Abstract
In spite of numerous research efforts, the exact etiology of autoimmune diseases remains largely unknown. Genetics and environmental factors, including xenobiotics, are believed to be involved in the induction of autoimmune disease. Some environmental chemicals, acting as haptens, can bind to a high-molecular-weight carrier protein such as human serum albumin (HSA), causing the immune system to misidentify self-tissue as an invader and launch an immune response against it, leading to autoimmunity. This study aimed to examine the percentage of blood samples from healthy donors in which chemical agents mounted immune challenges and produced antibodies against HSA-bound chemicals. The levels of specific antibodies against 12 different chemicals bound to HSA were measured by ELISA in serum from 400 blood donors. We found that 10% (IgG) and 17% (IgM) of tested individuals showed significant antibody elevation against aflatoxin-HSA adduct. The percentage of elevation against the other 11 chemicals ranged from 8% to 22% (IgG) and 13% to 18% (IgM). Performance of serial dilution and inhibition of the chemical–antibody reaction by specific antigens but not by non-specific antigens were indicative of the specificity of these antibodies. Although we lack information about chemical exposure in the tested individuals, detection of antibodies against various protein adducts may indicate chronic exposure to these chemical haptens in about 20% of the tested individuals. Currently the pathological significance of these antibodies in human blood is still unclear, and this protein adduct formation could be one of the mechanisms by which environmental chemicals induce autoimmune reactivity in a significant percentage of the population.
Keywords: haptens, autoimmunity, xenobiotics, chemicals, adducts
Introduction
The list of chemical agents capable of producing or enhancing autoimmune manifestations in an individual with a genetic predisposition is constantly growing (Schiraldi and Monestier, 2009). These low-molecular-weight chemicals that do not directly challenge the immune system are called ’haptens’, a term derived from the Greek meaning ’to fasten‘ which was coined by Landsteiner and Jacobs in 1936 (Landsteiner and Jacobs, 1936). Haptenic chemicals must bind to large carrier molecules such as proteins, lipoproteins, polysaccharides or other large molecules in order to become antigenic. This immunogenic capacity of chemicals depends proportionately on their ability to bind covalently to a high-molecular-weight carrier protein such as human serum albumin (HSA) under physiological conditions (Pichler, 2002; Divkovic et al., 2005).
Reactive organic compounds most often bind covalently through their electrophilic properties that react with protein nucleophilic groups, such as the amino, hydroxyl and thiol groups (Roberts and Lepoittevin, 1998). The binding of these compounds to different tissues frequently leads to sensitization after their inhalation, ingestion, or dermal contact. Examples of such reactive haptenic compounds are formaldehyde, toluene diisocyanate, trimellitic anhydride, phthalic anhydride, some benzene ring-containing compounds, ethylene tetrachloride, ethylene oxide, penicillin and other drugs (Griem et al., 1998). These organic haptens form covalent bonds by binding to a single amino acid side chain.
Sensitizing heavy metal chemicals such as mercury, nickel or cobalt react differently from organic compounds, oxidizing proteins and forming protein metal chelate complexes by undergoing multiple binding with several amino acid side chains of a protein. This interaction between metal ion and amino acids allows the electron-rich ligands to transfer part of their electron density to the positively charged metal ion in order to increase the stability of the metal protein complexes (Griem et al., 1998).
Different groups of chemicals that elicit adverse immune reactions are unable to bind to proteins when entering the body. However, they can bind to various tissue proteins after conversion to reactive metabolites by the hepatic or extrahepatic tissues or cells. These types of chemicals are considered prohaptens, as a pharmaco–toxicological phase is needed for their metabolic conversion into haptens (Liberato et al., 1981; Krasteva et al., 1993; Bourdi et al., 1994; Lecouer et al., 1994, 1996; Andersen et al., 1995; Eliasson and Kenna, 1996; Griem et al., 1998; Merk, 1998). Dihydralazine, halothane and tienilic acid are examples of prohaptens that are catalyzed by liver enzymes into reactive metabolites. These haptenic metabolites immediately manage to bind to proteins with which they come in contact, such as the cytochrome P450 enzyme; this is the enzyme that catalyzes the metabolism of these prohaptens into haptens. This binding of chemical reactive metabolites to a self-tissue enzyme results in the production of autoantibodies directed against the liver enzyme, the chemicals and also the neo-antigen which is the combination of the chemicals and the self-tissue enzyme [Vojdani et al., 1992; Bourdi et al., 1994; Lecouer et al., 1994, 1996; Eliasson and Kenna, 1996; Griem et al., 1998; Merk, 1998).
It does not always happen that the chemicals are metabolized in the liver and subsequently travel to distant extrahepatic sites such as the lung, skin or bone marrow. With some chemicals, the reactive metabolites are formed at the very site where the adverse immune reaction to xenobiotics occurs. One example of an organ with considerable metabolic and immunological capacity is the skin, which is often involved in immune reaction to xenobiotics after dermal application. This metabolic conversion of prohapten to hapten is done by dermal Langerhans cells which contain CYP1A isoenzyme. For example, urushiol, a chemical from poison ivy (Toxicodendron radicans) and poison oak (Toxicodendron diversilobum), can be oxidized in the skin forming quinones first, and then quinine adducts with proteins which can elicit specific T-cell responses against the neo-antigen (Liberato et al., 1981; Krasteva et al., 1993; Kalish et al., 1994).
Monocytes, macrophages, resident Langerhans cells and polymorphonuclear leukocytes are also involved in xenobiotic metabolism. For example, phagocytic cells are involved in the metabolism of procainamide, propylthiouracil and disodium gold from prohaptens to haptens first, and then to hapten-protein adducts in these cells. This hapten and hapten-adduct formation in phagocytic cells elicits a systemic immune reaction against these haptens as well as self-proteins such as nuclear and nucleolar proteins of white blood cells (Kalish et al., 1994; Goebel et al., 1995; Von Schmiedeberg et al., 1996).
Thus, small chemical molecules have the capacity to bind directly to self-proteins or indirectly after hepatic or extrahepatic conversion from prohaptens to haptens, generating hapten–protein adducts. This neoantigen formation can result in a systemic T-cell or antibody immune response against the haptens and self-proteins (Kubicka-Muranyi et al., 1993).
As early as 1965 it was shown that IgM antibodies showed considerably higher activity against various haptens other than IgG (Onoue et al., 1965). Furthermore, in a 1983 study with mice it was shown that repeated exposure to environmental stimulus or DNP-protein conjugate results in a significantly high titer of IgM anti-IgG production, but a much lower production of IgG or IgA against IgG, in a condition characterized as rheumatoid factor (RF) (Nemazee and Sato, 1983).
Haptenated proteins are known to be T-cell independent and can stimulate antibody formation even in the absence of antigen-presenting cells and help from T cells (Dintzis et al., 1989). In response to soluble haptenated molecules, a maximum anti-hapten IgM response occurs at an optimal molecular mass and hapten density independent of the chemical characteristics and molecular conformation of the carrier molecule (Dintzis et al., 1989). In some cases, T cells and their cytokines have been found to enhance a B-cell response to T-cell-independent antigens (Nordin and Schreier, 1982; Alderson et al., 1987). Indeed, researchers have reported that haptenated polymers bring up IgG as well as IgM responses against the haptens (Sharon et al., 1975).
In mice receiving multiple injections of beta-glucuronidase-PEG, IgG antibodies against the native protein and PEG (protein-independent) were produced with similar affinities against the native protein and the conjugate. Anti-beta-glucuronidase IgM antibodies were also produced which exhibit a high affinity against the conjugate, but did not recognize the native protein (Garay and Labaune, 2011).
Based on these mechanisms of action, the aim of this study was to examine the frequency of IgG- and IgM-specific antibody production against many haptenic chemicals with which the general population comes in contact on a daily basis, and subsequently to discuss the possible role these chemicals and corresponding antibodies may play in various autoimmune reactivities. As there was no information available regarding possible chemical exposure in the healthy blood donors, the use of methodologies described in this manuscript is highly recommended in subjects with known exposure to chemicals and their comparison to healthy control subjects.
Materials and Methods
Blood samples from 400 donors (200 males of different ethnicities aged 18 to 65 years old with a median age of 35.5 years and 200 females of different ethnicities aged 18 to 65 years old with a median age of 36.2 years) were purchased from Innovative Research Inc. (Southfield, MI, USA). Blood samples were obtained from healthy individuals who were qualified to donate blood based on a health questionnaire provided by the Food and Drug Administration (FDA). Each individual at the time of blood draw also did not exhibit any health complaints. Prior to shipping, each blood sample was tested according to FDA guidelines for the detection of hepatitis B surface antigen, antibodies to HIV, antibodies to hepatitis C, HIV-1 RNA, hepatitis C RNA and syphilis. No information was obtained about their home or work environment and hence possible exposure to environmental chemicals.
Proteins and Chemicals
HSA, bovine serum albumin (BSA), hemoglobin, formaldehyde, tolylene-2.4-diisocyanate, trimellitic anhydride, p-amino benzoic acid, bisphenol-A, tetrabromobisphenol-A, isopropyl benzoic acid, cyanoethyl benzoic acid, propyl, 4-hydroxy benzoic acid, permethrin, mercury chloride, nickel sulfate, cobalt acetate, cadmium chloride, lead acetate and arsenic oxide were purchased from Sigma Aldrich (St. Louis, MO, USA).
Preparation of Formaldehyde-Human Serum Albumin (F-HSA)
We chose HSA as a model protein as it is naturally abundant in serum and found in most tissues; it is monomeric and its sequence and three-dimensional structure have been well defined; and, it has been identified as a target of hapten binding in vivo (Chipinda et al., 2011; Hettick and Siegel, 2011).
F-HSA was prepared according to the method described by Patterson et al. (1989). Briefly, 1 mg of HSA in phosphate-buffered saline (PBS), pH 7.4, each separately, was exposed to 1 mg of formaldehyde. The mixture was incubated for 30 min at 37 °C and then extensively dialyzed against PBS. The F-HSA was sterilized with a 0.2-µm filter (Millipor Corp., Bedford, MA, USA). Electrophoretic and immunoelectrophoretic comparison of HSA with F-HSA was performed to determine conjugation occurrence. Conjugation was evidenced by altered mobility of F-HSA, when compared with HSA. Similar to formaldehyde, bisphenol-A and tetrabromobisphenol-A were bound to HSA and tested.
Preparation of Tolylene-2.4-Diisocyanate-Human Serum Albumin (TDI-HSA)
This preparation was similar to the methods of Pezzini et al. (1984). According to this method, 1 g of HSA was dissolved in 10 ml of a buffer solution containing potassium chloride (0.05 mol 1–1), sodium borate (0.05 mol 1–1), pH 9.4, and cooled to 4 °C. Dioxane (10 ml) containing 0.15 ml of tolylene-2.4-diisocyanate was then added dropwise while stirring over a period of 3 h, followed by the addition of 2 ml of ethanolamine, centrifugation, dialysis filtration and lysophilization. Similar to F-HSA, the conjugation was confirmed by electrophoresis and the determination of free amino groups present in the conjugate.
Preparation of Trimellitic Anhydride-Human Serum Albumin (TMA-HSA)
To prepare these conjugates, 25 mg of TMA was dissolved in 0.5 ml of dioxane and added dropwise to 25 mg of HSA, dissolved in 5 ml of cold 7% NaHCO3 in water. After stirring for 1 h at 4 °C, the conjugates were dialyzed against four changes of 0.1 M NaHCO3 and one change of buffer. Finally, the mixture was filtered and kept at –20 °C until used (Pien et al., 1988).
Preparation of Benzene Ring-HSA (DNP-HSA) Conjugates
For this preparation, 40 mg each of p-aminobenzoic acid, isopropyl benzoic acid, cyanoethyl benzoic acid, or permethrin was dissolved in 2 ml of 1 N HCl and cooled by immersion in an ice bath. In parallel, 1 g of HSA was dissolved in boric acid 0.16 M sodium chloride [0.15 M buffer pH 9.0 (pH was raised with NaOH)]. The beaker containing the solution of albumins was surrounded by an ice bath on a magnetic stirrer. The solution of diazonium salt was added dropwise, with rapid stirring, to the cold protein solution. After addition of each drop, the pH was readjusted to 9.0 to 9.5 with NaOH. After adding all the solution, the reaction was allowed to continue with slow stirring for at least an hour with further additions of NaOH solution, and maintenance of the pH at the range of 9.0 to 9.5. Unreacted small molecules were removed by extensive dialysis using a molecular cutoff of 8000 Dalton. Similar to preparation of DNP-HSA, DNP was conjugated to BSA as well as hemoglobin and used for specificity studies.
Preparation of Bisphenol-A, Tetrabromobisphenol-A, Tetrachloroethylene, and Parabens-HSA Conjugates
For this preparation, 1 g of HSA was dissolved in 100 ml of 0.01 M PBS, pH 7.4, to which 40 mg of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide HCl was added and kept on the stirrer for 10 min.
In a separate tube, 100 mg of N-hydroxysulfosuccinimide sodium salt was dissolved in 10 ml of distilled water and was added dropwise to the mixture. Next, 100 mg each of bisphenol-A, tetrabromobisphenol-A, tetrachloroethylene or propyl 4-hydroxybenzoate (parabens) was dissolved in 10 ml of 0.01 M PBS pH 7.4; each was separately added dropwise to the protein mixture. The mixtures were kept for 1 h at room temperature and then for 4 h at 4 °C. The unreacted small molecules were removed by dialysis using a molecular cutoff of 8000. Bisphenol-A was also bound to BSA and hemoglobin using methodology similar to that used for specificity studies.
Binding of Mercury, Mixed Heavy Metals to HSA
For this preparation, 100 mg of HSA was dissolved in 9 mL of buffer solution containing potassium chloride and sodium borate 0.05 ml l–1 and the pH was adjusted to 9.4 with 0.1 N NaOH. Then 25 mg of Thimerosal, mercury chloride or another heavy metal was dissolved in 1 ml of buffer and added dropwise to the HSA solution. The reaction mixture was stirred overnight, dialyzed against 0.1 M PBS using tubing with a cutoff of 8000 Dalton.
Conjugation of haptenic chemicals was confirmed by sodium dodecyl sulfate (SDS) gel electrophoresis and a shift in the HSA band (Vojdani et al., 2003). In addition, spectrographic analysis of the conjugate was undertaken. In all cases there was a marked increase in absorption from 230 to 260 nM, which indicated that haptenic chemicals became covalently linked to the HSA or protein carrier.
Detection of Chemical Antibodies by ELISA
HSA or various chemicals bound to HSA at a concentration of 1.0 mg ml–1 were diluted 1:100 in 0.1 M carbonate-bicarbonate buffer, pH 9.5, and 100 µl were added to each well of a polystyrene flat-bottom ELISA plate. Plates were incubated overnight at 4 °C and then washed three times with 200 µl Tris-buffered saline (TBS) containing 0.05% Tween 20, pH 7.4. The non-specific binding of immunoglobulins was prevented by adding 2% BSA in PBS, and incubated overnight at 4 °C.
Plates were washed as described above, and then serum samples diluted 1:100 in 0.1 M PBS Tween containing 2% BSA were added to duplicate wells and incubated for 1 h at room temperature. Plates were washed, and then alkaline phosphatase goat anti-human IgG or IgM F(ab')2 fragments (KPI, Gaithersburg, MD, USA) optimal dilution of 1:400-1:2000 in 1% HSA-TBS was added to each well; plates were incubated for an additional 1 h at room temperature. After washing five times with TBS-Tween buffer, the enzyme reaction was started by adding 100 µl of paranitrophenylphosphate in 0.1 ml of diethanolamine buffer 1 mg ml–1 containing 1 mM MgCl2 and sodium azide, pH 9.8. The reaction was stopped 45 min later with 50 µl of 1 N NaOH. The optical density (OD) was read at 405 nm by means of a microtiter reader. To detect non-specific binding, several control wells contained all reagents except human serum, or wells were coated with HSA followed by the addition of human serum and all other reagents to be used for specificity of the antigen-antibody reaction.
Determination of Specificity of Chemical Antibody Assay
For the determination of the specificity of the antigen–antibody reaction, serial dilutions of sera as well as inhibition studies were conducted.
-
Serial dilution
Different sera with high levels of IgG or IgM antibodies against each of the 12 tested chemicals were diluted serially from 1:100 to 1:3200 and then applied to ELISA plates coated with specific antigens. After completion of the ELISA procedure the recorded ODs were converted to different curves.
-
Inhibition studies
Different sera, each with a very high titer of antibody against a specific chemical, were used in inhibition studies. In different test tubes, 1 ml of 1:100 diluted serum sample was pre-incubated with 100 µl diluent containing 100 µg of HSA, formaldehyde-HSA, isocyanate-HSA, trimellitic anhydride-HSA, p-aminobenzoic acid-HSA, bisphenol-A-HSA, tetrabromobisphenol-A-HSA, mercury-HSA, or other chemicals. After mixing, the tubes were kept for 1 h in a 37 °C water bath followed by 4-h incubation at 4 °C, and then centrifuged at 3000 g for 10 min. The supernatant was used for measuring antibody levels against the various chemicals bound to HSA before and after absorption with different haptens bound to HSA.
-
Measurement of antibodies against chemicals bound to different carriers
Sera from 48 different subjects were tested simultaneously for the presence of IgG antibody against DNP-HSA, DNP-BSA, DNP-hemoglobin, BPA-HSA, BPA-BSA and BPA-hemoglobin.
Coefficients of Intra- and Inter-Assay Variation
Coefficients of intra-assay variation were calculated by running five samples eight times within a single assay. Coefficients of inter-assay variation were determined by measuring the same samples in six consecutive assays. This replicate testing established the validity of the ELISA assays, determined the appropriate dilution with minimal background, and detected serum IgG and IgM against different haptenic chemicals. Coefficients of intra- and inter-assay variations for IgG and IgM against all tested antigens and peptides were less than 15%.
Statistical Analysis
First, to study the pairwise associations between the OD of IgG antibody levels against the haptenic chemicals, we created a scatterplot matrix. Similarly, we also created a scatterplot matrix for visualizing the association between the IgM antibody levels against those chemicals. Next, we carried out a two-way cluster analysis of the Pearson's correlation coefficients between all pairs of the OD of the antibody levels (both IgG and IgM) against those haptenic chemicals. These statistical analyses were performed in the statistical software ‘R’ (http://www.r-project.org/).
Results
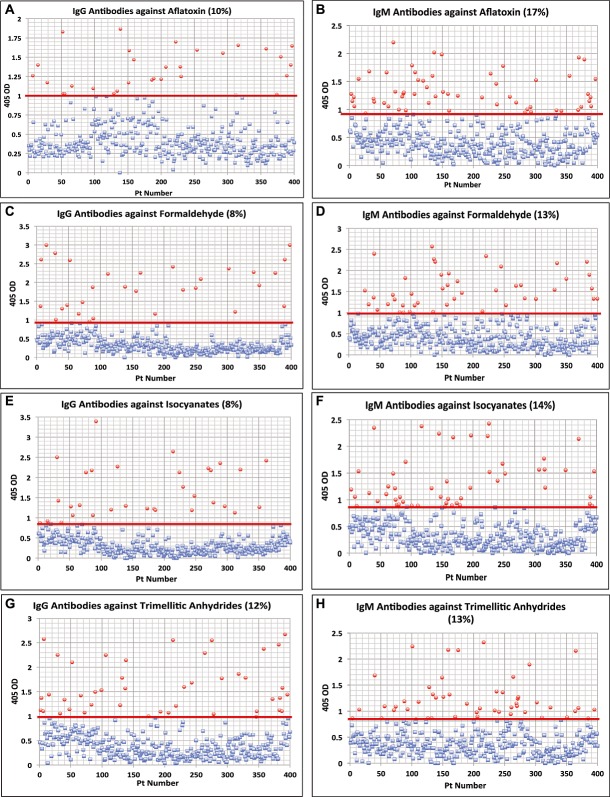
To examine protein adduct and neoantigen formation owing to exposure to very common chemicals, we measured antibodies against various chemicals bound to HSA and compared them with the level of antibodies produced against aflatoxin-HSA, to which many people are exposed via the consumption of different foods, including grains and plants (Wild et al., 1990). Aflatoxin is a known hapten that binds to human tissue, causing an autoimmune response. Levels for antibodies against the specific 11 chemicals that are comparable to antibody levels against aflatoxin would be a valid and significant indication of the possible danger of an autoimmune response. Sera from 400 blood donors were measured for the simultaneous presence of IgG and IgM antibodies against aflatoxin, formaldehyde, isocyanate, trimellitic anhydride, benzene ring compounds, bisphenol-A, tetrabromobisphenol-A, tetrachloroethylene, parabens, pyrethroids, mercury and mixed heavy metals (nickel, cobalt, cadmium, lead and arsenic). Results expressed as OD at 405 nm in the form of scattergrams are shown in Figs. 3. The OD of IgG antibody levels against aflatoxin ranged from 0–1.9 with a mean value of 0.50. At one standard deviation (2SD) above the mean or OD of 0.92, 10% of the samples exhibited IgG antibody against aflatoxin (Fig. 1A). For IgM antibodies at the same dilution of serum both the mean value and the percentage elevation above the mean were different from IgG values. The mean OD of IgM anti-aflatoxin was 0.522 with an elevation of 17%. The percentage of elevation for IgG and IgM combined was 7%.
Figure 3.
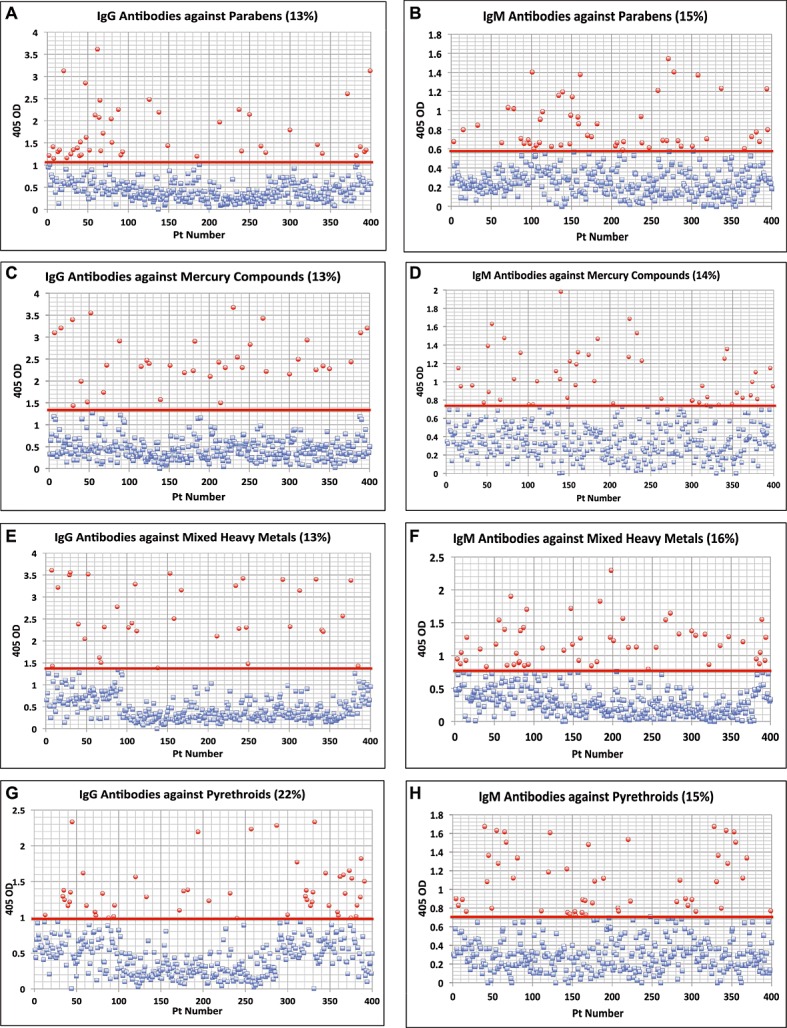
Results for chemical antibodies expressed as optical density (OD) at 405 nm in the form of scattergrams. (A) Parabens IgG. (B) Parabens IgM. (C) Mercury IgG. (D) Mercury IgM. (E) Mixed Heavy Metals IgG. (F) Mixed Heavy Metals IgM. (G) Pyrethroids IgG. (H) Pyrethroids IgM.
Figure 1.
Results for chemical antibodies expressed as optical density (OD) at 405 nm in the form of scattergrams. (A) Aflatoxin IgG. (B) Aflatoxin IgM. (C) Formaldehyde IgG. (D) Formaldehyde IgM. (E) Isocyanates IgG. (F) Isocyanates IgM. (G) Trimellitic & Phthalic Anhydride IgG. (H) Trimellitic & Phthalic Anhydride IgM.
In comparison to aflatoxin, levels of antibodies against formaldehyde were from 0–3.0 OD with a mean of 0.44 for IgG and 0–2.6 for IgM with a mean value of 0.53 (Fig. 1C and D). At 1SD above the mean the percentage elevation of antibodies against formaldehyde IgG was 8%, and for IgM 13%. Regarding tolylene-2.4-diisocyanate antibody, while the ODs varied from 0–3.4 for IgG and 0–2.4 for IgM, the percent elevation of IgG was 8% whereas IgM was 14% (Fig. 1E and F).
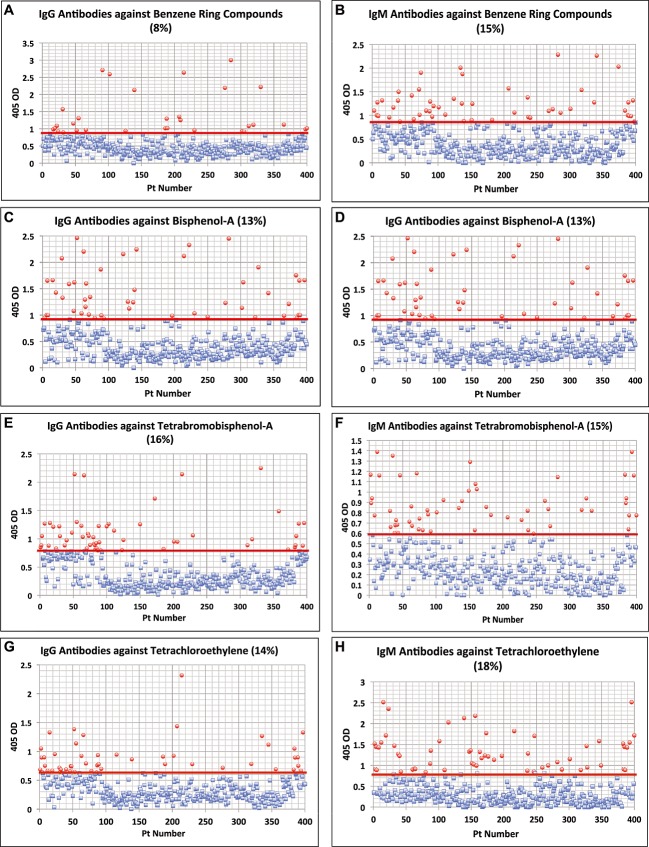
For trimellitic anhydride the OD for IgG antibodies varied from 0.1–2.7 with a mean of 0.50; for IgM it was 0.122.4 with a mean of 0.45. At 1SD above the mean 12% and 13% respectively of the samples exhibited high levels of antibodies (Fig. 1G and H). Similar calculations for antibodies against benzene ring compounds showed that 8% and 15% of specimens showed significant elevations respectively in IgG and IgM antibodies (Fig. 2A and B). Regarding the other chemicals, the percentage of elevation and distribution of IgG and IgM antibodies against bisphenol-A are shown in Fig. 2C and D, against tetrabromobisphenol-A in Fig. 2E and F, against tetrachloroethylene in Fig. 2G and H, parabens in Fig. 3A and B, mercury in Fig. 3C and D, mixed heavy metals in Fig. 3E and F, and pyrethroids in Fig. 3G and H. The percentage of elevation varied from 13% to 22% for IgG and from 14% to 18% for IgM
Figure 2.
Results for chemical antibodies expressed as optical density (OD) at 405 nm in the form of scattergrams. (A) Benzenes IgG. (B) Benzenes IgM. (C) Bisphenol-A IgG. (D) Bisphenol-A IgM. (E) Tetrabromobisphenol-A IgG. (F) Tetrabromobisphenol-A IgM. (G) Tetrachloroethylene IgG. (H) Tetrachloroethylene IgM.
Serial Dilution and Inhibition by Specific and Non-Specific Antigens
Data presented in Figs. 4–6 showed that in proportion to the dilution of sera a significant decline in the ODs or antibody (Ab) titers was observed. To demonstrate inhibition by specific but not by non-specific antigens, Ab-positive samples were evaluated in three different conditions. In the first tube, the Ab-positive sample was diluted with the standard serum diluent containing BSA. In the second tube the patient sample was also diluted with the same serum diluent but in which the appropriate specific chemical had been bound to HSA. In the third tube, the Ab-positive sample was diluted with serum diluent but treated with HSA alone. After comparing the ODs from each of the three tubes, the percentage of inhibition was calculated. The data presented in Tables 1 and 2 show that for IgG the percentage of inhibition by specific antigens was between 67% and 83%, whereas with non-specific antigens it was between 0% and 15% (P < 0.0001). The percentage of inhibition for IgM with specific antigens was between 60% and 79%, whereas with non-specific antigens it was 0–13% (P < 0.0001).
Figure 4.
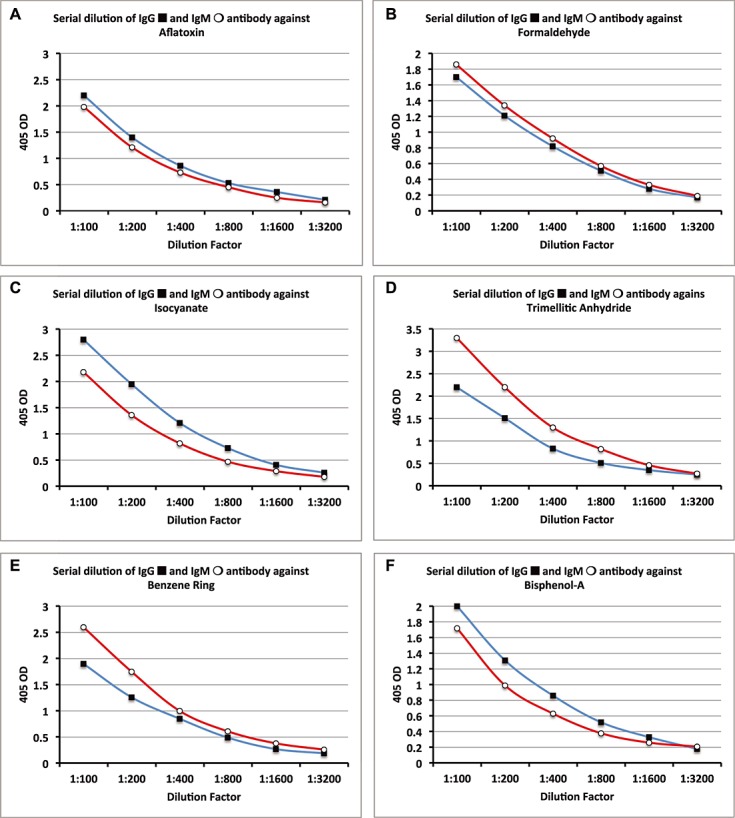
Serial dilutions for chemical antibodies expressed as optical density (OD) at 405 nm in the form of curves. (A) Aflatoxin IgG & IgM. (B) Formaldehyde IgG & IgM. (C) Isocyanates IgG & IgM. (D) Trimellitic Anhydride IgG & IgM. (E) Benzene Ring Compounds IgG & IgM. (F) Bisphenol-A IgG & IgM.
Figure 6.
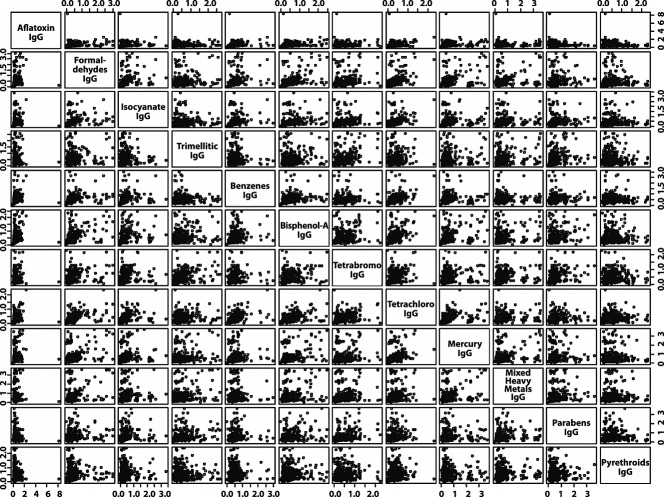
Scatterplot matrix of the optical density (OD) of the IgG antibody levels against the haptenic chemicals.
Table 1.
Inhibition of IgG antibody by specific and non-specific antigens
| Antigens | Baseline OD | OD and % inhibition by specific antigen | OD and % inhibition by non-specific antigen | ||
|---|---|---|---|---|---|
| Aflatoxin | 3.1 | 0.54 | (82.6) | 2.9 | (7) |
| Formaldehyde | 1.9 | 0.62 | (67) | 1.62 | (15) |
| Isocyanate | 2.1 | 0.68 | (68) | 1.82 | (13) |
| Trimellitic Anhydride | 3.3 | 0.81 | (75) | 2.96 | (10) |
| Benzene Ring Compounds | 2.63 | 0.56 | (79) | 2.51 | (5) |
| Bisphenol-A | 3.57 | 0.72 | (80) | 3.62 | (0) |
| Tetrabromobisphenol-A | 2.82 | 0.53 | (81) | 2.74 | (3) |
| Tetrachloroethylene | 2.18 | 0.44 | (80) | 1.97 | (10) |
| Mercury Compound | 2.65 | 0.57 | (79) | 2.57 | (3) |
| Mixed Heavy Metals | 2.95 | 0.83 | (72) | 3.1 | (0) |
| Parabens | 3.3 | 0.89 | (73) | 3.1 | (6) |
| Pyrethroids | 2.8 | 0.78 | (72) | 2.52 | (10) |
OD, optical density.
Table 2.
Inhibition of IgM antibody by specific and non-specific antigens
| Antigens | Baseline OD | OD and % inhibition by specific antigen | OD and % inhibition by non-specific antigen | ||
|---|---|---|---|---|---|
| Aflatoxin | 2.76 | 0.59 | (79) | 2.81 | (0) |
| Formaldehyde | 2.91 | 0.78 | (73) | 2.75 | (5) |
| Isocyanate | 2.15 | 0.63 | (71) | 1.96 | (9) |
| Trimellitic Anhydride | 3.1 | 0.88 | (72) | 2.97 | (4) |
| Benzene Ring Compounds | 3.4 | 1.23 | (64) | 3.16 | (7) |
| Bisphenol-A | 1.93 | 0.49 | (75) | 2.1 | (0) |
| Tetrabromobisphenol-A | 1.68 | .57 | (76) | 1.55 | (8) |
| Tetrachloroethylene | 3.3 | .98 | (70) | 2.95 | (11) |
| Mercury Compound | 2.81 | .95 | (66) | 2.71 | (4) |
| Mixed Heavy Metals | 3.45 | 1.26 | (63) | 3.1 | (10) |
| Parabens | 1.67 | .66 | (60) | 1.72 | (0) |
| Pyrethroids | 2.49 | .72 | (71) | 2.16 | (13) |
OD, optical density.
Figure 5.
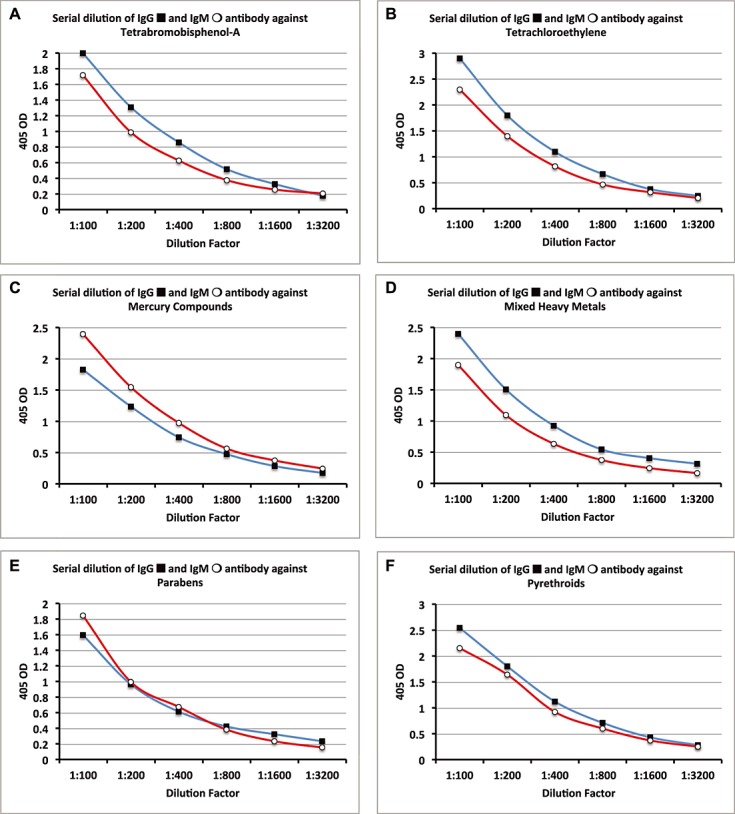
Serial dilutions for chemical antibodies expressed as optical density (OD) at 405 nm in the form of curves. (A) Tetrabromobisphenol-A IgG & IgM. (B) Tetrachloroethylene IgG & IgM. (C) Mercury Compounds IgG & IgM. (D) Mixed Heavy Metals IgG & IgM. (E) Parabens IgG & IgM. (F) Pyrethroids IgG & IgM.
Binding Haptens to Different Carrier Proteins
Results of IgG antibody measured against DNP and BPA bound to different carrier proteins (HSA, BSA and hemoglobin) in duplicate wells are shown in Table 3. These measurements were applied simultaneously to 48 different sera from healthy donors. Only data from 20 different subjects with low, medium and high levels of antibodies are presented.
Table 3.
IgG antibodies against bisphenol-A (BPA) and dinitrophenol (DNP) as haptens bound to three different carrier proteins [human serum albumin (HSA), bovine serum albumin (BSA) and hemoglobin] expressed as optical density (OD) at 405 nm, run in duplicate
| Sample number | BPA | Sample number | DNP | ||||
|---|---|---|---|---|---|---|---|
| BPA-HSA | BPA-BSA | BPA-HEM | DNP-HSA | DNP-BSA | DNP-HEM | ||
| 1 | 0.2, 0.21 | 0.3, 0.28 | 0.36. 0.41 | 1 | 0.31, 0.31 | 0.36, 0.4 | 0.38, 0.41 |
| 2 | 0.19, 0.18 | 0.25, 0.3 | 0.31, 0.35 | 2 | 0.35, 0.38 | 0.4, 0.38 | 0.36, 0.39 |
| 3 | 0.25, 0.27 | 0.28, 0.28 | 0.29, 0.32 | 3 | 0.43, 0.46 | 0.44, 0.47 | 0.53, 0.55 |
| 4 | 0.75, 0.78 | 0.88, 0.99 | 1.1, 1.0 | 4 | 0.7, 0.74 | 0.79, 0.87 | 0.81, 0.76 |
| 5 | 1.0, 1.0 | 1.0, 1.1 | 1.4, 1.3 | 5 | 0.85, 0.98 | 0.9, 1.0 | 1.2, 1.4 |
| 6 | 1.0, 1.1 | 1.2, 1.2 | 1.6, 1.8 | 6 | 1.0, 1.0 | 1.2, 1.1 | 1.5, 1.3 |
| 7 | 1.5, 1.5 | 1.8, 1.7 | 1.9, 1.7 | 7 | 1.0, 1.0 | 1.1, 1.0 | 1.3, 1.4 |
| 8 | 1.9, 1.8 | 2.0, 2.2 | 2.4, 2.2 | 8 | 1.0, 1.1 | 1.2, 1.2 | 1.5, 1.7 |
| 9 | 1.9, 2.0 | 2.0, 2.0 | 2.7, 2.8 | 9 | 1.5, 1.6 | 1.6, 1.7 | 1.9, 2.1 |
| 10 | 2.7, 2.7 | 2.7, 2.6 | 3.1, 3.2 | 10 | 1.9, 2.1 | 2.3, 2.2 | 2.4, 2.6 |
Statistical Analysis
Statistical analyses were performed to study the association between the OD of the antibody levels against the haptenic chemicals. The results of the analyses are shown in Figs. 8. Figure 6 presents the scatterplots of the OD of the IgG antibody levels against the haptenic chemicals. (M,N)-th box in Fig. 6 corresponds to the scatterplot of the variables shown in the M-th and N-th diagonal box. For example, the (1,4)-th box in Fig. 6 presents the scatterplot of the variables in the first and fourth diagonal box, i.e. aflatoxin IgG and trimellitic IgG. Similarly, we interpret all scatterplots in Fig. 6. From these scatterplots we see that some pairs of the IgG antibody levels are strongly associated (e.g. benzenes IgG and bisphenol-A IgG) and some pairs are not (e.g. bisphenol-A IgG and mercury IgG).
Figure 8.
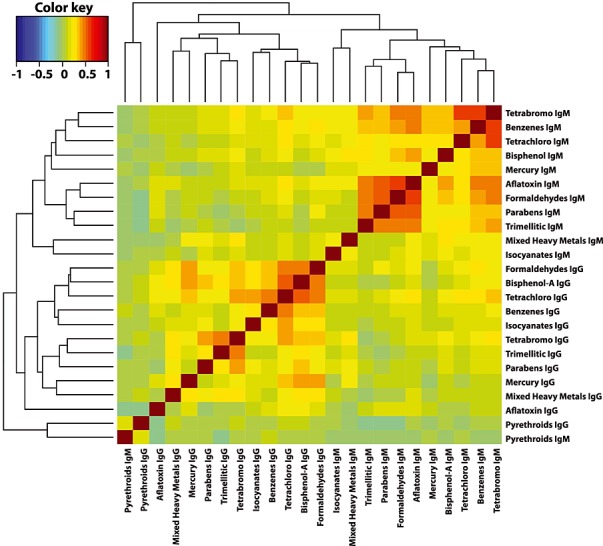
Two-way cluster analysis of the Pearson's correlation coefficients between all pairs of the optical density (OD) of the antibody levels (both IgG and IgM).
Figure 7 presents similar results about the OD of the IgM antibody levels. In this figure, we see that almost all pairs of the IgM antibody levels are somewhat associated. We performed a two-way cluster analysis of the Pearson's correlation coefficients between all pairs of the OD of the antibody levels (both IgG and IgM). Figure 8 presents the results. In Fig. 8, the darker the color the stronger the correlation between the variables in the corresponding row and column. In this figure, we see that all IgG and IgM antibody levels are clustered out perfectly except pyrethroids IgM. We also noted that most IgM antibody levels are strongly correlated among each other, some IgG antibody levels (e.g., bisphenol-A IgG and tetrachloroethylene IgG) are strongly correlated among each other, but the correlations among the IgG and IgM antibodies are weak in most pairs. However, pyrethroid IgM is very weakly correlated with other chemical IgM antibodies.
Figure 7.
Scatterplot matrix of the optical density (OD) of the IgM antibody levels against the haptenic chemicals.
Finally, further statistical analyses of the data were conducted by comparing females with males and age groups of 18–40 to 41–65 years. We performed two sample t-tests for the equality of the means of the two gender groups and two age groups separately. We found that none of these differences were significant. When levels of IgG antibody against each chemical were compared between females with males, the lowest P-value of 0.07 was obtained in the case of mercury. All other P-values were 0.1 or larger.
Discussion
The goal of this study was to measure the levels of IgG and IgM antibodies against various chemicals bound to HSA in so-called ’healthy’ blood donors. We acknowledge the limitations of this study regarding the lack of information on chemical exposure of the donors of the purchased blood samples, which were classified as healthy. We hold to the validity of our results in relation to the stated goal of our study. It was not within the parameters of our study to test the effects of age and gender with variables such as work environment. As such, we hope that the methodologies described in this manuscript will encourage other researchers to apply them to test groups with known exposure to environmental chemicals and a detailed medical history.
The industrialization of modern life is such that residues of industrial chemicals can now be detected in the air, soil, water and food systems in the most remote regions of the world (Loganathan and Kannan, 1994; Simonich and Hites, 1995; Thornton et al., 2002). It has come to the point that all humans are now exposed to synthetic pollutants in their food, drinking water and in the air, as well as in the ordinary things they use in everyday life (Environmental Protection Agency (US), 2000; Wu and Schaum, 2000; Thornton et al., 2002). Thus, even individuals regarded as nominally healthy have some measure, no matter how small, of exposure to environmental chemicals. Some of these chemicals resist metabolism and excretion and therefore accumulate in body tissues. A survey by the U.S. Environmental Protection Agency on the extent of these chemicals in human adipose tissue found some 700 contaminants that were identified and considered likely to be exogenous (Onstot et al., 1987; Thornton et al., 2002). Even human breast milk has been found to be contaminated with toxic chemicals such as parabens, octylphenols, bisphenols, dioxins, furans, dichlorophenylcloroethane (DDT), organochlorine cyclodienes, semi-volatile organohalogens, heavy metals, pesticides, volatile and other organic compounds including phthalates, as well as the highly carcinogenic and immunosuppressive aflatoxins (Jafarian-Dehkordi and Pourradi, 2013; Picone and Paolillo, 2013). Aflatoxins have long been recognized as significant environmental contaminants, and their metabolites react with cellular DNA and proteins to form covalent adducts (IARC, 1993). The detection and quantification of these adducts have been suggested as alternative methods to detect human exposure to these mycotoxins (Muller, 1980; Wild et al., 1992; Rea et al., 2003). Therefore, we wanted to investigate whether or not, as with aflatoxins, antibodies against other chemicals such as bisphenol-A, parabens, pyrethroids and others can be detected in the sera of blood donors. Data presented in Figs. 1–3 show that at 1SD above the mean the percentage of donors that showed elevation of antibodies against aflatoxin was 10% for IgG and 17% for IgM. Similarly, the percentage of individuals who exhibited a significant elevation of antibodies against the eleven other chemicals conjugated to HSA were 8–22% for IgG and 13–18% for IgM. As was described in the Materials and Methods, haptenated proteins induce mainly IgM and some IgG or IgA antibody production in mice; however, in this study we found three subgroups of indivduals, some of whom produced hapten-specific IgG or IgM antibodies, whereas others produced both IgG plus IgM antibody isotypes. This variation in individual immune reactivity against various haptenated proteins deserves further investigation. Detection of antibodies against these chemical adducts is an indication not only of exposure but of neo-antigen formation with tissue macromolecules (Garner, 1985). Figure 6 shows that there are strong associations between some pairs of the IgG antibody levels, whereas Fig. 7 shows that most pairs of the IgM antibody levels are strongly associated. Figure 8 shows that most IgM antibody levels are strongly correlated with each other, some IgG antibody levels (e.g. bisphenol-A IgG and benzene with tetrabromobisphenol-A IgG) are strongly correlated with each other, but the correlations among the IgG and IgM antibodies are weak in most pairs. The strength of the correlation between various pairs of IgG or IgM antibody levels may be related to the chemical structures of these compounds, the nature of the exposure, and/or the body burden (Perger and Szadkowski, 1994). For instance, by comparing the chemical structure of pyrethroids with those of other compounds, a notable difference can be seen. This may explain the relatively weak clustering of pyrethroid antibodies with other compounds bound to HSA. Another reason could be the inter-individual variations in the hepatic metabolism and body burden of pyrethroids.
Figure 9 sets out the commonly accepted schematic representation for the process of metabolism or detoxification of chemicals, excretion or adduct formation between haptenic chemicals and macromolecules. In this regard there is significant inter-individual variation in the effectiveness of the body's defensive responses and detoxification of chemicals (Perera, 1996).
Figure 9.
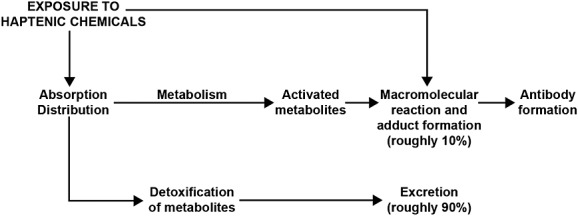
Simplified schematic for the metabolism of chemical compounds.
The rate of metabolism of chemicals or macromolecular reaction depends on a variety of factors: genetics, gender, age, nutritional status, health or disease can all affect the rate at which the reaction takes place (Garner, 1985). Based on this mechanism of action, many organic chemical carcinogens have been found to bind to cellular macromolecules after administration to animals. Consequently, measuring levels of these macromolecule adducts has been used as a risk assessment procedure in animal models (Sanborn et al., 1998). However, it is obviously inconceivable to inject large doses of radiolabeled compounds into human beings and then remove their tissues and organs to measure macromolecular binding levels. Therefore, the best choice would be to measure antibodies against macromolecule adducts in human blood, which is what was done in this study.
Starting around 25 years ago, it had been demonstrated that antibodies against albumin conjugates of formaldehyde, tolylene-2.4-diisocyanate, trimellitic anhydride and benzene ring compounds were found in individuals with long-term exposure to these chemicals (Thrasher et al., 1987, 1989, 1990; Wisnewski et al., 2004; Wisnewski, 2007). In a later study in our own lab (Vojdani et al., 1992) we demonstrated immune alteration and production of antibodies against various low-molecular-weight chemicals in 289 patients with a medical history of exposure for over 10 years to a mixture of chemicals through inhalation and skin contact with no protective devices. We concluded that the detection of IgG and/or IgM antibodies against formaldehyde, trimellitic anhydride, phthalic anhydride and benzene ring compounds is an indication of chronic exposure to these chemical haptens. Antibodies can be exclusively specific for their antigens and for many years have been used in clinical chemistry to measure low concentrations of analyzed substances in human body fluid. This specificity of antibodies detected against the 12 different chemicals used in this study was demonstrated by conducting serial dilution of serum and inhibition studies using specific and non-specific antigens (Figs. 1 and 2; Tables 3). The best demonstration of specificity for these chemical antibodies detected in the blood of healthy subjects was the decline in antibody titer in proportion to the dilution of sera and a significant inhibition in antibody level by chemicals bound to HSA but not by HSA or BSA alone. Furthermore, the binding of two different haptens (DNP and BPA) to three different carriers such as HSA, BSA and hemoglobin and the resultant demonstration of similar immune reactivity against two different haptens bound to various carriers is a further indication of antibody specificity against the hapten but not the carrier proteins (Table 3). As we indicated earlier (Vojdani et al., 1992), the pathological significance of these antibodies in human blood is still unclear. However, as the underlying mechanisms by which xenobiotics and their neo-antigen formation induce allergic and autoimmune reactions have been discussed in many research and review articles (Griem et al., 1998; McFadden et al., 2009; Wisnewski et al., 2010; Chipinda et al., 2011), the chemical reactivity kinetic studies suggest that the rate of protein binding and neo-antigen formation is the major determinant of allergic and autoimmune reactivities (Wild et al., 1990; Anitha et al., 2011). Indeed, based on this mechanism of action, exposure to various xenobiotics including heavy metals, organic solvents, halogenated aromatics, hydrocarbons, cosmetics, pesticides, drugs and others have been associated with autoimmune diseases (Shoenfeld and Isenberg, 1989; Pollard et al., 1997, 2005, 2010; Sanborn et al., 1998; Layland et al., 2004; Chen and Mikecz, 2005; Amital et al., 2006; Guzzi et al., 2008; Wang et al., 2008; Schiraldi and Monestier, 2009; Selmi and Gershwin, 2009; Reeves et al., 2009; Selmi et al., 2011; Barragan-Martinez et al., 2012; Pollard, 2012; Leung et al., 2013; Perricone et al., 2013). The titles of some of these articles, such as ’How can a chemical element elicit complex immunopathology? Lessons from mercury-induced autoimmunity‘ by Schiraldi and Monestier (2009), and ’Organic solvents as risk factors for autoimmune diseases‘ by Barragan-Martinez et al., (2012), or ’Toxicology of autoimmune diseases‘ by Pollard et al., (2010) are the best indication of progress made since our 1987 publication (Thrasher et al., 1987).
In spite of this elucidation of the mechanism of action of chemicals and their association with autoimmune reactivities, little is known about the reactivity of the above-mentioned chemicals and many others that, at very low doses, are known to be endocrine disruptors (Kavlock et al., 1996; Sax, 2010; Yang et al., 2011) to which the population at large are exposed on a daily basis. In fact, according to the 2003-2004 National Health and Nutrition Examination Survey conducted by the Centers for Disease Control and Prevention (CDC, 2004), detectable levels of BPA were found in 93% of 2517 urine samples from tested subjects 6 years and older. We still know little about metabolites and the body burden of many chemicals in humans, particularly in children, women and ethnic groups who are more exposed to many haptenic chemicals (Perera, 1996). For this reason, in this study an attempt was made to measure the degree of neo-antigen formation by these chemicals based on the specific antibodies produced against the chemicals bound to carrier proteins. This could be one of the mechanisms by which chemical as environmental triggers induce autoimmune reactivities that could be followed by autoimmune disease.
In spite of numerous research efforts, the etiology of autoimmune disease remains largely unknown. The current hypothesis states that autoimmunity results from the impact of environmental factors such as chemicals, infections and genetic background (Selmi et al., 2012).
We hope that the results of this study will encourage further research of an expanded nature with subjects that have more detailed information, such as confirmed history of chemical exposure and work environments as well as age and gender. As antibodies appear in the blood many years before the onset of autoimmune disease (Vojdani, 2008), the methodology described here for the detection of antibodies against chemical adducts in so-called healthy blood donors can be extended in the future to groups at high risk for autoimmune diseases; these methods can be used to guide these susceptible individuals toward lifestyles or protocols that involve avoiding exposure to the chemicals discussed in this manuscript, thus decreasing their risks of developing autoimmune disorders. More effort and resources need to be applied towards this research in connecting the level of macromolecular adducts and related antibodies formed against them to the risk of autoimmune disease, which afflicts 7–10% of the world population (National Institutes of Health, 2005). In addition, the method described should enable scientists to assess the levels of antibodies to these chemicals in chemically exposed individuals, and to learn the importance of chemicals in the etiology of autoimmune disorders, particularly in a subgroup of individuals who are more susceptible to autoimmune diseases.
Acknowledgments
Acknowledgment is given to Joel Bautista for the preparation of this manuscript for publication as well as for the creation of the tables and figures. Other figures were provided by Partha Mukherjee, who also performed the statistical analysis.
Disclosure
Dr Aristo Vojdani is the CEO of Immunosciences Lab., Inc. Dr Datis Kharrazian and Dr Partha Mukherjee have nothing to disclose.
Conflict of Interest
The Authors did not report any conflict of interest.
References
- Amital H, Gershwin ME, Shoenfeld Y. Reshaping the mosaic of autoimmunity. Semin. Arthritis Rheum. 2006;35:341–343. doi: 10.1016/j.semarthrit.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Anitha S, Waliyar F, Reddy AS, Rao R, Rao R, Kumar PL. Development of a simple enzyme-linked immunosorbent assay for quantitative estimation of aflatoxin B1 albumin adduct in humans. Curr. Sci. 2011;101:844–846. [Google Scholar]
- Alderson MR, Pike BL, Nossal GJV. Effects of antigens and lymphocytes on early activation of single hapten-specific B lymphocytes. J. Immunol. 1987;138:1056–1063. [PubMed] [Google Scholar]
- Andersen C, Hehr A, Robbin R, Hasan R, Athar M, Mukhtar H, Elmats CA. Metabolic requirements for induction of contact hypersensitivity to immunotoxic polycyclic hydrocarbons. J. Immunol. 1995;155:3530–3537. [PubMed] [Google Scholar]
- Barragan-Martinez C, Speck-Hernandez CA, Montoya-Ortiz G, Mantilla RD, Anaya JM, Rojas-Villarraga A. Organic solvents as risk factor for autoimmune disease: a systematic review and meta-analysis. PLoS One. 2012;7:e51506. doi: 10.1371/journal.pone.0051506. doi: 10.1371/journal.pone.0051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdi M, Tinel M, Beaune PH, Pessayre D. Interactions of dihydralazine with cytochromes P4501A: a possible explanation for the appearance of anti-cytochrome P4501A2 autoantibodies. Mol. Pharmacol. 1994;45:1287–1295. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES) Hyattsville, MD: Centers for Disease Control and Prevention; 2004. 2003. [Google Scholar]
- Chen M, Mikecz A. Xenobiotic-induced recruitment of autoantigens to nuclear proteasomes suggests a role for altered antigen processing in scleroderma. Ann. N. Y. Acad. Sci. 2005;1051:382–389. doi: 10.1196/annals.1361.080. [DOI] [PubMed] [Google Scholar]
- Chipinda I, Hettick JM, Siegel PD. Haptenation: chemical reactivity and protein binding. J. Allergy (Cairo) 2011;2011:839682. doi: 10.1155/2011/839682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dintzis RZ, Okajima M, Middleton MH, Greene G, Dintzis HM. The immunogenicity of soluble haptenated polymers is determined by molecular mass and hapten valence. J. Immunol. 1989;143:1239–1244. [PubMed] [Google Scholar]
- Divkovic M, Pease CK, Geberick GF, Basketter DA. Hapten-protein binding: from theory to practical application in the in vitro prediction of skin sensitization. Contact Dermatitis. 2005;53:189–200. doi: 10.1111/j.0105-1873.2005.00683.x. [DOI] [PubMed] [Google Scholar]
- Eliasson E, Kenna JG. Cytochrome P450 2E1 is a cell surface autoantigen in halothane hepatitis. Mol. Pharmacol. 1996;50:573–582. [PubMed] [Google Scholar]
- Environmental Protection Agency (US), National Center for Environmental Assessment. Exposure and Human Health Reassessment of 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) and Related Compounds, Part III (SAB Review Draft) Washington, DC: Environmental Protection Agency (US), National Center for Environmental Assessment; 2000. [Google Scholar]
- Garay RP, Labaune JP. Immunogenicity of polyethylene glycol (PEG) Open Conf. Proc. J. 2011;2:104–107. [Google Scholar]
- Garner RC. Assessment of carcinogen exposure in man. Carcinogenesis. 1985;6:1071–1078. doi: 10.1093/carcin/6.8.1071. [DOI] [PubMed] [Google Scholar]
- Goebel C, Kubicka-Muranyi M, Tonn T, Gonzalez J, Gleichmann E. Phagocytes render chemicals immunogenic: oxidation of gold(I) to the T cell-sensitizing gold(III) metabolite generated by mononuclear phagocytes. Arch. Toxicol. 1995;69:450–459. doi: 10.1007/s002040050198. [DOI] [PubMed] [Google Scholar]
- Griem P, Wulferink M, Sachs B, Gonzalez JB, Gleichmann E. Allergic and autoimmune reactions to xenobiotics: how do they arise? Immunol. Today. 1998;19:133–141. doi: 10.1016/s0167-5699(97)01219-x. [DOI] [PubMed] [Google Scholar]
- Guzzi G, Fogazzi GB, Cantu M, Minoia C, Ronchi A, Pigatto PD, Severi G. Mercury dental amalgam and renal autoimmunity. J. Environ. Pathol. Toxicol. Oncol. 2008;27:147–155. doi: 10.1615/jenvironpatholtoxicoloncol.v27.i2.70. [DOI] [PubMed] [Google Scholar]
- Hettick JM, Siegel PD. Determination of the toluene diisocyanate binding sites on human serum albumin by tandem mass spectrometry. Anal. Biochem. 2011;414:232–238. doi: 10.1016/j.ab.2011.03.035. [DOI] [PubMed] [Google Scholar]
- IARC. 1993;56 Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxin. International Agency for Research on Cancer (IARC Monographs on the Evaluation of Carcinogenic Risks of Chemicals to Humans, Volume. Lyon, France. [Google Scholar]
- Jafarian-Dehkordi A, Pourradi N. Aflatoxin M1 contamination of human breast milk in Isfahan. Iran. Adv. Biomed. Res. 2013;2:86. doi: 10.4103/2277-9175.122503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalish RS, Wood JA, LaPorte A. Processing of urushiol (poison ivy) hapten by both endogenous and exogenous pathways for presentation to T cells in vitro. J. Clin. Invest. 1994;93:2039–2047. doi: 10.1172/JCI117198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock RJ, Daston GP, DeRosa C, Fenner-Crisp P, Gray LE, Kaattari S, Lucier G, Luster M, Mac MJ, Maczka C, Miller R, Moore J, Rolland R, Scott G, Sheehan DM, Sinks T, Tilson HA. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a U.S. EPA-sponsored workshop. Environ. Health Perspect. 1996;104(Suppl 4):715–740. doi: 10.1289/ehp.96104s4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasteva M, Nicolas JF, Chabeau G, Garrigue JL, Bour H, Thivolet J, Schmitt D. Dissociation of allergenic and immunogenic functions in contact sensitivity to para-phenylenediamine. Int. Arch. Allergy Immunol. 1993;102:200–204. doi: 10.1159/000236573. [DOI] [PubMed] [Google Scholar]
- Kubicka-Muranyi M, Goebels R, Goebel C, Uetrecht J, Gleichmann E. T lymphocytes ignore procainamide, but respond to its reactive metabolites in peritoneal cells: demonstration by the adoptive transfer popliteal lymph node assay. Toxicol. Appl. Pharmacol. 1993;122:88–94. doi: 10.1006/taap.1993.1175. [DOI] [PubMed] [Google Scholar]
- Layland LE, Wulferink M, Dierkes S, Gleichmann E. Drug-induced autoantibody formation in mice: triggering by primed CD4 + CD25- T cells, prevention by primed CD4 + CD25+ T cells. Eur. J. Immunol. 2004;34:36–46. doi: 10.1002/eji.200324406. [DOI] [PubMed] [Google Scholar]
- Landsteiner K, Jacobs J. Studies on the sensitization of animals with simple chemical compounds. J. Exp. Med. 1936;64:625–639. doi: 10.1084/jem.64.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecouer S, Bonierbale E, Challine D, Gautier JC, Valadon P, Dansette PM, Catinot R, Ballet F, Mansuy D, Beaune PH. Specificity of in vitro covalent binding of tienilic acid metabolites to human liver microsomes in relationship to the type of hepatotoxicity: comparison with two directly hepatotoxic drugs. Chem. Res. Toxicol. 1994;7:434–442. doi: 10.1021/tx00039a023. [DOI] [PubMed] [Google Scholar]
- Lecouer S, Andre C, Beaune PH. Tienilic acid-induced autoimmune hepatitis: anti-liver and-kidney microsomal type 2 autoantibodies recognize a three-site conformational epitope on cytochrome P4502C9. Mol. Pharmacol. 1996;50:326–333. [PubMed] [Google Scholar]
- Leung PS, Wang J, Naiyanetr P, Kenny TP, Lam KS, Kurth MJ, Gershwin ME. Environment and primary biliary cirrhosis: electrophilic drugs and the induction of AM. J. Autoimmun. 2013;41:79–86. doi: 10.1016/j.jaut.2012.12.007. doi: 10.1016/j.jaut.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberato DJ, Byers VS, Dennick RG, Castagnoli N., Jr Regiospecific attack of nitrogen and sulfur nucleophiles on quinones derived from poison oak/ivy catechols (urushiols) and analogues as models for urushiol-protein conjugate formation. J. Med. Chem. 1981;34:28–33. doi: 10.1021/jm00133a007. [DOI] [PubMed] [Google Scholar]
- Loganathan BG, Kannan K. Global organochlorine contamination trends: an overview. Ambio. 1994;23:187–191. [Google Scholar]
- Merk HF. Skin metabolism. In: Lepoittevin JP, Basketter DA, Goosens A, Karlberg AT, editors. Allergic Contact Dermatitis. Berlin: Springer-Verlag; 1998. pp. 68–80. [Google Scholar]
- McFadden JP, White JML, Basketter DA, Kimber I. Does hapten exposure predispose to atopic disease? The hapten atopy hypothesis. Trends Immunol. 2009;30:67–74. doi: 10.1016/j.it.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Muller R. Calculation of average antibody affinity in anti-hapten sera from data obtained by competitive radioimmunoassay. J. Immunol. Methods. 1980;34:345–352. doi: 10.1016/0022-1759(80)90107-6. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. 2005. March Progress in Autoimmune Diseases Research. Report of The Autoimmmune Diseases Coordinating Committee.
- Nemazee DA, Sato VL. Induction of rheumatoid antibodies in the mouse: regulated production of autoantibody in the secondary humoral response. J. Exp. Med. 1983;158:529–545. doi: 10.1084/jem.158.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin AA, Schreier MH. T cell control of the antibody response to the T-independent antigen, DAGG-Ficoll. J. Immunol. 1982;129:557–562. [PubMed] [Google Scholar]
- Onoue K, Tanigaki N, Yagi Y, Pressman D. IgM and IgG anti-hapten antibody: hemolytic, hemagglutinating and precipitating activity. Exp. Biol. Med. (Maywood) 1965;120:340–346. doi: 10.3181/00379727-120-30531. [DOI] [PubMed] [Google Scholar]
- Onstot J, Ayling R, Stanley J. Characterization of HRGC/MS unidentified peaks from the analysis of human adipose tissue, Volume 1: technical approach. Washington (DC): US Environmental Protection Agency Office of Toxic Substances; 1987. (560/6-87-002a) [Google Scholar]
- Patterson R, Dykewicz MS, Evans R, Grammer LC, Greenberger PA, Harris KE, Lawrence ID, Pruzansky JJ, Roberts M, Shaughnessy MA, et al. IgG antibody against formaldehyde human serum proteins: a comparison with other IgG antibodies against inhalant proteins and reactive chemicals. J. Allergy Clin. Immunol. 1989;84:359–366. doi: 10.1016/0091-6749(89)90421-1. [DOI] [PubMed] [Google Scholar]
- Perera FP. Uncovering new clues to cancer risk. Sci. Am. 1996;274:54–55. doi: 10.1038/scientificamerican0596-54. 58–62. [DOI] [PubMed] [Google Scholar]
- Perger G, Szadkowski D. Toxicology of pyrethroids and their relevance to human health. Ann. Agric. Environ. Med. 1994;1:11–17. [Google Scholar]
- Perricone C, Agmon-Levin N, Shoenfeld Y. Novel pebbles in the mosaic of autoimmunity. BMC Med. 2013;11:101. doi: 10.1186/1741-7015-11-101. doi: 10.1186/1741-7015-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzini A, Rivera A, Paggiaro P. Specific IgE antibodies in twenty-eight workers with diisocyanate-induced bronchial asthma. Clin. Allergy. 1984;14:453–461. doi: 10.1111/j.1365-2222.1984.tb02229.x. [DOI] [PubMed] [Google Scholar]
- Pichler WJ. Pharmacological interaction of drugs withantigen-specific immune receptors: the p-I concept. Curr. Opin. Allergy Clin. Immunol. 2002;2:301–305. doi: 10.1097/00130832-200208000-00003. [DOI] [PubMed] [Google Scholar]
- Picone S, Paolillo P. Chemical contaminants in breast milk. Early Human Dev. 2013;89(Suppl. 4):S117–S118. [Google Scholar]
- Pien L, Zeiss R, Leach C, Hatoum NS, Levitz D, Garvin PJ, et al. Antibody response to trimellityl hemoglobin in trimellitic anhydride-induced lung injury. J. Allergy Clin. Immunol. 1988;82:1098–1103. doi: 10.1016/0091-6749(88)90149-2. [DOI] [PubMed] [Google Scholar]
- Pollard KM. Gender differences in autoimmunity associated with exposure to environmental factors. J. Autoimmun. 2012;38:J177–J186. doi: 10.1016/j.jaut.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard KM, Lee DK, Casiano CA, Bluthner M, Johnston MM, Tan EM. The autoimmunity-inducing xenobiotic mercury interacts with the autoantigen fibrillarin and modifies its molecular and antigenic properties. J. Immunol. 1997;158:3521–3528. [PubMed] [Google Scholar]
- Pollard KM, Hultman P, Kono DH. Immunology and genetics of induced systemic autoimmunity. Autoimmun. Rev. 2005;4:L282–288. doi: 10.1016/j.autrev.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Pollard KM, Hultman P, Kono DH. Toxicology of autoimmune diseases. Chem. Res. Toxicol. 2010;23:455–466. doi: 10.1021/tx9003787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea JW, Didriksen N, Simon TR, Pan Y, Fenyves EJ, Griffiths B. Effects of toxic exposure to molds and mycotoxins in building-related illnesses. Arch. Environ. Health. 2003;58:12–19. doi: 10.1080/00039896.2003.11879140. [DOI] [PubMed] [Google Scholar]
- Reeves WH, Lee PY, Weinstein JS, Satoh M, Lu L. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 2009;30:455–464. doi: 10.1016/j.it.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DW, Lepoittevin JP. Hapten-protein interactions. In: Lepoittevin JP, Basketter DA, Goosens A, Karlberg AT, editors. Allergic Contact Dermatitis. Berlin: Springer-Verlag; 1998. pp. 81–111. [Google Scholar]
- Sanborn JR, Gee SJ, Gilman SD, Sugawara Y, Jones AD, Rogers J, et al. Hapten synthesis and antibody development for polychlorinated dibenzo-p-dioxin immunoassays. J. Agric. Food Chem. 1998;46:2407–2416. [Google Scholar]
- Sax L. Polyethylene terephthalate may yield endocrine disruptors. Environ. Health Perspect. 2010;118:445–448. doi: 10.1289/ehp.0901253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiraldi M, Monestier M. How can a chemical element elicit complex immunopathology? Lessons from mercury-induced autoimmunity. Trends Immunol. 2009;30:502–509. doi: 10.1016/j.it.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Selmi C, Gershwin ME. The role of environmental factors in primary biliary cirrhosis. Trends Immunol. 2009;30:415–420. doi: 10.1016/j.it.2009.05.006. doi: 10.1016/j.it.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Selmi C, Leung PS, Sherr DH, Diaz M, Nyland JF, Monestier M, Rose NR, Gershwin ME. Mechanisms of environmental influence on human autoimmunity: a National Institute of Environmental Health Sciences expert panel workshop. J. Autoimmunity. 2012;39:272–284. doi: 10.1016/j.jaut.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Selmi C, Maria Papini A, Pugliese P, Claudia Alcaro M, Gershwin ME. Environmental pathways to autoimmune disease: the cases of primary biliary cirrhosis and multiple sclerosis. Arch. Med. Sci. 2011;7:368–380. doi: 10.5114/aoms.2011.23398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon R, McMaster PRB, Kask AM, Owens JD, Paul WE. DNP-Lys-Ficoll: a T-independent antigen which elicits both IgM and IgG anti-DNP antibody-secreting cells. J. Immunol. 1975;114:1585–1589. [PubMed] [Google Scholar]
- Shoenfeld Y, Isenberg DA. The mosaic of autoimmunity. Immunol. Today. 1989;10:123–126. doi: 10.1016/0167-5699(89)90245-4. [DOI] [PubMed] [Google Scholar]
- Simonich SL, Hites RA. Global distribution of persistent organochlorine compounds. Science. 1995;269:1851–1854. doi: 10.1126/science.7569923. [DOI] [PubMed] [Google Scholar]
- Thrasher JD, Broughton A, Madison R. Immune activation and autoantibodies in humans with long-term inhalation exposure to formaldehyde. Arch. Environ. Health. 1990;45:217–223. doi: 10.1080/00039896.1990.9940805. [DOI] [PubMed] [Google Scholar]
- Thrasher JD, Madison R, Broughton A, Gard Z. Building-related illness and antibodies to albumin conjugates of formaldehyde, toluene dissocyanate, and trimellitic anhydride. Am. J. Industrial Med. 1989;15:187–195. doi: 10.1002/ajim.4700150207. [DOI] [PubMed] [Google Scholar]
- Thrasher JD, Wojdani A, Heuser G, Cheung G. Evidence for formaldehyde antibodies and altered cellular immunity in subjects exposed to formaldehyde in mobile homes – a brief communication. Arch. Environ. Health. 1987;42:347–350. doi: 10.1080/00039896.1987.9934357. [DOI] [PubMed] [Google Scholar]
- Thornton JW, McCally M, Houlihan J. Biomonitoring of industrial pollutants: health and policy implications of the chemical body burden. Public Health Rep. 2002;117:315–323. doi: 10.1016/S0033-3549(04)50167-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojdani A. Antibodies as predictors of autoimmune diseases and cancer. Expert Opin. Med. Diagn. 2008;2:593–605. doi: 10.1517/17530059.2.6.593. [DOI] [PubMed] [Google Scholar]
- Vojdani A, Ghoneum M, Brautbar N. Immune alteration associated with exposure to toxic chemicals. Toxicol. Ind. Health. 1992;8:239–254. [PubMed] [Google Scholar]
- Vojdani A, Pangborn JB, Vojdani E, Cooper EL. Infections, toxic chemicals and dietary peptides binding to lymphocyte receptors and tissue enzymes are major instigators of autoimmunity in autism. Int. J. Immunopathol. Pharmacol. 2003;16:189–199. doi: 10.1177/039463200301600302. [DOI] [PubMed] [Google Scholar]
- Von Schmiedeberg S, Hanten U, Goebel C, Schuppe HC, Uetrecht J, Gleuchmann E. T cells ignore the parent drug propylthiouracil but are sensitized to a reactive metabolite generated in vivo. Clin. Immunol. Immunopathol. 1996;80:162–170. doi: 10.1006/clin.1996.0110. [DOI] [PubMed] [Google Scholar]
- Wang J, Kay AB, Fletcher J, Formica MK, McAlindon TE. Is lipstick associated with the development of systemic lupus erythematosus (SLE)? Clin. Rheumatol. 2008;27:1183–1187. doi: 10.1007/s10067-008-0937-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild CP, Hudson GJ, Sabbioni G, Chapot B, Hall AJ, Wogan GN, Whittle H, Montesano R, Groopman JD. Dietary intake of aflatoxins and the level of albumin-bound aflatoxin in peripheral blood in The Gambia, West Africa. Cancer Epidemiol. Biomark Prev. 1992;1:228–234. [PubMed] [Google Scholar]
- Wild CP, Jiang YZ, Sabbioni G, Chapot B, Montesano R. Evaluation of methods for quantitation of aflatoxin-albumin adducts and their application to human exposure assessment. Cancer Res. 1990;50:245–251. [PubMed] [Google Scholar]
- Wisnewski AV, Stowe MH, Cartier A, Liu Q, Liu J, Chen L, Redlich CA. Isocyanate vapor-induced antigenicity of human albumin. J. Allergy Clin. Immunol. 2004;113:1178–1184. doi: 10.1016/j.jaci.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Wisnewski AV. Developments in laboratory diagnostics for isocyanate asthma. Curr. Opin. Allergy Clin. Immunol. 2007;7:138–145. doi: 10.1097/ACI.0b013e3280895d22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnewski AV, Liu J, Redlich CA. Antigenic changes in human albumin caused by reactivity with the occupational allergen diphenylmethane diisocyanate. Anal. Biochem. 2010;400:251–258. doi: 10.1016/j.ab.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Schaum J. Exposure assessment of trichloroethylene. Environ. Health Perspect. 2000;108(S2):359–363. doi: 10.1289/ehp.00108s2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CZ, Yaniger SI, Jordan VC, Klein DJ, Bittner GD. Most plastic products releaqse estrogenic chemicals: a potential health problem can be solved. Environ. Health Perspect. 2011;119:989–996. doi: 10.1289/ehp.1003220. doi: 10.1289/ehp.1003220. [DOI] [PMC free article] [PubMed] [Google Scholar]