg decrease in weight gain. In agreement, studies in beef calves have shown that assistance required at calving is associated with reduced daily weight gain and weaning weight (36,37). Although assistance cannot be directly correlated with duration of calving, in most cases, the decision to assist is based on the duration of stage-II labor. However, other studies in dairy calves have found no effect of calving difficulty on weight gain (23,38). Calves in the current study were only followed to 14 d of age, rather than for the entire pre-weaning stage, which differs from other studies in which this parameter has been monitored.
Calf vitality may also be evaluated throughout the pre-weaning period by measuring standing, lying, and feeding behavior. Sick and depressed calves may have a decreased ability to rise, go to the feeder, and suck on the artificial teat as many times or as vigorously as healthy calves. Svensson and Jensen (39) found that the number of unrewarded visits to the automatic milk feeder (visits without receiving milk, as the calf had already consumed its allotment) was significantly reduced when calves were ill, which may be due to a reduction in appetite. Furthermore, De Paula Viera et al (40) found that hungry calves spent 1 h longer standing per day than calves that were fed to satiation. Thus, it is logical that longer standing times in pre-weaned calves indicate vigor, good health, appetite, and greater motivation to consume milk, which may lead to greater weight gain. This is supported by the current study, as calves that spent a longer time standing in the first 2 d of life, had greater weight gains.
In conclusion, the findings of this study suggest that difficult, prolonged, and stressful calving events result in physiological and behavioral characteristics in calves that can be associated with reduced newborn vitality. As the number of people required to extract the calf during delivery increased, the negative association between the duration of calving and blood pH at SR increased. Low pH at SR may indicate reduced calf vitality. Reduced vitality can be measured by observing the calf’s inability to attain SR and stand after birth, the lack of strength of the suckling reflex, and subsequently, standing and feeding behavior. Reduced calf vitality may have negative consequences on newborn behavior, absorption of colostral immunoglobulin, and weight gain.
Acknowledgments
The authors thank Boehringer Ingelheim Canada, the Little Mountain Veterinary Clinic (Chilliwack, British Columbia), Holberg Farm (Agassiz, British Columbia), and the Saskatoon Colostrum Co. Ltd. (Saskatoon, Ontario) for their support of this project, as well as summer students Tehya Read and Kailee Bear and the staff and students of the UBC Dairy Education and Research Centre for their hard work in making this project possible. This work was funded by the Saskatoon Colostrum Co. Ltd., Agriculture & Agri-Food Canada, and the Agriculture Adaptation Council through the Ontario Veal Association.
References
- 1.Mee JF. Prevalence and risk factors for dystocia in dairy cattle: A review. Vet J. 2008;176:93–101. doi: 10.1016/j.tvjl.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 2.United States Department of Agriculture (USDA) Heifer Calf Health and Management Practices on US Dairy Operations, 2007. Fort Collins: USDA; 2010. [Google Scholar]
- 3.Murray CF, Leslie KE. Newborn calf vitality: Risk factors, characteristics, assessment, resulting outcomes and strategies for improvement. Vet J. 2013;198:322–328. doi: 10.1016/j.tvjl.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Szenci O, Taverne MA, Bakonyi S, Erdodi A. Comparison between pre- and postnatal acid-base status of calves and their perinatal mortality. Vet Quart. 1988;10:140–144. doi: 10.1080/01652176.1988.9694161. [DOI] [PubMed] [Google Scholar]
- 5.Bleul U. Respiratory distress syndrome in calves. Vet Clin Food Anim. 2009;25:179–193. doi: 10.1016/j.cvfa.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Szenci O. Correlations between muscle tone and acid-base balance in newborn calves: Experimental substantiation of a simple new score system proposed for neonatal status diagnosis. Acta Vet Acad Sci Hung. 1982;30:79–84. [PubMed] [Google Scholar]
- 7.Grove-White D. Resuscitation of the newborn calf. In Pract. 2000;22:17–23. [Google Scholar]
- 8.Bleul U, Lejeune B, Schwantag S, Kahn W. Blood gas and acid-base analysis of arterial blood in 57 newborn calves. Vet Rec. 2007;161:688–691. doi: 10.1136/vr.161.20.688. [DOI] [PubMed] [Google Scholar]
- 9.Schuijt G, Taverne M. The interval between birth and sternal recumbency as an objective measure of the vitality of newborn calves. Vet Rec. 1994;135:111–115. doi: 10.1136/vr.135.5.111. [DOI] [PubMed] [Google Scholar]
- 10.Vermorel M, Vernet J, Dardillat C, Saido D, Demigne C, Davicco M. Energy metabolism and thermoregulation in the newborn calf; effect of calving conditions. Can J Anim Sci. 1989;69:113–122. [Google Scholar]
- 11.Eigenmann UJE, Zaremba W, Luetgebrune K. Untersuchungen über die kolostrumaufnahme und die immunglobulinabsorption bei kälbern mit und ohne gerburtsazidose. Berl Munch Tierarzt Wochenschr. 1983;96:109–113. [PubMed] [Google Scholar]
- 12.Boyd JW. Relationships between acid-base balance, serum composition and colostrum absorption in newborn calves. Br Vet J. 1989;145:249–256. doi: 10.1016/0007-1935(89)90077-8. [DOI] [PubMed] [Google Scholar]
- 13.Besser TE, Szenci O, Gay CC. Decreased colostral immunoglobulin absorption in calves with postnatal respiratory acidosis. J Am Vet Med Assoc. 1990;196:1239–1243. [PubMed] [Google Scholar]
- 14.Canadian Council for Animal Care (CCAC) CCAC Guidelines On: The Care and Use of Farm Animals in Research, Teaching and Testing. Ottawa: CCAC; 2009. [Google Scholar]
- 15.Chelack BJ, Morley PS, Haines DM. Evaluation of methods for dehydration of bovine colostrum for total replacement of normal colostrum in calves. Can Vet J. 1993;34:407–412. [PMC free article] [PubMed] [Google Scholar]
- 16.Lombard JE, Garry FB, Tomlinson SM, Garber LP. Impacts of dystocia on health and survival of dairy calves. J Dairy Sci. 2007;90:1751–1760. doi: 10.3168/jds.2006-295. [DOI] [PubMed] [Google Scholar]
- 17.Johanson J, Berger P. Birth weight as a predictor of calving ease and perinatal mortality in Holstein cattle. J Dairy Sci. 2003;86:3745–3755. doi: 10.3168/jds.S0022-0302(03)73981-2. [DOI] [PubMed] [Google Scholar]
- 18.Gregory K, Echternkamp S, Cundiff L. Effects of twinning on dystocia, calf survival, calf growth, carcass traits, and cow productivity. J Anim Sci. 1996;74:1223–1233. doi: 10.2527/1996.7461223x. [DOI] [PubMed] [Google Scholar]
- 19.Mee JF. PhD dissertation. Galway, Ireland: National University of Ireland; 1991. Bovine perinatal mortality and parturient problems in Irish dairy herds. [Google Scholar]
- 20.Szenci O. Role of acid-base disturbances in perinatal mortality of calves: A review. Vet Bull. 2003;73:7R–14R. [PubMed] [Google Scholar]
- 21.Szenci O, Gálfi P, Lajcsák A. Comparison of carbonic anhydrase in neonatal and maternal red blood cells with different levels of acidosis in newborn calves. Zentralblatt für Veterinärmedizin Reihe A. 1984;31:437–440. doi: 10.1111/j.1439-0442.1984.tb01302.x. [DOI] [PubMed] [Google Scholar]
- 22.Knowles TG, Edwards JE, Bazeley KJ, Brown SN, Butterworth A, Warriss RD. Changes in the blood biochemical and haematological profile of neonatal calves with age. Vet Rec. 2000;147:593–598. doi: 10.1136/vr.147.21.593. [DOI] [PubMed] [Google Scholar]
- 23.Barrier AC, Ruelle E, Haskell MJ, Dwyer CM. Effect of a difficult calving on the vigour of the calf, the onset of maternal behaviour, and some behavioural indicators of pain in the dam. Prev Vet Med. 2012;103:248–256. doi: 10.1016/j.prevetmed.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Diesch TJ, Mellor DJ, Stafford KJ, Ward RN. The physiological and physical status of single calves at birth in a dairy herd in New Zealand. NZ Vet J. 2004;52:250–255. doi: 10.1080/00480169.2004.36436. [DOI] [PubMed] [Google Scholar]
- 25.Drewry JJ, Quigley JD, Geiser DR, Welborn MG. Effect of high arterial carbon dioxide tension on efficiency of immunoglobulin G absorption in calves. Am J Vet Res. 1999;60:609–614. [PubMed] [Google Scholar]
- 26.Middleton P, Kelly AM, Brown J, Robertson M. Agreement between arterial and central venous values for pH, bicarbonate, base excess, and lactate. Emerg Med J. 2006;23:622–624. doi: 10.1136/emj.2006.035915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malatesha G, Singh NK, Bharija A, Rehani B, Goel A. Comparison of arterial and venous pH, bicarbonate, PCO2 and PO2 in initial emergency department assessment. Emerg Med J. 2007;24:569–571. doi: 10.1136/emj.2007.046979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasseur E, Rushen J, de Passille AM. Does a calf’s motivation to ingest colostrum depend on time since birth, calf vigor, or provision of heat? J Dairy Sci. 2009;92:3915–3921. doi: 10.3168/jds.2008-1823. [DOI] [PubMed] [Google Scholar]
- 29.Schulz J, Plischke B, Braun H. Sucking and drinking behavior as criteria of vitality in newborn calves. Tierarztl Prax. 1997;25:116–122. [PubMed] [Google Scholar]
- 30.Roy JHB. The Calf, Volume 1: Management of Health. 5th ed. Boston: Butterworth-Heinemann; 1990. [Google Scholar]
- 31.Vann RC, Holloway JW, Carstens GE, Boyd ME, Randel RD. Influence of calf genotype on colostral immunoglobulins in Bos taurus and Bos indicus cows and serum immunoglobulins in their calves. J Anim Sci. 1995;73:3044–3050. doi: 10.2527/1995.73103044x. [DOI] [PubMed] [Google Scholar]
- 32.Olson DP, Papasian CJ, Ritter RC. The effects of cold stress on neonatal calves. II. Absorption of colostral immunoglobulins. Can J Comp Med. 1980;44:19–23. [PMC free article] [PubMed] [Google Scholar]
- 33.Stott G, Wiersma F, Menefee B, Radwanski F. Influence of environment on passive immunity in calves. J Dairy Sci. 1976;59:1306–1311. doi: 10.3168/jds.S0022-0302(76)84360-3. [DOI] [PubMed] [Google Scholar]
- 34.Stott GH. Immunoglobulin absorption in calf neonates with special considerations of stress. J Dairy Sci. 1980;63:681–688. doi: 10.3168/jds.S0022-0302(80)82990-0. [DOI] [PubMed] [Google Scholar]
- 35.Murray CF. Characteristics, Risk Factors and Management Programs for Vitality of Newborn Dairy Calves. PhD thesis. 2014. A field study to evaluate the effects of meloxicam NSAID therapy and calving assistance on newborn calf vigor, improvement of health and growth in pre-weaned Holstein calves; pp. 109–136. [Google Scholar]
- 36.Bellows RA, Short RE, Staigmiller RB, Milmine WL. Effects of induced parturition and early obstetrical assistance in beef cattle. J Anim Sci. 1988;66:1073–1080. doi: 10.2527/jas1988.6651073x. [DOI] [PubMed] [Google Scholar]
- 37.Goonewardene LA, Wang Z, Price MA, Yang R-C, Berg RT, Makarechian M. Effect of udder type and calving assistance on weaning traits of beef and dairy × beef calves. Liv Prod Sci. 2003;81:47–56. [Google Scholar]
- 38.Heinrichs AJ, Heinrichs BS, Harel O, Rogers GW, Place NT. A prospective study of calf factors affecting age, body size, and body condition score at first calving of Holstein dairy heifers. J Dairy Sci. 2005;88:2828–2835. doi: 10.3168/jds.S0022-0302(05)72963-5. [DOI] [PubMed] [Google Scholar]
- 39.Svensson C, Jensen MB. Short communication: Identification of diseased calves by use of data from automatic milk feeders. J Dairy Sci. 2007;90:994–997. doi: 10.3168/jds.S0022-0302(07)71584-9. [DOI] [PubMed] [Google Scholar]
- 40.De Paula Vieira A, Guesdon V, de Passillé AM, von Keyserlingk MAG, Weary DM. Behavioural indicators of hunger in dairy calves. Appl Anim Behav Sci. 2008;109:180–189. [Google Scholar]