Abstract
Streptococcus suis is an important swine pathogen and a zoonotic agent causing meningitis and septicemia. Although serotype 2 is the most virulent type, serotype 14 is emerging, and understanding of its pathogenesis is limited. To study the role of the capsular polysaccharide (CPS) of serotype 14 as a virulence factor, we constructed knockout mutants devoid of either cps14B, a highly conserved regulatory gene, or neu14C, a gene coding for uridine diphospho-N-acetylglucosamine 2-epimerase, which is involved in sialic acid synthesis. The mutants showed total loss of the CPS with coagglutination assays and electron microscopy. Phagocytosis assays showed high susceptibility of mutant Δcps14B. An in vivo murine model was used to demonstrate attenuated virulence of this non-encapsulated mutant. Despite the difference in the CPS composition of different serotypes, this study has demonstrated for the first time that the CPS of a serotype other than 2 is also an important antiphagocytic factor and a critical virulence factor.
Résumé
Streptococcus suis est un important pathogène du porc et également un agent de zoonose, causant méningite et septicémie. Outre le sérotype 2, qui est le plus virulent, le sérotype 14 est en émergence, et les connaissances sur la pathogenèse de ce pathogène demeurent très limitées. Afin d’étudier le rôle de la capsule polysaccharidique du sérotype 14 comme facteur de virulence, nous avons construit un mutant déficient pour une protéine régulatrice hautement préservée, cps14B, et un mutant du gène codant pour l’enzyme uridine diphospho-N-acétylglucosamine 2-épimérase, neu14C, impliquée dans la synthèse de l’acide sialique. Les mutants présentent un phénotype non-encapsulé lors de la caractérisation par les tests de coagglutination et de microscopie électronique. Les tests de phagocytose ont permis de démontrer la grande susceptibilité à la phagocytose du mutant non-encapsulé Δcps14B. Ce même mutant a démontré une virulence atténuée lors d’une infection in vivo chez un modèle murin. Malgré les différences dans la composition chimique dans le matériel capsulaire des différents sérotypes, cette étude a démontré pour la première fois que la capsule d’un sérotype autre que le sérotype 2 a aussi des activités anti-phagocytaires, et elle est un facteur de virulence critique.
(Traduit par les auteurs)
Streptococcus suis, a Gram-positive bacterium, is an important swine pathogen causing meningitis, septicemia, endocarditis, arthritis, and other infections (1). It is responsible for economic losses to the swine industry and is considered an emerging zoonotic agent causing mainly meningitis and septic shock in humans (2). Thirty-five serotypes based on capsular epitopes have been described (1). Serotype 2 is considered the most virulent and has several reported virulence-associated factors implicated in infection (3). Among these factors, the capsular polysaccharide (CPS) is one of the most important, playing a crucial role in the infection (4). In fact, almost all studies in the literature on virulence factors have been done with serotype 2 strains (3).
In addition to serotype 2, serotype 14 is highly virulent and represents serious health and economic problems in several countries, such as Thailand (in humans) and those of the United Kingdom (in swine) (5,6). Swine and human cases due to this serotype have also been described in Canada (7,8). Recent chemical analyses of the CPS of serotypes 2 and 14 revealed differences in sugar composition. For example, no rhamnose residue was found in the serotype 14 CPS (9). A side chain containing sialic acid (coded by the genes neuA to neuD) and an α-2,6-sialyltransferase are present in the CPS of both serotypes (10). Bacterial sialic acid has been implicated as a virulence factor for several pathogens, such as the closely related group B Streptococcus (GBS) (11). However, mutants lacking genes involved in sialic acid synthesis were found to have poorly encapsulated or non-encapsulated phenotypes for GBS and S. suis serotype 2, respectively, which complicates the study of the sialic acid moiety as a virulence factor (12).
Since no studies had yet been carried out on the role of CPS as a virulence factor for any S. suis serotype other than serotype 2, we constructed a serotype 14 isogenic knockout mutant devoid of a highly conserved regulatory gene, cps14B. In addition, we studied the effect of the absence of sialic acid on the expression of the whole CPS of serotype 14 by constructing a mutant deficient in the neu14C gene, which codes for uridine diphospho-N-acetylglucosamine 2-epimerase, an enzyme involved in sialic acid synthesis.
The well-encapsulated S. suis serotype 14 reference strain DAN13730, isolated from a human case in The Netherlands (13), was used as the host strain for in-frame allelic deletion mutagenesis. The well-encapsulated virulent serotype 2 strain P1/7 and its previously obtained isogenic capsule-deficient mutant Δcps2F (14) were used for comparison purposes. The bacterial strains and plasmids used in this study are listed in Table I. The S. suis strains were grown in Todd Hewitt broth (THB) or agar (THA) (Becton-Dickinson, Sparks, Maryland, USA) at 37°C. Precise in-frame deletions in cps14B and neu14C (cps14Q) were achieved by using splicing-by-overlap- extension polymerase chain reaction (PCR), as previously described (16).
Table I.
Bacterial strains and plasmids used in this study
| Material | General characteristics | Source or reference number |
|---|---|---|
| Escherichia coli | ||
| TOP 10 | F-mrcA Δ(mrr-hsdRMS-mcrBC)φ80 lacZΔM5 ΔlacX74 recA1 araD139 Δ(ara-leu)7697 galU galK rpsL (StrR) endA1 nupG | Invitrogen, Burlington, Ontario |
| MC1061 | araD139 Δ(ara-leu)7697 ΔlacX74 galU galK hsdR2(rK− mK+) mcrB1 rpsL | 12 |
| Streptococcus suis | ||
| P1/7 | Wild-type, highly encapsulated serotype 2 strain isolated from a clinical swine case in the United Kingdom | 14 |
| DAN13730 | Wild-type, highly encapsulated serotype 14 strain isolated from a human case in The Netherlands | 13 |
| Δcps2F | Non-encapsulated isogenic mutant strain, with deletion of the cps2F gene, derived from strain P1/7 | 14 |
| Δcps14B | Non-encapsulated isogenic mutant strain, with deletion of the cps14B gene, derived from strain DAN13730 | This work |
| Δneu14C | Non-encapsulated isogenic mutant strain, with deletion of the neu14C gene, derived from DAN13730 | This work |
| Δcps14B/cps14B | Mutant Δcps14B complemented with pMX14B complementation vector | This work |
| Δneu14C/neu14C | Mutant Δneu14C complemented with pMXNEU14C complementation vector | This work |
| Plasmid | ||
| pCR2.1 | Apr, Kmr, oriR(f1) MCS oriR (ColE1) | Invitrogen |
| pSET4s | Thermosensitive vector for allelic replacement; replication functions of pG + host3 and pUC19, MCS, and lacZ of pUC19, SpR | 17 |
| pMX1 | Replication functions of pSSU1, MCS of pUC19, SpR, malX promoter of S. suis, derivative of pSET2 | 15 |
| p4Δcps14B | pSET4s carrying the construct for cps14B allelic replacement | This work |
| p4Δneu14C | pSET4s carrying the construct for neu14C allelic replacement | This work |
| pMX14B | pMX1 complementation vector carrying intact cps14B | This work |
| pMXNEU14C | pMX1 complementation vector carrying inact neu14C | This work |
Genomic DNA of S. suis was purified by InstaGene Matrix solution (BioRad Laboratories, Hercules, California, USA). The primers used for the construction of deletion alleles (Table II) were obtained from Integrated DNA Technologies (Coralville, Iowa, USA). The PCR reactions were carried out with iProof proofreading DNA polymerase (BioRad Laboratories) or with Taq DNA polymerase (Qiagen, Valencia, California, USA). Amplification products were purified with the QIAquick PCR purification kit (Qiagen) and sequenced with an ABI 310 automated DNA sequencer and the ABI PRISM dye terminator cycle sequencing kit (Applied Biosystems, Foster City, California, USA). Overlapping PCR products were cloned into plasmid pCR2.1 (Invitrogen, Burlington, Ontario), extracted with EcoRI, and recloned into the thermosensitive Escherichia coli–S. suis shuttle vector pSET4s digested with the same enzyme, which gave rise to the p4Δcps14B and p4Δneu14C mutation vectors (17). The recombinant plasmids were extracted and purified with the QIAprep Spin Miniprep kit (Qiagen). Restriction enzymes and DNA-modifying enzymes were purchased from Fisher Scientific (Ottawa, Ontario) and used according to manufacturer recommendations.
Table II.
Oligonucleotide primers used in this study
| Primera | Nucleotide sequence,b 5′–3′ | Construct |
|---|---|---|
| cps14B-ID1 | GACCAAGATAACATCACCGC | p4Δcps14B |
| cps14B-ID2 | GGTTGGCATTTGTCTACAGT | p4Δcps14B |
| cps14B-ID3 | ACCACTCCAAATACAAAACG | p4Δcps14B |
| cps14B-ID4 | GCTCGCGCTATATTCTCTTG | p4Δcps14B |
| cps14B-ID5 | CGACTTGGTGGGTGGAATTG | p4Δcps14B |
| cps14B-ID6 | TCTTCGATGTCCTGAGGACGGTCAACACTGCAGTTAAGAG | p4Δcps14B |
| cps14B-ID7 | CTCTTAACTGCAGTGTTGACCGTCCTCAGGACATCGAAGA | p4Δcps14B |
| cps14B-ID8 | GGTTTCTCCCAACCCTACTG | p4Δcps14B |
| neu14C-ID1 | CGGTGATGTTCATCTAGCACGG | p4Δneu14C |
| neu14C-ID2 | AGCGATCCCCCAGAATCAACAC | p4Δneu14C |
| neu14C-ID3 | CACAGCCGAAGAACAAACGCAG | p4Δneu14C |
| neu14C-ID4 | TGGACGCATGAGGACTTGAACC | p4Δneu14C |
| neu14C-ID5 | TCTCAGCTCGAAATGACTCGTC | p4Δneu14C |
| neu14C-ID6 | CATGGTTGAGGCCTGACGAGAGCCTGTCAC | p4Δneu14C |
| neu14C-ID7 | GTGACAGGCTCTCGTCAGGCCTCAACCATG | p4Δneu14C |
| neu14C-ID8 | AGGTCCCTGACTCCGTCAAC | p4Δneu14C |
| pCPS14BF_NcoI | AGCCATGGAGTCCGTACTTGTTTA | pMX14B |
| pCPS14BR_EcoRI | GTACGTGGAATTCCTAACATTGCC | pMX14B |
| pNEU14CF_PstI | TGAGCTGCAGCAAAATATTTGCCATAGTGC | pMXNEU14C |
| pNEU14CR_PstI | CATCTGCAGAGGTACCCGCTCCTAGAAAGG | pMXNEU14C |
Obtained from Integrated DNA Technologies, Coralville, Iowa, USA.
Restriction sites are underlined.
Final constructions of pSET4s, p4Δcps14B, and p4Δneu14C were electroporated into S. suis-competent cells with the Biorad Gene PulserXcell apparatus (BioRad Laboratories) under specific conditions: 12.5 kV/cm, 200 Ω, and 25 μF. Transformants were plated on THA supplemented with spectinomycin (THA + Sp) and incubated for 3 d at 28°C. Several Sp-resistant colonies were then subcultured on THA + Sp for 3 d at 28°C. The candidates were next cultured on THA + Sp and incubated at 37°C for 2 successive passages. Temperature- and Sp-resistant clones were successively cultured on THA and THA + Sp to obtain Sp-sensitive candidates. Deletion of the genes cps14B and neu14C was confirmed by PCR and sequence analysis.
For complemented mutants, intact cps14B and neu14C genes were amplified from genomic DNA of the wild-type strain with primers designed with restriction sites (Table II). The PCR products and pMX1 vectors were then digested with the appropriate restriction enzyme before ligation. Final constructions were cloned into E. coli MC1061. The E. coli strains were grown in Luria–Bertani broth or agar (Becton-Dickinson) at 37°C. When needed, antibiotics (Sigma-Aldrich Canada, Oakville, Ontario) were added to the culture medium at the following concentrations: for S. suis, spectinomycin at 100 μg/mL; for E. coli, kanamycin and spectinomycin at 50 μg/mL; and for E. coli, ampicillin at 100 μg/mL. Complementation of both mutants was achieved by electroporation with pMX14B and pMXNEU14C under the conditions mentioned previously.
Serotyping was carried out by coagglutination tests as described by Gottschalk et al (13), and positive results were recorded when a strong reaction was obtained within 1 min. Transmission electron microscopy (TEM) was carried out as described by Jacques et al (18) with a few modifications. Briefly, bacteria were grown to mid-logarithmic phase and resuspended in 0.1 M cacodylate buffer, pH 7.3, containing 2.5% (v/v) glutaraldehyde and 0.05% (w/v) ruthenium red. Ferritin was then added, to a final concentration of 1 mg/mL, and the suspension incubated for 30 min at room temperature. Afterwards the cells were immobilized in 3% (w/v) agar, washed 5 times in cacodylate buffer containing 0.05% ruthenium red, and fixed with 2% (v/v) osmium tetroxide for 2 h. Samples were washed and dehydrated in graded series of acetone, then washed twice in propylene oxide and embedded in Spurr low-viscosity resin (Sigma-Aldrich Canada). Thin sections were stained with uranyl acetate and lead citrate and examined with a transmission electron microscope (JEM 1230; JEOL, Tokyo, Japan) at 80 kV.
The J774A.1 murine macrophage-like cell line (American Type Culture Collection TIB 67; Rockville, Maryland, USA) was maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated fetal bovine serum and penicillin/streptomycin, 25 units/mL (Gibco, Burlington, Ontario). The cells were grown at 37°C with 5% CO2 until confluent. The cultures were then scraped and the cells washed twice with phosphate-buffered saline (PBS), pH 7.4, resuspended in antibiotic-free medium at 1 × 105 cells per well in a 24-well tissue culture plate (VWR CanLab, Montreal, Quebec), and incubated for 3 h at 37°C with 5% CO2 to allow cell adhesion. The cells were infected by removing the medium and adding 1 mL of a bacterial suspension, 1 × 107 colony-forming units (CFU)/mL in antibiotic-free medium; the multiplicity of infection (MOI) was thus 100. The infected cells were incubated for 60 min at 37°C with 5% CO2 to allow phagocytosis. The optimal incubation time and MOI were chosen according to the results of preliminary studies (data not shown). After incubation, cell monolayers were washed twice with PBS and incubated for 1 h with medium containing penicillin G (Sigma-Aldrich Canada), 5 μg/mL, and gentamicin (Gibco), 100 μg/mL, to kill extracellular bacteria. The cell monolayers were washed 3 times with PBS and lysed with sterile water. The presence of viable intracellular bacteria was determined by plating serial dilutions on THA. Each test was repeated 4 times in independent experiments.
A well-standardized S. suis serotype 2 murine model of infection (19) was adapted for the first time to serotype 14. Six-week-old female CD1 mice (Charles River Laboratories, Wilmington, Massachusetts, USA) were used, the experiments involving them being conducted in accordance with the guidelines and policies of the Canadian Council on Animal Care and the principles set forth in the Guide for the Care and the Use of Laboratory Animals by the Animal Welfare Committee of the University of Montreal. The 45 animals were divided into 3 groups of 15 animals each. On the day of the experiment, each animal was inoculated by intraperitoneal injection with 1.5 × 108 CFU of the S. suis serotype 14 wild-type strain DAN13730 or the mutant strain Δcps14B or with a vehicle (THB) as a control. The bacterial concentration was determined from preliminary trials with DAN13730 to establish a high but controlled mortality level (data not shown). The mice were examined at least 3 times daily for clinical signs of septic disease, such as depression, swollen eyes, rough coat hair, and lethargy, for 72 h after infection. Blood samples (5 μL) were collected from the tail vein at 12, 24, 48, and 72 h after infection and plated on THA for evaluation of bacteremia.
All data are expressed as mean ± standard error. For the in vitro experiments, data were analyzed for significance with the Mann–Whitney rank-sum test. For the in vivo virulence experiments, the Mantel–Cox log-rank test was used to evaluate the difference in mortality rate between the groups, and the Mann–Whitney rank-sum test was used to evaluate the difference in bacteremia between the groups. A P-value of less than 0.05 was considered significant.
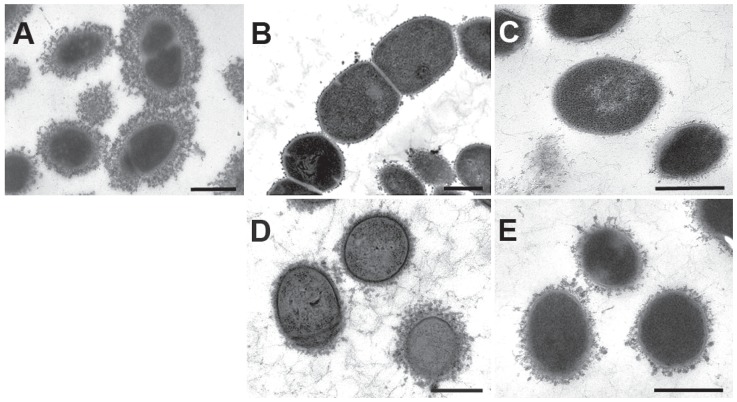
The coagglutination serotyping test is an easy and rapid method to indirectly observe the presence of the capsule in S. suis isolates. In this study, both Δcps14B and Δneu14C mutants showed a clear negative result: no agglutination reaction with any typing antiserum. The wild-type strain, DAN13730, showed a strong and fast reaction with serotype 14-specific antiserum. As depicted in Figure 1, TEM showed a thick capsule surrounding the wild-type strain (A) and a complete absence of CPS structure for both mutants (B, C). The gene deletions were complemented in Δcps14B/cps14B and Δneu14C/neu14C to partially restore CPS production, as expected (D, E). Smith et al (20) demonstrated the importance of the glycosyltransferase gene cps2EF and a gene involved in chain-length determination (cps2B) in the production of CPS for serotype 2, using mutants that resulted in a capsule-deficient phenotype. The non-encapsulated Δcps14B mutant showed that cps2B is also required for CPS synthesis in serotype 14. Lack of the sialic acid synthesis gene prevented CPS production in the Δneu14C mutant. This result suggests that, despite differences in CPS composition, sialylation of the S. suis serotype 14 CPS repeating units is crucial for CPS exportation or polymerization, as has been reported for serotype 2 (12). Unfortunately, it is still not possible to precisely study the role of sialic acid in the virulence of both serotypes.
Figure 1.
Transmission electron micrographs showing the expression of capsular polysaccharide (CPS) by the wild-type strain of Streptococcus suis serotype 14 and its derived mutants. The CPS was labelled with polycationic ferritin. The wild-type strain, DAN13730 (A), is surrounded by a thick capsule, whereas the Δcps14B and Δneu14C mutant strains (B and C, respectively) are non-encapsulated. The complemented strains Δcps14B/cps14B (D) and Δneu14C/neu14C (E) show an intermediate state of CPS production. Bars = 0.5 μm.
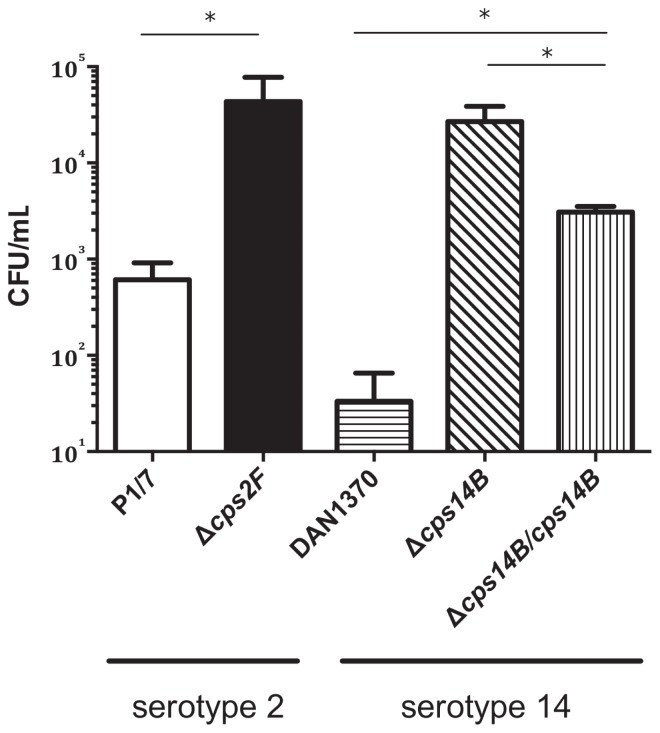
Since both mutants were non-encapsulated, further experiments were carried out with Δcps14B alone to investigate the role of CPS in the pathogenesis of S. suis serotype 14 infections. Bacterial clearance by phagocytic cells represents an important host mechanism for defending against bacterial infection. To evaluate the susceptibility to phagocytosis of the capsule-deficient mutant Δcps14B, bacteria were incubated with the J774A.1 murine macrophage-like cell line. As expected, the control serotype 2 strain P1/7 and its capsule-deficient mutant Δcps2F were poorly and highly internalized by macrophages, respectively (Figure 2). The wild-type serotype 14 strain DAN13730 was highly resistant to phagocytosis, and only a few bacteria were internalized. In contrast, the serotype 14 non-encapsulated mutant Δcps14B was significantly more internalized than its wild-type strain (P < 0.05). The Δcps14B/cps14B complemented strain, in which the CPS was partially restored, was less internalized than the Δcps14B mutant strain but still more phagocytosed than the wild-type strain (P < 0.05). These results are similar to those previously reported for serotype 2 strains with use of the RAW264.6 murine macrophage cell line and porcine alveolar macrophages (12,20,21). Overall, the high phagocytosis susceptibility of Δcps14B compared with the wild-type strain shows the essential role of the CPS in phagocytic resistance to S. suis serotype 14. Interestingly, the wild-type serotype 14 strain demonstrated even greater resistance to phagocytosis than the serotype 2 strain in this in vitro model (P < 0.05).
Figure 2.
Phagocytosis of S. suis serotypes 2 and 14 by murine macrophages. Results for the well-encapsulated virulent serotype 2 strain P1/7 and its previously obtained isogenic capsule-deficient mutant Δcps2F (14) are depicted for comparison only. Bacteria, 1 × 107 colony-forming units (CFU)/mL, were incubated for 60 min with J774A.1 murine macrophage-like cells, at a multiplicity of infection of 100, and gentamicin/penicillin G was used to kill any extracellular bacteria remaining after incubation. Intracellular counts were done after 3 washes and cell lysis with water. Results represent the mean count + the standard error in 4 independent experiments. Each asterisk indicates a significant difference between strains (P < 0.05) according to the Mann–Whitney rank-sum test.
The role of the CPS in the virulence of a serotype other than 2 was also demonstrated in vivo. In the first mouse model for a serotype 14 strain, we investigated the effect of capsule loss using an in vivo CD1 murine model of S. suis infection. All 15 mice inoculated with the wild-type strain presented severe clinical signs of infection and died within the first 36 h (Figure 3A). The animals died relatively fast and mainly from septicemia and septic shock. Reducing the dose by 1 log resulted in complete absence of clinical signs and death. In contrast to the results with serotype 2, no cases of meningitis were observed, suggesting that the 2 serotypes do not behave identically in this animal model. None of the mice inoculated with the non-encapsulated Δcps14B mutant strain died from the infection (P < 0.0001), and few clinical signs were observed. At 12 h after infection the mice inoculated with the wild-type strain had a mean blood bacterial burden of 2 × 108 CFU/mL, significantly greater (P < 0.001) than the burden of the mice inoculated with the Δcps14B mutant (Figure 3B). At 24 h after infection the few surviving mice infected with the wild-type strain had a blood bacterial burden similar to that at 12 h and also significantly greater (P < 0.001) than that of the mice inoculated with the Δcps14B mutant (data not shown). As Δcps14B is avirulent in our infection model, the in vivo results confirm the phagocytosis results and demonstrate the crucial role of CPS in the virulence of S. suis serotype 14.
Figure 3.
Survival (A) and blood bacterial burden (B) of CD1 mice inoculated by intraperitoneal injection with 1.5 × 108 CFU of DAN13730 or Δcps14B or with Todd Hewitt broth as a control. Bacterial counts were determined in serial dilutions of 5 μL of blood obtained from the tail vein 12 h after inoculation. The Mantel–Cox log-rank test revealed a significant difference (P < 0.0001) in survival rate between the groups: all mice receiving the wild-type strain died, whereas all those receiving the mutant strain survived, and all the control animals were unaffected. The Mann–Whitney rank-sum test showed a significant difference (P < 0.001) in blood bacterial burden between the mice inoculated with the wild-type strain and those inoculated with the mutant strain (asterisk).
In conclusion, whereas there are differences in CPS composition and structure between serotypes 2 and 14, this study has demonstrated that the CPS of serotype 14 possesses important antiphagocytic properties and is a critical virulence factor. Since serotypes 14 and 1 have not only epitopes in common but also highly similar cps clusters and highly similar CPS structures (9; unpublished observations), the serotype 1 CPS may play a role in virulence and phagocytosis similar to that of serotypes 2 and 14. Further studies are needed to confirm this theory. This is the first report on the role of the CPS of an S. suis serotype other than 2. As with serotype 2, it has so far been impossible to evaluate the specific role of sialic acid in the virulence of S. suis serotype 14 since no CPS is produced in the absence of this sugar. Although the mouse model used in this study may be used to evaluate the systemic virulence of S. suis serotype 14, other models of meningitis with this serotype must be standardized. Further characterization and investigation will be necessary to dissect other virulence factors that would explain why serotype 2 strains are more widespread globally and seem to be more virulent in the field than serotype 14 strains.
Acknowledgments
We thank Sonia Lacouture for her assistance. This work was supported by Natural Sciences and Engineering Research Council of Canada grant 154280 to Dr. Gottschalk and grant 342150-07 to Dr. Segura and partially by Grant-in-Aid for Young Scientists (B) (23480310) to Dr. Okura from the Japan Society for the Promotion of Science.
References
- 1.Gottschalk M. Streptococcus. In: Zimmerman J, Karriker L, Ramirez A, Schwartz K, Stevenson G, editors. Diseases of Swine. 10th ed. Ames, Iowa: Wiley-Blackwell Publishing; 2012. pp. 841–855. [Google Scholar]
- 2.Gottschalk M, Xu J, Calzas C, Segura M. Streptococcus suis: A new emerging or an old neglected zoonotic pathogen? Future Microbiol. 2010;5:371–391. doi: 10.2217/fmb.10.2. [DOI] [PubMed] [Google Scholar]
- 3.Fittipaldi N, Segura M, Grenier D, Gottschalk M. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol. 2012;7:259–279. doi: 10.2217/fmb.11.149. [DOI] [PubMed] [Google Scholar]
- 4.Gottschalk M, Segura M. The pathogenesis of the meningitis caused by Streptococcus suis: The unresolved questions. Vet Microbiol. 2000;76:259–272. doi: 10.1016/s0378-1135(00)00250-9. [DOI] [PubMed] [Google Scholar]
- 5.Kerdsin A, Oishi K, Sripakdee S, et al. Clonal dissemination of human isolates of Streptococcus suis serotype 14 in Thailand. J Med Microbiol. 2009;58:1508–1513. doi: 10.1099/jmm.0.013656-0. [DOI] [PubMed] [Google Scholar]
- 6.Heath PJ, Hunt BW. Streptococcus suis serotypes 3 to 28 associated with disease in pigs. Vet Rec. 2001;148:207–208. doi: 10.1136/vr.148.7.207. [DOI] [PubMed] [Google Scholar]
- 7.Gottschalk M, Lacouture S, Bonifait L, Roy D, Fittipaldi N, Grenier D. Characterization of Streptococcus suis isolates recovered between 2008 and 2011 from diseased pigs in Quebec, Canada. Vet Microbiol. 2013;162:819–825. doi: 10.1016/j.vetmic.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 8.Haleis A, Alfa M, Gottschalk M, Bernard K, Ronald A, Manickam K. Meningitis caused by Streptococcus suis serotype 14, North America. Emerg Infect Dis. 2009;15:350–352. doi: 10.3201/eid1502.080842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Calsteren MR, Gagnon F, Calzas C, et al. Structure determination of Streptococcus suis serotype 14 capsular polysaccharide. Biochem Cell Biol. 2013;91:49–58. doi: 10.1139/bcb-2012-0036. [DOI] [PubMed] [Google Scholar]
- 10.Van Calsteren MR, Gagnon F, Lacouture S, Fittipaldi N, Gottschalk M. Structure determination of Streptococcus suis serotype 2 capsular polysaccharide. Biochem Cell Biol. 2010;88:513–525. doi: 10.1139/o09-170. [DOI] [PubMed] [Google Scholar]
- 11.Wessels MR, Rubens CE, Benedi VJ, Kasper DL. Definition of a bacterial virulence factor: Sialylation of the group B streptococcal capsule. Proc Natl Acad Sci U S A. 1989;86:8983–8987. doi: 10.1073/pnas.86.22.8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lecours MP, Fittipaldi N, Takamatsu D, et al. Sialylation of Streptococcus suis serotype 2 is essential for capsule expression but is not responsible for the main capsular epitope. Microb Infect. 2012;14:941–950. doi: 10.1016/j.micinf.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Gottschalk M, Higgins R, Jacques M, Mittal KR, Henrichsen J. Description of 14 new capsular types of Streptococcus suis. J Clin Microbiol. 1989;27:2633–2636. doi: 10.1128/jcm.27.12.2633-2636.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lecours MP, Gottschalk M, Houde M, Lemire P, Fittipaldi N, Segura M. Critical role for Streptococcus suis cell wall modifications and suilysin in resistance to complement-dependent killing by dendritic cells. J Infect Dis. 2011;204:919–929. doi: 10.1093/infdis/jir415. [DOI] [PubMed] [Google Scholar]
- 15.Okura M, Osaki M, Fittipaldi N, Gottschalk M, Sekizaki T, Takamatsu D. The minor pilin subunit sgp2 is necessary for assembly of the pilus encoded by the srtG cluster of Streptococcus suis. J Bacteriol. 2011;193:822–831. doi: 10.1128/JB.01555-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warrens AN, Jones MD, Lechler RI. Splicing by overlap extension by PCR using asymmetric amplification: An improved technique for the generation of hybrid proteins of immunological interest. Gene. 1997;186:29–35. doi: 10.1016/s0378-1119(96)00674-9. [DOI] [PubMed] [Google Scholar]
- 17.Takamatsu D, Osaki M, Sekizaki T. Construction and characterization of Streptococcus suis–Escherichia coli shuttle cloning vectors. Plasmid. 2001;45:101–113. doi: 10.1006/plas.2000.1510. [DOI] [PubMed] [Google Scholar]
- 18.Jacques M, Gottschalk M, Foiry B, Higgins R. Ultrastructural study of surface components of Streptococcus suis. J Bacteriol. 1990;172:2833–2838. doi: 10.1128/jb.172.6.2833-2838.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dominguez-Punaro MC, Segura M, Plante MM, Lacouture S, Rivest S, Gottschalk M. Streptococcus suis serotype 2, an important swine and human pathogen, induces strong systemic and cerebral inflammatory responses in a mouse model of infection. J Immunol. 2007;179:1842–1854. doi: 10.4049/jimmunol.179.3.1842. [DOI] [PubMed] [Google Scholar]
- 20.Smith HE, Damman M, van der Velde J, et al. Identification and characterization of the CPS locus of Streptococcus suis serotype 2: The capsule protects against phagocytosis and is an important virulence factor. Infect Immun. 1999;67:1750–1756. doi: 10.1128/iai.67.4.1750-1756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng Y, Cao M, Shi J, et al. Attenuation of Streptococcus suis virulence by the alteration of bacterial surface architecture. Sci Rep. 2012;2:710. doi: 10.1038/srep00710. Epub 2012 Oct 5. [DOI] [PMC free article] [PubMed] [Google Scholar]